Composite Polymeric Cryogel Cartridges for Selective Removal of Cadmium Ions from Aqueous Solutions
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Composite Cryogel Cartridges
2.3. Characterization of MIP Composite Cryogel Cartridges
2.4. Removal of Cd(II) Ions from Aqueous Solutions
2.5. Selectivity Experiments
2.6. Reusability Studies and Removal Efficiency in Artificial Wastewater Sample
3. Results and Discussion
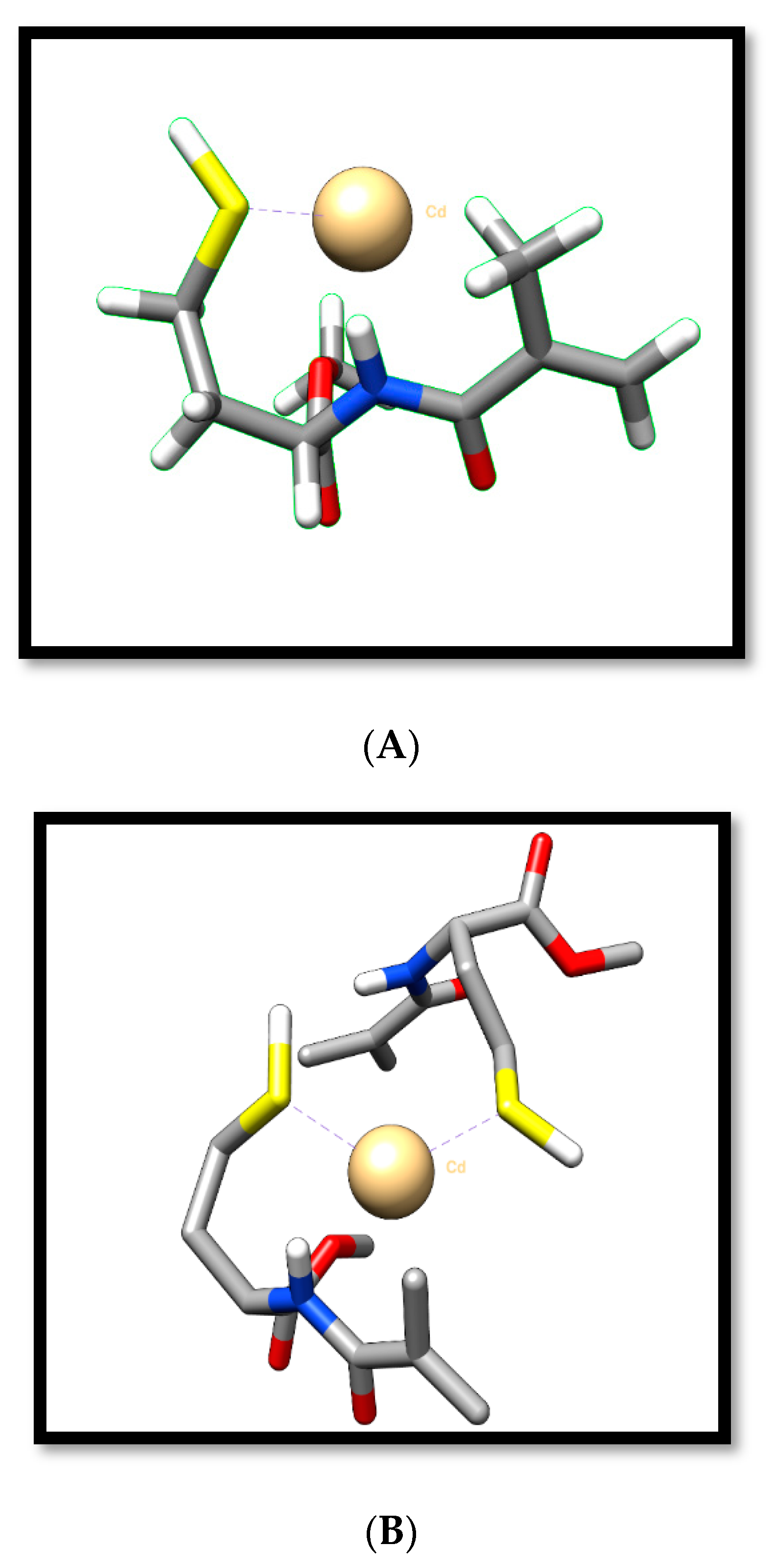
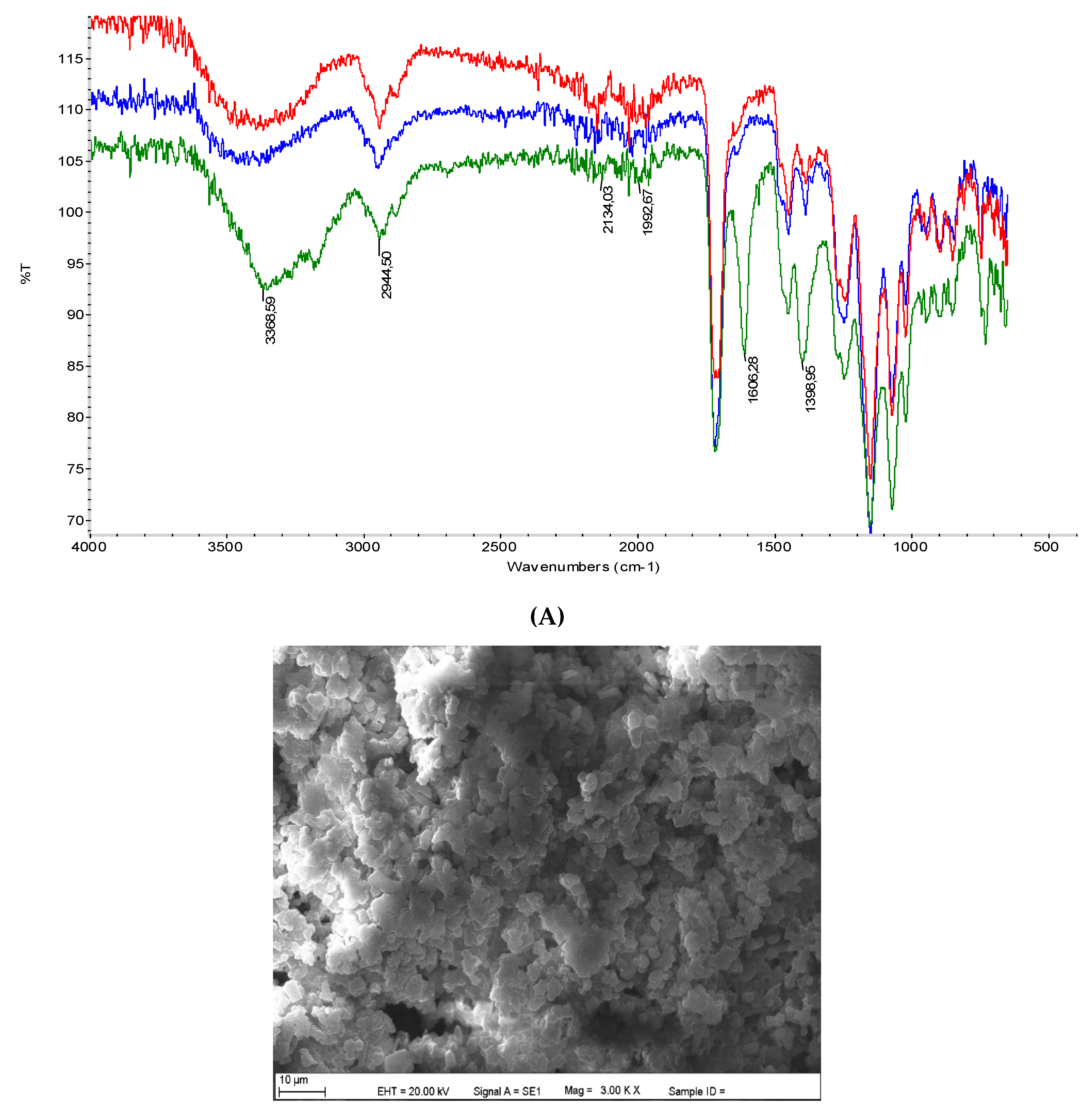
3.1. Characterization Studies
3.2. Optimization of Preparation and Binding Conditions
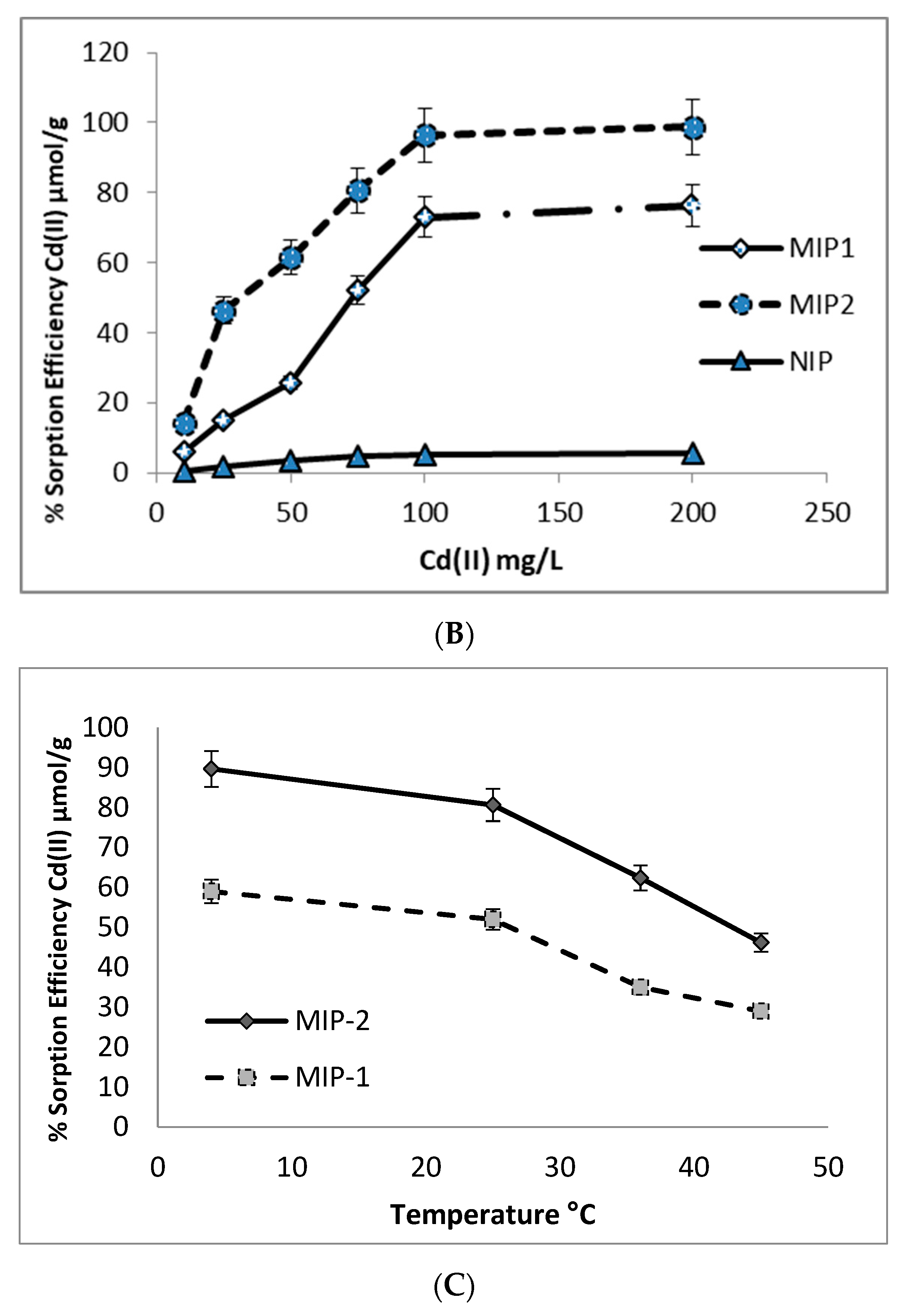
3.3. Adsorption of Cd(II) from Aqueous Solutions
3.4. Comparison of Adsorption Capacities for the Cd (II) ion of Various Cd (II)-Imprinted Polymers from Aqueous Samples
3.5. Selectivity Experiments
3.6. Reusability Studies and Removal Efficiency from an Environmental Water Sample
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.; Yang, X.; Wang, P.; Wang, Z.X.; Li, M.; Zhao, F.J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.; Yedjou, C.; Patlolla, A.; Sutton, D. Heavy metal Toxicity and theenvironment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Deng, X.J.; Lü, L.L.; Li, H.W.; Fang, L. The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2018, 183, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Alina, M.; Azrina, A.; Mohd, Y.A.S.; Mohd, Z.S.; Mohd, I.E.H.; Muhammad, R.R. Heavy metals (mercury, arsenic, cadmium, plumbum) in selected marine fish and shellfish along the Straits of Malacca. Int. Food Res. 2012, 19, 135–140. [Google Scholar]
- Karri, V.; Kumar, V.; Ramos, D.; Oliveira, E.; Schuhmacher, M. Comparative In Vitro Toxicity Evaluation of Heavy Metals (Lead, Cadmium, Arsenic, and Methylmercury) on HT-22 Hippocampal Cell Line. Biol. Trace Elem. Res. 2018, 184, 226–239. [Google Scholar] [CrossRef]
- Sağ, Y.; Kutsal, T. The selective biosorption of chromium (VI) and copper (II) ions from binary metal mixtures, R. Arrhizus. Process. Biochem. 1996, 31, 561–572. [Google Scholar] [CrossRef]
