Advances in the Research of Bioinks Based on Natural Collagen, Polysaccharide and Their Derivatives for Skin 3D Bioprinting
Abstract
:1. Introduction
2. Skin Damage and Wound Repair
3. Three-Dimensional Bioprinting
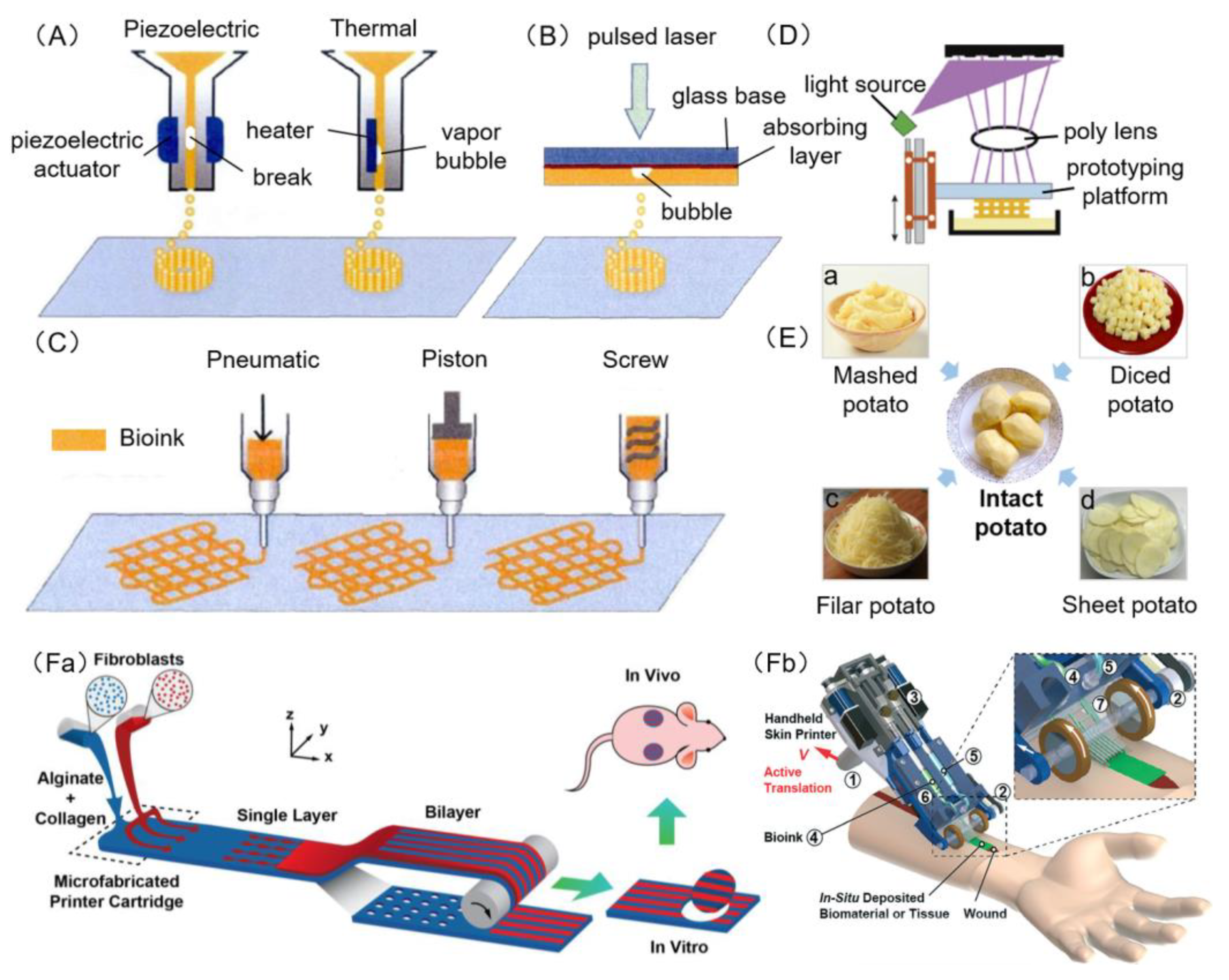
3.1. Inkjet Bioprinting
3.2. Laser Bioprinting
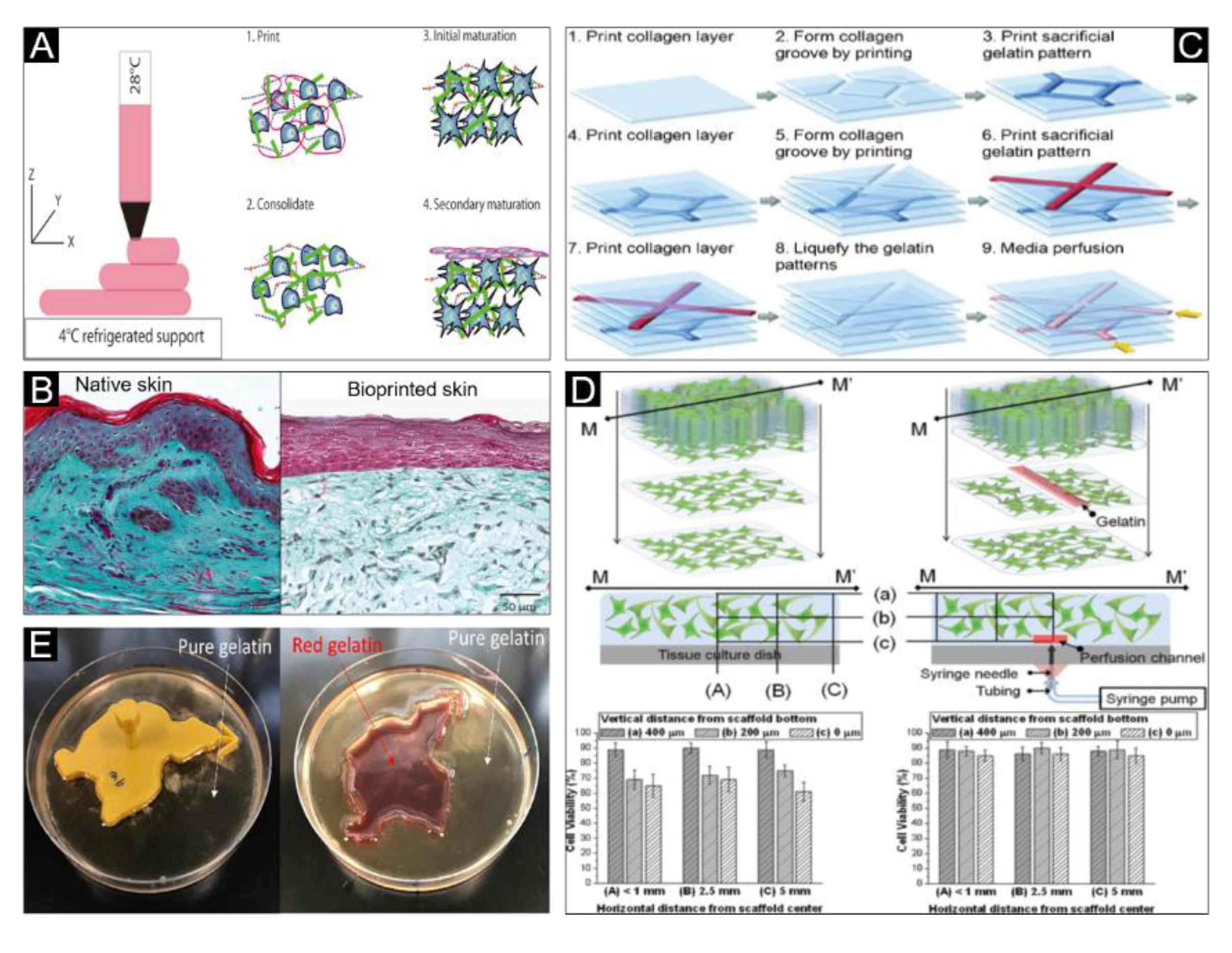
3.3. Extrusion Bioprinting
3.4. Stereolithography Bioprinting
3.5. Microfluidic Bioprinting
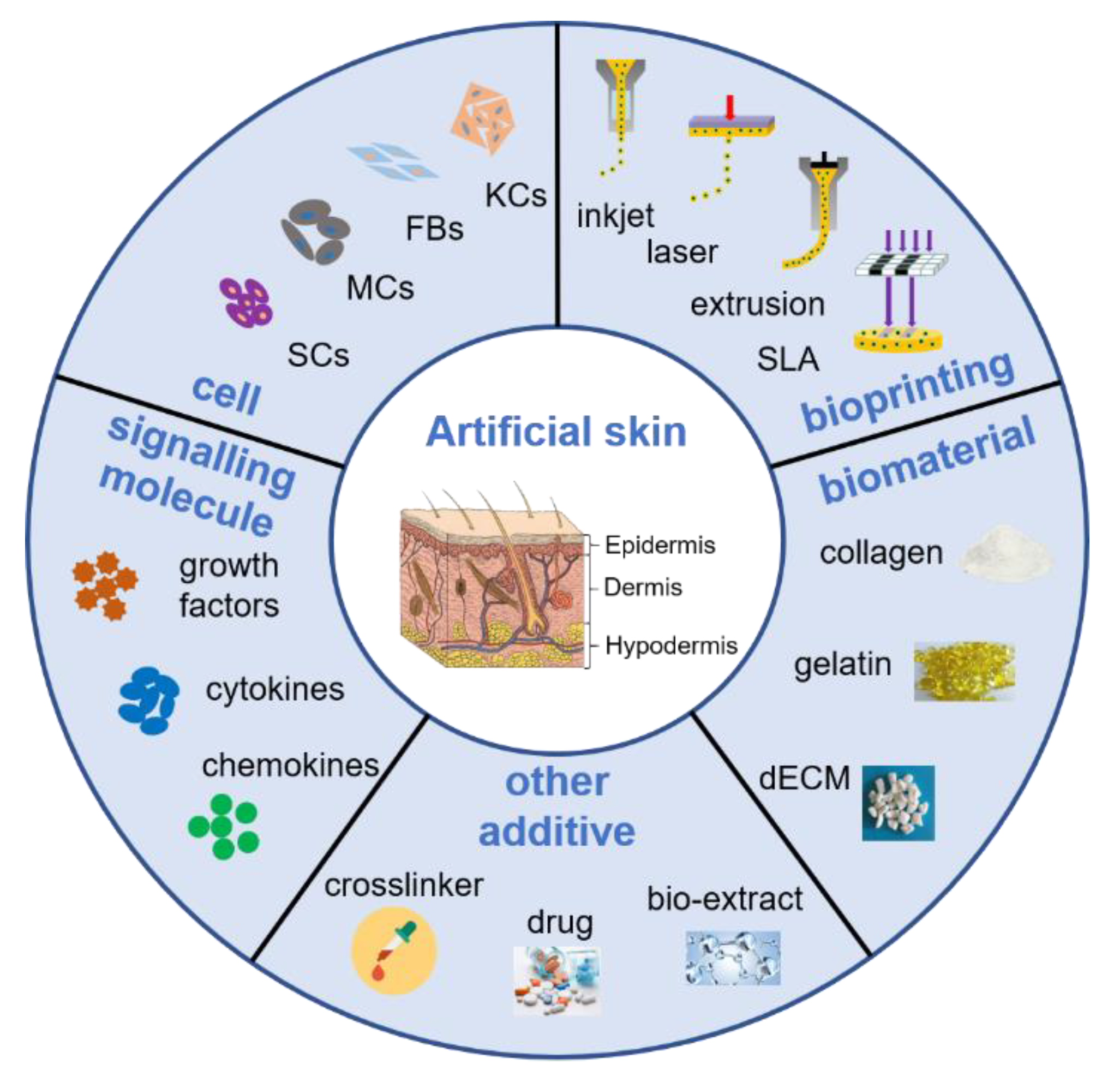
4. Bioink Components
4.1. Biomaterials
4.1.1. Collagen
4.1.2. Gelatin
4.1.3. Alginate
4.1.4. Fibrin
4.1.5. Hyaluronic Acid
4.1.6. Chitosan
4.1.7. Pectin
4.2. Cells
4.3. Growth Factors, Cytokines and Chemokines
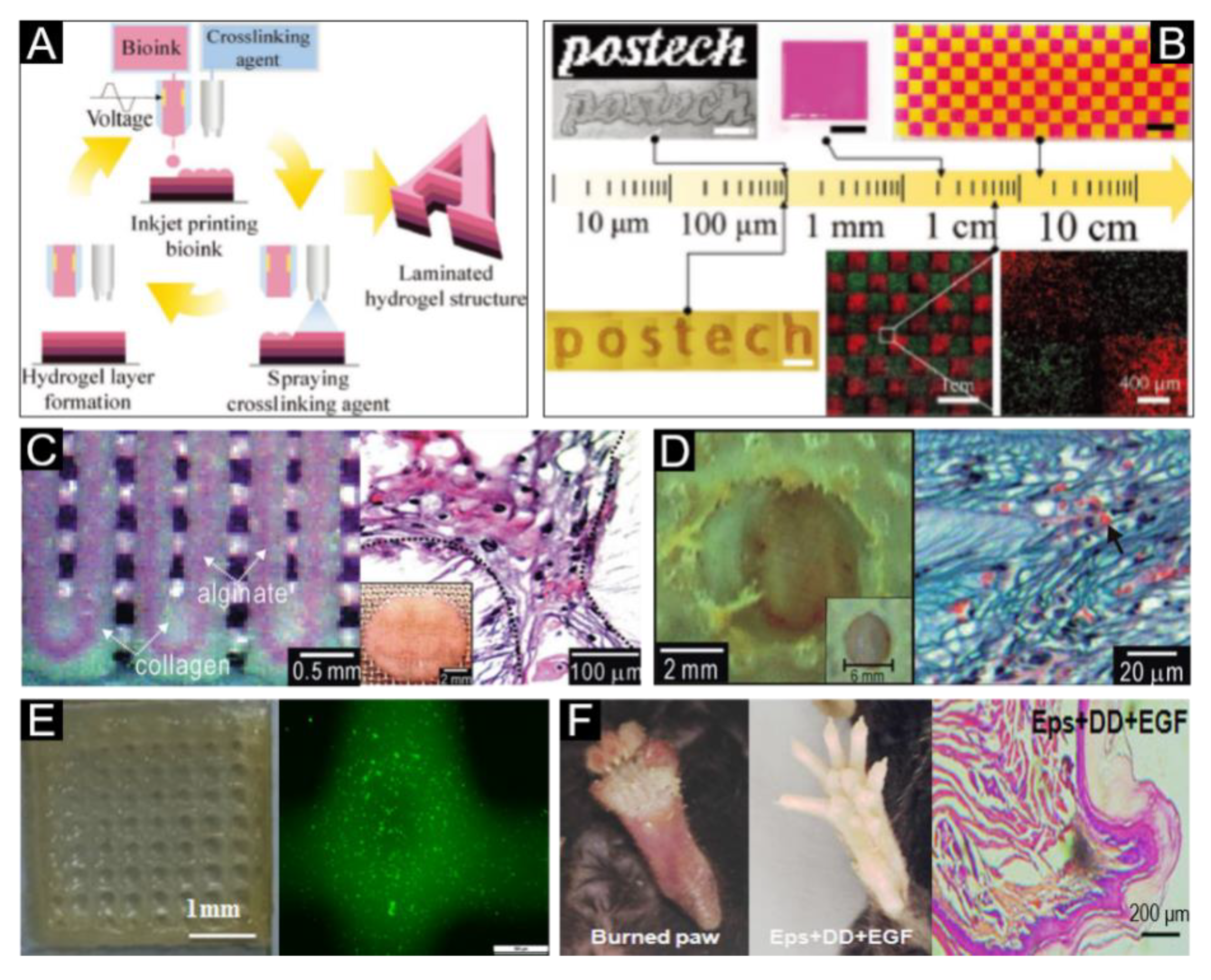
5. Classification of Bioink
5.1. Acellular Bioink
5.2. Cell-Encapsulating Bioink
6. Current Bioink Products for Skin Bioprinting
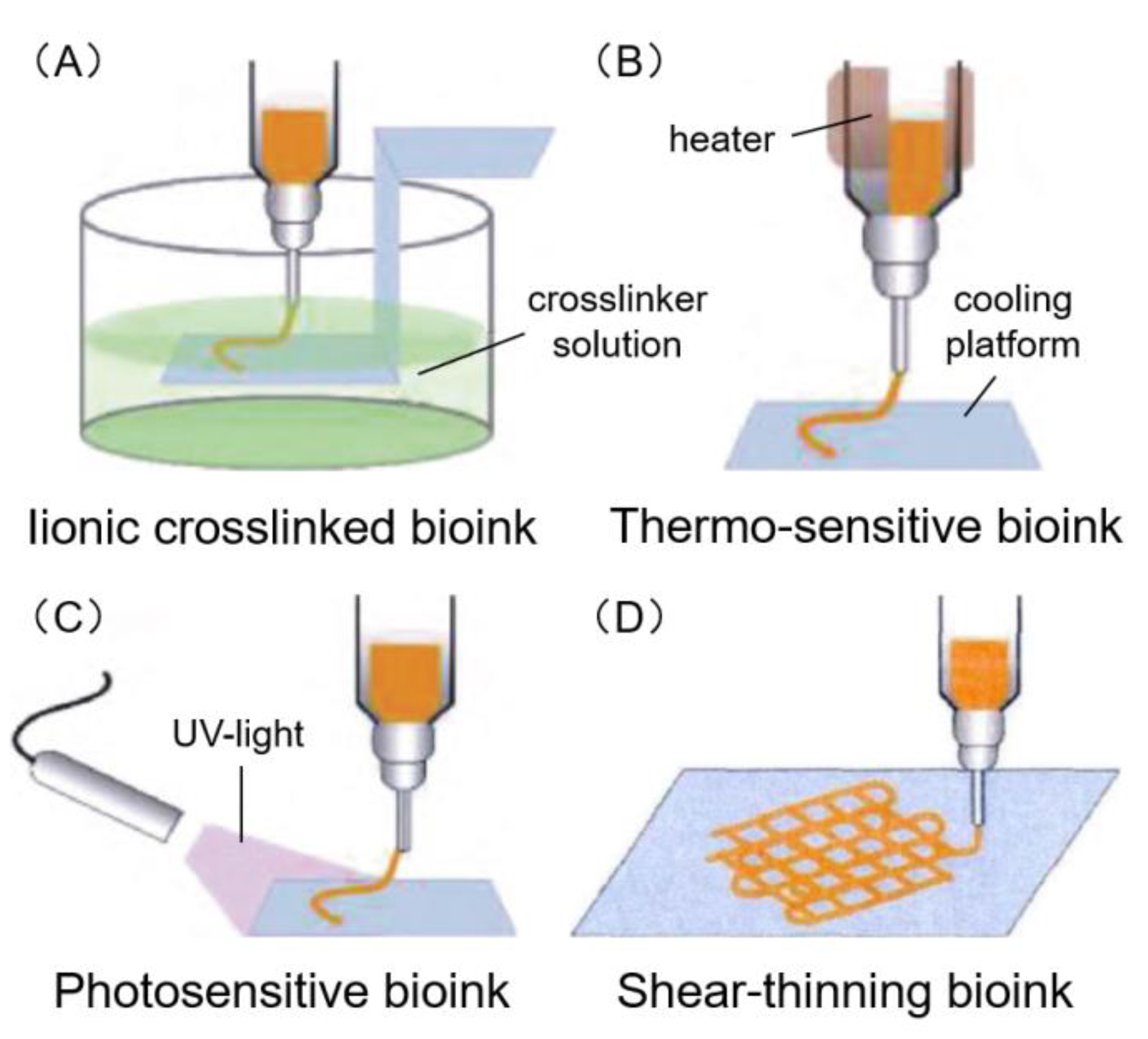
6.1. Ionic Crosslinked Bioink
6.2. Thermo-Sensitive Bioink
6.3. Photosensitive Bioink
6.4. Shear-Thinning Bioink
6.5. Decellularized Extracellular Matrix (dECM) Bioink
7. Discussion and Future Perspective
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Velasquillo, C.; Galue, E.A.; Rodriquez, L.; Ibarra, C.; Ibarra-Ibarra, L.G. Skin 3D bioprinting. Applications in cosmetology. J. Cosm. Dermatol. Sci. Appl. 2013, 3, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Coyer, F.; Gardner, A.; Doubrovsky, A.; Cole, R.; Ryan, F.M.; Allen, C.; McNamara, G. Reducing pressure injuries in critically ill patients by using a patient skin integrity care bundle (InSPiRE). Am. J. Crit. Care 2015, 24, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef]
- Yildirimer, L.; Thanh, N.T.; Seifalian, A.M. Skin regeneration scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012, 30, 638–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.F.; Barrias, C.C.; Granja, P.L.; Bartolo, P.J. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 2013, 8, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2009, 77–95. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Yao, B.; Hu, T.; Li, Z.; Huang, S.; Fu, X. Beyond 2D: 3D bioprinting for skin regeneration. Int. Wound J. 2019, 16, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Brock, W.D.; Bearden, W.; Tann, T., 3rd; Long, J.A. Autogenous dermis skin grafts in lower eyelid reconstruction. Ophthalmic. Plast. Reconstr. Surg. 2003, 19, 394–397. [Google Scholar] [CrossRef]
- Ali, J.M.; Catarino, P.; Dunning, J.; Giele, H.; Vrakas, G.; Parmar, J. Could sentinel skin transplants have some utility in solid organ transplantation? Transpl. Proc. 2016, 48, 2565–2570. [Google Scholar] [CrossRef]
- Pajardi, G.; Rapisarda, V.; Somalvico, F.; Scotti, A.; Russo, G.L.; Ciancio, F.; Sgro, A.; Nebuloni, M.; Allevi, R.; Torre, M.L.; et al. Skin substitutes based on allogenic fibroblasts or keratinocytes for chronic wounds not responding to conventional therapy: A retrospective observational study. Int. Wound J. 2016, 13, 44–52. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, Y.; Su, Y.; Cheng, T. Stem cells and their applications in skin-cell therapy. Trends Biotechnol. 2006, 24, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 2: Role of growth factors in normal and pathological wound healing: Therapeutic potential and methods of delivery. Adv. Skin Wound Care 2012, 25, 349–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, S.; Chou, C.F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater. Sci. Eng. C 2014, 34, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Fabrication and characterization of scaffold from cadaver goat-lung tissue for skin tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4032–4038. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Leu, M.C.; Mazumder, J.; Donmez, A. Additive manufacturing: Current State, future potential, gaps and needs, and recommendations. J. Manuf. Sci. Eng. 2015, 137, 014001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Moghaddam, K.M.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Bittner, S.M.; Guo, J.L.; Melchiorri, A.; Mikos, A.G. Three-dimensional Printing of Multilayered Tissue Engineering Scaffolds. Mater. Today 2018, 21, 861–874. