Combined Molecular Dynamics and DFT Simulation Study of the Molecular and Polymer Properties of a Catechol-Based Cyclic Oligomer of Polyether Ether Ketone
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussions
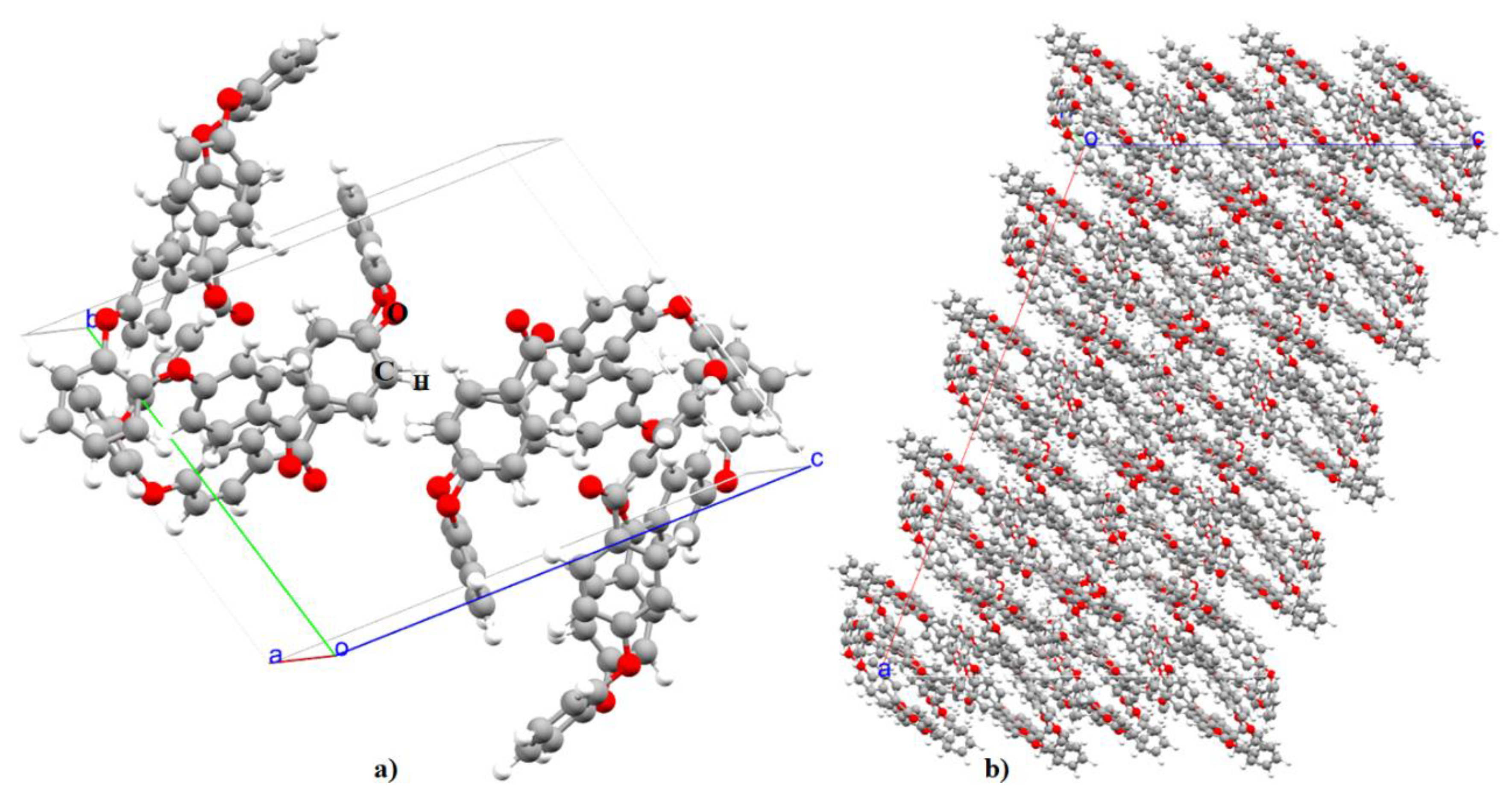
3.1. Lattice Properties
3.2. Polymer Properties
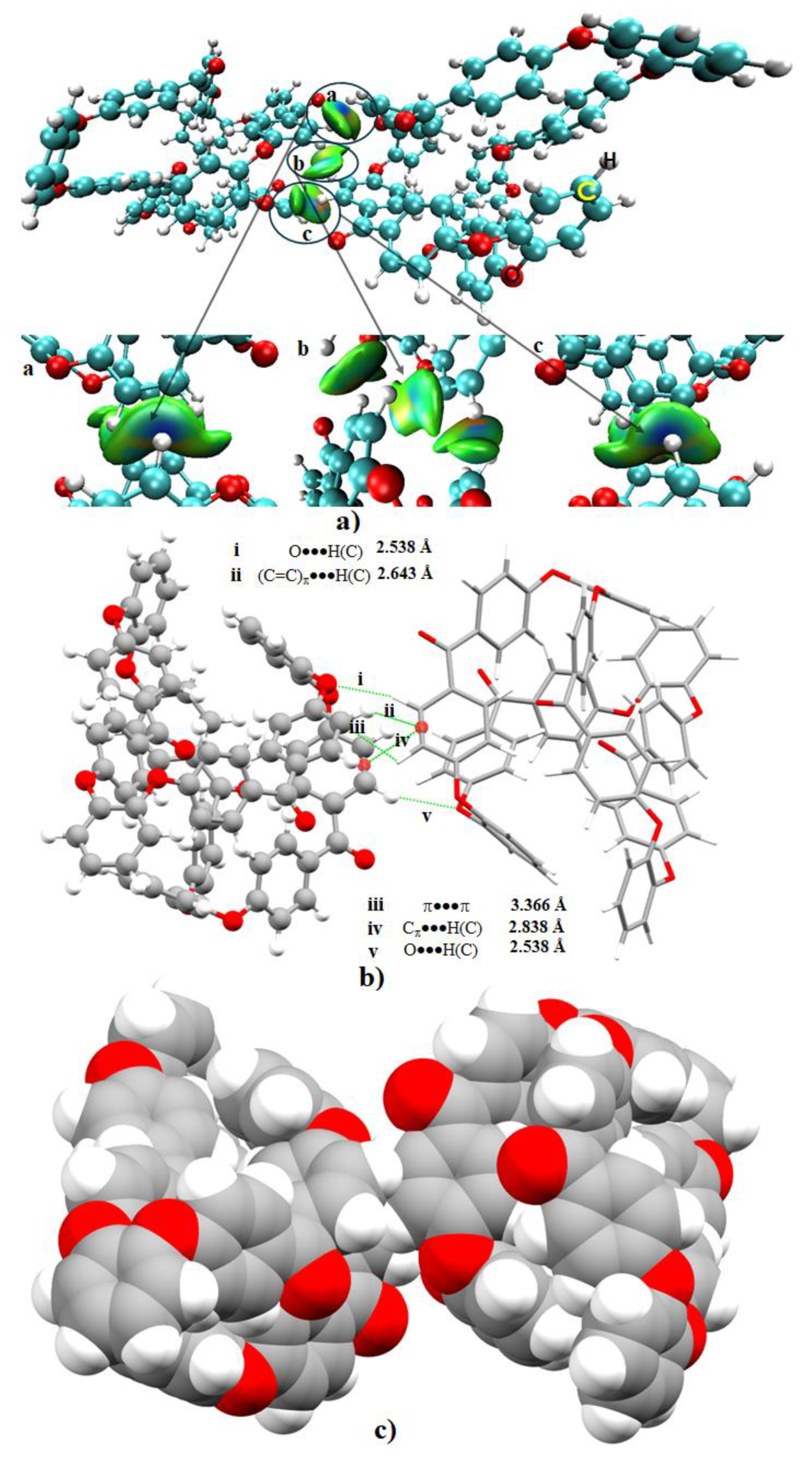
