Thermo-Responsive Antimicrobial Hydrogel for the In-Situ Coating of Mesh Materials for Hernia Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Preparation of HApN Hydrogels and Rheological Characterization
2.1.2. Mesh Coating
2.1.3. Release Kinetics
2.1.4. Cell Culture and Flow Cytometry
2.1.5. Preparation of Bacteria
2.1.6. Antibacterial Activity of the Hydrogel Coatings
2.2. In Vivo Preclinical Study
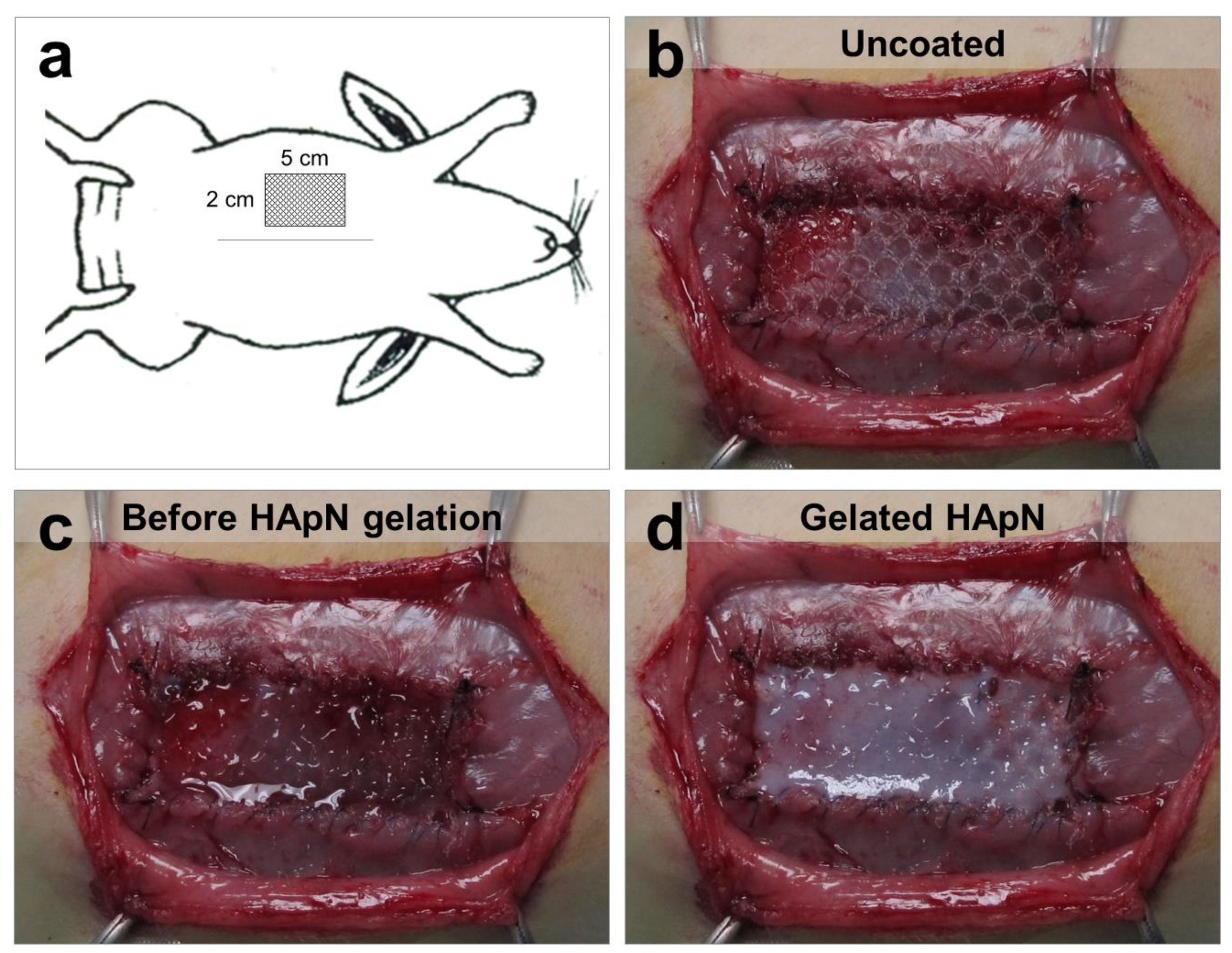
2.2.1. Experimental Design and Surgical Technique
2.2.2. Postsurgical Monitoring and Euthanasia
2.2.3. Macroscopic Observations and Sample Collection
2.2.4. Antibacterial Effectiveness of the Coated Implants
2.2.5. Histology and Immunohistochemistry
2.2.6. Scanning Electron Microscopy
2.3. Statistical Analysis
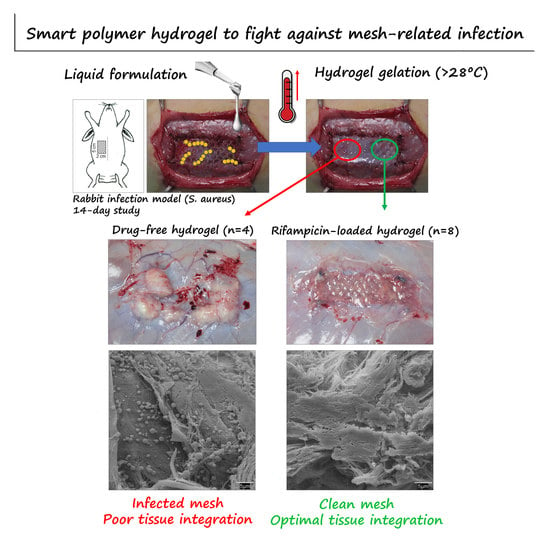
3. Results
3.1. In Vitro Characterization of the Formulated Hydrogels
3.2. In-Situ Coating of Implants
3.3. Postoperative Follow-Up
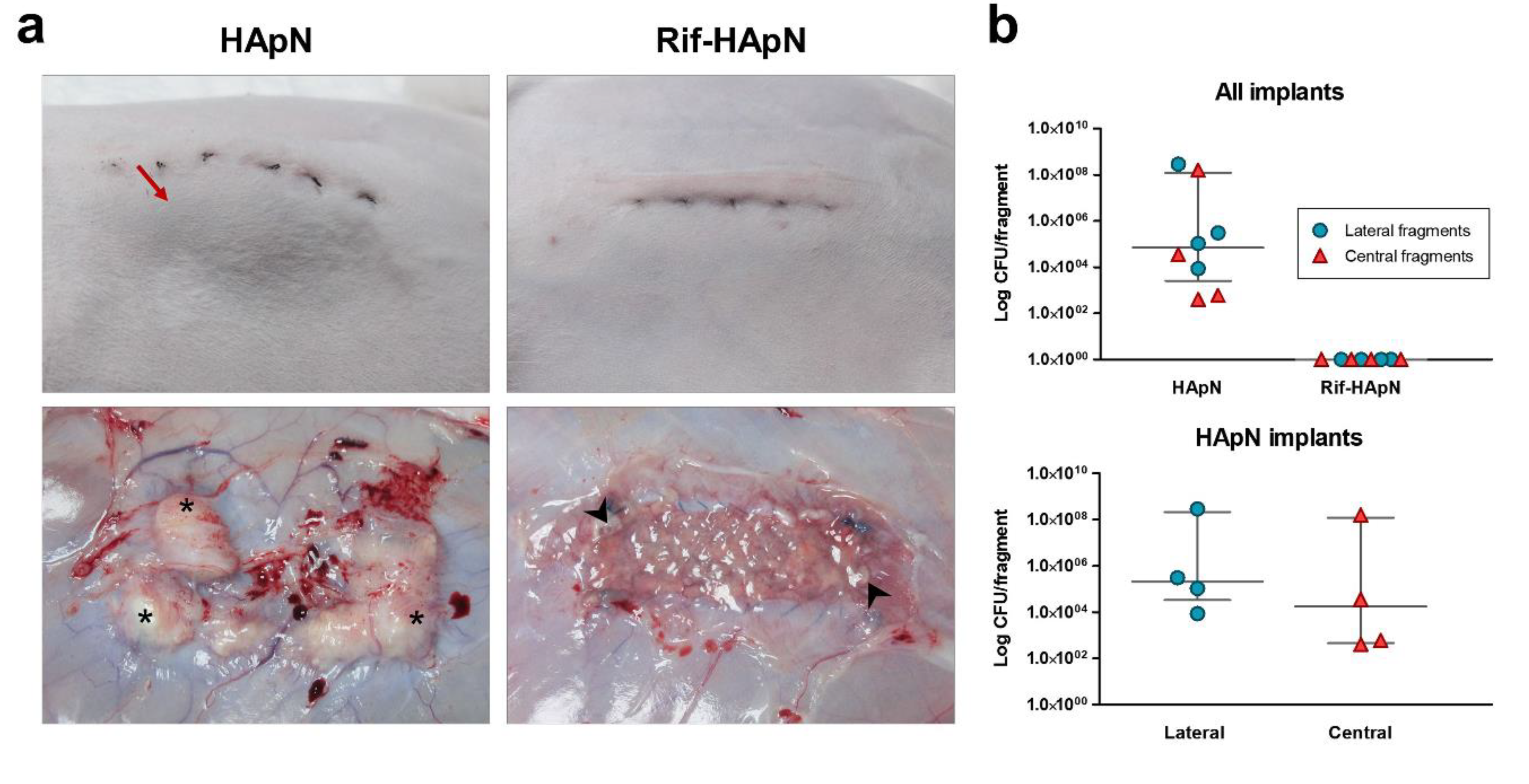
3.4. Macroscopic Outcomes at Euthanasia
3.5. Bacterial Adhesion to the Mesh
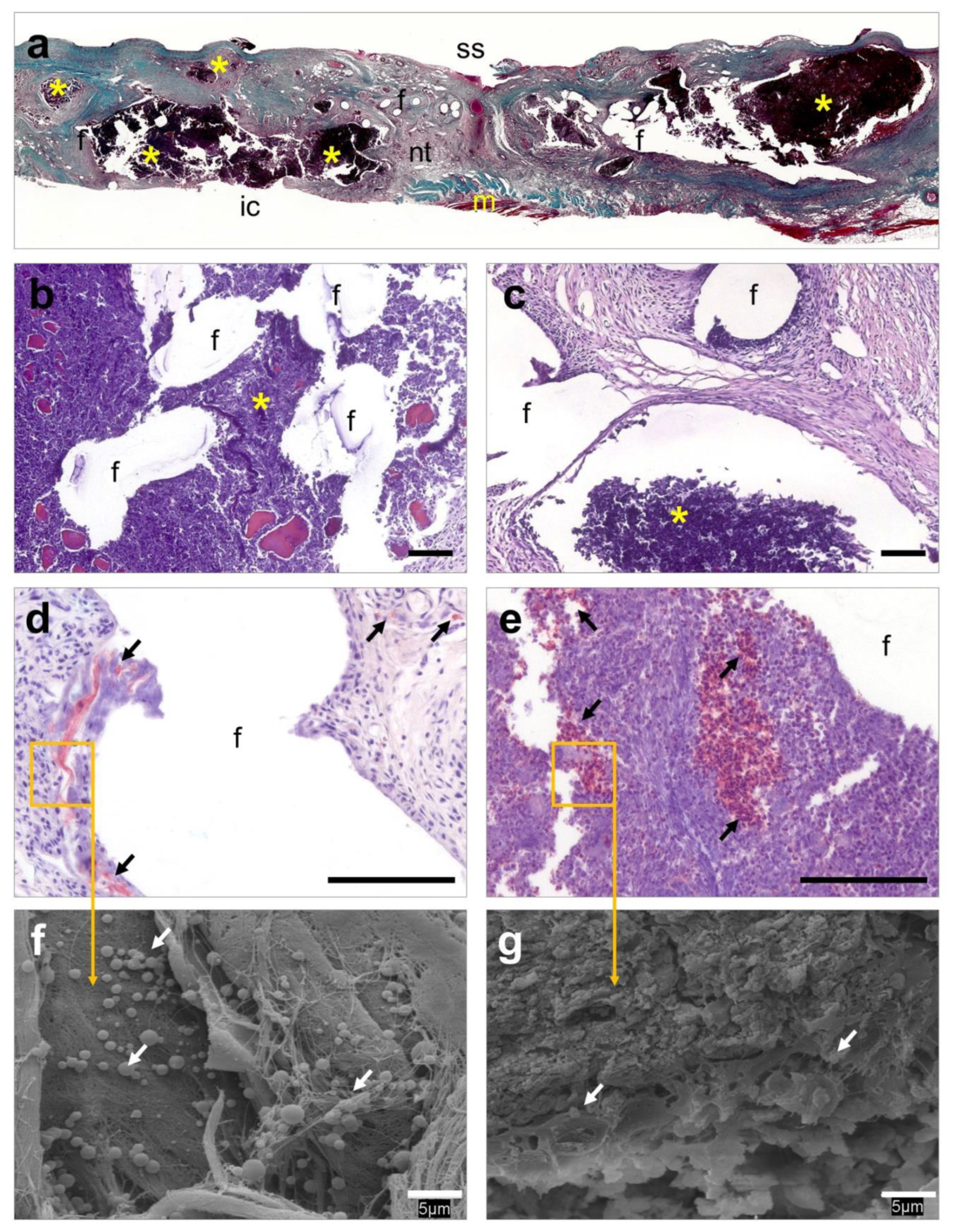
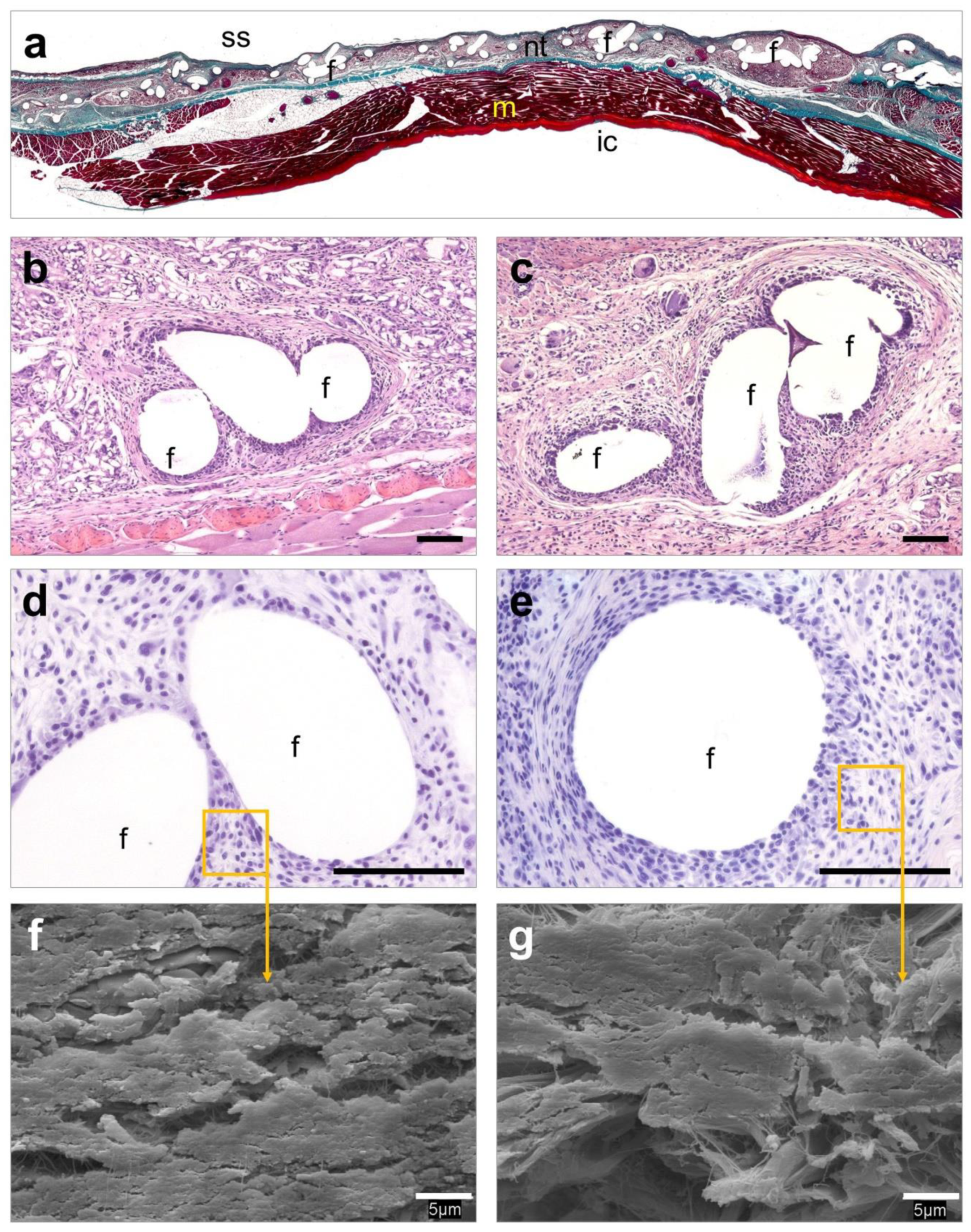
3.6. Histological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Gupta, N. Implantable medical devices treated with antimicrobial agents. In Antimicrobial Coatings and Modifications on Medical Devices, 1st ed.; Zhang, Z., Wagner, V., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 127–142. [Google Scholar]
