Self-Healing Thermoplastic Polyurethane Linked via Host-Guest Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
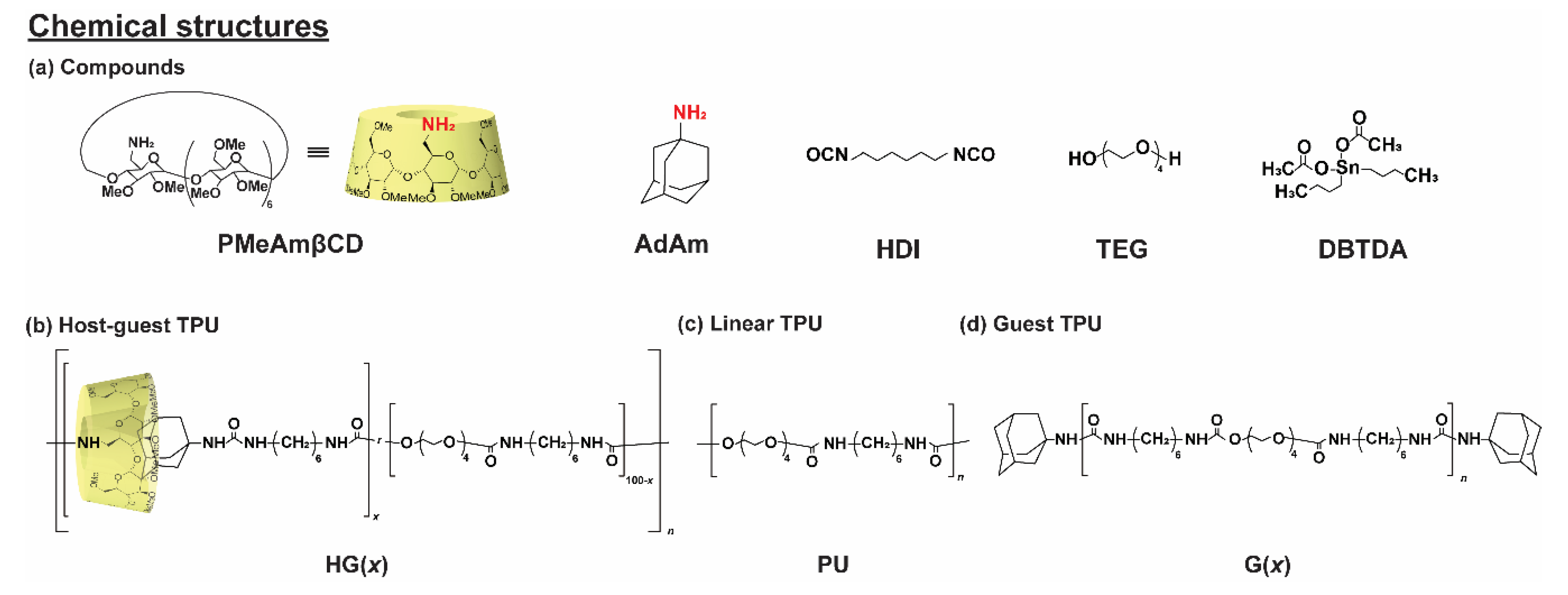
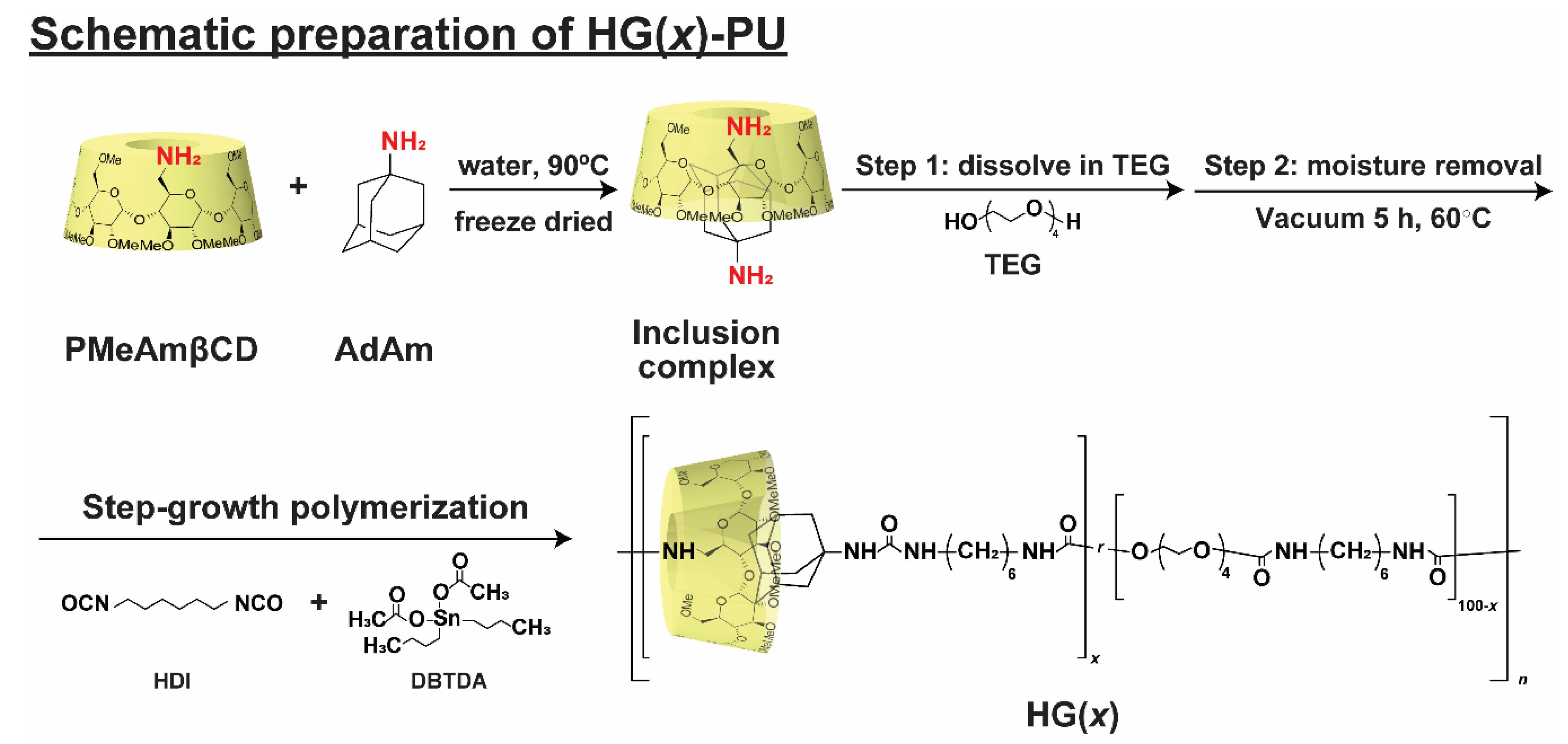
2.3. Preparation of the Host-Guest Thermoplastic Polyurethanes (HG(x))
3. Results and Discussion
3.1. Preparation of Thermoplastic Polyurethane Linked via Host-Guest Interactions
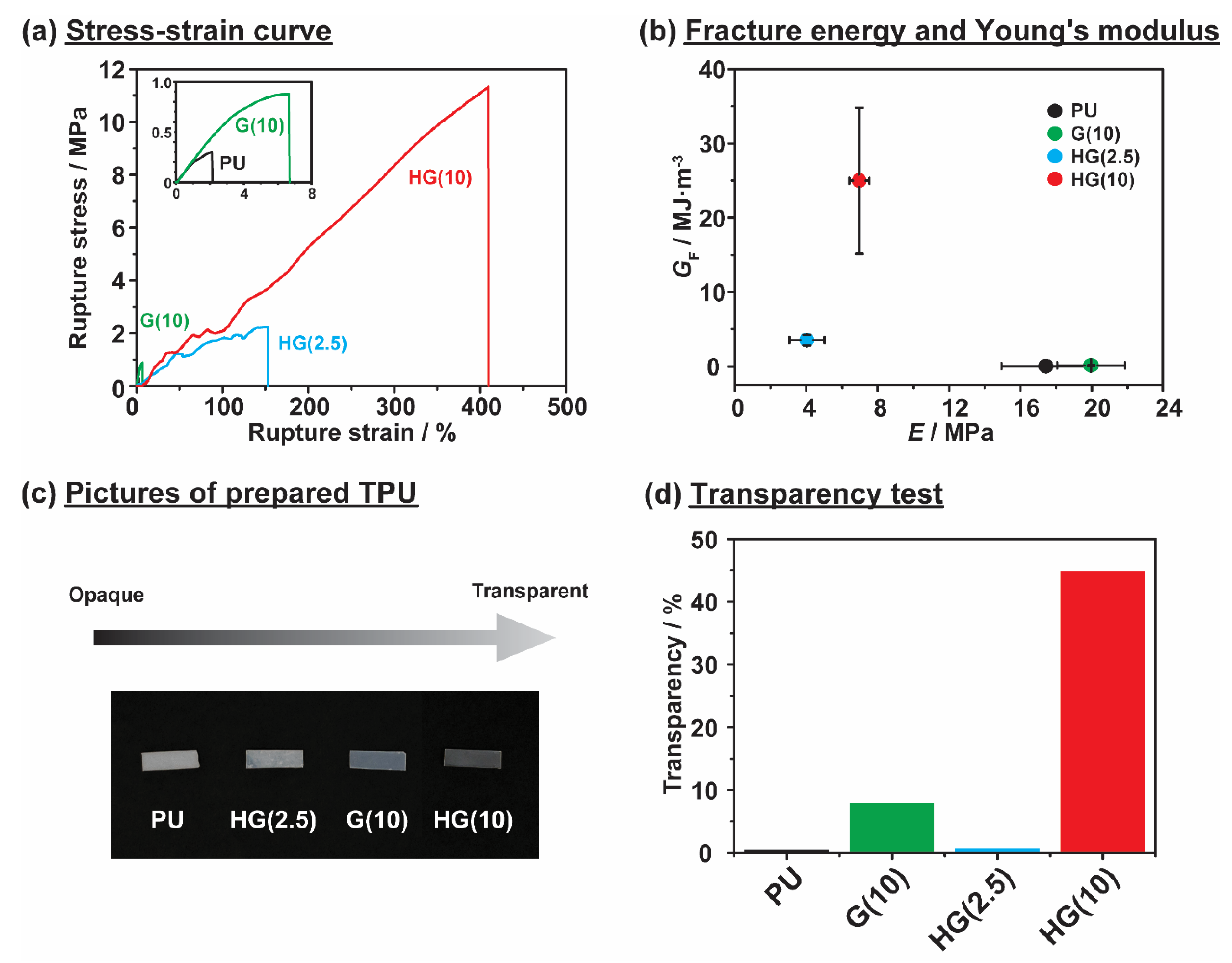
3.2. Mechanical and Transparency Properties of Thermoplastic Polyurethane Linked via Host-Guest Interactions
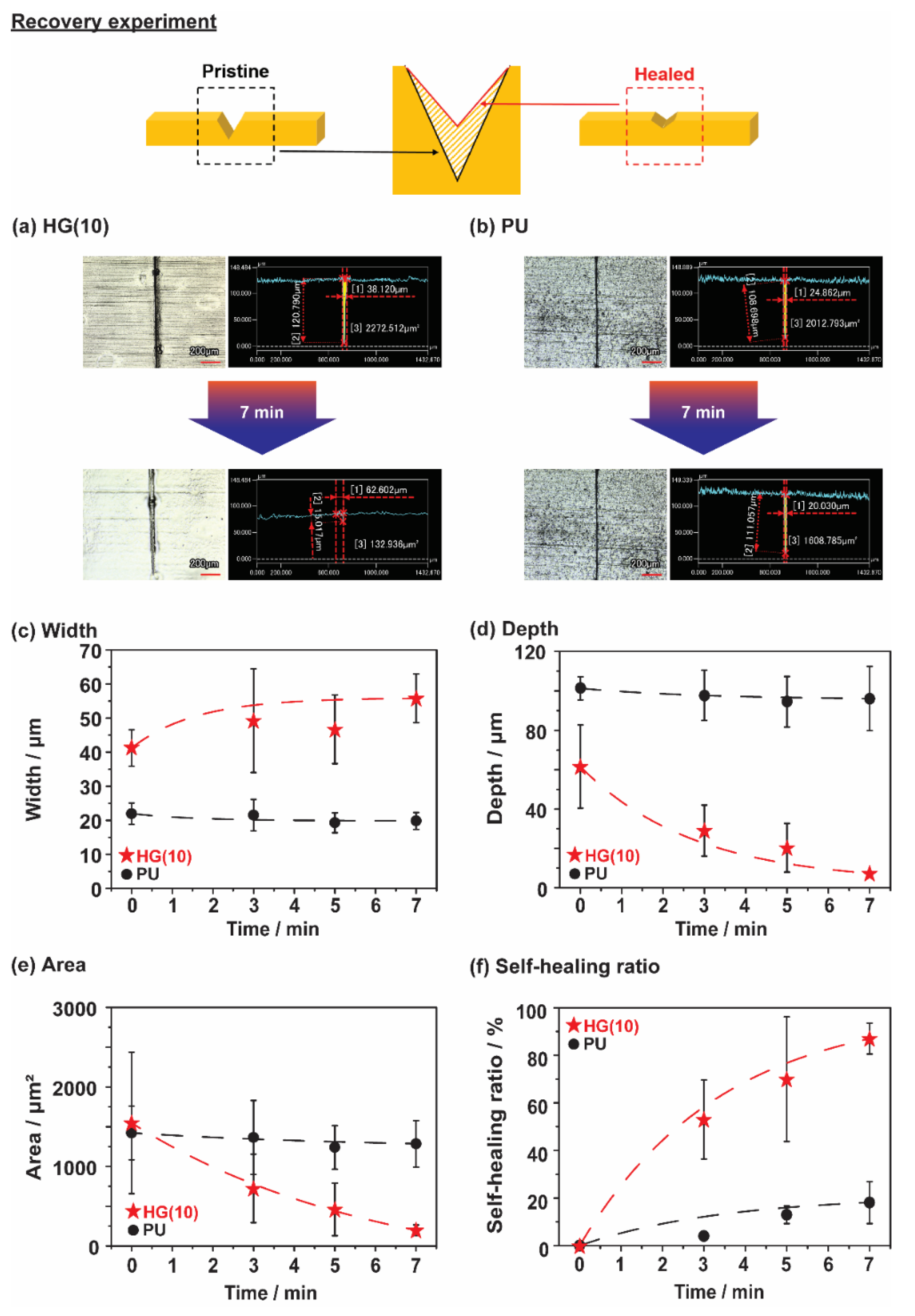
3.3. Fast Recovery of Thermoplastic Polyurethane Linked via Host-Guest Interactions
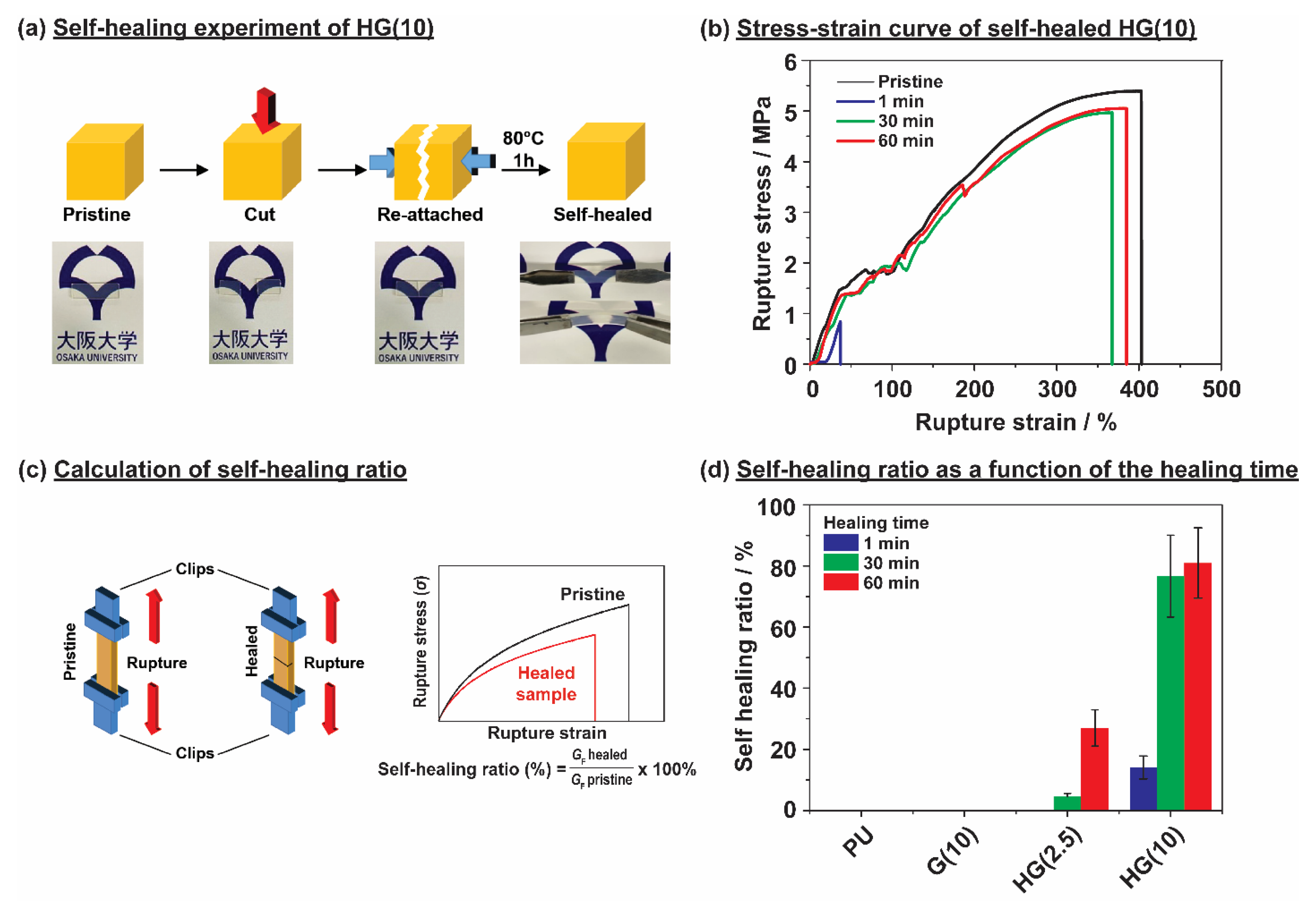
3.4. Self-Healing Properties of Thermoplastic Polyurethane Linked via Host-Guest Interactions
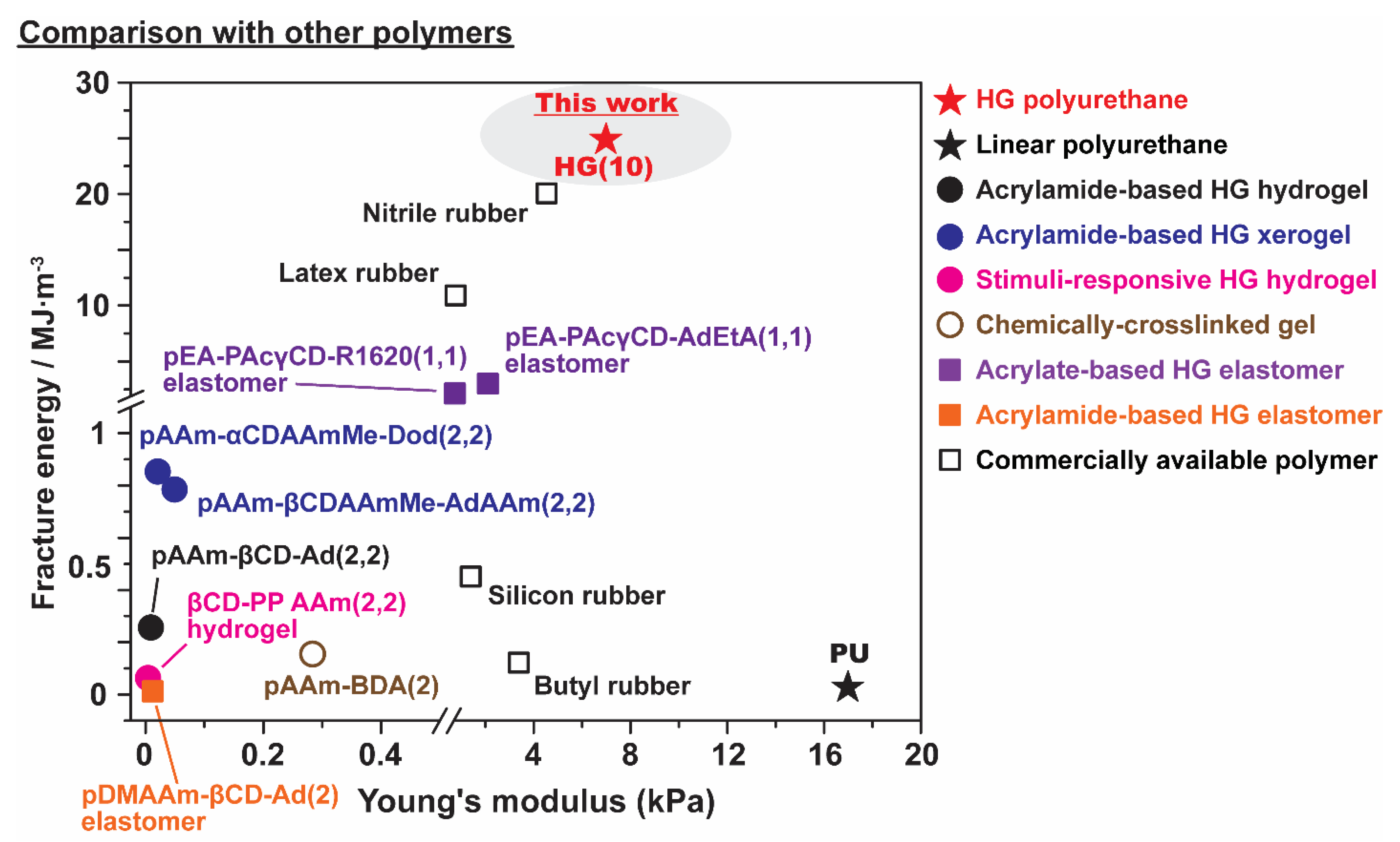
3.5. Comparison of Thermoplastic Polyurethane Linked by Host-Guest Interactions with Previously Reported Host-Guest Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Harada, A.; Kawaguchi, Y.; Hoshino, T. Supramolecular Polymers Formed by Modified Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2001, 41, 115–121. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally Re-mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Chappell, D.; Colquhoun, H.M.; Hayes, W. Healable polymeric materials: A tutorial review. Chem. Soc. Rev. 2010, 39, 1973–1985. [Google Scholar] [CrossRef]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional Supramolecular Polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef]