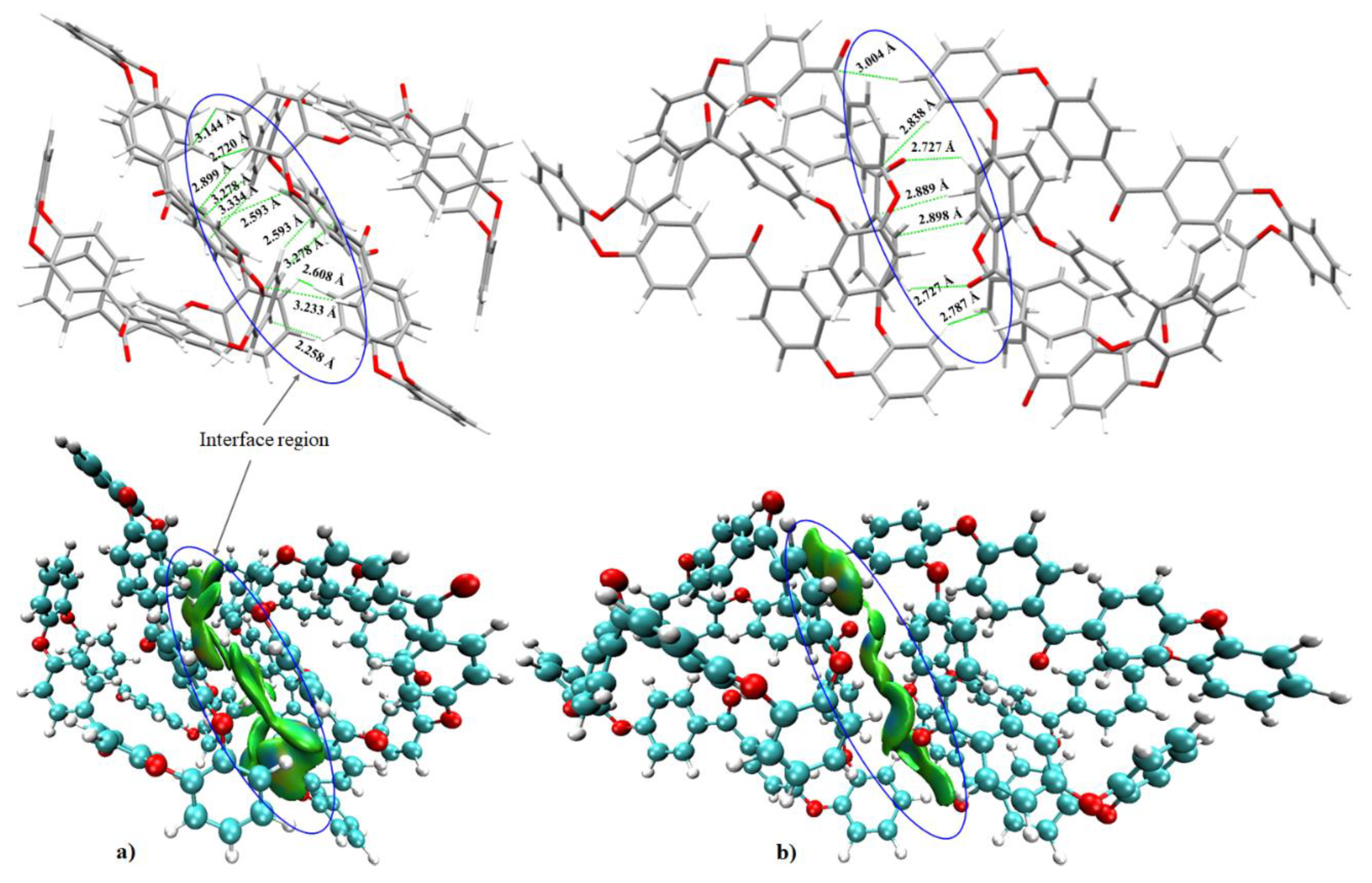
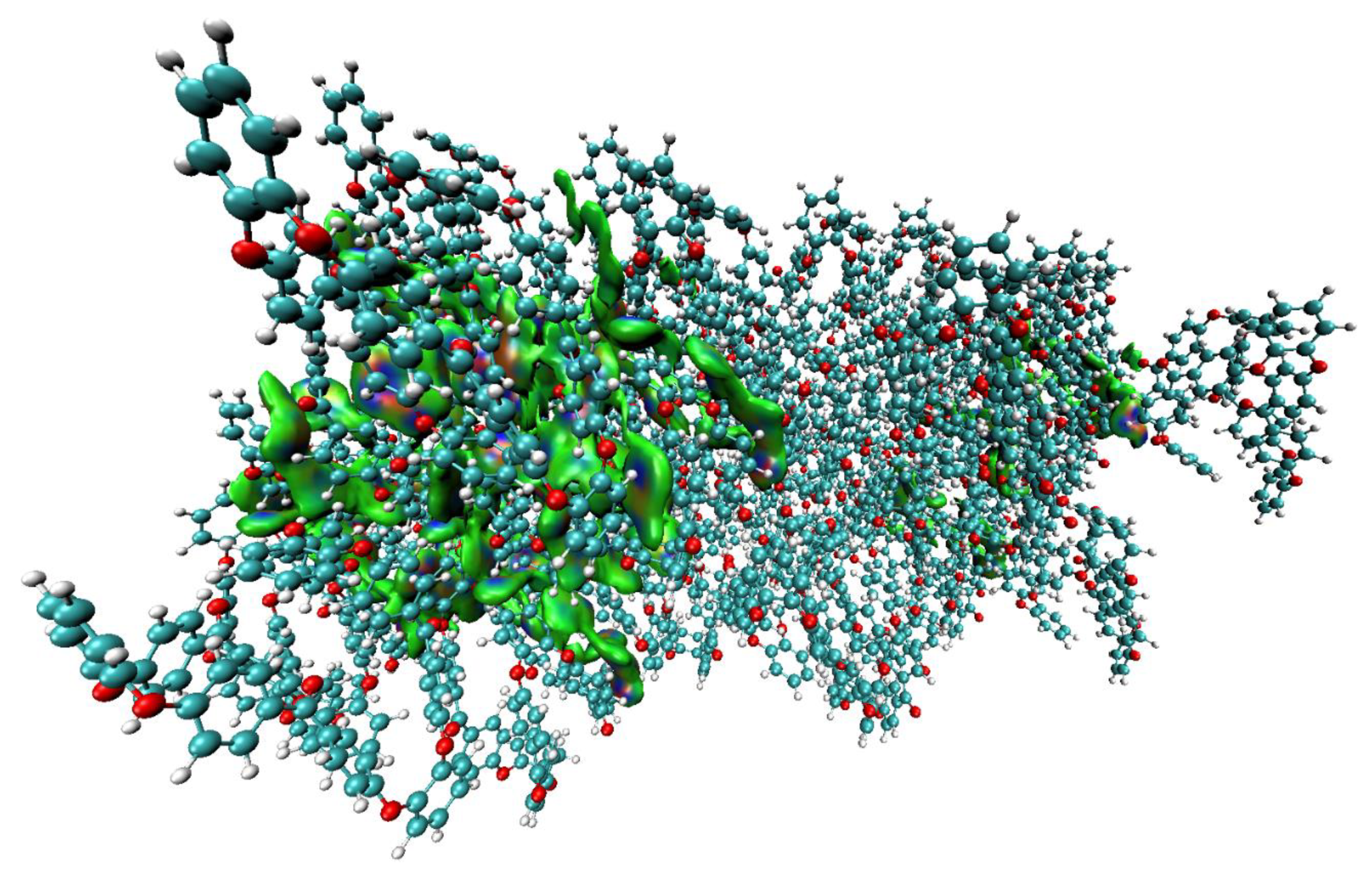
3.3. Nature of Noncovalent Interactions and Complex Stabilities
4. Conclusions
- (i)
- MD and DFT simulations results suggest that the packing density, volume, and lattice constant of o-PEEK predicted using these methods are not only consistent with each other but also with the experiment.