- Yavuz, O.; Altunkaynak, Y.; Guzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, J.; Li, H.; Zhang, X.; Shi, H. Removal of cadmium (II) from aqueous solution by granular activated carbon supported magnesium hydroxide. J. Taiwan Inst. Chem. Eng. 2016, 61, 287–291. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Ehteshami, M.; Dahrazma, B. Isotherm and kinetic studies for the biosorption of cadmium from aqueous solution by Alhajimaurorum seed. Process Saf. Environ. Prot. 2015, 98, 374–382. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metal uptake from contaminated water. J. Hazard. Mater. 2003, 9, 219–224. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- Yaacoubi, H.; Zidani, O.; Mouflih, M.; Gourai, M.; Sebti, S. Removal of cadmium from water using natural phosphate as adsorbent. Procedia Eng. 2014, 83, 386–393. [Google Scholar] [CrossRef]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Attar, K.; Bouazza, D.; Miloudi, H.; Tayeb, A.; Boos, A.; Sastre, A.M.; Demey, H. Cadmium removal by a low-cost magadiite-based material: Characterization and sorption applications. J. Environ. Chem. Eng. 2018, 6, 5351–5360. [Google Scholar] [CrossRef]
- Demey, H.; Barron-Zambrano, J.; Mhadhbi, T.; Miloudi, H.; Yang, Z.; Ruiz, M.; Sastre, A.M. Boron Removal from Aqueous Solutions by Using a Novel Alginate-Based Sorbent: Comparison with Al2O3 Particles. Polymers 2019, 11, 1509. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking Water Quality, 4th ed.; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 2011. [Google Scholar]
- Campbell, P.G.C. Cadmium a priority pollutant. Environ. Chem. 2006, 3, 387–388. [Google Scholar] [CrossRef]
- Luo, C.S.; Huang, S. Adsorption of copper ion with metal hydroxide from ammonia solution. Sep. Sci. Technol. 1993, 28, 1253. [Google Scholar] [CrossRef]
- Mustafa, S.; Baloch, N.; Muhammad, S.; Malik, Y.; Khan, T.; Bibi, M.; Qadir, A.; Razaque, G.; Baloch, I.A. Determination of trace and heavy metals in drinking water of Jhal Magsi district of Balochistan, Pakistan. Pure Appl. Biol. 2017, 6, 9–17. [Google Scholar] [CrossRef]