- Lockhart, K.; Dunn, D.; Teo, S.; Ng, J.Y.; Dhillon, M.; Teo, E.; van Driel, M.L. Mesh versus non-mesh for inguinal and femoral hernia repair. Cochrane Database Syst. Rev. 2018, 9, CD011517. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbons, R.J.; Forse, R.A. Clinical practise. Groin hernias in adults. N. Engl. J. Med. 2015, 372, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Montgomery, A.; Kallinowski, F.; Köckerling, F. Evidence for replacement of an infected synthetic by a biological mesh in abdominal wall hernia repair. Front. Surg. 2016, 2, 67. [Google Scholar] [CrossRef] [PubMed]
- Bower, C.; Roth, J.S. Economics of abdominal wall reconstruction. Surg. Clin. Am. N. Am. 2013, 93, 1241–1253. [Google Scholar] [CrossRef]
- Guillaume, O.; Teuschl, A.H.; Gruber-Blum, S.; Fortelny, R.H.; Redl, H.; Petter-Puchner, A. Emerging trends in abdominal wall reinforcement: Bringing bio-functionality to meshes. Adv. Healthc. Mater. 2015, 4, 1763–1789. [Google Scholar] [CrossRef]
- Guillaume, O.; Pérez-Tanoira, R.; Fortelny, R.; Redl, H.; Moriarty, T.F.; Richards, R.G.; Eglin, D.; Petter Puchner, A. Infections associated with mesh repairs of abdominal wall hernias: Are antimicrobial biomaterials the longed-for solution? Biomaterials 2018, 167, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Wiegering, A.; Sinha, B.; Spor, L.; Klinge, U.; Steger, U.; Germer, C.T.; Dietz, U.A. Gentamicin for prevention of intraoperative mesh contamination: Demonstration of high bactericide effect (in vitro) and low systemic bioavailability (in vivo). Hernia 2014, 18, 691–700. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020. [Google Scholar] [CrossRef]