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the field of 3D biomaterial printing. Biomed. Mater. 2016, 11, 014102. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Frueh, F.S.; Menger, M.D.; Lindenblatt, N.; Giovanoli, P.; Laschke, M.W. Current and emerging vascularization strategies in skin tissue engineering. Crit. Rev. Biotechnol. 2017, 37, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Ahn, G.; Min, K.H.; Kim, C.; Lee, J.S.; Kang, D.; Won, J.Y.; Cho, D.W.; Kim, J.Y.; Jin, S.; Yun, W.S.; et al. Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Sci. Rep. 2017, 7, 8624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Cubo, N.; Garcia, M.; Del Canizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derr, K.; Zou, J.; Luo, K.; Song, M.J.; Sittampalam, G.S.; Zhou, C.; Michael, S.; Ferrer, M.; Derr, P. Fully three-dimensional bioprinted skin equivalent constructs with validated morphology and barrier function. Tissue Eng. Part C Methods 2019, 25, 334–343. [Google Scholar] [CrossRef]
- Ding, H.; Chang, R.C. Simulating image-guided in situ bioprinting of a skin graft onto a phantom burn wound bed. Addit. Manuf. 2018, 22, 708–719. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Development of polyelectrolyte chitosan-gelatin hydrogels for skin bioprinting. Procedia CIRP 2016, 49, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Xiong, L.; Hu, Y.; Li, W.; Chen, Z.; Liu, K.; Zhang, X. Three-dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering. Polym. Eng. Sci. 2018, 58, 1782–1790. [Google Scholar] [CrossRef]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.H.; Fischer, K.; Edminster, K.; Park, J.K.; Yoo, S.S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Singh, D.; Han, S.S. 3D printing of scaffold for cells delivery: Advances in skin tissue engineering. Polymers 2016, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [Green Version]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; Van Weeren, P.R.; Dhert, W.J.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef]
- Wang, L.; Shelton, R.M.; Cooper, P.R.; Lawson, M.; Triffitt, J.T.; Barralet, J.E. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003, 24, 3475–3481. [Google Scholar]
- Diao, J.; Liu, J.; Wang, S.; Chang, M.; Wang, X.; Guo, B.; Yu, Q.; Yan, F.; Su, Y.; Wang, Y. Sweat gland organoids contribute to cutaneous wound healing and sweat gland regeneration. Cell Death Dis. 2019, 10, 238. [Google Scholar]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast Reconstr Aesthet. Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [Google Scholar] [CrossRef]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bayat, A.; Granja, P.L.; Bártolo, P.J. Advances in bioprinted cell-laden hydrogels for skin tissue engineering. Biomanuf. Rev. 2017, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [PubMed]
- Amini-Nik, S.; Glancy, D.; Boimer, C.; Whetstone, H.; Keller, C.; Alman, B.A. Pax7 expressing cells contribute to dermal wound repair, regulating scar size through a beta-catenin mediated process. Stem Cells 2011, 29, 1371–1379. [Google Scholar] [PubMed]
- Horch, R.E.; Jeschke, M.G.; Spilker, G.; Herndon, D.N.; Kopp, J. Treatment of second degree facial burns with allografts--preliminary results. Burns 2005, 31, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.; Currie, L.; Martin, R. A guide to biological skin substitutes. Br. J. Plast. Surg. 2002, 55, 185–193. [Google Scholar] [CrossRef]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar]
- Volk, S.W.; Iqbal, S.A.; Bayat, A. Interactions of the Extracellular Matrix and Progenitor Cells in Cutaneous Wound Healing. Adv. Wound Care 2013, 2, 261–272. [Google Scholar]