- Järup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Pavel, M.E.; Baudin, E.; Öberg, K.E.; Hainsworth, J.D.; Voi, M.; Rouyrre, N.; Peeters, M.; Gross, D.J.; Yao, J.C. Efficacy of everolimus plus octreotide LAR in patients with advanced neuroendocrine tumor and carcinoid syndrome: Final overall survival from the randomized, placebo-controlled phase 3 RADIANT-2 study. Ann. Oncol. 2017, 28, 1569–1575. [Google Scholar] [CrossRef]
- Donpunha, W.; Kukongviriyapan, U.; Sompamit, K.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effect of ascorbic acid on cadmium-induced hypertension and vascular dysfunction in mice. BioMetals 2011, 24, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Şarkaya, K.; Bakhshpour, M.; Denizli, A. Ag+ ions imprinted cryogels for selective removal of silver ions from aqueous solutions. Sep. Sci. Technol. 2018, 54, 2993–3004. [Google Scholar] [CrossRef]
- Yoshikawa, M. Molecularly imprinted polymeric membranes. Bioseparation 2002, 10, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Haginaka, J.; Kagawa, C. Uniformly sized molecularly imprinted polymer for d- chlorpheniramine. Evaluation of retention and molecular recognition properties in an aqueous mobile phase. J. Chromatogr. A 2002, 948, 77–84. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Göktürk, I.; Bereli, N.; Denizli, A. Molecularly imprinted cryogel cartridges for the selective recognition of tyrosine. Biotechnol. Prog. 2020, e3006. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Yan, H.; Ho Row, K. Characteristics of a Monolithic Molecularly Imprinted Column and Its Application for Chromatographic Separation. Ind. Eng. Chem. 2007, 13, 552–557. [Google Scholar]
- Göktürk, I.; Üzek, R.; Uzun, L.; Denizli, A. Synthesis of a specific monolithic column with artificial recognition sites for L-glutamic acid via cryo-crosslinking of imprinted nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1133–1140. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Cao, W.; Ji, X.; Wang, J.; Yan, Y.; Zhong, T.; Wang, Y. Synthesis of Molecularly Imprinted Cryogels to Deplete Abundant Proteins from Bovine Serum. Polymers 2018, 10, 97. [Google Scholar] [CrossRef]
