CO2 Separation with Polymer/Aniline Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
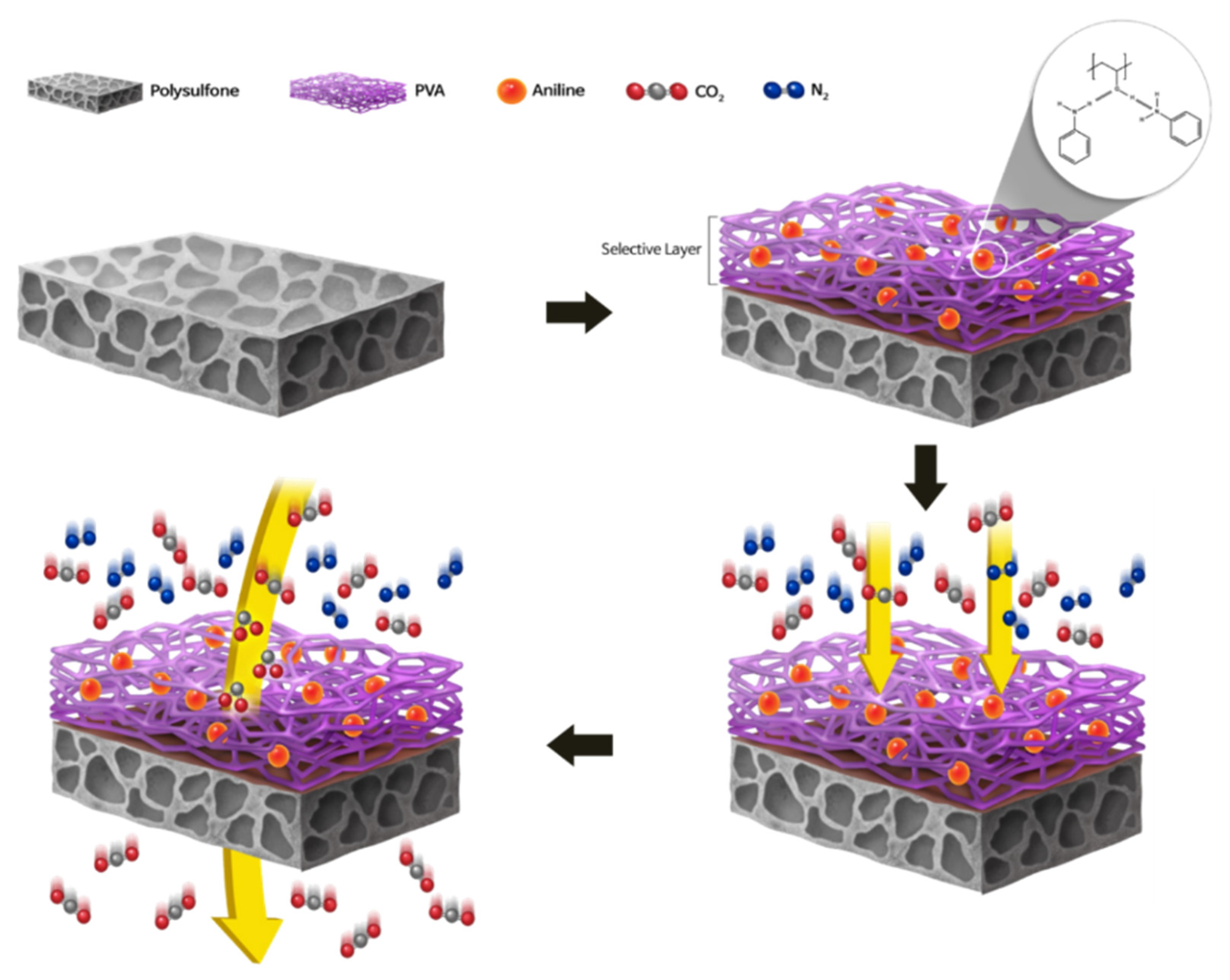
2.2. Preparation of Membrane
2.3. Permeance Measurements
2.4. Characterization
3. Results
3.1. Scanning Electron Microscopy (SEM) Images of the Membrane
3.2. Thermogravimetric Analysis (TGA)
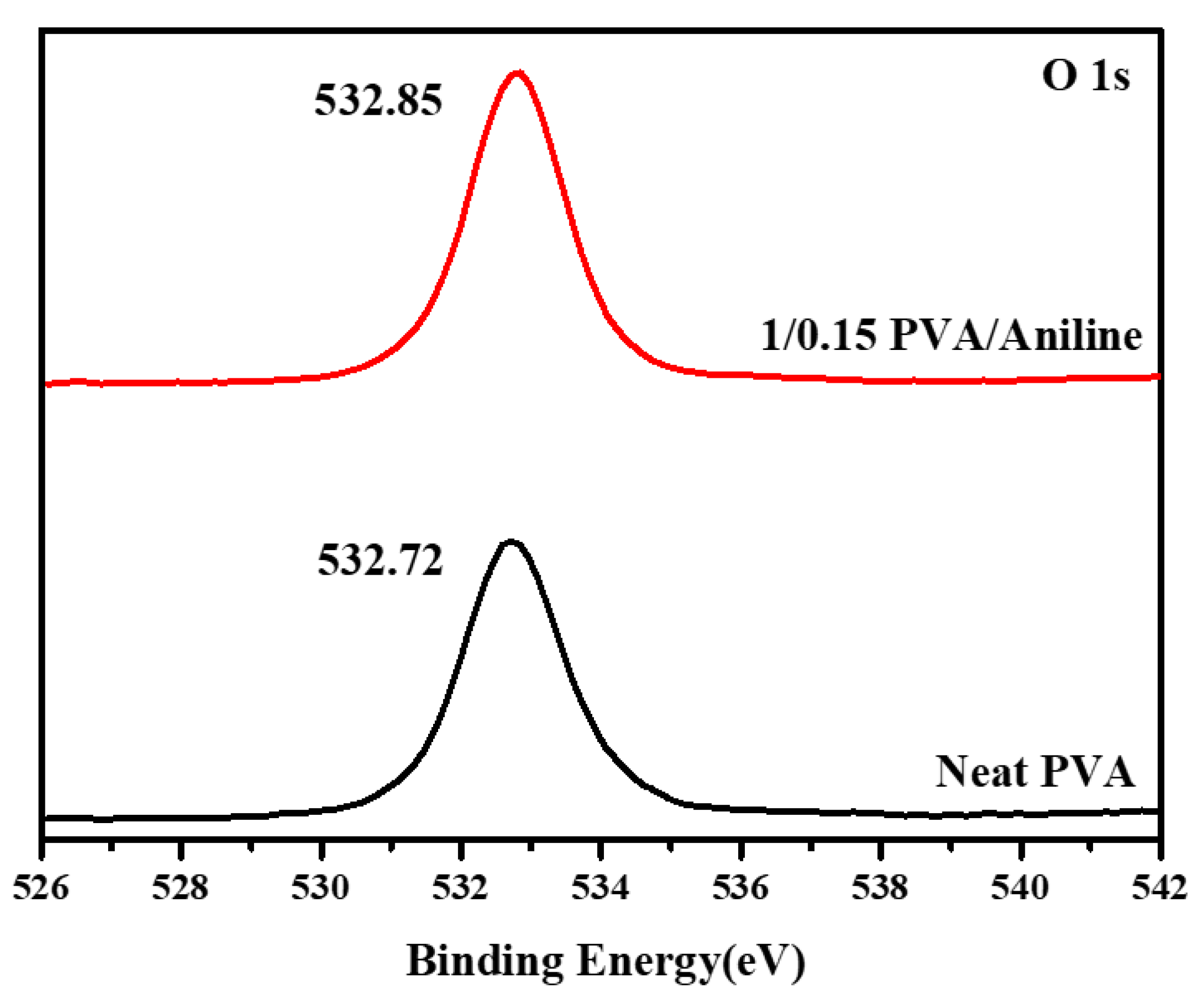
3.3. X-ray Photoelectron Spectroscopy (XPS)
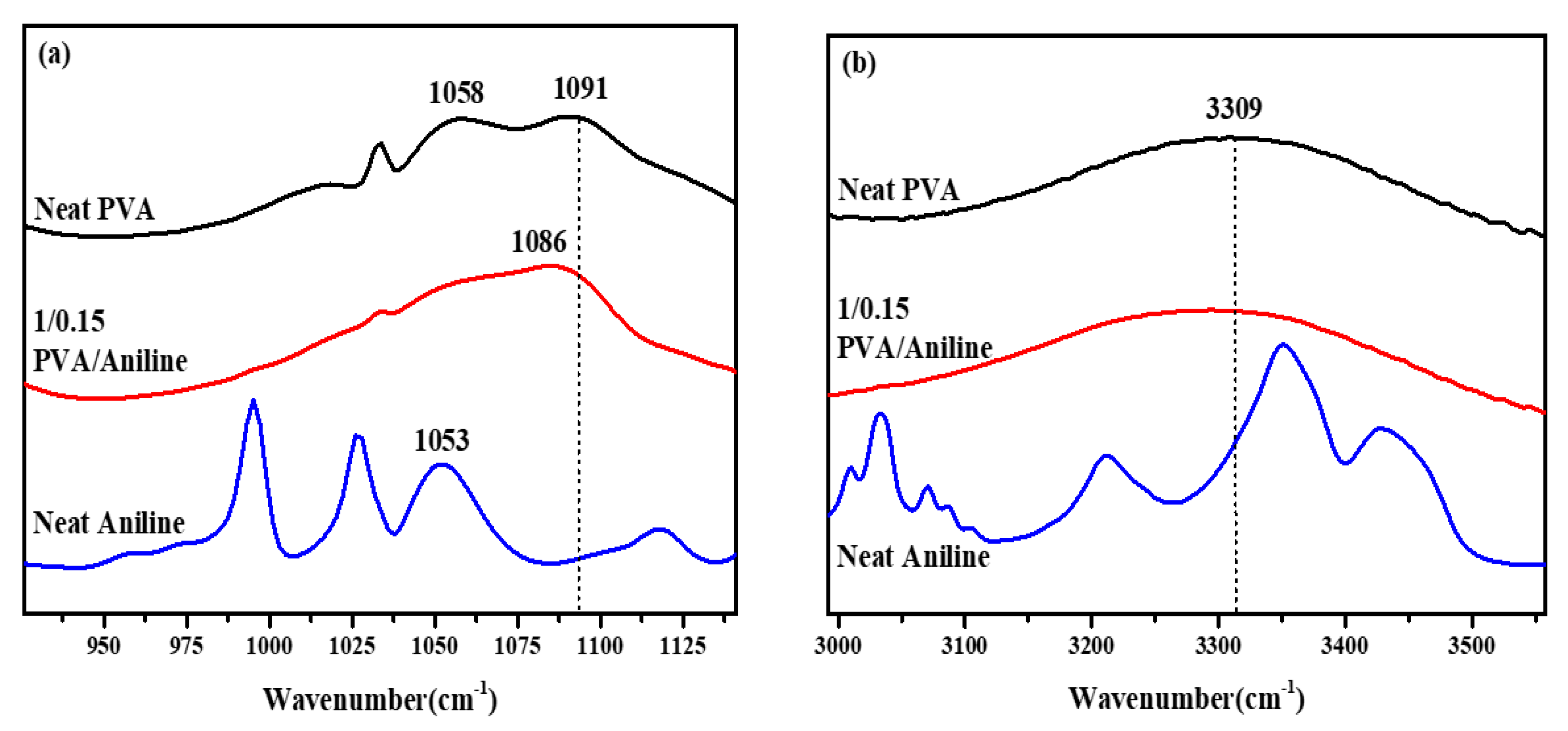
3.4. FT-IR
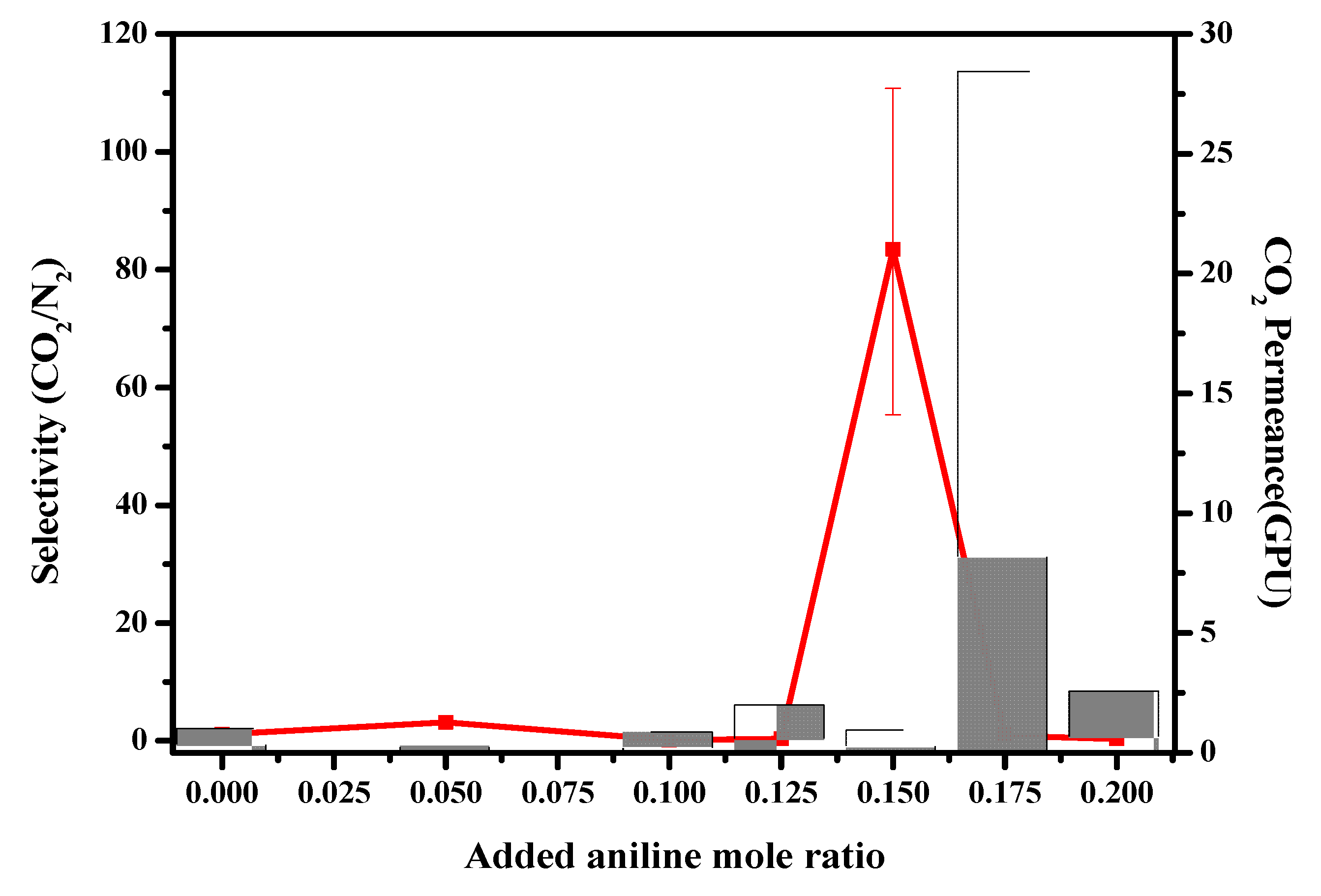
3.5. Separation Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niall, M.; Nick, F.; Antoine, B.; Jason, H.; Amparo, G.; George, J.; Claire, S.A.; Charlotte, K.W.; Nilay, S.; Paul, F. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar]
- Bo, L.; Congyong, T.; Xuewen, L.; Bin, W.; Rongfei, Z. High-performance SAPO-34 membranes for CO2 separations from simulated flue gas. Microporous Mesoporous Mater. 2020, 292, 109712. [Google Scholar]
- Dennis, Y.C.L.; Giorgio, C.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar]
- Vahab, M.; Masoud, R.; Bahman, Z. Energy saving in carbon dioxide hydrate formation process using Boehmite nanoparticles. Korean J. Chem. Eng. 2019, 36, 1859–1868. [Google Scholar]
