Influence of Cross-Linking Degree on Hydrodynamic Behavior and Stimulus-Sensitivity of Derivatives of Branched Polyethyleneimine
Abstract
1. Introduction
2. Materials and Methods
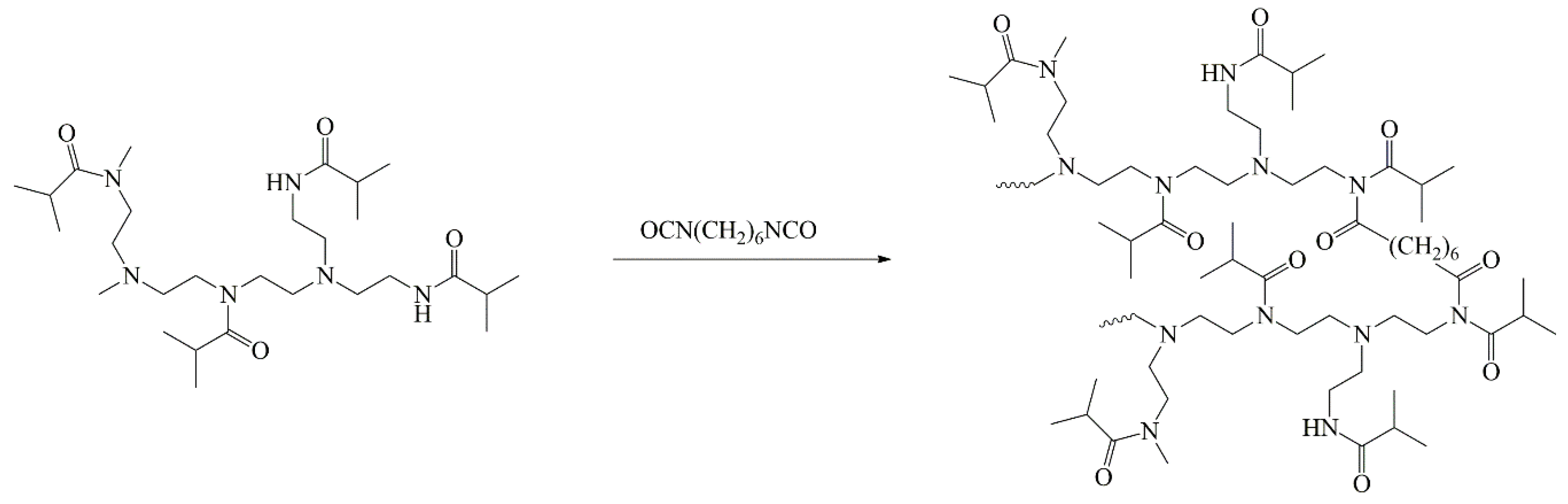
2.1. Synthesis of Partially Cross-linked Poly(ethyleneimine)
2.2. Determination of Hydrodynamic Characteristics
3. Results
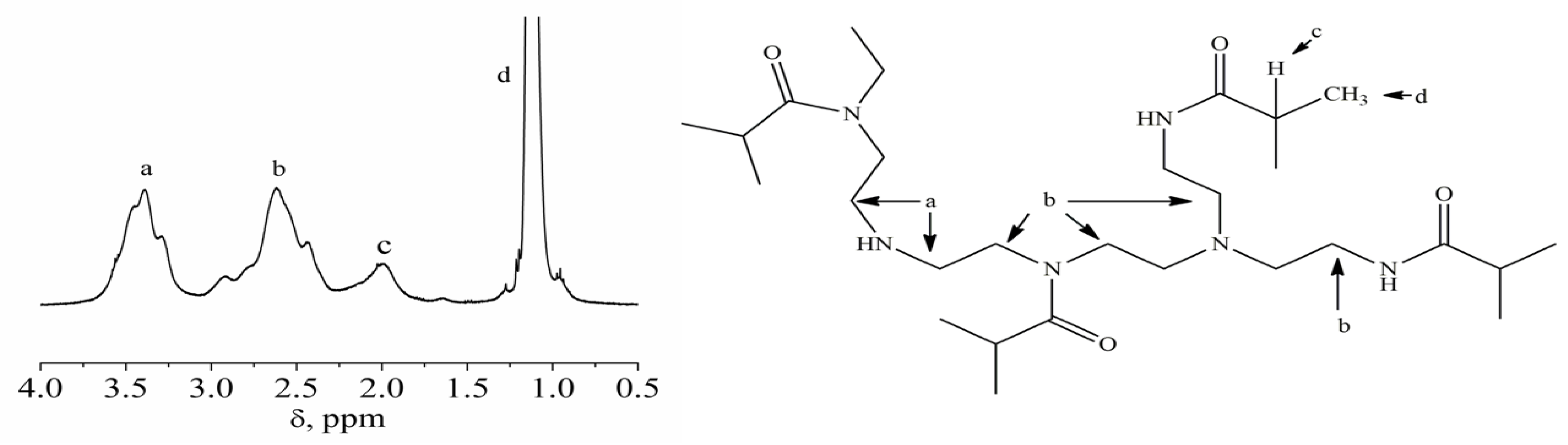
3.1. Structure and Hydrodynamic Behavior of PEI-n in Dilute Chloroform Solution
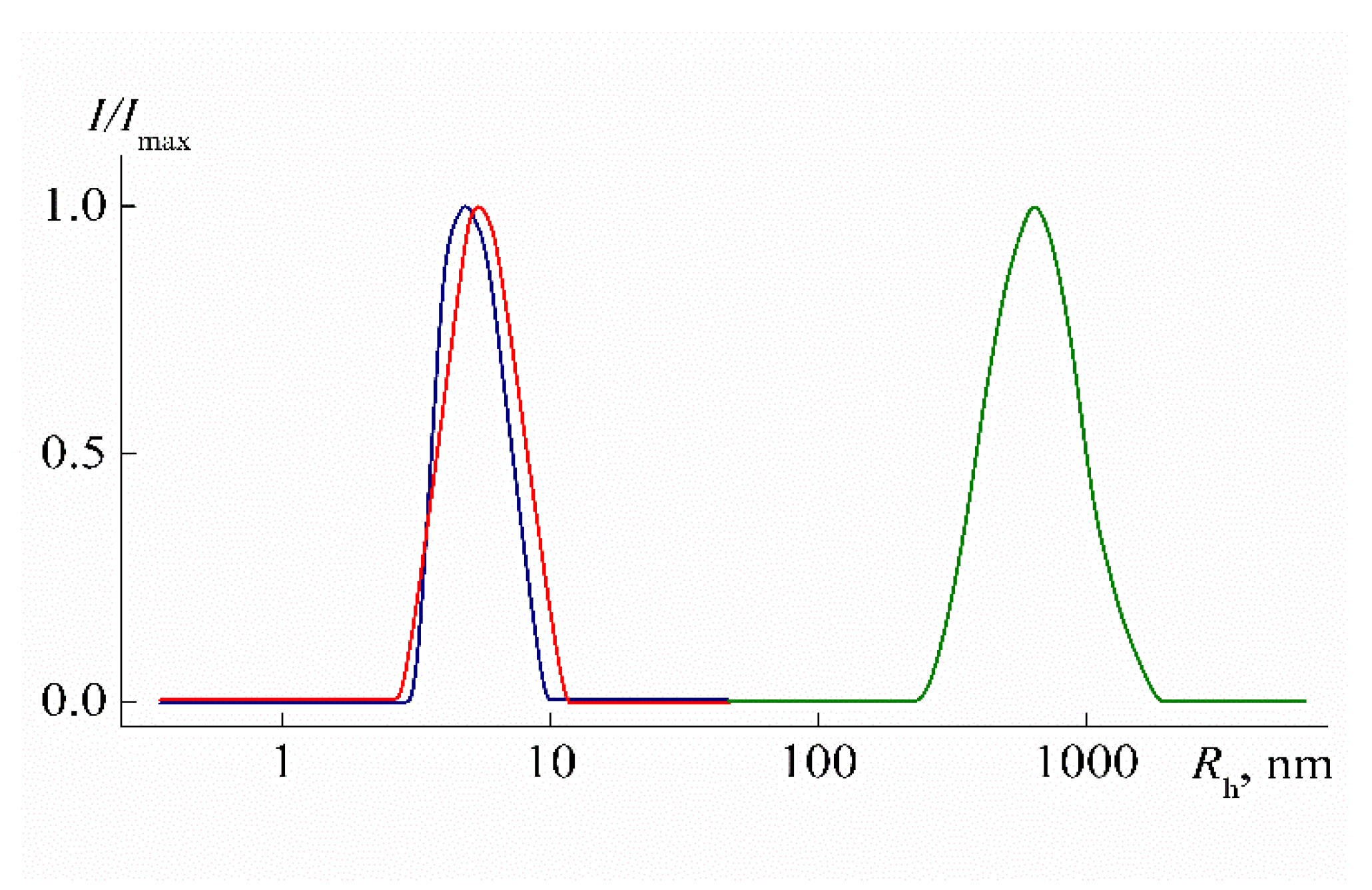
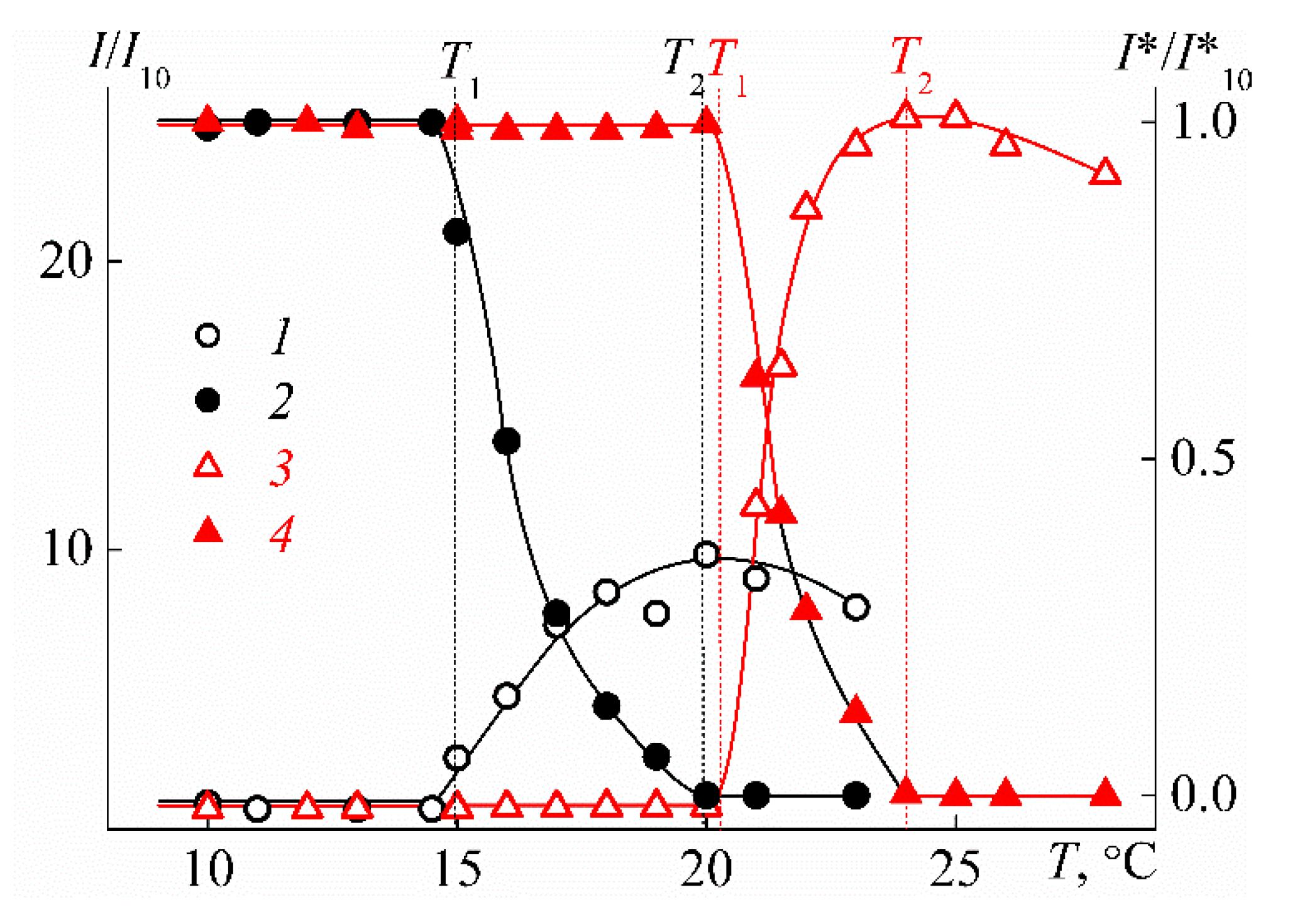
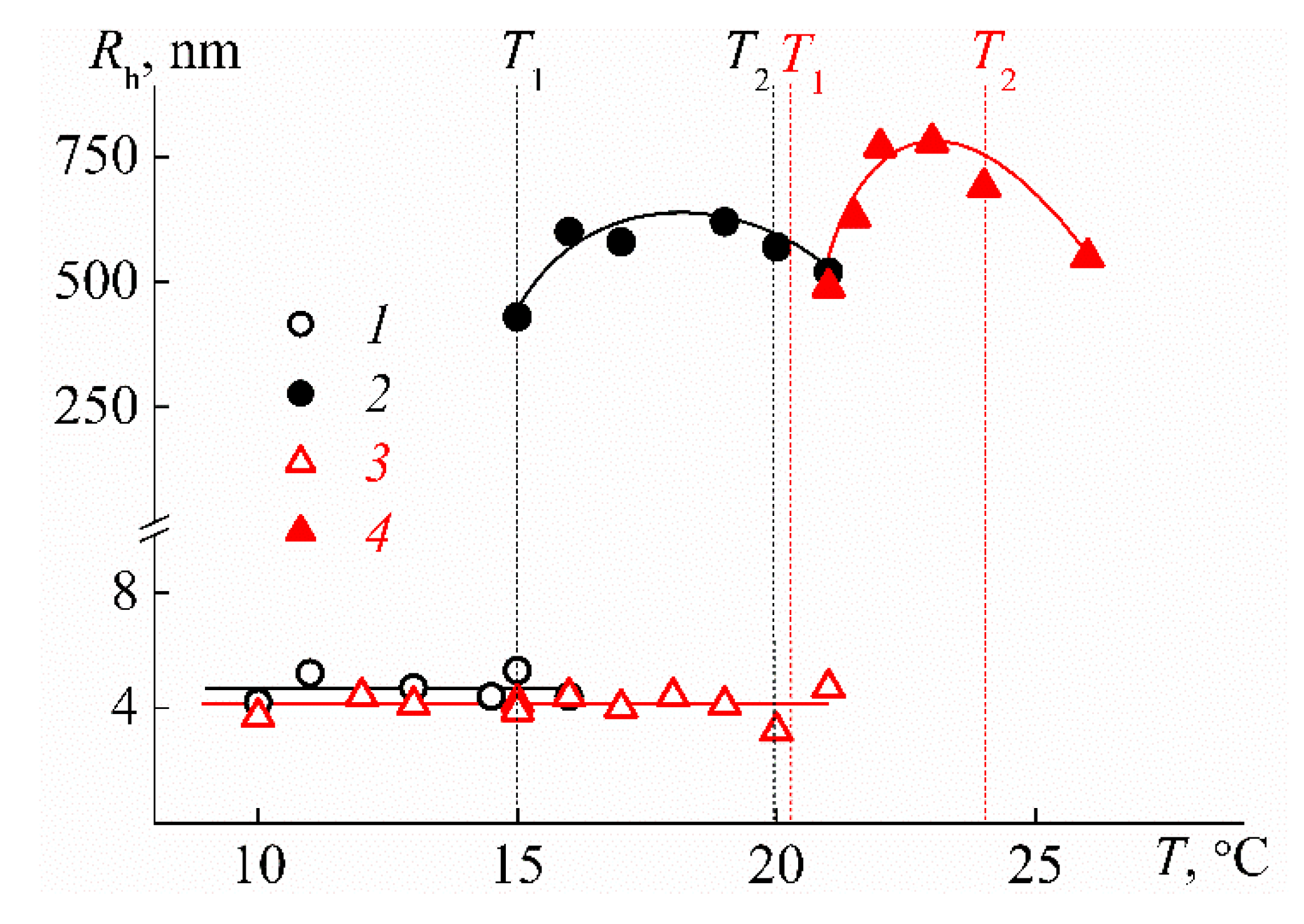
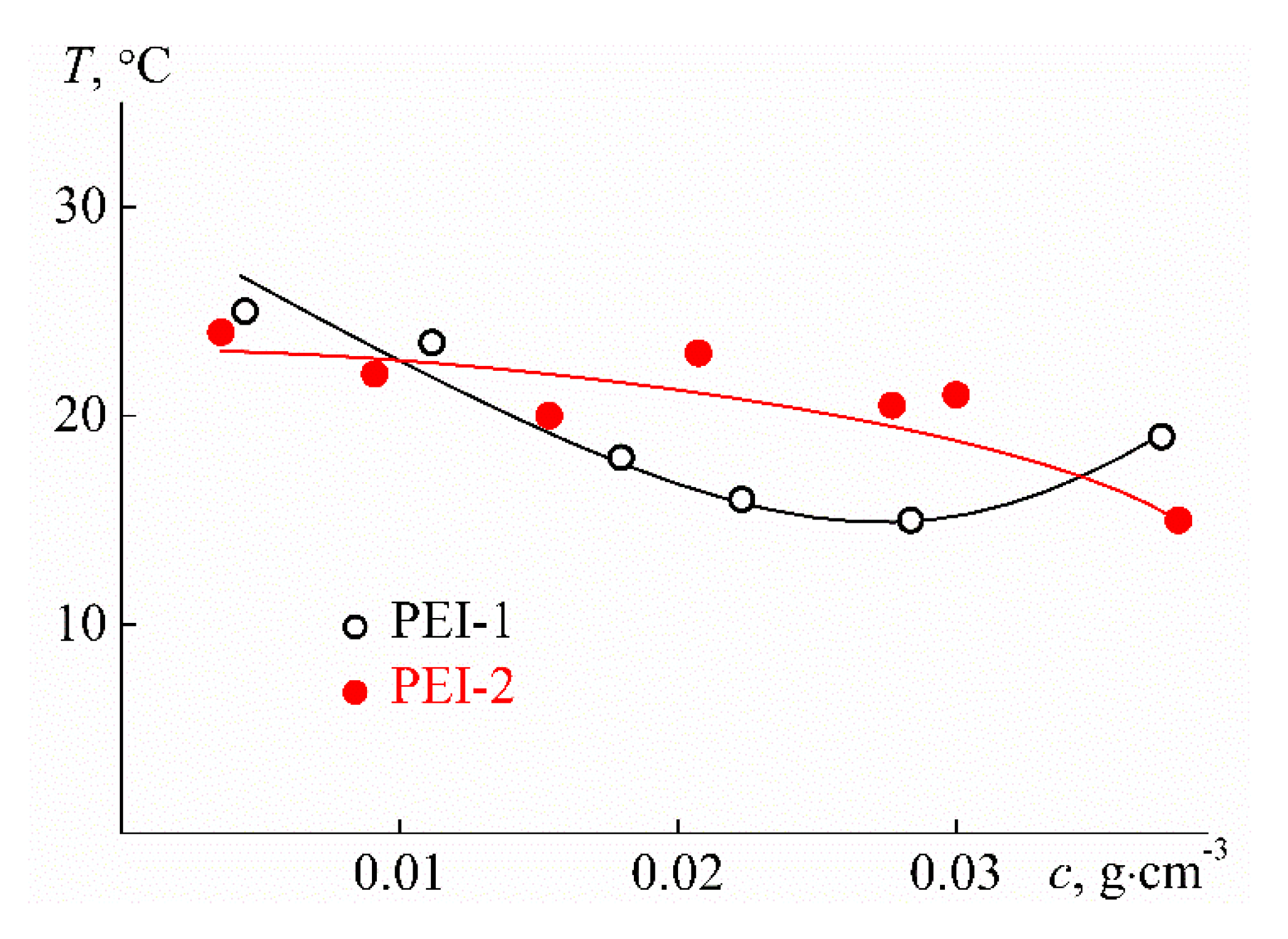
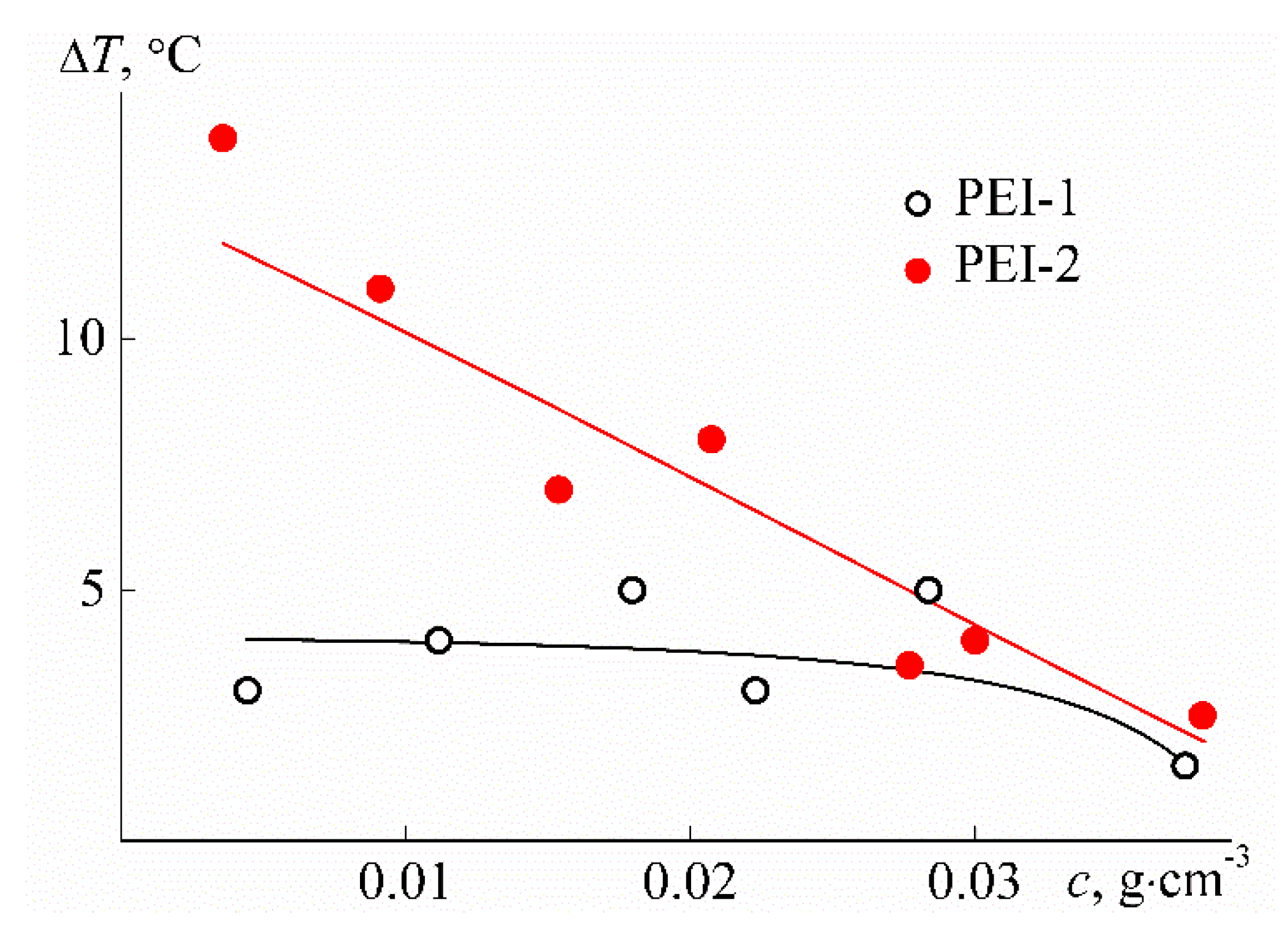
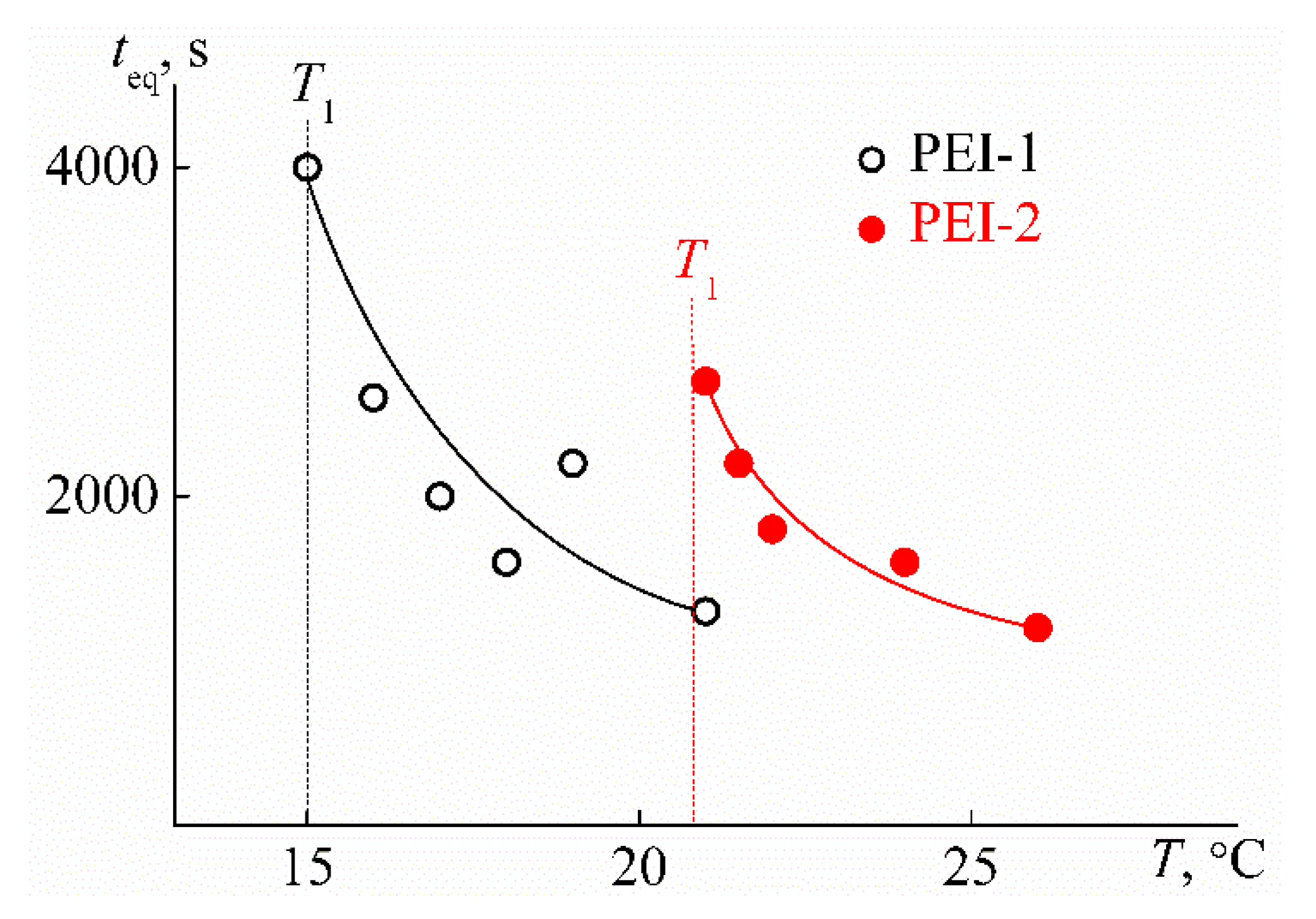
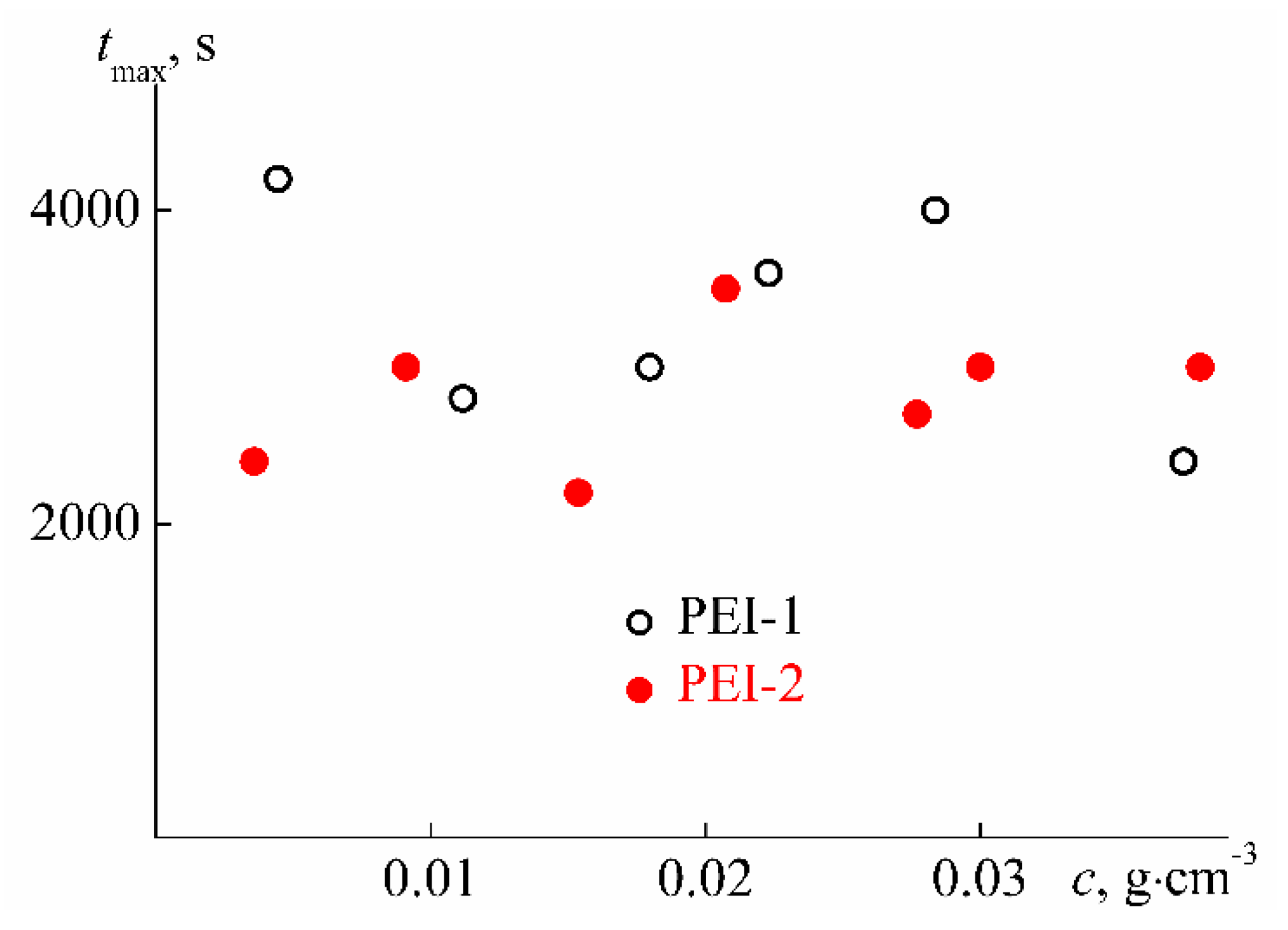
3.2. Behavior of PEI-n in Aqueous Solution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reif, M.; Jordan, R. α,ω-Functionalized poly(2-oxazoline)s bearing hydroxyl and amino functions. Macromol. Chem. Phys. 2011, 212, 1815–1824. [Google Scholar] [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design strategies for functionalized poly(2-oxazoline)s and derived materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef]
- Sedlacek, O.; Hoogenboom, R. Drug delivery systems based on poly(2-oxazoline)s and poly(2-oxazine)s. Adv. Therap. 2020, 3, 1900168. [Google Scholar] [CrossRef]
- Schlaad, H.; Diehl, C.; Gress, A.; Meyer, M.; Demirel, A.L.; Nur, Y.; Bertin, A. Poly(2-oxazoline)s as smart bioinspired polymers. Macromol. Rapid Commun. 2010, 31, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release. 1999, 60, 149–160. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2008, 109, 259–302. [Google Scholar] [CrossRef]
- Wagner, E.; Kloeckner, J. Gene delivery using polymer therapeutics. Adv. Polym. Sci. 2006, 192, 135–173. [Google Scholar]
- Lungwitz, U.; Breunig, M.; Blunk, T.; Gopferich, A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 2005, 60, 247–266. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, J.; Wen, S.; Peng, C.; Shen, M.; Shi, X. Effect of surface charge of polyethyleneimine-modified multiwalled carbon nanotubes on the improvement of polymerase chain reaction. Nanoscale 2011, 3, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Ustinova, T.M.; Yuidin, M.A.; Vengerovich, N.G.; Stepanov, A.V.; Gadzikovskii, S.V. Comparative analysis of polyethyleneimine efficiency for improvement of plasmid DNA bioavailability. Bull. Exp. Biol. Med. 2018, 164, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.K.; Wang, H.F.; Yan, X.P. A sensitive and selective resonance light scattering bioassay for homocysteine in biological fluids based on target-involved assembly of polyethyleneimine-capped Ag-nanoclusters. Chem. Commun. 