- Furth, M.E.; Atala, A.; Van Dyke, M.E. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 2007, 28, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-responsive drug release from smart polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Pé, *!!! REPLACE !!!*; rez-Kö, *!!! REPLACE !!!*; hler, B.; Linardi, F.; Pascual, G.; Belló, *!!! REPLACE !!!*; n, J.M.; Eglin, D.; Guillaume, O. Efficacy of antimicrobial agents delivered to hernia meshes using an adaptable thermo-responsive hyaluronic acid-based coating. Hernia 2019. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob. Agents Ther. Chemother. 2019, 63, e01746-18. [Google Scholar] [CrossRef]
- Reinbold, J.; Hierlemann, T.; Urich, L.; Uhde, A.K.; Müller, I.; Weindl, T.; Vogel, U.; Schlensak, C.; Wendel, H.P.; Krajewski, S. Biodegradable rifampicin-releasing coating of surgical meshes for the prevention of bacterial infections. Drug Des. Devel. Ther. 2017, 11, 2753–2762. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Benito-Martínez, S.; García-Moreno, F.; Rodríguez, M.; Pascual, G.; Bellón, J.M. Preclinical bioassay of a novel antibacterial mesh for the repair of abdominal hernia defects. Surgery 2020, 167, 598–608. [Google Scholar] [CrossRef]
- Majumder, A.; Scott, J.R.; Novitsky, Y.W. Evaluation of the antimicrobial efficacy of a novel rifampin/minocycline-coated, noncrosslinked porcine acellular dermal matrix compared with uncoated scaffolds for soft tissue repair. Surg. Innov. 2016, 23, 442–455. [Google Scholar] [CrossRef]