- Rossi, A.; Appelt-Menzel, A.; Kurdyn, S.; Walles, H.; Groeber, F. Generation of a three-dimensional full thickness skin equivalent and automated wounding. J. Vis. Exp. 2015, 96, e52576. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, T.; Tanishima, H.; Tamagawa, K.; Sakaguchi, S.; Shono, Y.; Tsubakihara, H.; Tabuse, K.; Kinoshita, Y. A wound protector shields incision sites from bacterial invasion. Surg. Infect. 2010, 11, 501–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Leng, L.; Amini-Nik, S.; Ba, Q.; Jeschke, M.; Günther, A. Skin printer: Microfluidic approach for skin regeneration and wound dressings. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Freiburg, Germany, 27–31 October 2013. [Google Scholar]
- Zoller, N.; Valesky, E.; Butting, M.; Hofmann, M.; Kippenberger, S.; Bereiter-Hahn, J.; Bernd, A.; Kaufmann, R. Clinical application of a tissue-cultured skin autograft: An alternative for the treatment of non-healing or slowly healing wounds? Dermatology 2014, 229, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Jank, B.J.; Goverman, J.; Guyette, J.P.; Charest, J.M.; Randolph, M.; Gaudette, G.R.; Gershlak, J.R.; Purschke, M.; Javorsky, E.; Nazarian, R.M.; et al. Creation of a bioengineered skin flap scaffold with a perfusable vascular pedicle. Tissue Eng. Part A 2017, 23, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verseijden, F.; Van Sluijs, S.J.P.; Farrell, E.; Van Neck, J.W.; Hovius, S.E.; Hofer, S.O.; Van Osch, G.J. Prevascular structures promote vascularization in engineered human adipose tissue constructs upon implantation. Cell Transpl. 2010, 19, 1007–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frueh, F.S.; Spater, T.; Lindenblatt, N.; Calcagni, M.; Giovanoli, P.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Adipose tissue-derived microvascular fragments improve vascularization, lymphangiogenesis, and integration of dermal skin substitutes. J. Investig. Dermatol. 2017, 137, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, H.H.; Darwood, A.R.; Shaunak, S.; Kulatilake, P.; El-Hilly, A.A.; Mulki, O.; Baskaradas, A. Three-dimensional printing in surgery: A review of current surgical applications. J. Surg. Res. 2015, 199, 512–522. [Google Scholar] [CrossRef]
- Gutierrez-Rivera, A.; Pavon-Rodriguez, A.; Cormenzana, P.; Sanz-Jaka, J.P.; Izeta, A. A protocol for enrichment of CD34+ stromal cell fraction through human skin disaggregation and magnetic separation. J. Dermatol. Sci. 2010, 59, 60–62. [Google Scholar] [CrossRef]
- Hennessy, R.; Markey, M.K.; Tunnell, J.W. Impact of one-layer assumption on diffuse reflectance spectroscopy of skin. J. Biomed. Opt. 2015, 20, 27001. [Google Scholar] [CrossRef] [Green Version]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A review of three-dimensional printing in tissue engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christenson, E.M.; Soofi, W.; Holm, J.L.; Cameron, N.R.; Mikos, A.G. Biodegradable fumarate-based PolyHIPEs as tissue engineering scaffolds. Biomacromolecules 2007, 8, 3806–3814. [Google Scholar] [CrossRef] [PubMed]
- Unkovskiy, A.; Spintzyk, S.; Axmann, D.; Engel, E.-M.; Weber, H.; Huettig, F. Additive manufacturing: A comparative analysis of dimensional accuracy and skin texture reproduction of auricular prostheses replicas. J. Prosthodont. 2019, 28, e460–e468. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Gunther, A. Handheld skin printer: In situ formation of planar biomaterials and tissues. Lab. Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, Q.; Liu, A.; Sun, M.; Fu, J. 3D bioprinting: From structure to function. J. Zhejiang Univ. (Eng. Sci.) 2019, 53, 407–419. [Google Scholar]
- Calvert, P. Materials science printing cells. Science 2007, 318, 208–209. [Google Scholar] [CrossRef]
- Derby, B. Bioprinting: Inkjet printing proteins and hybrid cell-containing materials and structures. J. Mater. Chem. 2008, 18, 5717–5721. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Koch, L.; Gruene, M.; Unger, C.; Chichkov, B. Laser assisted cell printing. Curr. Pharm. Biotechnol. 2013, 14, 91–97. [Google Scholar]
