Intelligent Poly(N-Isopropylmethacrylamide) Hydrogels: Synthesis, Structure Characterization, Stimuli-Responsive Swelling Properties, and Their Radiation Decomposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method of p(NiPMAm) Hydrogel Synthesis
2.3. Structural Confirmation of p(NiPMAm) Hydrogels
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Scanning Electron Microscopy Analysis
2.3.3. Swelling Study
2.3.4. Residual Reactants Analysis
2.3.5. Gamma-Ray Irradiation of Hydrogel
2.3.6. Gel Permeation Chromatography Analysis of Hydrogel after Radiolysis
2.3.7. Static Headspace Gas Chromatography Mass Spectrometry and Gas Chromatography/Flame Ionization Detection Analysis of Hydrogel after Radiolysis
3. Results and Discussion
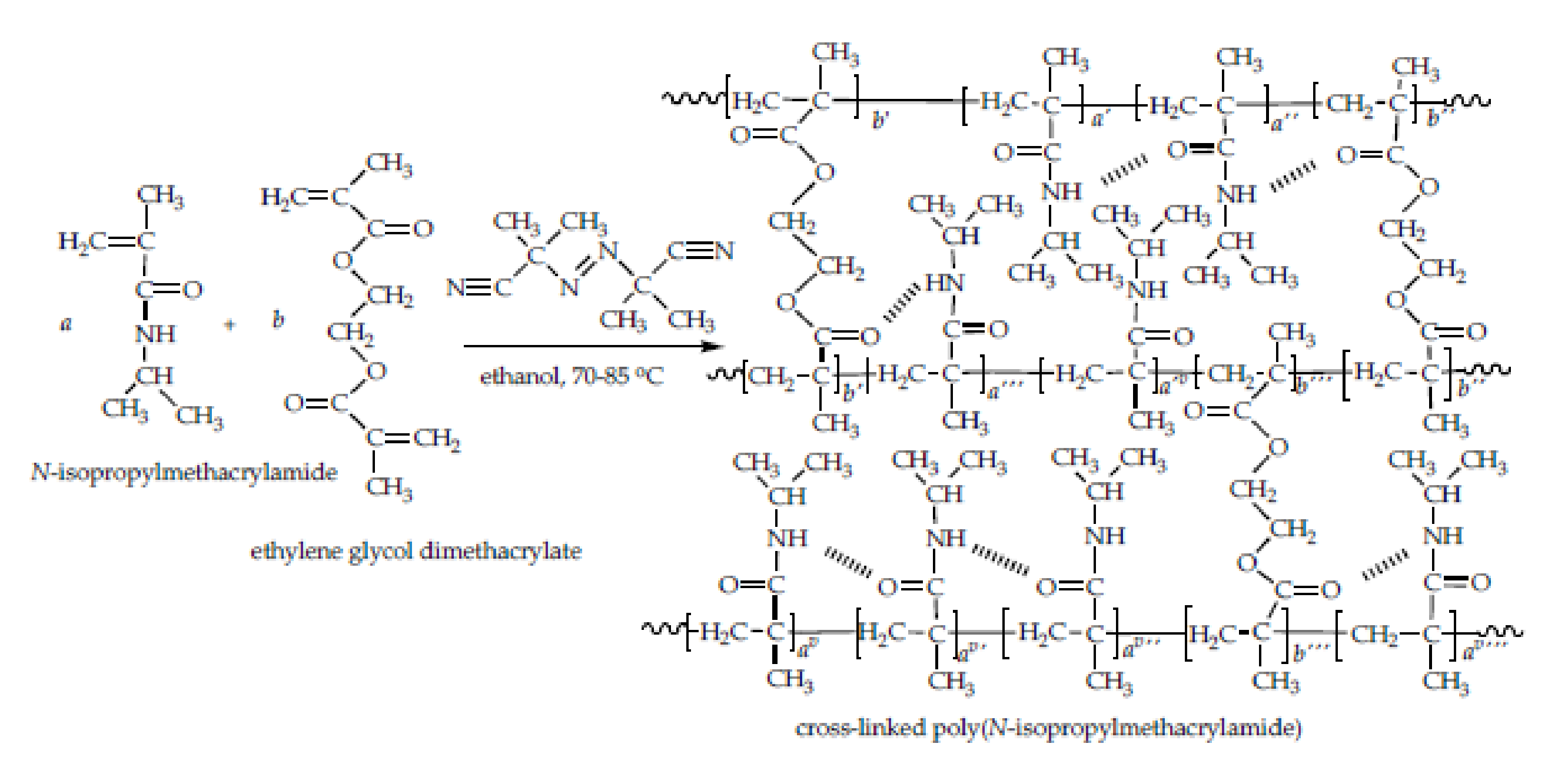
3.1. Synthesis of Homopolymeric Poly(N-Isopropylmethacrylamide) Hydrogel
3.2. Structural Characterization of Synthesized Poly(N-isopropylmethacrylamide) Hydrogels
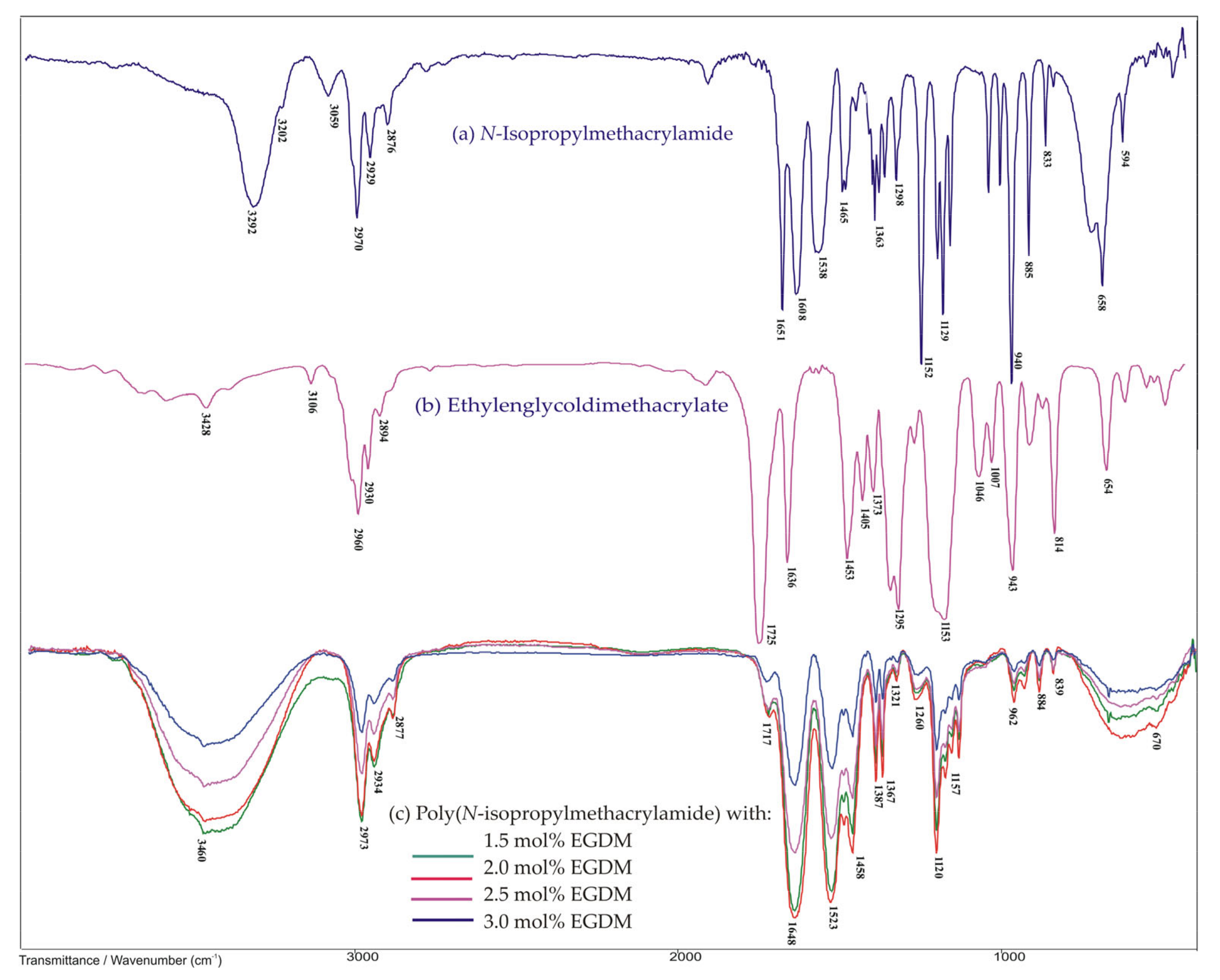
3.2.1. FTIR Spectroscopy Analysis
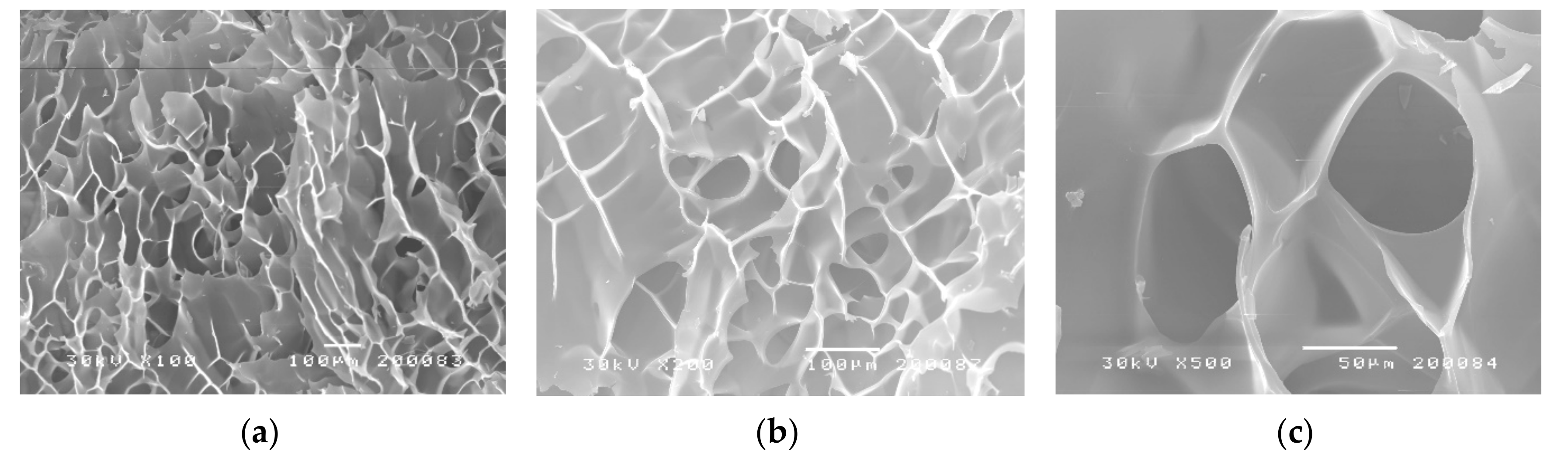
3.2.2. Scanning Electron Microscopy Analysis
3.3. Residual Reactant Analysis
3.4. Swelling Study
3.4.1. Equilibrium Hydrogel Swelling at 20 °C and pH = 2.0
3.4.2. Equilibrium Hydrogel Swelling at 20 °C and pH = 7.4
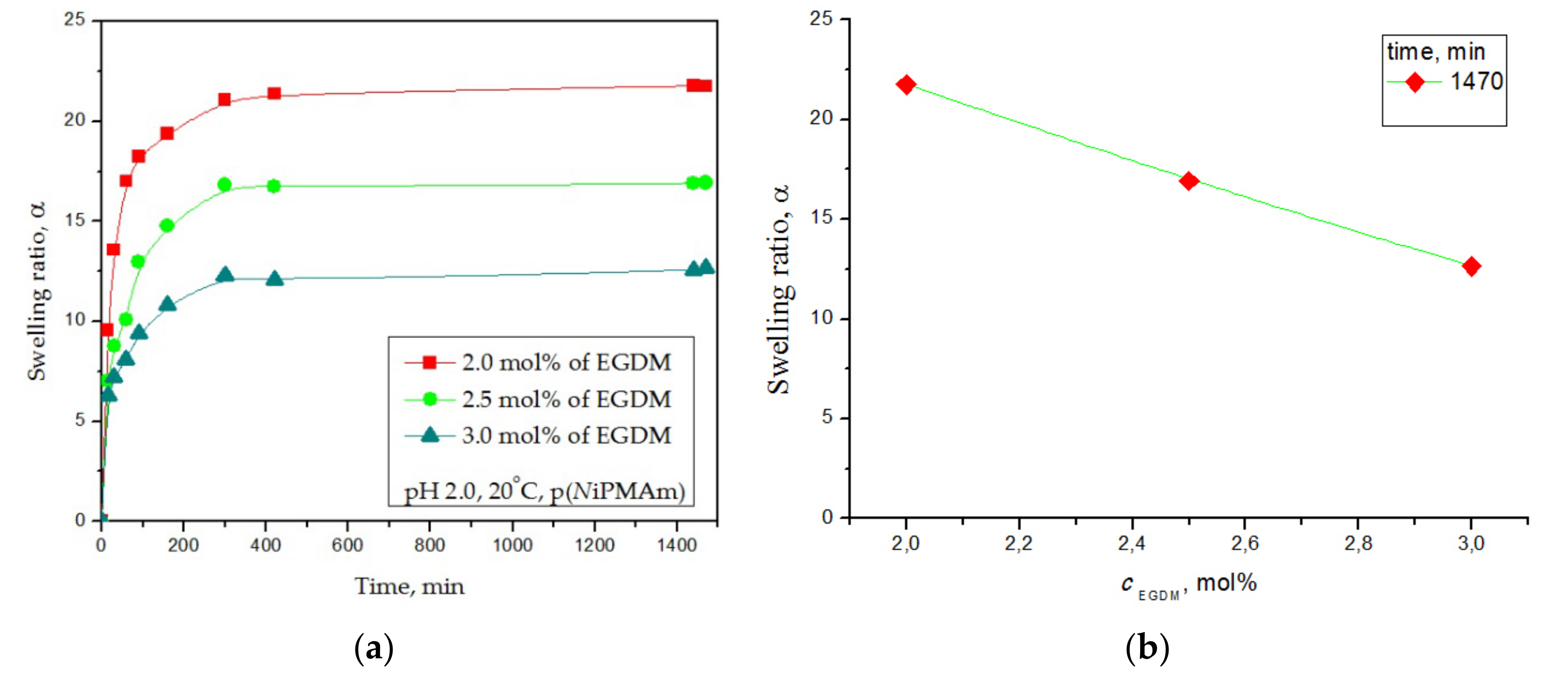
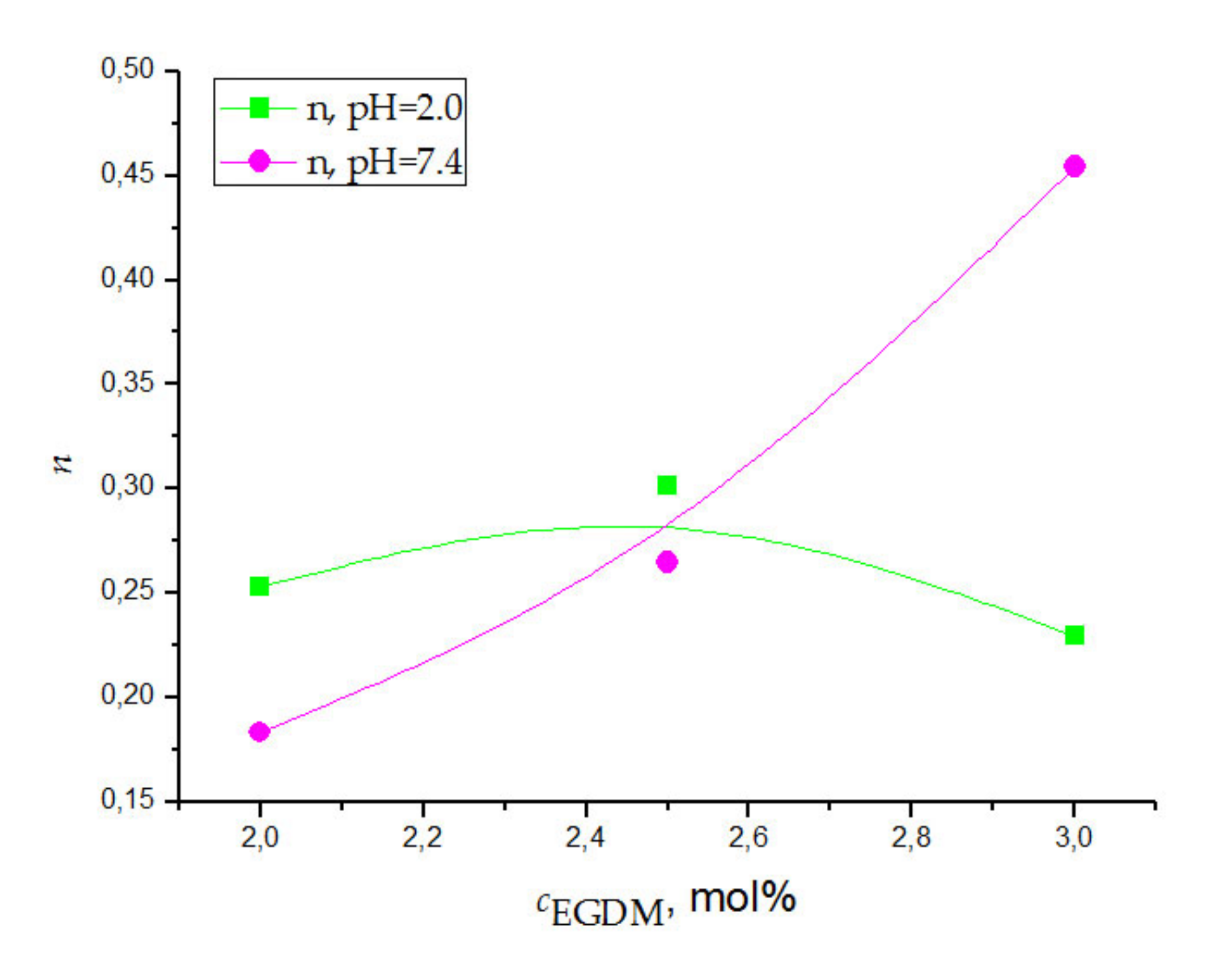
3.4.3. Swelling Kinetic Analysis
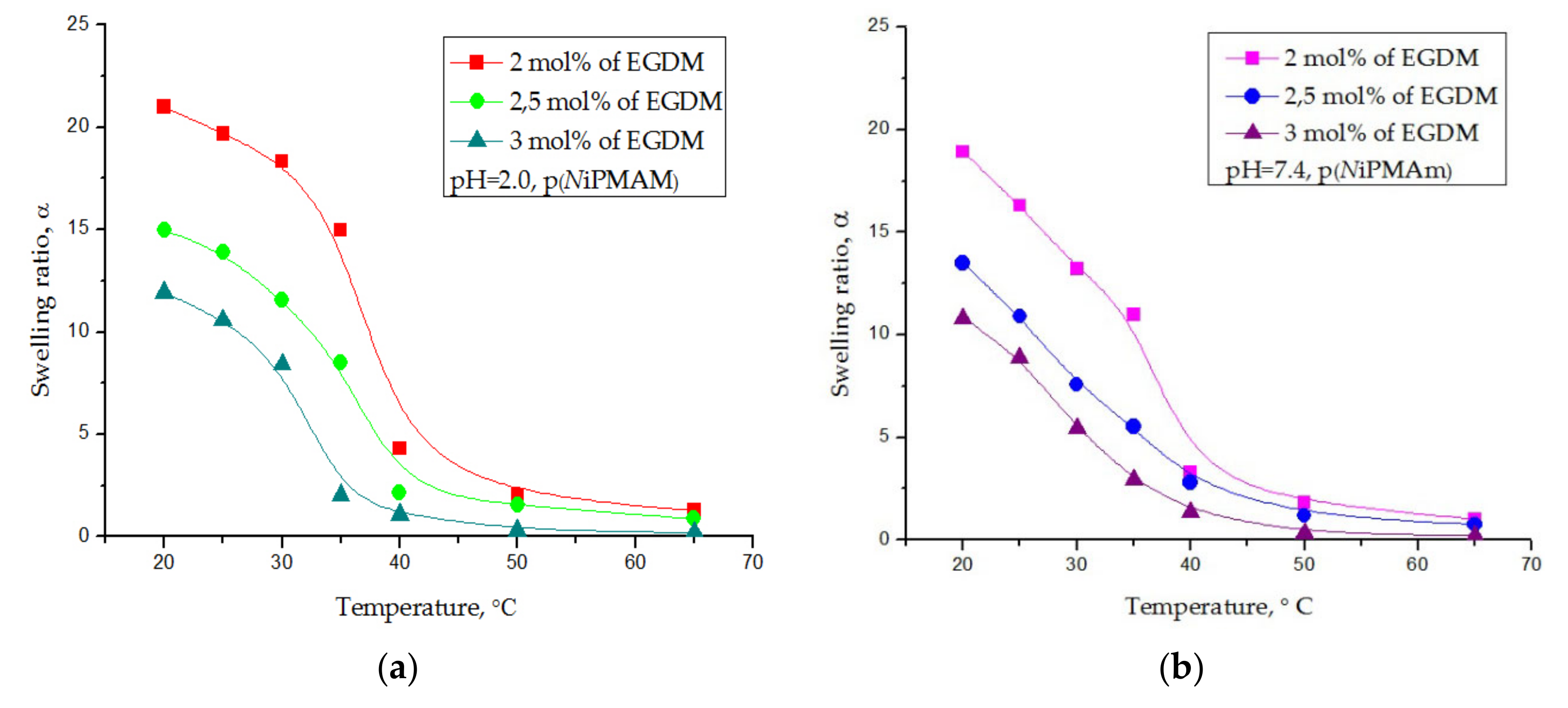
3.4.4. Thermosensitivity Testing
3.5. Gel Permeation Chromatography Analysis of Hydrogel after Radiolysis
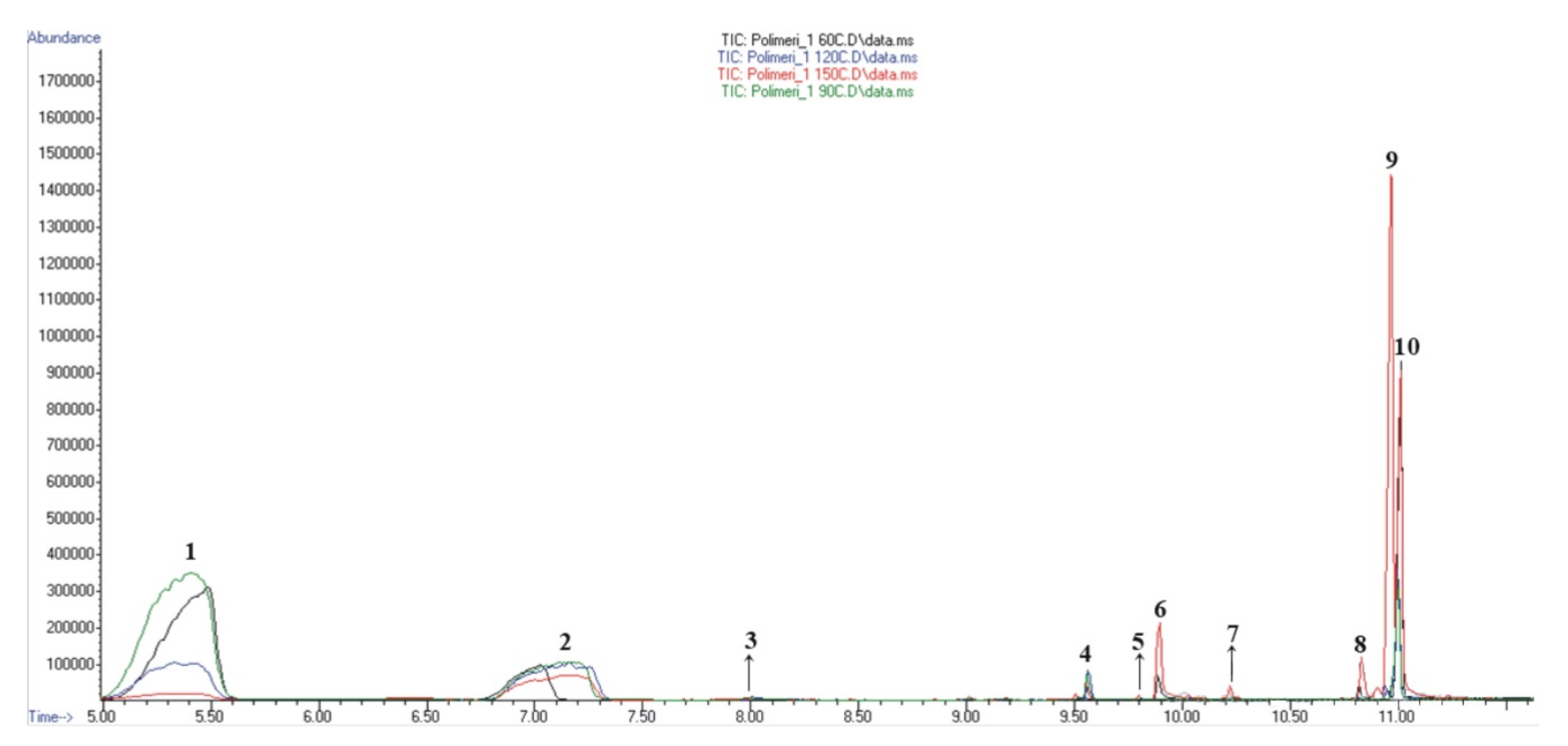
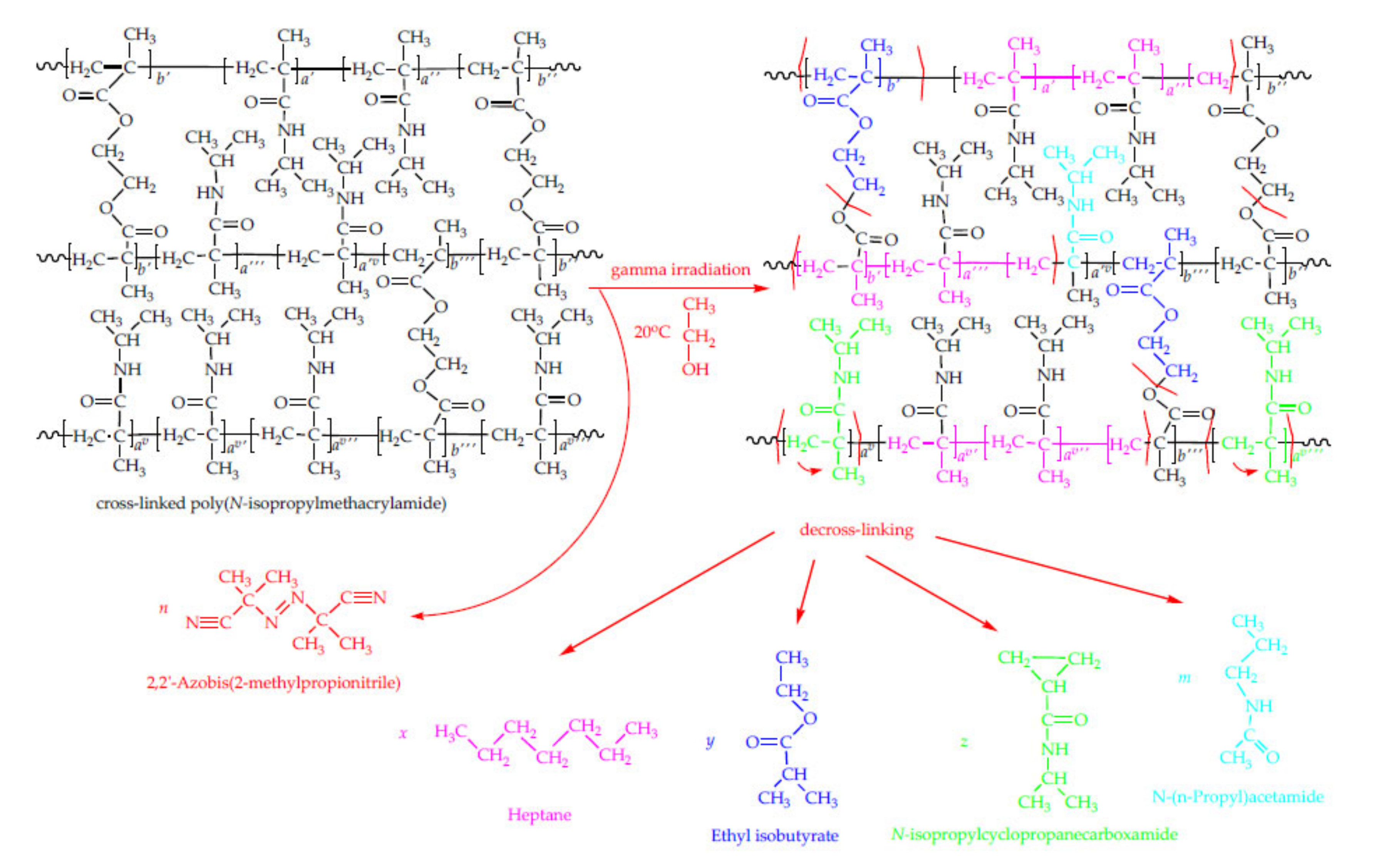
3.6. HSS-GC/MS and HSS-GC/FID Analysis of Hydrogel Sample after Radiolysis

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Petrović, S.D.; Keservani, R.K. Smart hydrogels for pharmaceutical applications. In Novel Approaches for Drug Delivery; Keservani, R.K., Sharma, A.K., Kesharwani, R.K., Eds.; IGI Global: Hershey, PA, USA, 2016; pp. 278–310. ISBN 9781522507512. e-ISBN:9781522507529. [Google Scholar] [CrossRef]
- Vakıflı, A.; Demirel, G.B.; Caykara, T. Swelling characteristics of poly(N-isopropylmethacrylamide-co-itaconic acid) gels prepared in various conditions. J. Appl. Polym. Sci. 2010, 117, 817–823. [Google Scholar] [CrossRef]