- Liang, M.; Tingyu, Y.; Yu, W.; Xiaoqing, Y.; Jinrong, Y.; Shuai, Z.; Qiang, L.; Jianbin, Z. CO2 capture and preparation of spindle-like CaCO3 crystals for papermaking using calcium carbide residue waste via an atomizing approach. Korean J. Chem. Eng. 2019, 36, 1432–1440. [Google Scholar]
- Mohammdad, H.N.; Shahryar, B.; Reza, A. CO2 separation over light gases for nano-composite membrane comprising modified polyurethane with SiO2 nanoparticles. Korean J. Chem. Eng. 2019, 36, 763–779. [Google Scholar]
- Anoar, A.K.; Gopinath, H.; Asit, K.S. Kinetic effect and absorption performance of piperazine activator into aqueous solutions of 2-amino-2-methyl-1-propanol through post-combustion CO2 capture. Korean J. Chem. Eng. 2019, 36, 1090–1101. [Google Scholar]
- Gregory, P.K.; Alan, L.C. Shaped silica-polyethyleneimine composite sorbents for CO2 capture via adsorption. Energy Procedia 2017, 114, 2219–2227. [Google Scholar]
- Akbar, A.; Ramyakrishna, P.; Sajid, H.S.; Shahnawaz, P.; Muhammad, S.; Khalid, H.T. Graphene-based membranes for CO2 separation. Mater. Sci. Energy Technol. 2019, 2, 83–88. [Google Scholar]
- Liang, Y.; Shahpar, F.; Mattias, G.; Jonas, H. Ultra-thin MFI membranes with different Si/Al ratios for CO2/CH4 separation. Microporous Mesoporous Mater. 2019, 284, 258–264. [Google Scholar]