- (ii)
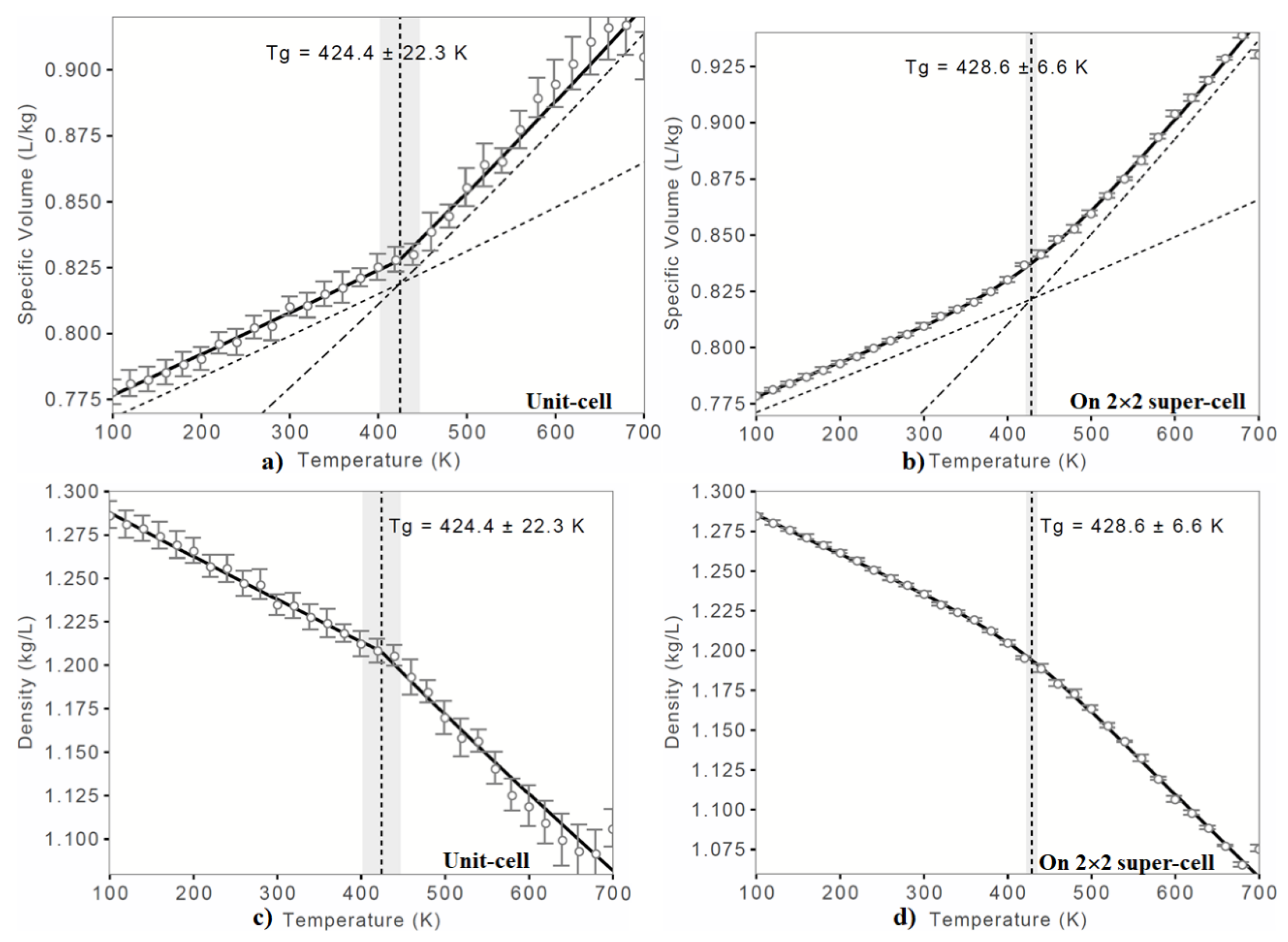
- COMPASS II force field coupled MD simulations could adequately predict the Tg of the o-PEEK system close to the experiment. This was true regardless of the size of the semi-crystalline system examined. The result also suggested that the glassy state of the semi-crystalline polymer system might be explained using relatively small-scale MD simulations.
- (iii)
- The compactness of the structure of the o-PEEK oligomer was recognized to be driven by the vast number of intramolecular interactions. These were identified and characterized to be O···H(C), Cπ···H(C), (C=C)π···H(C), C6(π)···H(C) and π···π.
- (iv)
- It was found that the local spatial arrangement between o-PEEK oligomers in the semi-crystalline system is controlled by a number of coordination modes. These modes appeared in various flavors, including intermolecular contacts composed of O···H(C), (C=C)π···H(C), Cπ···H(C), (C)H···H(C), (C=C)π···O(C), and (C6)π···H(C). Clearly, the stability of any of the five binary systems examined is controlled not only by the degree to which the energy of any individual interaction dominates, but also by the number of various such intermolecular interactions involved.