- Priest, J.H.; Murray, S.L.; Nelson, R.J.; Hoffman, A.S. Lower critical solution temperatures of aqueous copolymers of N-isopropylacrylamide and other N-substituted acrylamides. In Reversible Polymeric Gels and Related Systems; Russo, P.S., Ed.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 1987; Volume 350, pp. 255–264. ISBN 9780841214156. e-ISBN: 9780841211940. [Google Scholar] [CrossRef]
- Bokias, G.; Hourdet, D.; Iliopoulos, I. Positively charged amphiphilic polymers based on poly(N-isopropylacrylamide): Phase behavior and shear-induced thickening in aqueous solution. Macromolecules 2000, 33, 2929–2935. [Google Scholar] [CrossRef]
- Hu, X.; Tong, Z.; Lyon, A. One-pot synthesis of microcapsules with nanoscale inclusions. Macromol. Rapid Commun. 2011, 32, 1461–1466. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X. Collapse kinetics for individual poly(N-isopropylmethacrylamide) chains. Polymer 2010, 51, 897–901. [Google Scholar] [CrossRef]
- Netopilík, M.; Bohdanecký, M.; Chytrý, V.; Ulbrich, K. Cloud point of poly(N-isopropylmethacrylamide) solutions in water: Is it really a point? Macromol. Rapid Commun. 1997, 18, 107–111. [Google Scholar] [CrossRef]
- Kubota, K.; Hamano, K.; Kuwahara, N.; Fujishige, S.; Ando, I. Characterization of poly(N-isopropylmethacrylamide) in water. Polym. J. 1990, 22, 1051–1057. [Google Scholar] [CrossRef]
- Tang, Y.; Ding, Y.; Zhang, G. Role of methyl in the phase transition of poly(N-isopropylmethacrylamide). J. Phys. Chem. B 2008, 112, 8447–8451. [Google Scholar] [CrossRef] [PubMed]
- Djokpé, E.; Vogt, W. N-isopropylacrylamide and N-sopropylmethacryl-amide: Cloud points of mixtures and copolymers. Macromol. Chem. Phys. 2001, 202, 750–757. [Google Scholar] [CrossRef]
- Kirsh, Y.E.; Yanul, N.A.; Popkov, Y.M. Temperature behavior of thermo-responsive poly-N-vinylcaprolactam and poly-N-isopropylmethacrylamide in aqueous solutions involving organic solutes. Eur. Polym. J. 2002, 38, 403–406. [Google Scholar] [CrossRef]