2011, 47, 3817–3819. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhao, Q.; An, X.; Zhu, J.; Hou, W.; Li, K.; Huang, Y.; Shen, M.; Zhu, W.; Shi, X. Multifunctional PEGylated multiwalled carbon nanotubes for enhanced blood pool and tumor MR imaging. Adv. Healthc. Mater. 2014, 3, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Signori, A.M.; Santos, K.O.; Eising, R.; Albuquerque, B.L.; Giacomelli, F.C.; Domingos, J.B. Formation of catalytic silver nanoparticles supported on branched polyethyleneimine derivatives. Langmuir 2010, 26, 17772–17779. [Google Scholar] [CrossRef]
- Chertok, B.; David, A.E.; Yang, V.C. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials 2010, 31, 6317–6324. [Google Scholar] [CrossRef]
- Shi, X.; Gong, H.; Li, Y.; Wang, C.; Cheng, L.; Liu, Z. Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials 2013, 34, 4786–4793. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, G.-P.; Xu, F.-J. Efficient poly(N-3-hydroxypropyl) aspartamide—based carriers via ATRP for gene delivery. ACS Appl. Mater. Interfaces 2013, 5, 1840–1848. [Google Scholar] [CrossRef]
- Subramani, C.; Ofir, Y.; Patra, D.; Jordan, B.J.; Moran, I.W.; Park, M.H.; Carter, K.R.; Rotello, V.M. Nanoimprinted polyethyleneimine: A multimodal template for nanoparticle assembly and immobilization. Adv. Funct. Mater. 2009, 19, 2937–2942. [Google Scholar] [CrossRef]
- Wen, S.; Zheng, F.; Shen, M.; Shi, X. Surface modification and PEGylation of branched polyethyleneimine for improved biocompatibility. J. Appl. Polym. Sci. 2013, 128, 3807–3813. [Google Scholar] [CrossRef]
- Oskuee, R.K.; Dehshahri, A.; Shier, W.T.; Ramezani, M. Alkylcarboxylate grafting to polyethylenimine: A simple approach to producing a DNA nanocarrier with low toxicity. J. Gene Med. 2009, 11, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Pun, S.H.; Bellocq, N.C.; Liu, A.; Jensen, G.; Machemer, T.; Quijano, E.; Schluep, T.; Wen, S.; Engler, H.; Heidel, J.; et al. Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconjugate Chem. 2004, 15, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, A.; Uchegbu, I.F.; Schätzlein, A.G. PEI-based vesicle-polymer hybrid gene delivery system with improved biocompatibility. Int. J. Pharm. 2004, 274, 41–52. [Google Scholar] [CrossRef]
- Thomas, M.; Klibanov, A.M. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14640–14645. [Google Scholar] [CrossRef]