- Obata, K.; El-Tamer, A.; Koch, L.; Hinze, U.; Chichkov, B.N. High-aspect 3D two-photon polymerization structuring with widened objective working range (WOW-2PP). Light Sci. Appl. 2013, 2, e116. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jungst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Sun, W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater. Sci. Eng. C 2007, 27, 469–478. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-printed microfluidics. Angew. Chem. Int. Edit. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stabil. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Min, D.; Lee, W.; Bae, I.H.; Lee, T.R.; Croce, P.; Yoo, S.S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2018, 27, 453–459. [Google Scholar] [CrossRef]
- Chandrasekaran, A.R.; Venugopal, J.; Sundarrajan, S.; Ramakrishna, S. Fabrication of a nanofibrous scaffold with improved bioactivity for culture of human dermal fibroblasts for skin regeneration. Biomed. Mater. 2011, 6, 015001. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Ullah, M.W.; Ullah, I.; Ou, H.; Zhang, W.; Xiong, L.; Zhang, X. Cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosa for potential applications in skin tissue engineering. Biofabrication 2019, 11, 035023. [Google Scholar] [CrossRef]
- Huang, S.; Yao, B.; Xie, J.; Fu, X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 2016, 32, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Lee, M.H.; Kim, M.J.; Min, K.H.; Ahn, G.; Han, J.S.; Jin, S.; Yun, W.S.; Shim, J.H. A potential dermal substitute using decellularized dermis extracellular matrix derived bio-ink. Artif. Cells Nanomed. Biotechnol. 2019, 47, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A 2013, 101, 272–284. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Chidambarampadmavathy, K.; Cirés, S.; Heimann, K. Review of sustainable methane mitigation and biopolymer production. Crit. Rev. Env. Sci. Technol. 2014, 45, 1579–1610. [Google Scholar]
- Nitta, S.K.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprint. 2016, 2. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, T.L.; Zhang, H.B.; Yin, R.X.; Yang, S.M.; Wei, J.; Zhang, W.J. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed. Mater. 2018, 13, 035008. [Google Scholar] [CrossRef]
- Yang, K.; Shi, Y.; Zhang, H.; Yin, R.; Yang, S.; Zhang, W. Bioink of multi-biomaterials with tyrosine reinforcement for 3D bioprinting of skin constructs. Adv. Biol. Sci. Res. 2017, 4, 140–145. [Google Scholar]
- Yoon, S.; Park, J.A.; Lee, H.R.; Yoon, W.H.; Hwang, D.S.; Jung, S. Inkjet-spray hybrid printing for 3D freeform fabrication of multilayered hydrogel structures. Adv. Healthc. Mater. 2018, 7, e1800050. [Google Scholar] [PubMed]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [PubMed]
- Khew, S.T.; Tong, Y.W. The specific recognition of a cell binding sequence derived from type I collagen by Hep3B and L929 cells. Biomacromolecules 2007, 8, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Abas, M.; Biswas, N.; Banerjee, P.; Ghosh, N.; Rawat, A.; Khanna, S.; Roy, S.; Sen, C.K. A modified collagen dressing induces transition of inflammatory to reparative phenotype of wound macrophages. Sci. Rep. 2019, 9, 14293. [Google Scholar]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.C.; Lacour, S.P.; Raffoul, W.; Di Summa, P.G. Extracellular matrix components in peripheral nerve repair: How to affect neural cellular response and nerve regeneration? Neural Regen. Res. 2014, 9, 1943–1948. [Google Scholar]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajangam, T.; An, S.S. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int. J. Nanomed. 2013, 8, 3641–3662. [Google Scholar]
- Hakam, M.S.; Imani, R.; Abolfathi, N.; Fakhrzadeh, H.; Sharifi, A.M. Evaluation of fibrin-gelatin hydrogel as biopaper for application in skin bioprinting: An in-vitro study. Biomed. Mater. Eng. 2016, 27, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a Natural polysaccharide to complex networks. Soft Matter. 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Li, Q.; Yang, L.; Liu, H. Chemically modified magnetic chitosan microspheres for Cr(VI) removal from acidic aqueous solution. Particuology 2016, 26, 79–86. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011, 2011, 1–29. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef]
- Munarin, F.; Petrini, P.; Tanzi, M.C.; Barbosa, M.A.; Granja, P.L. Biofunctional chemically modified pectin for cell delivery. Soft Matter. 2012, 8, 4731. [Google Scholar] [CrossRef]