- Zhang, L.P.; Noda, I.; Wu, Y. Quantitative comparison of reversibility in thermal-induced hydration of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide) in aqueous solutions by “concatenated” 2D correlation analysis. Vib. Spectrosc. 2012, 60, 200–205. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. Poly(N-isopropylacrylamide-co-N -isopropylmethacrylamide) thermo-responsive microgels as self-regulated drug delivery system. Macromol. Chem. Phys. 2016, 217, 2525–2533. [Google Scholar] [CrossRef]
- Fujishige, S.; Kubota, K.; Ando, I. Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J. Phys. Chem. 1989, 93, 3311–3313. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Sanporean, C.G.; Christiansen, J.D. Modeling the effects of temperature and pH on swelling of stimuli-responsive gels. Eur. Polym. J. 2015, 73, 278–296. [Google Scholar] [CrossRef]
- Wedel, B.; Hertle, Y.; Wrede, O.; Bookhold, J.; Hellweg, T. Smart homopolymer microgels: Influence of the monomer structure on the particle properties. Polymers 2016, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Duracher, D.; Elaissari, A.; Pichot, C. Characterization of cross-linked poly(N-isopropylmethacrylamide) microgel latexes. Colloid Polym. Sci. 1999, 277, 905–913. [Google Scholar] [CrossRef]
- Cors, M.; Wiehemeier, L.; Oberdisse, J.; Hellweg, T. Deuteration-induced volume phase transition temperature shift of PNIPMAM microgels. Polymers 2019, 11, 620. [Google Scholar] [CrossRef]
- Alenichev, I.; Sedláková, Z.; Ilavský, M. Swelling and mechanical behavior of charged poly(N-isopropylmethacrylamide) and poly(N-isopropylacrylamide) networks in water/ethanol mixtures. Cononsolvency effect. Polym. Bull. 2007, 58, 191–199. [Google Scholar] [CrossRef]
- Dybal, J.; Trchová, M.; Schmidt, P. The role of water in structural changes of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide) studied by FTIR, Raman spectroscopy and quantum chemical calculations. Vib. Spectrosc. 2009, 51, 44–51. [Google Scholar] [CrossRef]
- Ruiz-Rubio, L.; Álvarez, V.; Lizundia, E.; Vilas, J.L.; Rodríguez, M.; León, L.M. Influence of α-methyl substitutions on interpolymer complexes formation between poly(meth)acrylic acids and poly(N-isopropyl (meth) acrylamide)s. Colloid Polym. Sci. 2015, 293, 1447–1455. [Google Scholar] [CrossRef]