- Lloyd, M.R. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar]
- Zhongde, D.; Jing, D.; Luca, A.; Saravanan, J.; Liyuan, D. Thin-film-composite hollow fiber membranes containing amino acid salts as mobile carriers for CO2 separation. J. Membr. Sci. 2019, 578, 61–68. [Google Scholar]
- Babul, P.; Bishnupada, M. Preparation and characterization of CO2 selective facilitated transport membrane composed of chitosan and poly(allylamine) blend for CO2/N2 separation. J. Ind. Eng. Chem. 2018, 66, 419–429. [Google Scholar]
- Babul, P.; Bishnupada, M. Moisture responsive and CO2 selective biopolymer membrane containing silk fibroin as a green carrier for facilitated transport of CO2. J. Membr. Sci. 2018, 550, 416–426. [Google Scholar]
- Han, Y.; Wu, D.; Ho, W.W. Nanotube-reinforced facilitated transport membrane for CO2/N2 separation with vacuum operation. J. Membr. Sci. 2018, 567, 261–271. [Google Scholar] [CrossRef]
- Zhongde, D.; Hesham, A.; Luca, A.; Jing, D.; Marco, G.B.; Liyuan, D. Nafion/PEG hybrid membrane for CO2 separation: Effect of PEG on membrane micro-structure and performance. Sep. Purif. Technol. 2019, 214, 67–77. [Google Scholar]