- Cho, S.H.; White, S.R.; Braun, P.V. Self-Healing Polymer Coatings. Adv. Mater. 2009, 21, 645–649. [Google Scholar] [CrossRef]
- Jin, J.; Cai, L.; Jia, Y.-G.; Liu, S.; Chen, Y.; Ren, L. Progress in self-healing hydrogels assembled by host–guest interactions: Preparation and biomedical applications. J. Mater. Chem. B 2019, 7, 1637–1651. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef]
- Kang, J.; Tok, J.B.H.; Bao, Z. Self-healing soft electronics. Nat. Electron. 2019, 2, 144–150. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Yu, Z.; Lan, Y.; Abell, C.; Scherman, O.A. Biomimetic Supramolecular Polymer Networks Exhibiting both Toughness and Self-Recovery. Adv. Mater. 2017, 29, 1604951. [Google Scholar] [CrossRef]
- Oh, J.Y.; Rondeau-Gagné, S.; Chiu, Y.-C.; Chortos, A.; Lissel, F.; Wang, G.-J.N.; Schroeder, B.C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, 411–415. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, e1602076. [Google Scholar] [CrossRef]
- Shibayama, M. Structure-mechanical property relationship of tough hydrogels. Soft Matter 2012, 8, 8030–8038. [Google Scholar] [CrossRef]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sato, K.; Nakajima, T.; Hisamatsu, T.; Nonoyama, T.; Kurokawa, T.; Gong, J.P. Phase-Separation-Induced Anomalous Stiffening, Toughening, and Self-Healing of Polyacrylamide Gels. Adv. Mater. 2015, 27, 6990–6998. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U.