- Pascual, G.; Sotomayor, S.; Rodríguez, M.; Pérez-Köhler, B.; Kühnhardt, A.; Fernández-Gutiérrez, M.; San Román, J.; Bellón, J.M. Cytotoxicity of cyanoacrylate-based tissue adhesives and short-term preclinical in vivo biocompatibility in abdominal hernia repair. PLoS ONE 2016, 11, e0157920. [Google Scholar] [CrossRef]
- Bellón, J.M.; Rodríguez, M.; Pérez-Köhler, B.; Pérez-López, P.; Pascual, G. The New Zealand white rabbit as a model for preclinical studies addressing tissue repair at the level of the abdominal wall. Tissue Eng. Part C Methods 2017, 23, 863–880. [Google Scholar] [CrossRef]
- Kim, Y.J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Huang, H.J.; Tsai, Y.L.; Lin, S.H.; Hsu, S.H. Smart polymers for cell therapy and precision medicine. J. Biomed. Sci. 2019, 26, 73. [Google Scholar] [CrossRef]
- Kuckling, D. Stimuli-responsive gels. Gels 2018, 4, e60. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chi-Leung Hui, P. Review of stimuli-responsive polymers in drug delivery and textile application. Molecules 2019, 24, e2547. [Google Scholar] [CrossRef]
- Bischofberger, I.; Calzolari, D.C.E.; de los Rios, P.; Jelezarov, I.; Trappe, V. Hydrophobic hydration of poly-N-isopropyl acrylamide: A matter of the mean energetic state of water. Sci. Rep. 2014, 4, 4377. [Google Scholar] [CrossRef]
- Ter Boo, G.J.A.; Arens, D.; Metsemakers, W.J.; Zeiter, S.; Richards, R.G.; Grijpma, D.W.; Eglin, D.; Moriarty, T.F. Injectable gentamicin-loaded thermo-responsive hyaluronic acid derivative prevents infection in a rabbit model. Acta Biomater. 2016, 43, 185–194. [Google Scholar] [CrossRef]