- Appelhans, D.; Komber, H.; Quadir, M.A.; Richter, S.; Schwarz, S.; van der Vlist, J.; Aigner, A.; Muller, M.; Loos, K.; Seidel, J.; et al. Hyperbranched PEI with various oligosaccgaride architectures: Synthesis, characterization, ATP complexation, and cellular uptake properties. Biomacromolecules 2009, 10, 1114–1124. [Google Scholar] [CrossRef]
- Peng, Q.; Zhong, Z.; Zhuo, R. Disulfide cross-linked polyethylenimines (PEI) prepared via thiolation of low molecular weight PEI as highly efficient gene vectors. Bioconjugate Chem. 2008, 19, 499–506. [Google Scholar] [CrossRef]
- Tong, W.; Cao, X.; Wen, S.; Guo, R.; Shen, M.; Wang, J.; Shi, X. Enhancing the specificity and efficiency of polymerase chain reaction using polyethyleneimine-based derivatives and hybrid nanocomposites. Int. J. Nanomed. 2012, 7, 1069–1078. [Google Scholar]
- Gabrielson, N.P.; Pack, D.W. Acetylation of polyethylenimine enhances gene delivery via weakened polymer/DNA interactions. Biomacromolecules 2006, 7, 2427–2435. [Google Scholar] [CrossRef]
- Griffiths, P.C.; Alexander, C.; Nilmini, R.; Pennadam, S.S.; King, S.M.; Heenan, R.K. Physicochemical characterization of thermoresponsive poly(N-isopropylacrylamide)−poly(ethylene imine) graft copolymers. Biomacromolecules 2008, 9, 1170–1178. [Google Scholar] [CrossRef]
- Dinçer, S.; Tuncel, A.; Pişkin, E. A potential gene delivery vector: N-isopropylacrylamide-ethyleneimine block copolymers. Macromol. Chem. Phys. 2002, 203, 14601465. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Chiang, H.Z.; Wang, C.H.; Juang, T.M. Nonviral gene carriers based on diblock copolymers of poly(2-ethyl-2-oxazoline) and linear polyethylenimine. Bioconjugate Chem. 2006, 17, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.; Mok, Y.; Nakayama, D.; Jang, S.; Lee, S.; Kim, T.; Lee, Y. Introduction of pH-sensitive upper critical solution temperature (UCST) properties into branched polyethylenimine. Polymer 2013, 54, 5338–5344. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Shen, Z. Thermoresponsive hyperbranched polyethylenimines with isobutyramide functional groups. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1177–1184. [Google Scholar] [CrossRef]
- Tenkovtsev, A.V.; Kurlykin, M.P.; Amirova, A.I.; Krasova, A.S.; Kirila, T.U.; Filippov, A.P. Thermosensitive nanoparticles based on polyethylenimine as promising materials for biomedical applications. Russ. J. Gen. Chem. 2020. In Press. [Google Scholar]
- Einhorn, A.; Holland, F. Ueber die Acylirung der Alkohole und Phenole in Pyridinlösung. Justus Liebigs Ann. Chem. 1898, 301, 95–115. [Google Scholar] [CrossRef]