- Gregory, K.K.; Jennifer, R.W.; Qinnan, Z.; Ruilan, G. Studies of the synergistic effects of crosslink density and crosslink inhomogeneity on crosslinked PEO membranes for CO2 selective separations. J. Membr. Sci. 2017, 544, 25–34. [Google Scholar]
- Sander, R.R.; Michel, H.K.; Kitty, N.; Matthias, W. Poly(ethylene glycol) and poly(dimethyl siloxane): Combining their advantages into efficient CO2 gas separation membranes. J. Membr. Sci. 2010, 352, 126–135. [Google Scholar]
- Shoji, H.; Tatsuo, M.; Tomohiro, S.; Masahiro, T.; Hideto, M.; Kazunori, N.; Misa, H.; Fukiko, K.; Masahiro, G. CO2 separation facilitated by task-specific ionic liquids using a supported liquid membrane. J. Membr. Sci. 2008, 314, 1–4. [Google Scholar]
- Sonia, Z.; Muhamad, I.S.; David, M. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar]
- Haiyang, Z.; Hailong, T.; Jinli, Z.; Ruili, G.; Xueqin, L. Facilitated transport membranes with an amino acid salt for highly efficient CO2 separation. Int. J. Greenh. Gas Control 2018, 78, 85–93. [Google Scholar]
- Sun, L.B.; Kang, Y.H.; Shi, Y.Q.; Jiang, Y.; Liu, X.Q. Highly Selective Capture of the Greenhouse Gas CO2 in Polymers. ACS Sustain. Chem. Eng. 2015, 3, 3077–3085. [Google Scholar]