- Neves, S.C.; Gomes, D.B.; Sousa, A.; Bidarra, S.J.; Petrini, P.; Moroni, L.; Barrias, C.C.; Granja, P.L. Biofunctionalized pectin hydrogels as 3D cellular microenvironments. J. Mater. Chem. B 2015, 3, 2096–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.F.; Barrias, C.C.; Bartolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef]
- Gruene, M.; Pflaum, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Wilhelmi, M.; Haverich, A.; Chichkov, B.N. Adipogenic differentiation of laser-printed 3D tissue grafts consisting of human adipose-derived stem cells. Biofabrication 2011, 3, 015005. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byme, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Singh, R.; Chen, C.; Phelps, R.G.; Elston, D.M. Stem cells in the skin and their role in oncogenesis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 542–549. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Itsykson, P.; Turetsky, T.; Pera, M.F.; Reinhatrz, E.; Itzik, A.; Ben-Hur, T. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1134–1140. [Google Scholar] [CrossRef]
- Ou, K.L.; Yu, C.H.; Chan, Y.H.; Cheng, W.J. Method for three dimensional printing artificial skin. U.S. Patent 2018/0105781A1, 19 April 2018. [Google Scholar]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, S.; Iwabuki, H.; Morimoto, C.; Hieda, M.; Inoue, H.; Matsushita, N. Membrane-anchored growth factors, the epidermal growth factor family: Beyond receptor ligands. Cancer Sci. 2008, 99, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Matsuoka, Y.; Funahashi, A.; Kitano, H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 2005, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, V.B.; Besner, G.E. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 2007, 25, 253–263. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Cardinali, G.; Aspite, N.; Picardo, M.; Marchese, C.; Torrisi, M.R.; Mancini, P. Cortactin involvement in the keratinocyte growth factor and fibroblast growth factor 10 promotion of migration and cortical actin assembly in human keratinocytes. Exp. Cell Res. 2007, 313, 1758–1777. [Google Scholar] [CrossRef]
- Di Vita, G.; Patti, R.; D’Agostino, P.; Caruso, G.; Arcara, M.; Buscemi, S.; Bonventre, S.; Ferlazzo, V.; Arcoleo, F.; Cillari, E. Cytokines and growth factors in wound drainage fluid from patients undergoing incisional hernia repair. Wound Repair Regen. 2006, 14, 259–264. [Google Scholar] [CrossRef]
- Sogabe, Y.; Abe, M.; Yokoyama, Y.; Ishikawa, O. Basic fibroblast growth factor stimulates human keratinocyte motility by Rac activation. Wound Repair Regen. 2006, 14, 457–462. [Google Scholar] [CrossRef]
- Goldberg, M.T.; Han, Y.P.; Yan, C.; Shaw, M.C.; Garner, W.L. TNF-alpha suppresses alpha-smooth muscle actin expression in human dermal fibroblasts: An implication for abnormal wound healing. J. Investig. Dermatol. 2007, 127, 2645–2655. [Google Scholar] [CrossRef] [Green Version]
- Kopecki, Z.; Luchetti, M.M.; Adams, D.H.; Strudwick, X.; Mantamadiotis, T.; Stoppacciaro, A.; Gabrielli, A.; Ramsay, R.G.; Cowin, A.J. Collagen loss and impaired wound healing is associated with c-Myb deficiency. J. Pathol. 2007, 211, 351–361. [Google Scholar] [CrossRef]
- Riedel, K.; Riedel, F.; Goessler, U.R.; Germann, G.; Sauerbier, M. TGF-beta antisense therapy increases angiogenic potential in human keratinocytes in vitro. Arch. Med. Res. 2007, 38, 45–51. [Google Scholar] [CrossRef]
- Uutela, M.; Wirzenius, M.; Paavonen, K.; Rajantie, I.; He, Y.; Karpanen, T.; Lohela, M.; Wiig, H.; Salven, P.; Pajusola, K.; et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood 2004, 104, 3198–3204. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, B.; Sun, W.; Zhao, W.; Zhao, Y.; Dai, J. The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials 2006, 27, 5708–5714. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, F.; Zambruno, G.; Brogelli, L.; Sera, F.; Lacal, P.M.; Pesce, M.; Capogrossi, M.C.; Failla, C.M.; Napolitano, M.; Odorisio, T. Placenta growth factor in diabetic wound healing: Altered expression and therapeutic potential. Am. J. Pathol. 2006, 169, 1167–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colwell, A.S.; Phan, T.T.; Kong, W.; Longaker, M.T.; Lorenz, P.H. Hypertrophic scar fibroblasts have increased connective tissue growth factor expression after transforming growth factor-beta stimulation. Plast. Reconstr. Surg. 2005, 116, 1387–1390. [Google Scholar] [CrossRef]