- Kelly, A.M.; Wiesbrock, F. Strategies for the Synthesis of Poly(2-Oxazoline)-Based Hydrogels. Macromol. Rapid Commun. 2012, 33, 1632–1647. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers; Plenum: New York, NY, USA, 1989. [Google Scholar]
- Rueb, C.J.; Zukoski, C.F. Rheology of suspensions of weakly attractive particles: Approach to gelation. J. Rheol. 1998, 42, 1451–1476. [Google Scholar] [CrossRef]
- Yamakawa, H. Modern Theory of Polymer Solutions; Harper and Row: New York, NY, USA, 1971. [Google Scholar]
- Filippov, A.P.; Belyaeva, E.V.; Tarabukina, E.B.; Amirova, A.I. Behavior of hyperbranched polymers in solutions. Polym. Sci. Ser. C 2011, 53, 107–117. [Google Scholar] [CrossRef]
- Bodnár, I.; Silva, A.S.; Deitcher, R.W.; Weisman, N.E.; Kim, Y.H.; Wagner, N.J. Structure and rheology of hyperbranched and dendritic polymers. I. Modification and characterization of poly(propyleneimine) dendrimers with acetyl groups. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 857–873. [Google Scholar] [CrossRef]
- Jeong, M.; Mackay, M.E.; Vestberg, R.; Hawker, C.J. Intrinsic viscosity variation in different solvents for dendrimers and their hybrid copolymers with linear polymers. Macromolecules 2001, 34, 4927–4936. [Google Scholar] [CrossRef]
- Simonova, M.A.; Tarasova, E.V.; Dudkina, M.M.; Tenkovtsev, A.V.; Filippov, A.P. Synthesis and hydrodynamic and conformation properties of star-shaped polystyrene with calix [8] arene core. Int. J. Polym. Anal. Charact. 2019, 24, 87–95. [Google Scholar] [CrossRef]
- Terao, K.; Hokajo, T.; Nakamura, Y.; Norisuye, T. Solution properties of polymacromonomers consisting of polystyrene. 3. Viscosity behavior in cyclohexane and toluene. Macromolecules 1999, 32, 3690–3694. [Google Scholar] [CrossRef]
- Park, H., II; Choi, E.-J. Characterization of branched polyethyleneimine by laser light scattering and viscometry. Polymer 1996, 37, 313–319. [Google Scholar] [CrossRef]
- Freire, J.J. Conformational properties of branched polymers: Theory and simulations. In Branched Polmers II; Roovers, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 35–112. [Google Scholar]
- Burchard, W. Solution properties of branched macromolecules. In Branched Polymers II; Roovers, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 113–194. [Google Scholar]
- Satoh, T.; Imai, T.; Ishihara, H.; Maeda, T.; Kitajyo, Y.; Sakai, Y.; Kaga, H.; Kaneko, N.; Ishii, F.; Kakuchi, T. Synthesis, branched structure, and solution property of hyperbranched d-glucan and d-galactan. Macromolecules 2005, 38, 4202–4210. [Google Scholar] [CrossRef]