- Berndt, I.; Pedersen, J.S.; Lindner, P.; Richtering, W. Structure of doubly temperature sensitive core-shell microgels based on poly-N-Isopropylacrylamide and poly-N-isopropylmethacrylamide. Progr. Colloid. Polym. Sci. 2006, 133, 35–40. [Google Scholar] [CrossRef]
- Brändel, T.; Dirksen, M.; Hellweg, T. Tuning the swelling properties of smart multiresponsive core-shell microgels by copolymerization. Polymers 2019, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, A.; Pospíšil, H.; Sedlakova, Z.; Pleštil, J.; Ilavský, M. Phase transition in swollen gels Part 32. Temperature transition in charged poly(N-isopropylmethacrylamide) hydrogels in water and aqueous NaCl solutions. Phys. Chem. Chem. Phys. 2002, 4, 4360–4367. [Google Scholar] [CrossRef]
- Jung, S.C.; Bae, Y.C. The effects of interaction energy on the volume phase transition of N-isopropylacrylamide-co-N-isopropylmethacrylamide nano-sized gel particles: Applicability of molecular simulation technique. Polymer 2009, 50, 4957–4963. [Google Scholar] [CrossRef]
- Barbu, E.; Verestiuc, L.; Iancu, M.; Jatariu, A.; Lungu, A.; Tsibouklis, J. Hybrid polymeric hydrogels for ocular drug delivery: Nanoparticulate systems from copolymers of acrylic acid-functionalized chitosan and N-isopropylacrylamide or 2-hydroxyethyl methacrylate. Nanotechnology 2009, 20, 225108. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Chua, P.C.; Kelland, M.A.; Ishitake, K.; Satoh, K.; Kamigaito, M.; Okamoto, Y. Kinetic hydrate inhibition of poly(N-isopropylmethacrylamide)s with different tacticities. Energy Fuels 2012, 26, 3577–3585. [Google Scholar] [CrossRef]
- Hu, X.; Tong, Z.; Lyon, L.A. Synthesis and physicochemical properties of cationic microgels based on poly(N-isopropylmethacrylamide). Colloid. Polym. Sci. 2011, 289, 333–339. [Google Scholar] [CrossRef]
- Von Nessen, K.; Karg, M.; Hellweg, T. Thermoresponsive poly-(N-isopropylmethacrylamide) microgels: Tailoring particle size by interfacial tension control. Polymer 2013, 54, 5499–5510. [Google Scholar] [CrossRef]
- Callinan, T.D. Polymer synthesis by gamma radiation. J. Electrochem. Soc. 1956, 103, 292. [Google Scholar] [CrossRef]
- Pushplata, R.G.; Suri, G.; Talwar, M.; Seshadri, G.; Alam, M.S.; Khandal, R.K. Gamma irradiation as an environment friendly process for synthesis of hydrogels. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 16223–16231. [Google Scholar] [CrossRef]