- (v)
- Analysis of the stabilization energies of the binary complex models suggested that the overall energy strengths would vary between –3.85 and –32.61 kcal mol−1 and are controlled by the nature of the spatial arrangement between the oligomers. The energy of each complex system was realized not simply by a single interaction between the oligomers, but was rather a collection of several contacts that determine overall stability.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mouritz, A.P. Introduction to Aerospace Materials; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 268–302. ISBN 978-1-85573-946-8. [Google Scholar] [CrossRef]
- Kausar, A. Role of Thermosetting Polymer in Structural Composite. Am. J. Polymer Sci. Eng. 2017, 5, 1–12. [Google Scholar]
- Hameed, N.; Salim, N.V.; Walsh, T.R.; Wiggins, J.S.; Ajayan, P.M.; Fox, B.L. Ductile thermoset polymers via controlling network flexibility. Chem. Commun. 2015, 51, 9903–9906. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Tolle, T.B. Carbon nanotube and epoxy composites for military applications. In Carbon Nanotechnology; Dai, L., Ed.; Elsevier: Dayton, OH, USA, 2006; pp. 633–675. Available online: https://www.elsevier.com/books/carbon-nanotechnology/dai/978-0-444-51855-2 (accessed on 15 April 2020).
- Tominaga, Y.; Shimamoto, D.; Hotta, Y. Curing Effects on Interfacial Adhesion between Recycled Carbon Fiber and Epoxy Resin Heated by Microwave Irradiation. Materials 2018, 11, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M.T.; Hassan, A. Recent advances in epoxy resin, natural fiber-reinforced epoxy composites and their applications. J. Reinf. Plast. Compos. 2015, 35, 447–470. [Google Scholar] [CrossRef]
- Pearson, R.A. Thermosetting Plastics. In Applied Polymer Science: 21st Century; Craver, C.D., Carraher, C.E., Jr., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 197–207. [Google Scholar] [CrossRef]
- Ouarhim, W.; Zari, N.; Bouhfid, R.; Qaiss, A.E. Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites. In Mechanical Performance of Natural Fibers–Based Thermosetting Composites; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Boca Raton, FL, USA, 2019; pp. 43–60. [Google Scholar]
- Biron, M. Thermoplastics: Economic Overview. In Material Selection for Thermoplastic Parts Practical and Advanced Information; William Andrew: Norwich, NY, USA, 2016; pp. 77–111. [Google Scholar]
- Baetens, R. High performance thermal insulation materials for buildings. In Nanotechnology in Eco-Efficient Construction Materials, Processes and Applications; Woodhead Publishing (USA) Series in Civil and Structural Engineering; Woodhead Publishing: Boca Raton, FL, USA, 2013; pp. 188–206, ISBN-13 978-0857095442. [Google Scholar]
- Beland, S. High Performance Thermoplastic Resins and their Composites, 1st ed.; William Andrew: Norwich, NY, USA, 1990; ISBN 9780815512783. [Google Scholar]
- Park, S.-J.; Seo, M.-K.C. 6-Element and Processing. In Interface Science and Technology; Academic Press: Cambridge, MA, USA, 2011; Volume 18, pp. 431–499. ISBN 9780123750495. [Google Scholar]
- Lee, L.H.; Vanselow, J.J.; Schneider, N.S. Effects of mechanical drawing on the structure and properties of peek. Polym. Eng. Sci. 1988, 28, 181–187. [Google Scholar] [CrossRef]
- McKeen, L.W. Polyether Ether Ketone (PEEK). In Fluorinated Coatings and Finishes Handbook; William Andrew: Norwich, NY, USA, 2006; ISBN 9780815515227. Available online: https://www.sciencedirect.com/book/9780815515227/fluorinated-coatings-and-finishes-handbook (accessed on 15 April 2020).
- Fortney, A.; Fossum, E. Soluble, semi-crystalline PEEK analogs based on 3,5-difluorobenzophenone: Synthesis and characterization. Polymer 2012, 53, 2327–2333. [Google Scholar] [CrossRef]
- King, M.A.; Blundell, D.J.; Howard, J.; Colbourn, E.A.; Kendrick, J. Modelling Studies of Crystalline PEEK. Mol. Simul. 1989, 4, 3–13. [Google Scholar] [CrossRef]
- Rudnik, E. Compostable Polymer Properties and Packaging Applications. In Plastic Films in Food Packaging; William Andrew: Norwich, NY, USA, 2013; Available online: https://doi.org/10.1016/B978-1-4557-3112-1.00013-2 (accessed on 15 April 2020).