- Rietveld, I.B.; Smit, J.A.M. Colligative and viscosity properties of poly(propylene imine) dendrimers in methanol. Macromolecules 1999, 32, 4608–4614. [Google Scholar] [CrossRef]
- Dworak, A.; Trzebicka, B.; Kowalczuk, A.; Tsvetanov, C.; Rangelov, S. Polyoxazolines—mechanism of synthesis and solution properties. Polymery 2014, 59, 88–94. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Kohjiya, S.; Tsutsumi, K.; Okamoto, Y. On the intrinsic viscosity of poly(macromonomer)s. Macromolecules 1994, 27, 1662–1664. [Google Scholar] [CrossRef]
- Von Harpe, A.; Petersen, H.; Li, Y.; Kissel, T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J. Control. Release 2000, 69, 309–322. [Google Scholar] [CrossRef]
- Mori, H.; Seng, D.C.; Lechner, H.; Zhang, M.; Muller, A.H.E. Synthesis and characterization of branched polyelectrolytes. 1. Preparation of hyperbranched poly(acrylic acid) via self-condensing atom transfer radical copolymerization. Macromolecules 2002, 35, 9270–9281. [Google Scholar] [CrossRef]
- Mori, H.; Walther, A.; André, X.; Lanzendorfer, M.G.; Muller, A.H.E. Synthesis of highly branched cationic polyelectrolytes via self-condensing atom transfer radical copolymerization with 2-(diethylamino)ethyl methacrylate. Macromolecules 2004, 37, 2054–2066. [Google Scholar] [CrossRef]
- Filippov, A.P.; Romanova, O.A.; Vinogradova, L.V. Molecular and hydrodynamic characteristics of star-shaped polystyrenes with one or two fullerene (C60) molecules as a branching center. Polym. Sci. Ser. A 2010, 52, 221–227. [Google Scholar] [CrossRef]
- Amirova, A.I.; Golub, O.V.; Meshkov, I.B.; Migulin, D.A.; Muzafarov, A.M.; Filippov, A.P. Solution behavior of hyperbranched polymethylsilsesquioxane with intramolecular cycles. Int. J. Polym. Anal. Char. 2015, 20, 268–276. [Google Scholar] [CrossRef]
- Shpyrkov, A.A.; Tarasenko, I.I.; Pankova, G.A.; Il’ina, I.E.; Tarasova, E.V.; Tarabukina, E.B.; Vlasov, G.P.; Filippov, A.P. Molecular mass characteristics and hydrodynamic and conformational properties of hyperbranched poly-l-lysines. Polym. Sci. Ser. A 2009, 51, 250–258. [Google Scholar] [CrossRef]
- Gubarev, A.S.; Monnery, B.D.; Lezov, A.A.; Sedlacek, O.; Tsvetkov, N.V.; Hoogenboom, R.; Filippov, S.K. Conformational properties of biocompatible poly(2-ethyl-2-oxazoline)s in phosphate buffered saline. Polym. Chem. 2018, 9, 2232–2237. [Google Scholar] [CrossRef]
- Iatrou, H.; Pitsikalis, M.; Hadjichristidis, N. Hyperbranched architectures. In Modification and Blending of Synthetic and Natural Macromolecules; Ciardelli, F., Penczek, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 175, pp. 73–89. [Google Scholar]