- Coqueret, X. Obtaining high performance polymeric materials by irradiation. In Radiation Chemistry—From Basic to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis Cedex A, France, 2008; pp. 131–151. ISBN 978-2-7598-0024-7. [Google Scholar]
- Buxton, G.V. An overview of the radiation chemistry of liquids. In Radiation Chemistry—From Basic to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis Cedex A, France, 2008; pp. 3–17. ISBN 978-2-7598-0024-7. [Google Scholar]
- Flores-Rojas, G.G.; López-Saucedo, F.; Bucio, E. Gamma-irradiation applied in the synthesis of metallic and organic nanoparticles: A short review. Radiat. Phys. Chem. 2020, 169, 107962. [Google Scholar] [CrossRef]
- Tinoco, D.; Ortega, A.; Burillo, G.; Islas, L.; García-Uriostegui, L. Different hydrogel architectures synthesized by gamma radiation based on chitosan and N,N-dimethylacrylamide. MRS Commun. 2018, 8, 617–623. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, Z.P.; Sun, P.C.; He, B.L.; Zhu, X.X. Synthesis and characterization of thermo-and pH-sensitive hydrogels based on Chitosan-grafted N-isopropylacrylamide via γ-radiation. Radiat. Phys. Chem. 2005, 74, 26–30. [Google Scholar] [CrossRef]
- Spasojević, J.; Radosavljević, A.; Krstić, J.; Mitrić, M.; Popović, M.; Rakočević, Z.; Kalagasidis-Krušić, M.; Kačarević-Popović, Z. Structural characteristics and bonding environment of Ag nanoparticles synthesized by gamma irradiation within thermo-responsive poly(N-isopropylacrylamide) hydrogel. Polym. Compos. 2017, 38, 1014–1026. [Google Scholar] [CrossRef]
- Alla, S.G.; Sen, A.M.; El-Naggar, A.W.M. Swelling and mechanical properties of superabsorbent hydrogels based on Tara gum/acrylic acid synthesized by gamma radiation. Carbohydr. Polym. 2012, 89, 478–485. [Google Scholar] [CrossRef]
- Maolin, Z.; Hongfei, H.; Yoshii, F.; Makuuchi, K. Effect of kappa-carrageenan on the properties of poly(N-vinyl pyrrolidone)/kappa-carrageenan blend hydrogel synthesized by γ-radiation technology. Radiat. Phys. Chem. 2000, 57, 459–464. [Google Scholar] [CrossRef]
- Ammar, N.E.B.; Saied, T.; Mejri, A.; Hosni, F.; Mnif, A.; Hichem, A.H. Study of agar proportions effect on a gamma ray synthesized hydrogel. J. Mater. Sci. Eng A 2016, 3, 88–100. [Google Scholar] [CrossRef][Green Version]