- Yönem, A.; Çakir, B.; Güler, S.; Azal, Ö.; Çorakçi, A. Effects of granulocyte-colony stimulating factor in the treatment of diabetic foot infection. Diabetes Obes. Metab. 2001, 3, 332–337. [Google Scholar] [CrossRef]
- Raja, R.; Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef] [Green Version]
- Christopherson, K., II; Hromas, R. Chemokine regulation of normal and pathologic immune responses. Stem Cells 2001, 19, 388–396. [Google Scholar] [CrossRef]
- Florin, L.; Maas-Szabowski, N.; Werner, S.; Szabowskl, A.; Angel, P. Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J. Cell Sci. 2005, 118, 1981–1989. [Google Scholar]
- Dipietro, L.A.; Reintjes, M.G.; Low, Q.E.H.; Levi, B.; Gamelli, R.L. Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound Repair Regen. 2001, 9, 28–33. [Google Scholar]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ikada, Y.; Tabata, Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J. Biomater. Sci. Polym. Ed. 2001, 12, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.A.; Enemchukwu, N.O.; Fiore, V.F.; Sy, J.C.; Murthy, N.; Sulchek, T.A.; Barker, T.H.; Garcia, A.J. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 2012, 24, 62, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Fedorovich, N.E.; Swennen, I.; Girones, J.; Moroni, L.; Van Bilitterswijk, C.A.; Schacht, E.; Alblas, J.; Dhert, W.J.A. Evaluation of photocrosslinked lutrol hydrogel for tissue printing applications. Biomacromolecules 2009, 10, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [PubMed] [Green Version]
- Wang, H.; Heilshorn, S.C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar]
- Datta, S.; Sarkar, R.; Vyas, V.; Bhutoria, S.; Barui, A.; Roy Chowdhury, A.; Datta, P. Alginate-honey bioinks with improved cell responses for applications as bioprinted tissue engineered constructs. J. Mater. Res. 2018, 33, 2029–2039. [Google Scholar] [CrossRef]
- Kim, G.; Ahn, S.; Kim, Y.; Cho, Y.; Chun, W. Coaxial structured collagen–alginate scaffolds: Fabrication, physical properties, and biomedical application for skin tissue regeneration. J. Mater. Chem. 2011, 21, 6165. [Google Scholar]
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of bio-active patches based on pectin for the treatment of ulcers and wounds using 3D-bioprinting technology. Pharmaceutics 2020, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Pourchet, L.J.; Thepot, A.; Albouy, M.; Courtial, E.J.; Boher, A.; Blum, L.J.; Marquette, C.A. Human skin 3D bioprinting using scaffold-free approach. Adv. Healthc. Mater. 2017, 6, 1601101. [Google Scholar]
- Lee, W.; Lee, V.; Polio, S.; Keegan, P.; Lee, J.H.; Fischer, K.; Park, J.K.; Yoo, S.S. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol. Bioeng. 2010, 105, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted skin recapitulates normal collagen remodeling in full-thickness wounds. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef] [PubMed]
- Admane, P.; Gupta, A.C.; Jois, P.; Roy, S.; Chandrasekharan Lakshmanan, C.; Kalsi, G.; Bandyopadhyay, B.; Ghosh, S. Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting 2019, 15, e00051. [Google Scholar] [CrossRef]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering. Mater. Horiz. 2018, 5, 1100–1111. [Google Scholar] [CrossRef]
- Kwak, H.; Shin, S.; Lee, H.; Hyun, J. Formation of a keratin layer with silk fibroin-polyethylene glycol composite hydrogel fabricated by digital light processing 3D printing. J. Ind. Eng. Chem. 2019, 72, 232–240. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Rimann, M.; Bono, E.; Annaheim, H.; Bleisch, M.; Graf-Hausner, U. Standardized 3D bioprinting of soft tissue models with human primary cells. J. Lab. Autom. 2016, 21, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Lee, J.S.; Gao, G.; Cho, D.W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar] [CrossRef]