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures—Synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef]
- Lezov, A.V.; Mel’nikov, A.B.; Polushina, G.E.; Antonov, E.A.; Novitskaya, M.E.; Ryumtsev, E.I.; Boiko, N.I.; Ponomarenko, S.A.; Shibaev, V.P.; Rebrov, E.A.; et al. Self-assembling of terminal mesogenic groups in carbosilane dendrimer molecules. Dokl. Chem. 2001, 381, 313–316. [Google Scholar] [CrossRef]
- Lezov, A.V.; Mel’nikov, A.B.; Polushina, G.E.; Ponomarenko, S.A.; Boiko, N.I.; Kossmehl, E.; Ryumtsev, E.I.; Shibaev, V.P. Dualism in hydrodynamic behavior of liquid crystalline carbosilane dendrimers in dilute solutions. Dokl. Physic.Chem. 1998, 362, 338–342. [Google Scholar]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. A hydrodynamic invariant of polymeric molecules. Russ. Chem. Rev. 1982, 51, 975–993. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic invariant of polymer-molecules. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 3447–3486. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Roy, R.; Michailova, N.A.; Ortega, P.C.; Perez, M.A. Sedimentation, translational diffusion, and viscosity of lactosylated polyamidoamine dendrimers. Progr. Colloid Polym. Sci. 1999, 113, 150–157. [Google Scholar]
- Jikei, M.; Chon, S.-H.; Kakimoto, M.; Kawauchi, S.; Imase, T.; Watanebe, J. Synthesis of Hyperbranched aromatic polyamide from aromatic diamines and trimesic Acid. Macromolecules 1999, 32, 2061–2064. [Google Scholar] [CrossRef]
- Monticelli, O.; Mariani, A.; Voit, B.; Komber, H.; Mendichi, R.; Pitto, V.; Tabuani, D.; Russo, S. Hyperbranched aramids by the A2 + B3 versus AB2 approach: Influence of the reaction conditions on structural development. High Perform. Polym. 2001, 13, S45–S59. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhu, H.; Yin, Q.; Liu, H.; Hu, Y. Effect of composition of PDMAEMA-b-PAA block copolymers on their pH- and temperature-responsive behaviors. Langmuir 2013, 29, 1024–1034. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, S.Y.; Chung, I.S. Unprecedented lower critical solution temperature behavior of polyimides in organic media. Macromolecules 2014, 47, 8846–8849. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behaviorin alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Kirila, T.; Smirnova, A.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Self-organization in aqueous solutions of thermosensitive star-shaped and linear gradient copolymers of 2-ethyl-2-oxazoline and 2-isopropyl-2-oxazoline. Colloid. Polym. Sci. 2020, 298, 1–12, In Press. [Google Scholar] [CrossRef]