- Urošević, M.Z.; Nikolić, L.B.; Ilić-Stojanović, S.S.; Nikolić, V.; Petrović, S.M.; Zdravković, A.S. Hydrogels based on N-isopropylmethacrylamide and N-isopropylacrylamide. Adv. Technol. 2018, 7, 79–91. [Google Scholar] [CrossRef]
- Urošević, M.Z.; Nikolić, L.B.; Ilić-Stojanović, S.; Zdravković, Z.; Nikolić, V.D. Synthesis and characterization of poly(N-isopropylmethacrylamide-co-N-isopropylacrylamide) copolymers. Hem. Ind. 2020, 74, 103–117. [Google Scholar] [CrossRef]
- Wu, Y.; Meersman, F.; Ozaki, Y. A novel application of hybrid two-dimensional correlation infrared spectroscopy: Exploration of the reversibility of the pressure-and temperature-induced phase separation of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide) in aqueous solution. Macromolecules 2006, 39, 1182–1188. [Google Scholar] [CrossRef]
- Kostic, M.; Pejcic, A.; Igic, M.; Gligorijevic, N. Adverse reactions to denture resin materials. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5298–5305. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Davis, P.K.; Zielinski, J.M.; Danner, R.P.; Duda, J.L. Application of free-volume theory to self diffusion of solvents in polymers below the glass transition temperature: A review. J. Polym. Sci. Pol. Phys. 2011, 49, 1629–1644. [Google Scholar] [CrossRef]
- Ramiah, K.V.; Puranik, P.G. Hydrogen bonding in amides. Proc. Indian Acad. Sci. A 1962, 56, 96–102. [Google Scholar] [CrossRef]
- Mikshiev, V.Y.; Pozharskii, A.F.; Filarowski, A.; Novikov, A.S.; Antonov, A.S.; Tolstoy, P.M.; Vovk, M.A.; Khoroshilova, O.V. How strong is hydrogen bonding to amide nitrogen? Chem. Phys. Chem. 2020, 21, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Wack, H.; Lectka, T. Strong hydrogen bonding to the amide nitrogen atom in an “amide proton sponge”: Consequences for structure and reactivity. Angew. Chem. Int. Ed. 1999, 38, 798–800. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Analytical Pyrolysis of Synthetic Organic Polymers, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 368–369. ISBN 9780444512925. e-ISBN 9780080455860. [Google Scholar]