- Zhai, Y.H.; Liu, Y.W.; Chang, X.J.; Chen, S.B.; Huang, X.P. Selective solid-phase extraction of trace cadmium (II) with an ionic imprinted polymer prepared from a dual-ligand monomer. Anal. Chim. Acta 2007, 593, 123–128. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Kitikova, N.V.; Shashkova, I.L.; Oleksiienko, O.V.; Levchuk, I.; Sillanpää, M. Using of phosphatized dolomite for treatment of real mine water from metal ions. J. Water Process. Eng. 2016, 9, 246–253. [Google Scholar] [CrossRef]
- He, Y.; Lai, Z.; Yan, T.; He, X.; Lu, Z.; Lv, S.; Li, F.; Fan, X.; Zhang, H. Effect of Cd2+ on early hydration process of magnesium phosphatecement and its leaching toxicity properties. Constr. Build. Mater. 2019, 209, 32–40. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Srivastav, V.; Kitikov, N.V.; Shashkov, I.L.; Sillanpää, M. Non-apatite Ca-Mg phosphate sorbent for removal of toxic metal ions fromaqueous solutions. J. Environ. Chem. Eng. 2017, 5, 2010–2017. [Google Scholar] [CrossRef]
- Tran, H.N.; Nguyen, H.C.; Woo, S.H.; Nguyen, T.V.; Vigneswaran, S.; Hosseini-Bandegharaei, A.; Rinklebe, J.; Sarmah, A.K.; Ivanets, A.; Dotto, G.L.; et al. Removal of various contaminants from water by renewable lignocellulose-derived biosorbents: A comprehensive and critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2155–2219. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Topcu, A.A.; Bereli, N.; Alkan, H.; Denizli, A. Poly(Hydroxyethyl Methacrylate) Immunoaffinity Cryogel Column for the Purification of Human Immunoglobulin M. Gels 2020, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Denizli, A. Supermacroporous Composite Cryogels in Biomedical Applications. Gels 2019, 5, 20. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Idil, N.; Percin, I.; Denizli, A. Biomedical Applications of Polymeric Cryogels. Appl. Sci. 2019, 9, 553. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Yavuz, H.; Denizli, A. Controlled release of mitomycin C from PHEMAH–Cu (II) cryogel membranes, Artif. Cells. Nanomed. Biotechnol. 2018, 46, 946–954. [Google Scholar]