- Amirova, A.; Rodchenko, S.; Milenin, S.; Tatarinova, E.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Influence of a hydrophobic core on thermoresponsive behavior of dendrimer-based star-shaped poly(2-isopropyl-2-oxazoline) in aqueous solutions. J. Polym. Res. 2017, 24, 124. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(Nisopropylacrylamide). J. Macromol. Sci. Part A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Uyama, H.; Kobayashi, S. A novel thermosensitive polymer—Poly(2-iso-propyl-2-oxazoline). Chem. Lett. 1992, 9, 1643–1646. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Kronek, J.; Bosowska, K.; Trzebicka, B.; Dworak, A. Star poly (2-ethyl-2-oxazoline)s—Synthesis and thermosensitivity. Polym. Int. 2011, 7, 1001–1009. [Google Scholar] [CrossRef]
- Park, J.-S.; Kataoka, K. Precise control of lower critical solution temperature of thermosensitive poly(2-isopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline as a hydrophilic comonomer. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Amirova, A.I.; Dudkina, M.M.; Tenkovtsev, A.V.; Filippov, A.P. Self-assembly of star-shaped poly(2-isopropyl-2-oxazoline) in aqueous solutions. Colloid Polym. Sci. 2015, 293, 239–248. [Google Scholar] [CrossRef]
- Trzebicka, B.; Szweda, R.; Kosowski, D.; Szweda, D.; Otulakowski, Ł.; Haladjova, E.; Dworak, A. Thermoresponsive polymer-peptide/protein conjugates. Prog. Polym. Sci. 2017, 68, 35–76. [Google Scholar] [CrossRef]
- Amirova, A.; Rodchenko, S.; Filippov, A. Time dependence of the aggregation of star-shaped poly(2-isopropyl-2-oxazolines) in aqueous solutions. J. Polym. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Kudryavtseva, A.A.; Kurlykin, M.P.; Tarabukina, E.B.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of thermosensitive graft-copolymers with aromatic polyester backbone and poly-2-ethyl-2-oxazoline side chains in aqueous solutions. Int. J. Polym. Anal. Charact. 2017, 22, 526–533. [Google Scholar] [CrossRef]
- De la Rosa, V.R.; Nau, W.M.; Hoogenboom, R. Tuning temperature responsive poly(2-alkyl-2-oxazoline)s by supramolecular host-guest interactions. Org. Biomol. Chem. 2015, 13, 3048–3057. [Google Scholar] [CrossRef]
- Zaccone, A.; Crassous, J.J.; Béri, B.; Ballauff, M. Quantifying the reversible association of thermosensitive nanoparticles. Phys. Rev. Lett. 2011, 107, 168303. [Google Scholar] [CrossRef]