- 2,2’-Azobis(2-Methylpropionitrile), Chemical Properties, Uses, Production, Suppliers. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2494212.htm (accessed on 10 May 2018).
- Alexander, P.; Charlesby, A.; Ross, M. The degradation of solid polymethylmethacrylate by ionizing radiation. Proc. R. Soc. Lond. A 1954, 223, 392–404. [Google Scholar] [CrossRef]
- Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Radiation processing of biodegradable polymer hydrogel from cellulose derivatives. In Proceedings of the Takasaki Symposium on Radiation Processing of Natural Polymers (No. JAERI-CONF-2001-005), Takasaki, Japan, 23–24 November 2000; Tamikazu, K., Yasunari, M., Eds.; Japan Atomic Energy Research Institute: Tokyo, Japan, 2001; Volume 33, pp. 89–100. [Google Scholar]
- Wündrich, K. The dependence of the radiation-induced polyisobutylene degradation on molecular mobilities. Eur. Polym. J. 1974, 10, 341–346. [Google Scholar] [CrossRef]
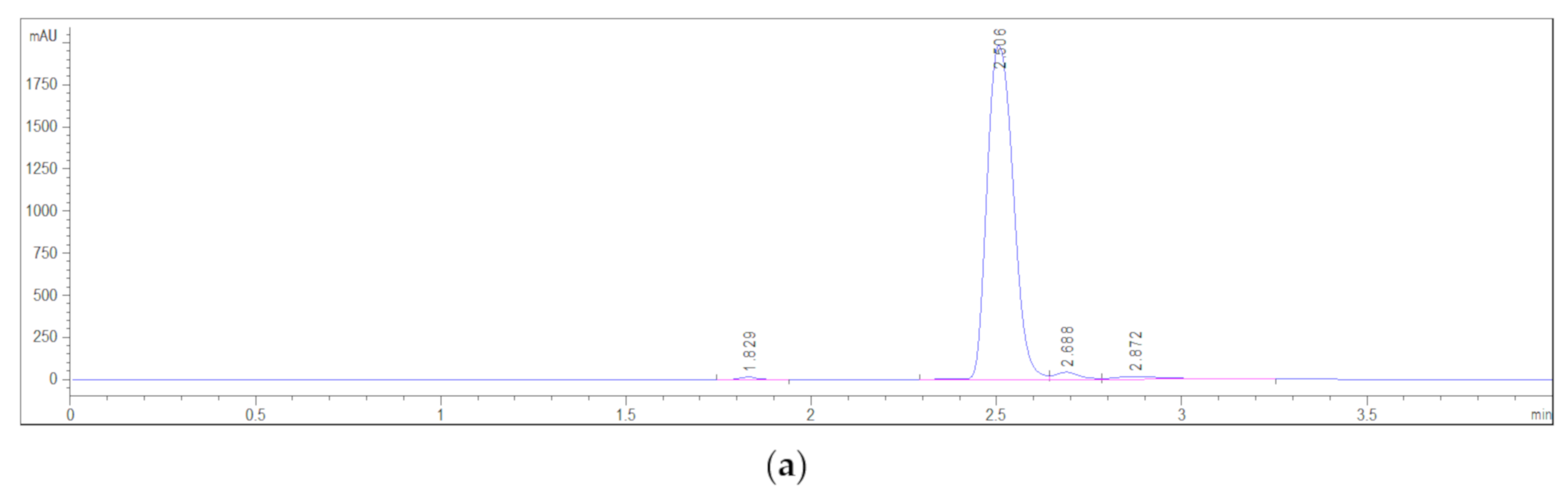


| p(NiPMAm), mol% of EGDM | Mass, g | A, mAU·s | NiPMAm, mg/g | NiPMAm, % |
|---|---|---|---|---|
| 1.5 | 0.115 | 9166.1 | 59.023 | 6.039 |
| 2.0 | 0.207 | 9932.9 | 35.774 | 3.688 |
| 2.5 | 0.258 | 10138.7 | 29.455 | 3.059 |
| 3.0 | 0.357 | 10743.9 | 22.616 | 2.367 |
| p(NiPMAm), mol% of EGDM | pH 2.0 | pH 7.4 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | k, min−1/2 | D⋅104, cm2/min | R2 | n | k, min−1/2 | D⋅104, cm2/min | R2 | |
| 2.0 | 0.253 | 0.250 | 7.423 | 0.950 | 0.183 | 0.249 | 5.115 | 0.966 |
| 2.5 | 0.302 | 0.184 | 7.591 | 0.991 | 0.265 | 0.218 | 3.716 | 0.996 |
| 3.0 | 0.229 | 0.262 | 8.083 | 0.991 | 0.454 | 0.077 | 3.403 | 0.972 |
| Elution Time, min | Figure | ||||
|---|---|---|---|---|---|
| 5.30–7.60 | 1.148⋅102 | 2.040⋅102 | 3.180⋅103 | 1.777 | S3 |
| 5.30–5.80 | 7.040⋅102 | 7.324⋅102 | 7.645⋅102 | 1.040 | S4 |
| 5.80–6.36 | 3.049⋅102 | 3.217⋅102 | 3.376⋅102 | 1.055 | S5 |
| 6.36–6.85 | 1.155⋅102 | 1.215⋅102 | 1.275⋅102 | 1.052 | S6 |
| No. | tret., min | Compound | Molecular Formula | Method of Identification | Prob. (%) | Area, % | |||
|---|---|---|---|---|---|---|---|---|---|
| 60 °C | 90 °C | 120 °C | 150 °C | ||||||
| 1. | 5.03–5.62 | Heptane and related compounds | MS | 76.6 | 76.3 | 77.4 | 49.3 | 1.9 | |