- Ben-Haida, A.; Colquhoun, H.M.; Hodge, P.; Williams, D.J. Synthesis of a Catechol-Based Poly(ether ether ketone) (“o-PEEK”) by Classical Step-Growth Polymerization and by Entropically Driven Ring-Opening Polymerization of Macrocyclic Oligomers. Macromolecules 2006, 39, 6467–6472. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation; Academic Press, Inc.: Orlando, FL, USA, 2001; ISBN 0122673514. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.S.; Li, S.L.; Truhlar, D.G. Perspective: Kohn-Sham density functional theory descending a staircase. J. Chem. Phys. 2016, 145, 130901. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero-de-la-Roza, A.; Johnson, E.R.; DiLabio, G.A. Halogen Bonding from Dispersion-Corrected Density-Functional Theory: The Role of Delocalization Error. J. Chem. Theory Comput. 2014, 10, 5436–5447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Piquemal, J.-P.; Hénon, E. The Independent Gradient Model: A New Approach for Probing Strong and Weak Interactions in Molecules from Wave Function Calculations. ChemPhysChem 2018, 19, 724–735. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Halogen Bonding: A Halogen-Centered Noncovalent Interaction Yet to Be Understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burk, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [Green Version]
- Bučko, T.; Lebègue, S.; Hafner, J.; Ángyán, J.G. Tkatchenko-Scheffler van der Waals correction method with and without self-consistent screening applied to solids. Phys. Rev. B 2013, 87, 064110. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Zeitschrift für Kristallographie-Crystalline Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Dassault Systèmes. BIOVIA Materials Studio; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
- Dassault Systèmes. BIOVIA, BIOVIA Workbook, Release 2019; BIOVIA Pipeline Pilot, Release 2019; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pople, J.A. The Lennard-Jones lecture. Intermolecular binding. Faraday Discuss. 1982, 73, 7–17. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Revision B. 01, Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting non-covalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K.K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Omnexus–The Material Selection Platform, Density of Plastics: Technical Properties. Available online: https://omnexus.specialchem.com/polymer-properties/properties/density (accessed on 1 July 2019).
- Napolitano, S.; Glynos, E.; Tito, N.B. Glass transition of polymers in bulk, confined geometries, and near interfaces. Rep. Prog. Phys. 2017, 80, 036602. [Google Scholar] [CrossRef]
- Watt, S.W. A molecular dynamics simulation of the melting points and glass transition temperatures of myo- and neo-inositol. J. Chem. Phys. 2004, 121, 9565. [Google Scholar] [CrossRef]
- Lua, Y.-Y.; Shua, Y.-J.; Liua, N.; Shub, Y.; Wanga, K.; Wua, Z.-K.; Wanga, X.-C.; Ding, X.-Y. Theoretical simulations on the glass transition temperatures andmechanical properties of modified glycidyl azide polymer. Comput. Mat. Sci. 2017, 139, 132–139. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, X.; He, Z.; Lan, F.; Liu, H. The glass transition temperature measurements of polyethylene: Determined by using molecular dynamic method. RSC Adv. 2016, 6, 12053. [Google Scholar] [CrossRef]
- WhiteJane, R.P.; Lipson, E.G. Polymer Free Volume and Its Connection to the Glass Transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-Order Transition Temperatures and Related Properties of Polystyrene. I. Influence of Molecular Weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. The glass temperature and related properties of polystyrene. Influence of molecular weight. J. Poly. Sci. 1954, 14, 315–319. [Google Scholar] [CrossRef]