- Yoon, K.W.; Kim, H.; Kang, Y.S.; Kang, S.W. 1-Butyl-3-methylimidazolium tetrafluoroborate/zinc oxide composite membrane for high CO2 separation performance. Chem. Eng. 2017, 320, 50–54. [Google Scholar] [CrossRef]
- Lee, W.G.; Kang, S.W. Highly selective poly(ethylene oxide)/ionic liquid electrolyte membranes containing CrO3 for CO2/N2 separation. Chem. Eng. 2019, 356, 312–317. [Google Scholar] [CrossRef]
- Jeon, H.; Kang, S.W. Enhanced CO2 transport through rod-shaped Al2O3 nanoparticles for ionic liquid composite membranes. Polym. Compos. 2019, 40, 2954–2958. [Google Scholar] [CrossRef]
- Yoon, K.W.; Kang, S.W. Highly permeable and selective CO2 separation membrane to utilize 5-hydroxyisophthalic acid in poly(ethylene oxide) matrix. Chem. Eng. 2018, 334, 1749–1753. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, Y.R.; Kang, Y.S.; Kang, S.W. Enhanced CO2 separation performance of polymer composite membranes through the synergistic effect of 1,3,5-benzenetricarboxylic acid. Chem. Eng. 2015, 279, 273–276. [Google Scholar] [CrossRef]
- Choi, Y.; Kang, S.W. Effect of 4-hydroxybenzoic acid on CO2 separation performance of poly(ethylene oxide) membrane. Macromol. Res. 2016, 24, 1111–1114. [Google Scholar] [CrossRef]