- Ter Boo, G.J.; Schmid, T.; Zderic, I.; Nehrbass, D.; Camenisch, K.; Richards, R.G.; Grijpma, D.W.; Moriarty, T.F.; Eglin, D. Local application of a gentamicin-loaded thermo-responsive hydrogel allows for fracture healing upon clearance of a high Staphylococcus aureus load in a rabbit model. Eur. Cell Mater. 2018, 35, 151–164. [Google Scholar] [CrossRef]
- Kahramanca, Ş.; Kaya, O.; Azılı, C.; Celep, B.; Gökce, E.; Küçükpınar, T. Does topical rifampicin reduce the risk of surgical field infection in hernia repair? Ulus Cerrahi Derg. 2013, 29, 54–58. [Google Scholar]
- Brown, R.H.; Subramanian, A.; Hwang, C.S.; Chang, S.; Awad, S.S. Comparison of infectious complications with synthetic mesh in ventral hernia repair. Am. J. Surg. 2013, 205, 182–187. [Google Scholar] [CrossRef]
- Baker, E.H.; Lepere, D.; Lundgren, M.P.; Greaney, P.J.; Ehrlich, D.A.; Copit, S.E.; Murphree, A.L.; Canfield, A.J.; Parker, G.; Iannitti, D.A. Early clinical outcomes of a novel antibiotic-coated, non-crosslinked porcine acellular dermal graft after complex abdominal wall reconstruction. J. Am. Coll. Surg. 2016, 223, 581–586. [Google Scholar] [CrossRef]
- Cobb, W.S.; Paton, B.L.; Novitsky, Y.W.; Rosen, M.J.; Kercher, K.W.; Kuwada, T.S.; Heniford, B.T. Intra-abdominal placement of antimicrobial-impregnated mesh is associated with noninfectious fever. Am. Surg. 2006, 72, 1205–1208. [Google Scholar] [PubMed]