-I. Design and Fabrication of a High-Strength Hydrogel with Ideally Homogeneous Network Structure from Tetrahedron-like Macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Kondo, S.; Hiroi, T.; Han, Y.-S.; Kim, T.-H.; Shibayama, M.; Chung, U.-I.; Sakai, T. Reliable Hydrogel with Mechanical “Fuse Link” in an Aqueous Environment. Adv. Mater. 2015, 27, 7407–7411. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Synthesis of a tubular polymer from threaded cyclodextrins. Nature 1993, 364, 516–518. [Google Scholar] [CrossRef]
- Nakahata, M.; Mori, S.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-Healing Materials Formed by Cross-Linked Polyrotaxanes with Reversible Bonds. Chem 2016, 1, 766–775. [Google Scholar] [CrossRef]
- Harada, A. Cyclodextrin-Based Molecular Machines. Acc. Chem. Res. 2001, 34, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Hayashi, Y.; Ito, K. From topological gels to slide-ring materials. J. Appl. Polym. Sci. 2014, 131, 40509. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133. [Google Scholar] [CrossRef] [PubMed]
- Reutenauer, P.; Buhler, E.; Boul, P.J.; Candau, S.J.; Lehn, J.-M. Room Temperature Dynamic Polymers Based on Diels–Alder Chemistry. Chem.—A Eur. J. 2009, 15, 1893–1900. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Burattini, S.; Colquhoun, H.M.; Fox, J.D.; Friedmann, D.; Greenland, B.W.; Harris, P.J.F.; Hayes, W.; Mackay, M.E.; Rowan, S.J. A self-repairing, supramolecular polymer system: Healability as a consequence of donor-acceptor π-π stacking interactions. Chem. Commun. 