- Erdem, Ö.; Saylan, Y.; Andaç, M.; Denizli, A. Molecularly imprinted polymers for removal of metal ions: An alternative treatment method. Biomimetics 2018, 3, 38. [Google Scholar] [CrossRef]
- Aşır, S.; Uzun, L.; Türkmen, D.; Say, R.; Denizli, A. Ion-selective imprinted superporous monolith for cadmium removal from human plasma. Sep. Sci. Technol. 2005, 40, 3167–3185. [Google Scholar] [CrossRef]
- Sutirman, A.Z.; Sanagia, M.M.; Karim, K.J.; Ibrahim, W.A.W.; Jume, B.H. Equilibrium, kinetic and mechanism studies of Cu(II) and Cd(II) ions adsorption by modified chitosan beads. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tamahkar, E.; Bakhshpour, M.; Andaç, M.; Denizli, A. Ion imprinted cryogels for selective removal of Ni(II) ions from aqueous solutions. Sep. Pur. Technol. 2017, 179, 36–44. [Google Scholar] [CrossRef]
- Metcallf, L.; Eddy, H.P. Wastewater Engineering, 3rd ed.; Mc Graw Hill: New York, NY, USA, 1991; pp. 48–126. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, J.F.; Mckay, G. Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Res. 2001, 35, 605–612. [Google Scholar] [CrossRef]
- Lia, M.; Feng, C.; Li, M.; Zeng, Q.; Gan, Q.; Yang, H. Synthesis and characterization of a surface-grafted Cd(II) ion-imprinted polymer for selective separation of Cd(II) ion from aqueous solution. Appl. Surf. Sci. 2015, 332, 463–472. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Meng, M.; Liu, Z.; Ni, L.; Meng, X.; Qiu, J. RAFT-mediated microemulsion polymerization to synthesize a novel high-performance graphene oxide-based cadmium imprinted polymer. Chem. Eng. J. 2016, 302, 609–618. [Google Scholar] [CrossRef]