- Kirchhoff, P.; Hoffmann, H.; Köckerling, F.; Adolf, D.; Bittner, R.; Staerkle, R.F. Is antibiotic prophylaxis mandatory in laparoscopic incisional hernia repair? Data from the herniamed registry. Int. J. Surg. 2018, 58, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kockerling, F. Antibiotic prophylaxis in laparoendoscopic hernia surgery. Int. J. Abdom. Wall Hernia Surg. 2018, 1, 9–12. [Google Scholar]
- Alston, D.; Parnell, S.; Hoonjan, B.; Sebastian, A.; Howard, A. Conservative management of an infected laparoscopic hernia mesh: A case study. Int. J. Surg. Case Rep. 2013, 4, 1035–1037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trunzo, J.A.; Ponsky, J.L.; Jin, J.; Williams, C.P.; Rosen, M.J. A novel approach for salvaging infected prosthetic mesh after ventral hernia repair. Hernia 2009, 13, 545–549. [Google Scholar] [CrossRef]


| Macroscopic Outcomes Relative to Infection | ||
|---|---|---|
| Outcome | HApN (n = 4) | Rif-HApN (n = 8) |
| Skin necrosis | 0 | 0 |
| Fistula | 0 | 0 |
| Edema | 0 | 0 |
| Abscess with purulent material | 1 (>50% implant surface) 3 (<50% implant surface) | 0 |
| Macroscopic Outcomes Relative to Tissue Repair | ||
| HApN (n = 4) | Rif-HApN (n = 8) | |
| Superficial vascularization | 2 (Moderate) 2 (Severe) | 6 (Normal) 2 (Moderate) |
| Thrombosis | 0 | 0 |
| Encapsulation | 3 (Moderate to thick) 1 (Very thick) | 2 (Non-encapsulated) 6 (Thin to moderate) |
| Mesh integration | 4 (Partial integration) | 8 (Complete integration) |
| HApN | Rif-HApN | |||
|---|---|---|---|---|