2009, 6717–6719. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Wie, J.J.; Greenland, B.W.; Burattini, S.; Hayes, W.; Colquhoun, H.M.; Mackay, M.E.; Rowan, S.J. High-Strength, Healable, Supramolecular Polymer Nanocomposites. J. Am. Chem. Soc. 2012, 134, 5362–5368. [Google Scholar] [CrossRef] [PubMed]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Yan, X.; Huang, F. Supramolecular polymers constructed by orthogonal self-assembly based on host–guest and metal–ligand interactions. Chem. Soc. Rev. 2015, 44, 815–832. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, J.; Guan, Z.; Zhang, Q. A Three-Armed Polymer with Tunable Self-Assembly and Self-Healing Properties Based on Benzene-1,3,5-tricarboxamide and Metal–Ligand Interactions. Macromol. Rapid Commun. 2019, 40, 1800909. [Google Scholar] [CrossRef]
- Rao, Y.-L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.-C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable Self-Healing Polymeric Dielectrics Cross-Linked Through Metal–Ligand Coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, F.; Dong, S.; Huang, F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem. Soc. Rev. 2012, 41, 1621–1636. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kakuta, T.; Yamagishi, T.-A. Applications of Pillar[n]arene-Based Supramolecular Assemblies. Angew. Chem. Int. Ed. 2019, 58, 2197–2206. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-Healing Supramolecular Gels Formed by Crown Ether Based Host–Guest Interactions. Angew. Chem. Int. Ed. 2012, 51, 7011–7015. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Dynamic Macromolecular Material Design—The Versatility of Cyclodextrin-Based Host–Guest Chemistry. Angew. Chem. Int. Ed. 2017, 56, 8350–8369. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Harada, A. Construction of Supramolecular Polymers with Alternating α-, β-Cyclodextrin Units Using Conformational Change Induced by Competitive Guests. J. Am. Chem. Soc. 2004, 126, 11418–11419. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.A.; Mozhdehi, D.; Guan, Z. Enhancing Mechanical Performance of a Covalent Self-Healing Material by Sacrificial Noncovalent Bonds. J. Am. Chem. Soc. 2015, 137, 4846–4850. [Google Scholar] [CrossRef]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef]
- Ducrot, E.; Chen, Y.; Bulters, M.; Sijbesma, R.P.; Creton, C. Toughening Elastomers with Sacrificial Bonds and Watching Them Break. Science 2014, 344, 186–189. [Google Scholar] [CrossRef]
- Martinez, R.V.; Fish, C.R.; Chen, X.; Whitesides, G.M. Elastomeric Origami: Programmable Paper-Elastomer Composites as Pneumatic Actuators. Adv. Funct. Mater. 2012, 22, 1376–1384. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Supramolecular self-healing materials from non-covalent cross-linking host–guest interactions. Chem. Commun. 2020, 56, 4381–4395. [Google Scholar] [CrossRef]