- Ashouri, N.; Mohammadi, A.; Hajiaghaee, R.; Shekarchi, M.; Khoshayand, M.R. Preparation of a new nanoparticle Cd (II)-imprinted polymer and its application for selective separation of cadmium (II) ions from aqueous solutions and determination via inductively coupled plasma optical emission spectrometry, Desalin. Water Treat. 2016, 57, 14280–14289. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Xing, J. Selective adsorption behavior of Cd(II) ion imprinted polymers synthesized by microwave-assisted inverse emulsion polymerization: Adsorption performance and mechanism. J. Hazard. Mater. 2017, 321, 103–110. [Google Scholar] [CrossRef]
- Mehdini, A.; Mehrabi, H.; Jabbari, A. Polythionine grafted onto magnetic SBA-15 for the removal of cadmium ions from aqueous solutions: Isothermal and kinetic studies. New J. Chem. 2019, 43, 5581–5591. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Y.; Li, Y.; Dolgorma, A.; Fang, H.; Guo, L.; Huang, J.; Yang, J. A Novel Magnetic Cd(II) Ion-Imprinted Polymer as a Selective Sorbent for the Removal of Cadmium Ions from Aqueous Solution. J. Inorg. Organomet. Polym. 2019, 29, 1874–1885. [Google Scholar] [CrossRef]
- Tabaklı, B.; Topçu, A.A.; Döker, S.; Uzun, L. Particle-assisted ion-imprinted cryogels for selective CdII Ion removal. Ind. Eng. Chem. Res. 2015, 54, 1816–1823. [Google Scholar] [CrossRef]
- Wang, X.; Gil Min, B. Cadmium sorption properties of poly(vinyl alcohol)/hydroxy-apatite cryogels: I. kinetic and isotherm studies. Fibers Polym. 2007, 43, 99–104. [Google Scholar]


| Composite Cryogel Cartridges | Swelling Degree H2O g/Polymer |
|---|---|
| MIP1 | 8.35 ± 0.12 |
| MIP2 | 8.72 ± 0.15 |
| NIP | 7.58 ± 0.11 |
| Polymer | MAC mmol | Cd(II) mmol | Q μmol/g Polymer |
|---|---|---|---|
| MIP1 | 0.2 | 0.2 | 76.35 |
| MIP2 | 0.4 | 0.2 | 98.89 |
| NIP | 0.2 | - | 5.74 |
| Composite Cryogel Cartridge | EXPERIMENTAL | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Q (μmol/g) | Qmax (μmol/g) | b (mL/μmol/g) | R2 | KF | 1/n | R2 | |
| MIP2 | 98.89 | 97.79 | 9.2 | 0.996 | 44.7 | 0.851 | 0.983 |
| MIP1 | 76.35 | 38.1 | 1.93 | 0.992 | 181.4 | 0.646 | 0.901 |
| Monomer | Polymerization Method | Adsorption Capacity | Reference |
|---|---|---|---|
| Allyl thiourea | Surface | 0.37 mmol/g | [47] |