| Value | Lateral (CFU) | Central (CFU) | Lateral (CFU) | Central (CFU) |
| Minimum | 8.60 × 103 | 4.00 × 102 | 0 | 0 |
| 25% Percentile | 3.30 ×104 | 4.50 × 102 | 0 | 0 |
| Median | 2.05 × 105 | 1.78 × 104 | 0 | 0 |
| 75% Percentile | 2.16 × 108 | 1.20 × 108 | 0 | 0 |
| Maximum | 2.88 × 108 | 1.60 × 108 | 0 | 0 |
| Mean | 7.21 × 107 | 4.00 × 107 | 0 | 0 |
| Std. Deviation | 1.44 × 108 | 7.99 × 107 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Köhler, B.; Pascual, G.; Benito-Martínez, S.; Bellón, J.M.; Eglin, D.; Guillaume, O. Thermo-Responsive Antimicrobial Hydrogel for the In-Situ Coating of Mesh Materials for Hernia Repair. Polymers 2020, 12, 1245. https://doi.org/10.3390/polym12061245
Pérez-Köhler B, Pascual G, Benito-Martínez S, Bellón JM, Eglin D, Guillaume O. Thermo-Responsive Antimicrobial Hydrogel for the In-Situ Coating of Mesh Materials for Hernia Repair. Polymers. 2020; 12(6):1245. https://doi.org/10.3390/polym12061245
Chicago/Turabian StylePérez-Köhler, Bárbara, Gemma Pascual, Selma Benito-Martínez, Juan Manuel Bellón, David Eglin, and Olivier Guillaume. 2020. "Thermo-Responsive Antimicrobial Hydrogel for the In-Situ Coating of Mesh Materials for Hernia Repair" Polymers 12, no. 6: 1245. https://doi.org/10.3390/polym12061245
APA StylePérez-Köhler, B., Pascual, G., Benito-Martínez, S., Bellón, J. M., Eglin, D., & Guillaume, O. (2020). Thermo-Responsive Antimicrobial Hydrogel for the In-Situ Coating of Mesh Materials for Hernia Repair. Polymers, 12(6), 1245. https://doi.org/10.3390/polym12061245