- Oh, J.H.; Kang, Y.S.; Kang, S.W. Poly(vinylpyrrolidone)/KF electrolyte membranes for facilitated CO2 transport. Chem. Commun. 2013, 49, 10181–10183. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.H.; Ji, D.; Kang, S.W. Facilitated CO2 Transport and Barrier Effect through Ionic Liquid Modified with Cyanuric Chloride. RSC Adv. 2014, 4, 16917–16919. [Google Scholar] [CrossRef]




| Peak (cm−1) | Area (%) | |
|---|---|---|
| Neat PVA | 3277.43 | 74.17 |
| 1/0.15 PVA/aniline composite membrane | 3268.59 | 85.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Kang, S.W. CO2 Separation with Polymer/Aniline Composite Membranes. Polymers 2020, 12, 1363. https://doi.org/10.3390/polym12061363
Lee HJ, Kang SW. CO2 Separation with Polymer/Aniline Composite Membranes. Polymers. 2020; 12(6):1363. https://doi.org/10.3390/polym12061363
Chicago/Turabian StyleLee, Hwa Jin, and Sang Wook Kang. 2020. "CO2 Separation with Polymer/Aniline Composite Membranes" Polymers 12, no. 6: 1363. https://doi.org/10.3390/polym12061363
APA StyleLee, H. J., & Kang, S. W. (2020). CO2 Separation with Polymer/Aniline Composite Membranes. Polymers, 12(6), 1363. https://doi.org/10.3390/polym12061363