- Michael, S.; Sorg, H.; Peck, C.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Ahn, S.H.; Yoon, H.; Kim, G.H.; Kim, Y.Y.; Lee, S.H.; Chun, W. Designed three-dimensional collagen scaffolds for skin tissue regeneration. Tissue Eng. Part C 2010, 16, 813–820. [Google Scholar] [CrossRef]
- Xue, S.L.; Liu, K.; Parolini, O.; Wang, Y.; Deng, L.; Huang, Y.C. Human acellular amniotic membrane implantation for lower third nasal reconstruction: A promising therapy to promote wound healing. Burns Trauma 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Man, Y.; Li, W.; Li, L.; Xu, J.; Parungao, R.; Wang, Y.; Zheng, S.; Nie, Y.; Liu, T.; et al. 3D Bio-printing of CS/Gel/HA/Gr hybrid osteochondral scaffolds. Polymers 2019, 11, 1601. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Dhanasopon, A.P.; Rofail, F.; Smith, H.; Wu, B.M.; Shemin, R.; Beygui, R.E.; MacLellan, W.R. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 2008, 29, 2907–2914. [Google Scholar] [CrossRef] [Green Version]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef] [Green Version]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilic, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Macadangdang, J.; Leung, W.; Laflamme, M.A.; Kim, D.H. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol. Adv. 2017, 35, 77–94. [Google Scholar] [CrossRef]




| Parameters | Inkjet | Laser | Extrusion | Stereolithography | Microfluidic |
|---|---|---|---|---|---|
| Printing process | Serial (drop by drop) | Serial (dot by dot) | Serial (line by line) | Parallel and continuous (projection based) | Organ-on-a-chip |
| Cost | Low | High | Medium | Low | Low |
| Cell viability | >85% | >95% | 40–80% | >85% | >80% |
| Print speed | Fast | Medium | Slow | Fast | Fast |
| Supported viscosities | 3.5–12 mPa/s | 1–300 mPa/s | 30–6 × 107 mPa/s | No limitation | 0–30 Pa/s |
| Resolution | High | High | Medium | High | High |
| Cell density | Low | Medium | High | Medium | High |
| Representative materials | alginate, collagen | collagen, alginate | alginate, GelMA, collagen | GelMA | collagen, alginate, gelatin |
| References | [18,30,69,78,79] | [18,69,80,81] | [18,69,82,83] | [69,74,75,84] | [51,65,77,85] |
| Hydrogels | Crosslinking Method | Crosslinking Time | References |
|---|---|---|---|
| Collagen | hydrophobic bonding | 0.5–60 min | [87,95,96,97] |
| Gelatin | temperature/glutaraldehyde | minutes to hours | [29,34,98] |
| Alginate | CaCl2 | seconds | [36,87,99,100] |
| Fibrin | thrombin | seconds | [101,102,103,104] |
| Hyaluronic acid | thiol group crosslink/UV light | 15–30 min/s | [35,65,87,105,106] |
| Chitosan | pH | 5–50 min | [87,92,107,108,109] |
| Pectin | thiol group crosslink | 15–30 min | [110,111,112,113,114,116] |
| Signaling Molecules | Cell | Function |
|---|---|---|
| EGF | PLs, MPs, FBs, KCs | Reepithelialization, Angiogenesis [124,125,126] |
| FGF | KCs, MCs, FBs, ECs, SMCs, CCs | Granulation tissue formation, Reepithelialization, Tissue remodeling [127,128,129] |
| TGF-β | PLs, KCs, MPs, FBs | Inflammation, Angiogenesis, Granulation tissue formation, Reepithelialization, Tissue remodeling [130,131,132] |
| PDGF | PLs, KCs, MPs, ECs, FBs | Inflammation, Granulation tissue formation, Angiogenesis, Reepithelialization, Tissue remodeling [133,134] |
| VEGF | PLs, NPs, MPs, ECs, SMCs, FBs | Inflammation, Angiogenesis, Granulation tissue formation [135] |
| CTGF | FBs | Granulation tissue formation, Reepithelialization, Tissue remodeling [136] |
| CM-CSF | KCs | Inflammation, Angiogenesis, Reepithelialization [137] |
| IL-1 | NPs, MOs, MPs, KCs | Inflammation, Reepithelialization [138] |
| IL-6 | NPs, MPs | Inflammation, Reepithelialization [128] |
| TNF-α | NPs, MPs | Inflammation, Reepithelialization [128] |
| CXCL1 | NPs | Reepithelialization [139] |
| CXCL8 | KCs, LCs | Reepithelialization, Tissue remodeling [139] |
| CXCL10 | KCs | Inflammation [140] |
| CXCL12 | ECs, KCs, MFBs | Inflammation, Angiogenesis, Reepithelialization [140] |