- Nomimura, S.; Osaki, M.; Park, J.; Ikura, R.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-Healing Alkyl Acrylate-Based Supramolecular Elastomers Cross-Linked via Host–Guest Interactions. Macromolecules 2019, 52, 2659–2668. [Google Scholar] [CrossRef]
- Okano, N.; Osaki, M.; Ikura, R.; Takashima, Y.; Yamaguchi, H.; Harada, A. Bulk Copolymerization of Host–Guest Monomers with Liquid-Type Acrylamide Monomers for Supramolecular Materials Applications. ACS Appl. Polym. Mater. 2020, 2, 1553–1560. [Google Scholar] [CrossRef]
- Engels, H.-W.; Pirkl, H.-G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile Materials and Sustainable Problem Solvers for Today’s Challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef] [PubMed]
- Sui, T.; Baimpas, N.; Dolbnya, I.P.; Prisacariu, C.; Korsunsky, A.M. Multiple-length-scale deformation analysis in a thermoplastic polyurethane. Nat. Commun. 2015, 6, 6583. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-Y.; Zhang, Q.; Yu, C.-Y.; Rao, S.-J.; Yang, S.; Tian, H.; Qu, D.-H. An Ultrastrong and Highly Stretchable Polyurethane Elastomer Enabled by a Zipper-Like Ring-Sliding Effect. Adv. Mater. 2020, 32, 2000345. [Google Scholar] [CrossRef]
- Sen, A.K.; Damewood, J. Coated Textiles: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of molecular weight and molecular weight distribution on mechanical properties of polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- Oprea, S.; Timpu, D.; Oprea, V. Design-properties relationships of polyurethanes elastomers depending on different chain extenders structures. J. Polym. Res. 2019, 26, 117. [Google Scholar] [CrossRef]