| Graphene Oxide | Surface | 0.77 mmol/g | [48] |
| 2-vinylpyridine | Precipitation polymerization | 0.14 mmol/g | [49] |
| β-cyclodextrin | Emulsion | 0.95 mmol/g | [50] |
| Thionine | Surface | 0.309 mmol g | [51] |
| Aminoethyl chitosan | Emulsion | 0.23 mmol/g | [52] |
| p(HEMA-co-MAC) | Emulsion | 26.6 µmol/g | [41] |
| p(HEMA-co-MAC) | Chemisorption | 0.05 µmol/g | [53] |
| p(HEMA-co-MAC)/IIP-Cd | Chemisorption | 0.28 µmol/g | [53] |
| p(PVA-co-HA) | Metal complexation | 0.47 mmol/g | [54] |
| Na-magadiite | hydrothermal medium condition | 0.57 mmol/g | [14] |
| Na-magadiite-Cyanex 272 | hydrothermal medium condition | 0.44 mmol/g | [14] |
| MIP2* | Emulsion | 98.33 µmol/g | This work |
| NIP | MIP1 | MIP2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Metal ions | Kd | k | Kd | k | K’ | Kd | k | K’ |
| Cd(II) | 293.6 | - | 156.6 | - | - | 377.7 | - | - |
| Pb(II) | 240.5 | 1.22 | 7.25 | 21.59 | 17.68 | 9.61 | 39.27 | 32.16 |
| Zn(II) | 253.1 | 1.16 | 5.22 | 29.67 | 25.58 | 8.23 | 45.86 | 39.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huseynli, S.; Bakhshpour, M.; Qureshi, T.; Andac, M.; Denizli, A. Composite Polymeric Cryogel Cartridges for Selective Removal of Cadmium Ions from Aqueous Solutions. Polymers 2020, 12, 1149. https://doi.org/10.3390/polym12051149
Huseynli S, Bakhshpour M, Qureshi T, Andac M, Denizli A. Composite Polymeric Cryogel Cartridges for Selective Removal of Cadmium Ions from Aqueous Solutions. Polymers. 2020; 12(5):1149. https://doi.org/10.3390/polym12051149
Chicago/Turabian StyleHuseynli, Sabina, Monireh Bakhshpour, Tahira Qureshi, Muge Andac, and Adil Denizli. 2020. "Composite Polymeric Cryogel Cartridges for Selective Removal of Cadmium Ions from Aqueous Solutions" Polymers 12, no. 5: 1149. https://doi.org/10.3390/polym12051149
APA StyleHuseynli, S., Bakhshpour, M., Qureshi, T., Andac, M., & Denizli, A. (2020). Composite Polymeric Cryogel Cartridges for Selective Removal of Cadmium Ions from Aqueous Solutions. Polymers, 12(5), 1149. https://doi.org/10.3390/polym12051149