- Briatico-Vangosa, F.; Rink, M. Dilatometric Behavior and Glass Transition in a Styrene-Acrylonitrile Copolymer. J. Poly. Sci. Part B Poly. Phys. 2005, 43, 1904–1913. [Google Scholar] [CrossRef]
- Liu, Y.; Bhandari, B.; Zhou, W. Glass Transition and Enthalpy Relaxation of Amorphous Food Saccharides: A Review. J. Agric. Food Chem. 2006, 54, 5701–5717. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Tatiana, F.; Drebushchaka, N.; Tantardini, C. Seeking the best model for non-covalent interactions within the crystal structure of meloxicam. Comput. Theor. Chem. 2019, 1157, 47–53. [Google Scholar]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.H.; Michael, P.; Rana, S. Reversible Self-Healing Carbon-Based Nanocomposites for Structural Applications. Polymers 2019, 11, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. Significance of hydrogen bonding and other noncovalent interactions in determining octahedral tilting in the CH3NH3PbI3 hybrid organic-inorganic halide perovskite solar cell semiconductor. Sci. Rep. 2019, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Levitt, M.; Perutz, M.F. Aromatic rings act as hydrogen bond acceptors. J. Mol. Biol. 1988, 201, 751–754. [Google Scholar] [CrossRef]
- Varadwaj, P.R.V.A.; Peslherbe, G.H. An electronic structure theory investigation of the physical chemistry of the intermolecular complexes of cyclopropenylidene with hydrogen halides. J. Comput. Chem. 2012, 33, 2073–2082. [Google Scholar] [CrossRef]
- Perutz, M.F.; Perutz, M.F. The role of aromatic rings as hydrogen-bond acceptors in molecular recognition. Philos. Trans. R. Soc. Lond. Ser. A Phys. Eng. Sci. 1993, 345, 105–112. [Google Scholar]
- Varadwaj, A.; Varadwaj, P.R.; Yamashita, K. Hybrid organic–inorganic CH3NH3PbI3 perovskite building blocks: Revealing ultra-strong hydrogen bonding and mulliken inner complexes and their implications in materials design. J. Comput. Chem. 2017, 38, 2802–2818. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.; Garza, J.; Dixon, D.A.; Hay, B.P. How Strong Is the Cα−H···OC Hydrogen Bond? J. Am. Chem. Soc. 2000, 122, 4750–4755. [Google Scholar] [CrossRef]



| Property | DFT b | MD b | Expt. c | % Change(DFT) d | % Change(MD) d |
|---|---|---|---|---|---|
| ρp(g/cm3) | 1.395 | 1.364 | 1.328 | +5.1 | +2.2 |
| V(cm3) | 2744.5 | 2808.5 | 2884.6 | –4.9 | –2.6 |
| N | 272 | 272 | 272 | ||
| Lattice Constants | |||||
| a/Å | 14.142 | 14.281 | 14.328 | –1.3 | –0.3 |
| b/Å | 14.142 | 14.281 | 14.328 | –1.3 | –0.3 |
| c/Å | 16.463 | 16.612 | 17.525 | –6.5 | –5.5 |
| α/deg | 107.7 | 107.9 | 110.5 | –2.6 | –2.4 |
| β/deg | 107.7 | 107.9 | 110.5 | –2.6 | –2.4 |
| γ/deg | 63.2 | 62.6 | 61.4 | +2.8 | +1.9 |
| Glass Transition Temperature c | |||||
| Tg/K | 424.4 ± 22.3 | 418.2 | |||
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varadwaj, P.R. Combined Molecular Dynamics and DFT Simulation Study of the Molecular and Polymer Properties of a Catechol-Based Cyclic Oligomer of Polyether Ether Ketone. Polymers 2020, 12, 1054. https://doi.org/10.3390/polym12051054
Varadwaj PR. Combined Molecular Dynamics and DFT Simulation Study of the Molecular and Polymer Properties of a Catechol-Based Cyclic Oligomer of Polyether Ether Ketone. Polymers. 2020; 12(5):1054. https://doi.org/10.3390/polym12051054
Chicago/Turabian StyleVaradwaj, Pradeep R. 2020. "Combined Molecular Dynamics and DFT Simulation Study of the Molecular and Polymer Properties of a Catechol-Based Cyclic Oligomer of Polyether Ether Ketone" Polymers 12, no. 5: 1054. https://doi.org/10.3390/polym12051054
APA StyleVaradwaj, P. R. (2020). Combined Molecular Dynamics and DFT Simulation Study of the Molecular and Polymer Properties of a Catechol-Based Cyclic Oligomer of Polyether Ether Ketone. Polymers, 12(5), 1054. https://doi.org/10.3390/polym12051054