- Ward, T.C. Molecular weight and molecular weight distributions in synthetic polymers. J. Chem. Educ. 1981, 58, 867. [Google Scholar] [CrossRef][Green Version]
- Kakuta, T.; Takashima, Y.; Harada, A. Highly Elastic Supramolecular Hydrogels Using Host-Guest Inclusion Complexes with Cyclodextrins. Macromolecules 2013, 46, 4575–4579. [Google Scholar] [CrossRef]
- Kim, S.-M.; Jeon, H.; Shin, S.-H.; Park, S.-A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior Toughness and Fast Self-Healing at Room Temperature Engineered by Transparent Elastomers. Adv. Mater. 2018, 30, 1705145. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, X.; Urban, M.W. Chemical and physical aspects of self-healing materials. Prog. Polym. Sci. 2015, 49, 34–59. [Google Scholar] [CrossRef]
- Hornat, C.C.; Urban, M.W. Entropy and interfacial energy driven self-healable polymers. Nat. Commun. 2020, 11, 1028. [Google Scholar] [CrossRef]
- Lee, M.W.; An, S.; Yoon, S.S.; Yarin, A.L. Advances in self-healing materials based on vascular networks with mechanical self-repair characteristics. Adv. Colloid Interface Sci. 2018, 252, 21–37. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Harada, A. Highly Flexible, Tough, and Self-Healing Supramolecular Polymeric Materials Using Host-Guest Interaction. Macromol. Rapid Commun. 2016, 37, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Sawa, Y.; Iwaso, K.; Nakahata, M.; Yamaguchi, H.; Harada, A. Supramolecular Materials Cross-Linked by Host-Guest Inclusion Complexes: The Effect of Side Chain Molecules on Mechanical Properties. Macromolecules 2017, 50, 3254–3261. [Google Scholar] [CrossRef]