| CCL2 | KCs | Inflammation [141] |
| Materials | Cell Encapsulation | Bio-Factors/Crosslinker | Bioink Type | Bioprinting Technology | Ref. |
|---|---|---|---|---|---|
| Alg, Gel | FBs | FBS/CaCl2, EDC, NHS | Ionic, thermo-sensitive | Extrusion | [29] |
| Alg, CNF, GelMA, fibrinogen | FBs | FBS/CaCl2, thrombin | Ionic, photosensitive | Inkjet | [93] |
| Gel, Alg | EPC, DH | FBS, EGF/CaCl2 | Ionic, thermo-sensitive | Extrusion | [82] |
| Alg, honey | FBs | FBS/CaCl2, | Ionic | Extrusion | [149] |
| Col, Alg | FBs, KCs | FBS, KGS/CaCl2, EDC | Ionic | Extrusion | [150] |
| Pectin, CS, CD, propolis | FBs | FBS | Ionic | Extrusion | [151] |
| Gel, Alg | FBs | FBS/CaCl2 | Thermo-sensitive, ionic | Inkjet | [27] |
| Fibrinogen, Gel | FBs | FBS/Thrombin | Thermo-sensitive | - | [104] |
| Gel, Alg, fibrinogen | FBs, KCs | CaCl2, thrombin | Thermo-sensitive, ionic | Extrusion | [152] |
| Col, Gel | FBs | FBS, FGS/NaHCO3 | Thermo-sensitive | Inkjet | [153] |
| Fibrinogen, Gel, Gly, HA | KCs, MCs, FBs, pACs, FDPC, DMEC | FBS, EGF, FGF, VEGF, IGF/Thrombin | Thermo-sensitive | Extrusion | [154] |
| Fibroin, Gel | FBs, KCs | FBS, FGF, EGF/Ty | Thermo-sensitive | Extrusion | [155] |
| Pectin | FBs, KCs | FBS/CA | Photosensitive | Extrusion | [114] |
| Pectin | FBs | CaCl2, MA | Photosensitive, ionic | Extrusion | [156] |
| Fibroin, PEG4A | FBs, KCs | FBS/NVP | Photosensitive | Stereolithography | [157] |
| GelMA, PEG | FBs | FBS/NVP | Photosensitive | Stereolithography | [158] |
| PEG | FBs, KCs | FBS | Photosensitive | Inkjet | [159] |
| Col | FBs, KCs | FBS/NaHCO3 | Shear thinning | Inkjet | [20] |
| Col | FBs, KCs | FBS, FGS, KGS/NaHCO3 | Shear thinning | Inkjet | [30] |
| Col, Alg | FBs, KCs | - | Shear thinning | Microfluidic | [51] |
| Col, Alg, fibrinogen, HA | FBs, KCs | CaCl2, thrombin | Shear thinning, ionic | Microfluidic | [65] |
| GelMA, Col | FBs, KCs, MCs | FBS/Ty | Shear thinning, photosensitive | Extrusion | [91] |
| CS, HA, Col | KCs | Ty | Shear thinning | Extrusion | [92] |
| Col, Alg | FBs, KCs | FBS/CaCl2 | Shear thinning, ionic | Laser | [83] |
| Col, Gel | FBs, KCs | FBS, KGS | Shear thinning, thermo-sensitive | Extrusion, inkjet | [160] |
| Col | FBs, KCs, MCs | FBS, KGS, MGS/NaHCO3 | Shear thinning | Laser | [79] |
| Col | FBs, KCs, MCs | FBS, KGS, MGS, FGS | Shear thinning | Inkjet | [116] |
| Col | FBs, KCs | FBS | Shear thinning | Laser | [161] |
| Col | FBs, KCs | FBS, FGS, KGS/EDC | Shear thinning | Extrusion | [162] |
| Col, fibrinogen | AFS, MSCs | FBS/Thrombin | Shear thinning | Extrusion | [94] |
| dECM | FBs, KCs, ASC, EPC, MNC | FBS, KGS, VEGF, FGF, EGF, IGF | dECM, thermo-sensitive | Inkjet, extrusion | [122] |
| dECM | FBs | FBS | dECM, thermo-sensitive | Inkjet | [84] |
| dECM | FBs | FBS | dECM, thermo-sensitive | Extrusion | [23] |
| dECM | FBs | FBS/EDC | dECM, thermo-sensitive | Extrusion | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zheng, S.; Hu, X.; Li, L.; Li, W.; Parungao, R.; Wang, Y.; Nie, Y.; Liu, T.; Song, K. Advances in the Research of Bioinks Based on Natural Collagen, Polysaccharide and Their Derivatives for Skin 3D Bioprinting. Polymers 2020, 12, 1237. https://doi.org/10.3390/polym12061237
Xu J, Zheng S, Hu X, Li L, Li W, Parungao R, Wang Y, Nie Y, Liu T, Song K. Advances in the Research of Bioinks Based on Natural Collagen, Polysaccharide and Their Derivatives for Skin 3D Bioprinting. Polymers. 2020; 12(6):1237. https://doi.org/10.3390/polym12061237
Chicago/Turabian StyleXu, Jie, Shuangshuang Zheng, Xueyan Hu, Liying Li, Wenfang Li, Roxanne Parungao, Yiwei Wang, Yi Nie, Tianqing Liu, and Kedong Song. 2020. "Advances in the Research of Bioinks Based on Natural Collagen, Polysaccharide and Their Derivatives for Skin 3D Bioprinting" Polymers 12, no. 6: 1237. https://doi.org/10.3390/polym12061237
APA StyleXu, J., Zheng, S., Hu, X., Li, L., Li, W., Parungao, R., Wang, Y., Nie, Y., Liu, T., & Song, K. (2020). Advances in the Research of Bioinks Based on Natural Collagen, Polysaccharide and Their Derivatives for Skin 3D Bioprinting. Polymers, 12(6), 1237. https://doi.org/10.3390/polym12061237