- Ye, J.; Xu, J.; Hu, J.; Wang, X.; Zhang, G.; Liu, S.; Wu, C. Comparative study of temperature-induced association of cyclic and linear poly(N-isopropylacrylamide) chains in dilute solutions by laser light scattering and stopped-flow temperature jump. Macromolecules 2008, 41, 4416–4422. [Google Scholar] [CrossRef]
- Adelsberger, J.; Grillo, I.; Kulkarni, A.; Sharp, M.; Bivigou-Koumba, A.M.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Kinetics of aggregation in micellar solutions of thermoresponsive triblock copolymers – influence of concentration, start and target temperatures. Soft Matter 2013, 9, 1685–1699. [Google Scholar] [CrossRef]
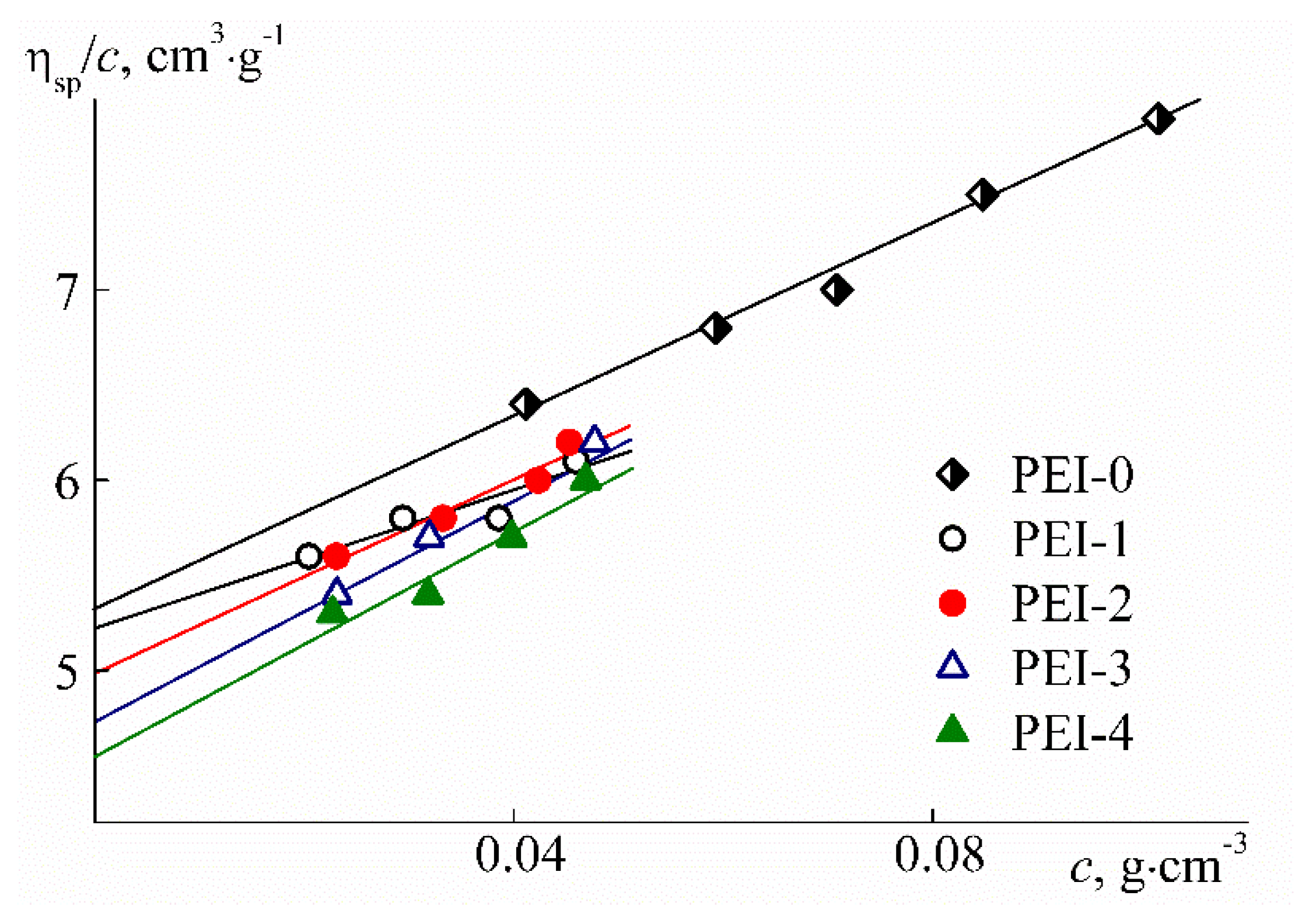
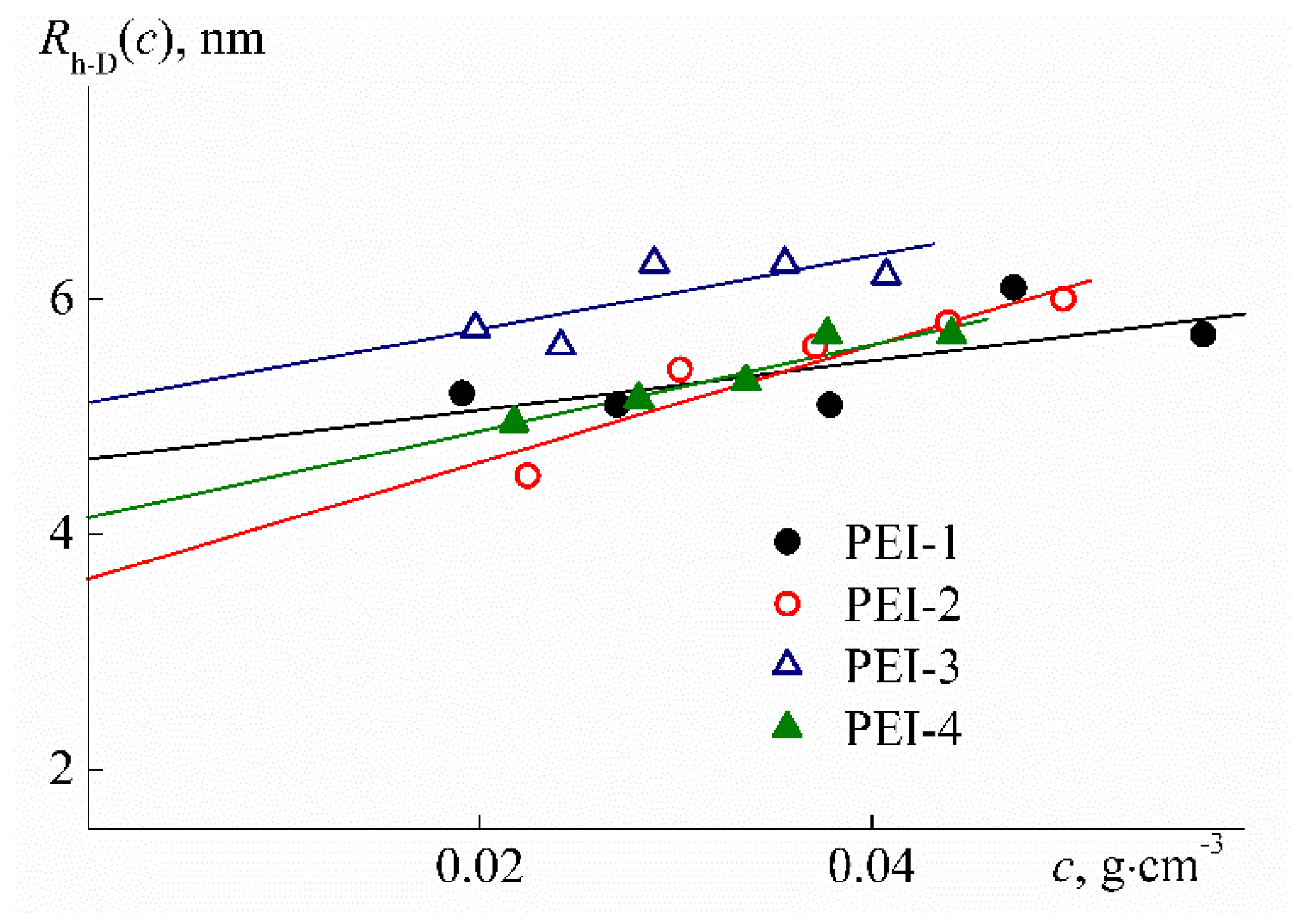
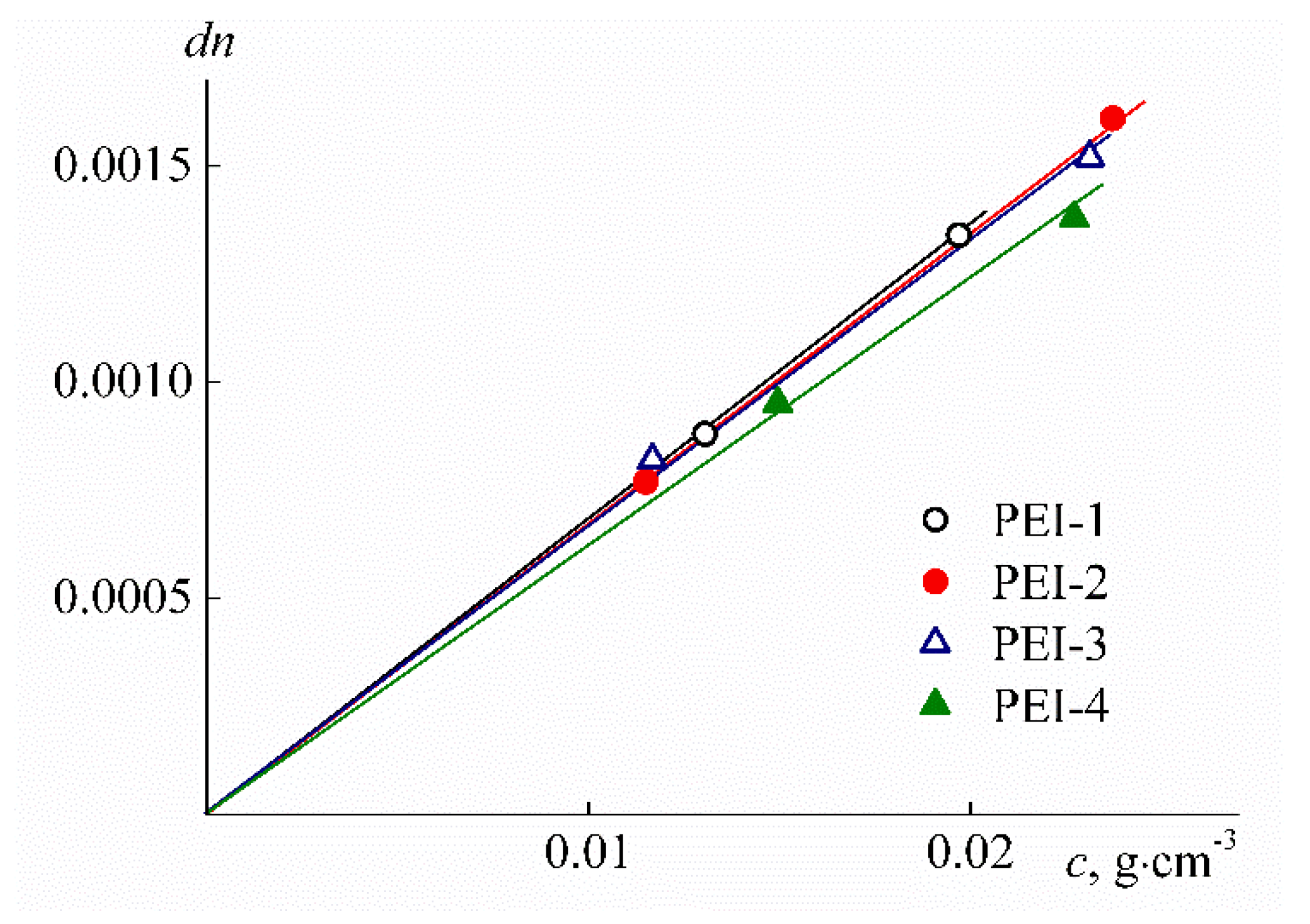
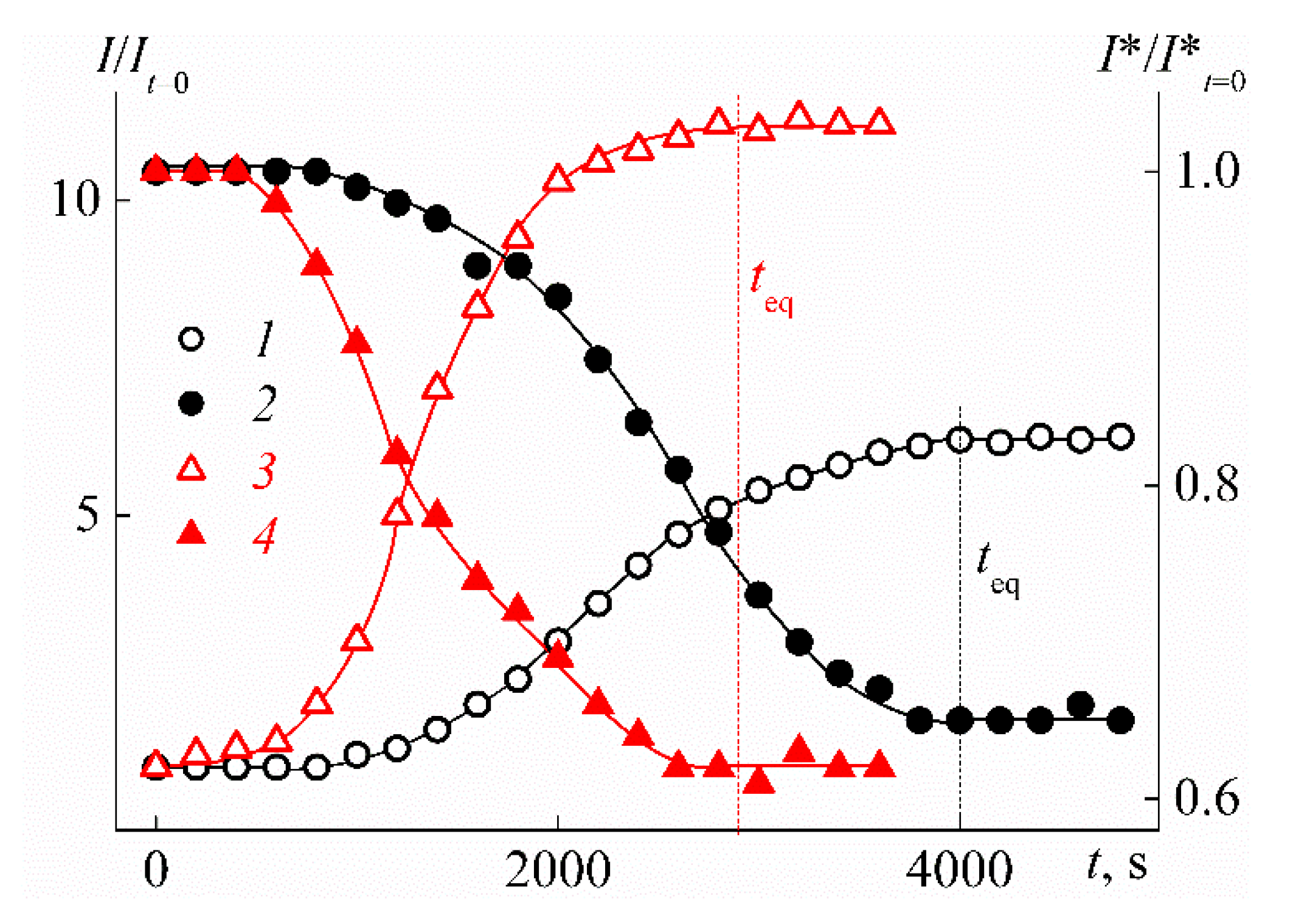
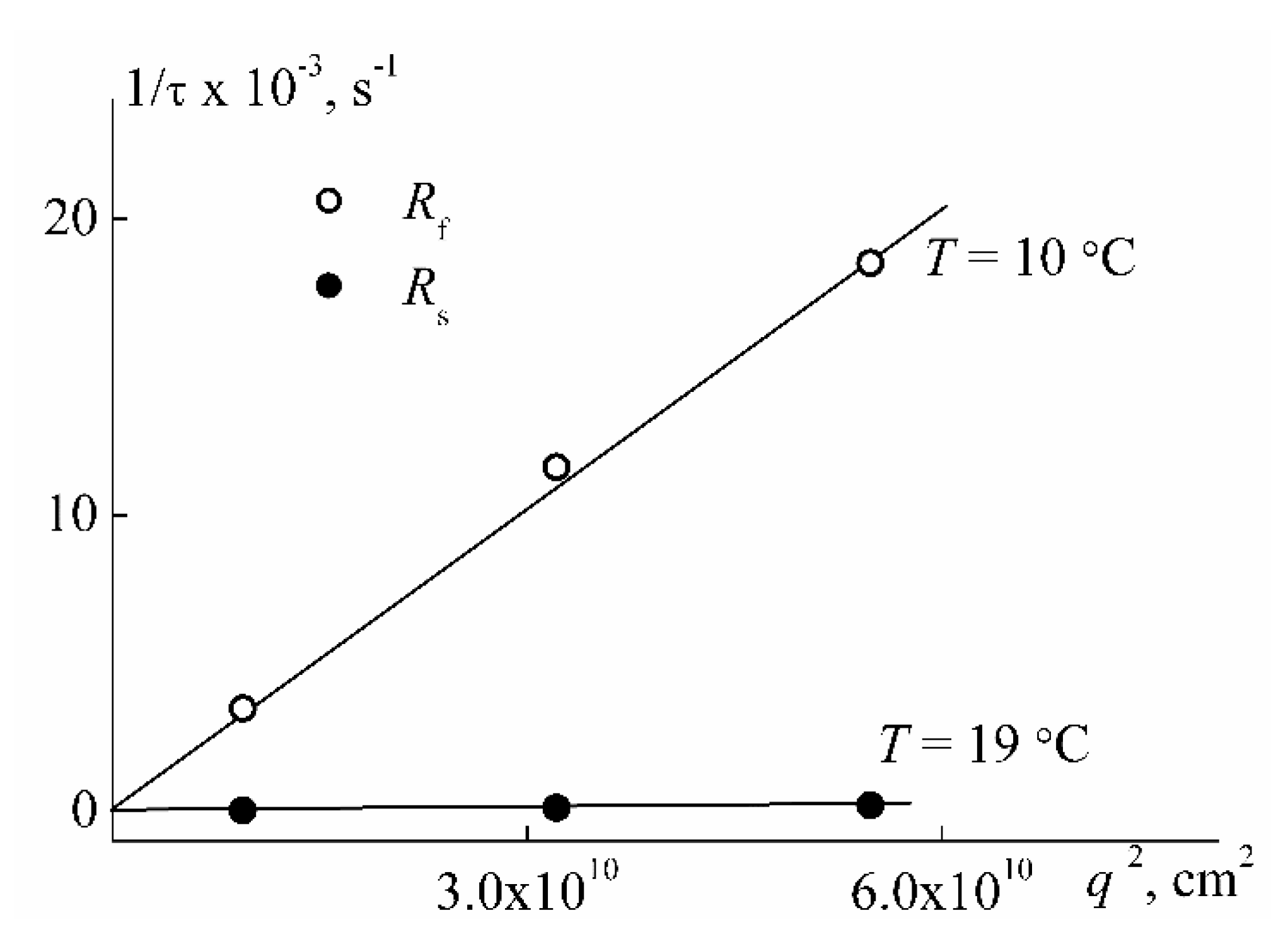
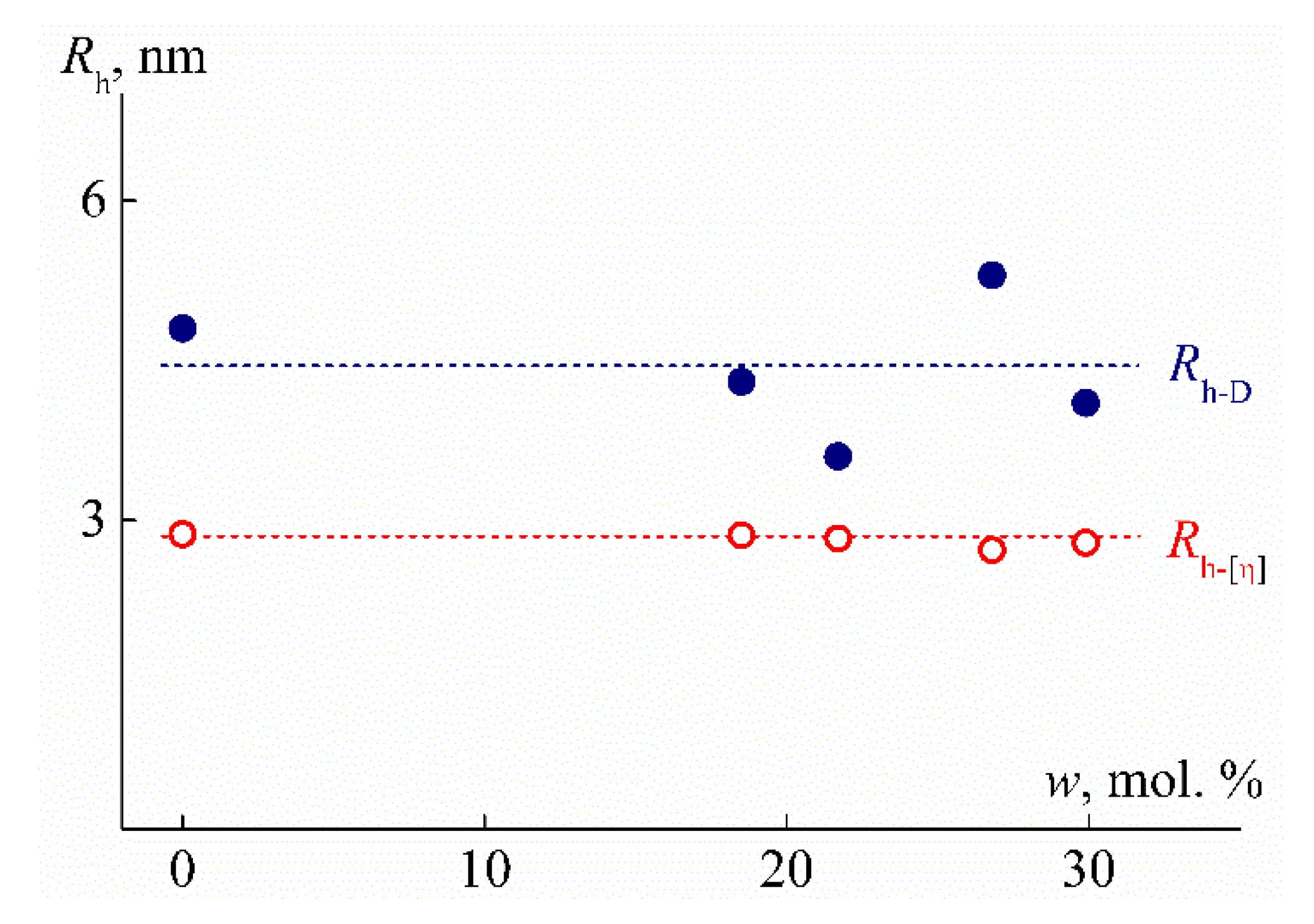
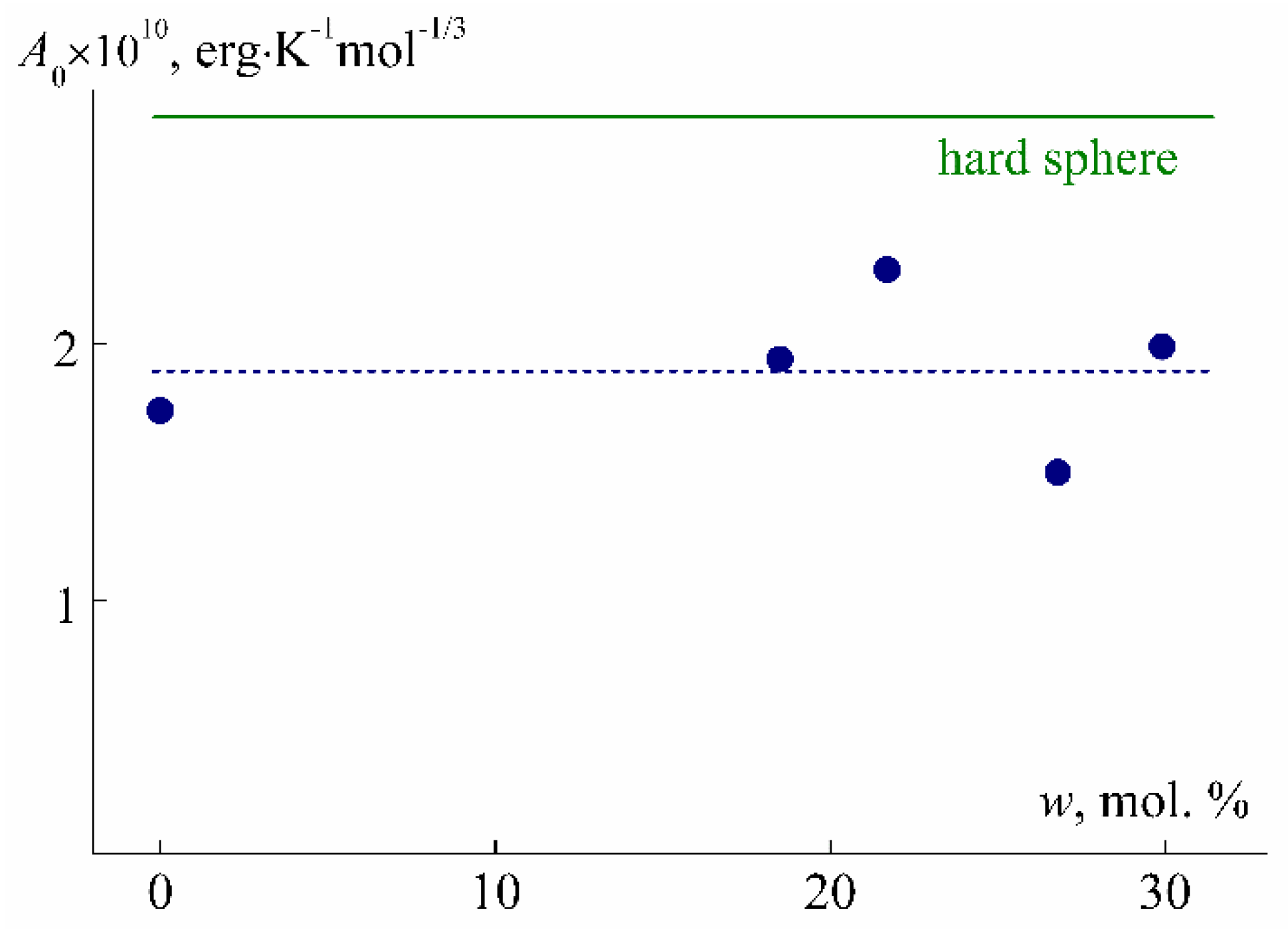
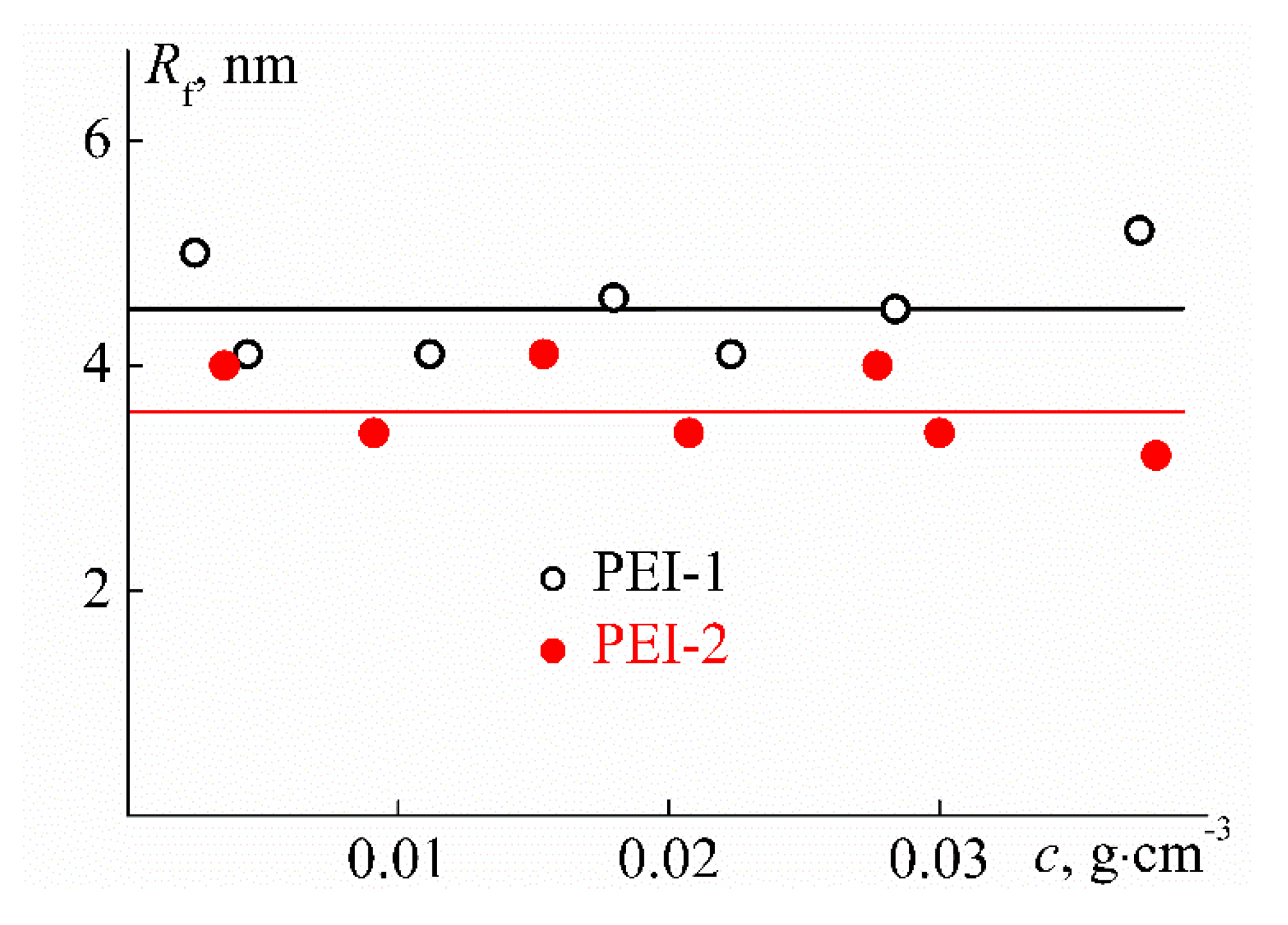
| Sample | w, mol % | Mw, G mol−1 | [η], cm3 g−1 | dn/dc, cm3 g−1 | Rh-D, nm | Rh-η, nm | A0 × 10−10, Erg K−1mol−1/3 |
|---|---|---|---|---|---|---|---|
| PEI-0 | 0 | 28,000 | 5.3 | 0.0964 | 4.8 | 2.9 | 1.7 |
| PEI-1 | 18.5 | 28,000 | 5.2 | 0.0679 | 4.3 | 2.9 | 1.9 |
| PEI-2 | 21.7 | 28,000 | 5.1 | 0.0677 | 3.6 | 2.8 | 2.3 |
| PEI-3 | 26.8 | 27,000 | 4.8 | 0.0666 | 5.3 | 2.7 | 1.5 |
| PEI-4 | 29.9 | 30,000 | 4.5 | 0.0616 | 4.1 | 2.8 | 2.0 |
| PEI-5 | 37.9 | insoluble | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirova, A.; Kirila, T.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Influence of Cross-Linking Degree on Hydrodynamic Behavior and Stimulus-Sensitivity of Derivatives of Branched Polyethyleneimine. Polymers 2020, 12, 1085. https://doi.org/10.3390/polym12051085
Amirova A, Kirila T, Kurlykin M, Tenkovtsev A, Filippov A. Influence of Cross-Linking Degree on Hydrodynamic Behavior and Stimulus-Sensitivity of Derivatives of Branched Polyethyleneimine. Polymers. 2020; 12(5):1085. https://doi.org/10.3390/polym12051085
Chicago/Turabian StyleAmirova, Alina, Tatyana Kirila, Mikhail Kurlykin, Andrey Tenkovtsev, and Alexander Filippov. 2020. "Influence of Cross-Linking Degree on Hydrodynamic Behavior and Stimulus-Sensitivity of Derivatives of Branched Polyethyleneimine" Polymers 12, no. 5: 1085. https://doi.org/10.3390/polym12051085
APA StyleAmirova, A., Kirila, T., Kurlykin, M., Tenkovtsev, A., & Filippov, A. (2020). Influence of Cross-Linking Degree on Hydrodynamic Behavior and Stimulus-Sensitivity of Derivatives of Branched Polyethyleneimine. Polymers, 12(5), 1085. https://doi.org/10.3390/polym12051085