- Takashima, Y.; Yonekura, K.; Koyanagi, K.; Iwaso, K.; Nakahata, M.; Yamaguchi, H.; Harada, A. Multifunctional Stimuli-Responsive Supramolecular Materials with Stretching, Coloring, and Self-Healing Properties Functionalized via Host–Guest Interactions. Macromolecules 2017, 50, 4144–4150. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Sinawang, G.; Osaki, M.; Zheng, Y.; Yamaguchi, H.; Harada, A.; Takashima, Y. Self-Healing Thermoplastic Polyurethane Linked via Host-Guest Interactions. Polymers 2020, 12, 1393. https://doi.org/10.3390/polym12061393
Jin C, Sinawang G, Osaki M, Zheng Y, Yamaguchi H, Harada A, Takashima Y. Self-Healing Thermoplastic Polyurethane Linked via Host-Guest Interactions. Polymers. 2020; 12(6):1393. https://doi.org/10.3390/polym12061393
Chicago/Turabian StyleJin, Changming, Garry Sinawang, Motofumi Osaki, Yongtai Zheng, Hiroyasu Yamaguchi, Akira Harada, and Yoshinori Takashima. 2020. "Self-Healing Thermoplastic Polyurethane Linked via Host-Guest Interactions" Polymers 12, no. 6: 1393. https://doi.org/10.3390/polym12061393
APA StyleJin, C., Sinawang, G., Osaki, M., Zheng, Y., Yamaguchi, H., Harada, A., & Takashima, Y. (2020). Self-Healing Thermoplastic Polyurethane Linked via Host-Guest Interactions. Polymers, 12(6), 1393. https://doi.org/10.3390/polym12061393