| 2. | 6.76–7.34 | Ethyl isobutyrate and related compounds | MS | 78.3 | 13.9 | 19.2 | 42.4 | 38.9 | |
| 3. | 8.01 | 2-hydroxypropanenitrile | C3H5NO | MS | 95.8 | - | 0.2 | 0.6 | - |
| 4. | 9.51 | 1-methyl-1.2.4-triazole | C3H5N3 | MS | 66.2 | 0.2 | 0.7 | 0.2 | 0.6 |
| 5. | 9.80 | 4-ethoxy-2-pentanone | C7H14O2 | MS | 97.0 | - | - | - | 0.2 |
| 6. | 9.89 | N-(n-propyl)acetamide | C5H11NO | MS | 80.9 | 0.7 | - | - | 5.1 |
| 7. | 10.23 | 2-pentanone | C5H10O | MS | 53.1 | - | - | - | 0.5 |
| 8. | 10.82 | Cyanuric acid | C3H3N3O3 | MS | 83.2 | 0.4 | - | - | 2.1 |
| 9. | 10.95 | N-Isopropylcyclopropanecarboxamide | C7H13NO | MS | 81.0 | 0.2 | - | 0.7 | 29.9 |
| 10. | 11.01 | 2.2’-azobis(2-methylpropionitrile) | C8H12N4 | MS | 64.7 | 8.3 | 2.5 | 5.2 | 18.3 |
| Total identified (%) | 100.0 | 100.0 | 98.4 | 97.5 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilić-Stojanović, S.; Urošević, M.; Nikolić, L.; Petrović, D.; Stanojević, J.; Najman, S.; Nikolić, V. Intelligent Poly(N-Isopropylmethacrylamide) Hydrogels: Synthesis, Structure Characterization, Stimuli-Responsive Swelling Properties, and Their Radiation Decomposition. Polymers 2020, 12, 1112. https://doi.org/10.3390/polym12051112
Ilić-Stojanović S, Urošević M, Nikolić L, Petrović D, Stanojević J, Najman S, Nikolić V. Intelligent Poly(N-Isopropylmethacrylamide) Hydrogels: Synthesis, Structure Characterization, Stimuli-Responsive Swelling Properties, and Their Radiation Decomposition. Polymers. 2020; 12(5):1112. https://doi.org/10.3390/polym12051112
Chicago/Turabian StyleIlić-Stojanović, Snežana, Maja Urošević, Ljubiša Nikolić, Djordje Petrović, Jelena Stanojević, Stevo Najman, and Vesna Nikolić. 2020. "Intelligent Poly(N-Isopropylmethacrylamide) Hydrogels: Synthesis, Structure Characterization, Stimuli-Responsive Swelling Properties, and Their Radiation Decomposition" Polymers 12, no. 5: 1112. https://doi.org/10.3390/polym12051112
APA StyleIlić-Stojanović, S., Urošević, M., Nikolić, L., Petrović, D., Stanojević, J., Najman, S., & Nikolić, V. (2020). Intelligent Poly(N-Isopropylmethacrylamide) Hydrogels: Synthesis, Structure Characterization, Stimuli-Responsive Swelling Properties, and Their Radiation Decomposition. Polymers, 12(5), 1112. https://doi.org/10.3390/polym12051112






