Relationship between the Ionization Degree and the Inter-Polymeric Aggregation of the Poly(maleic acid-alt-octadecene) Salts Regarding Time
Abstract
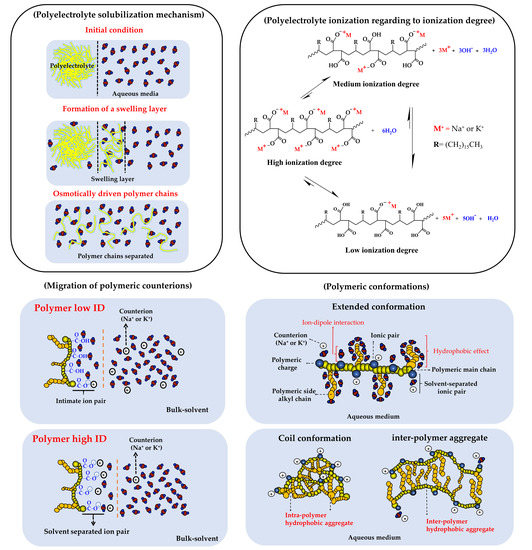
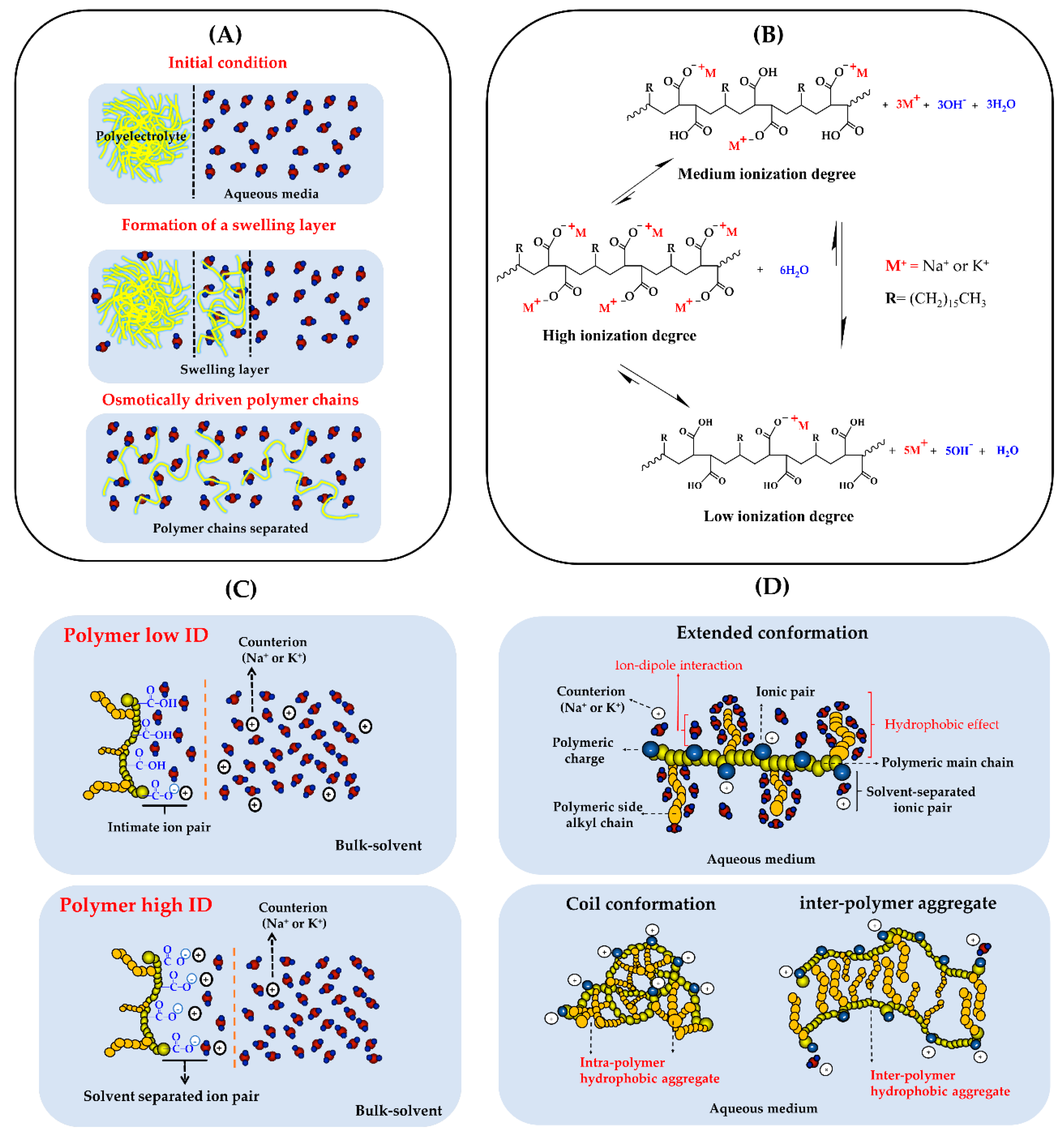
1. Introduction
2. Material and Methods
2.1. Materials
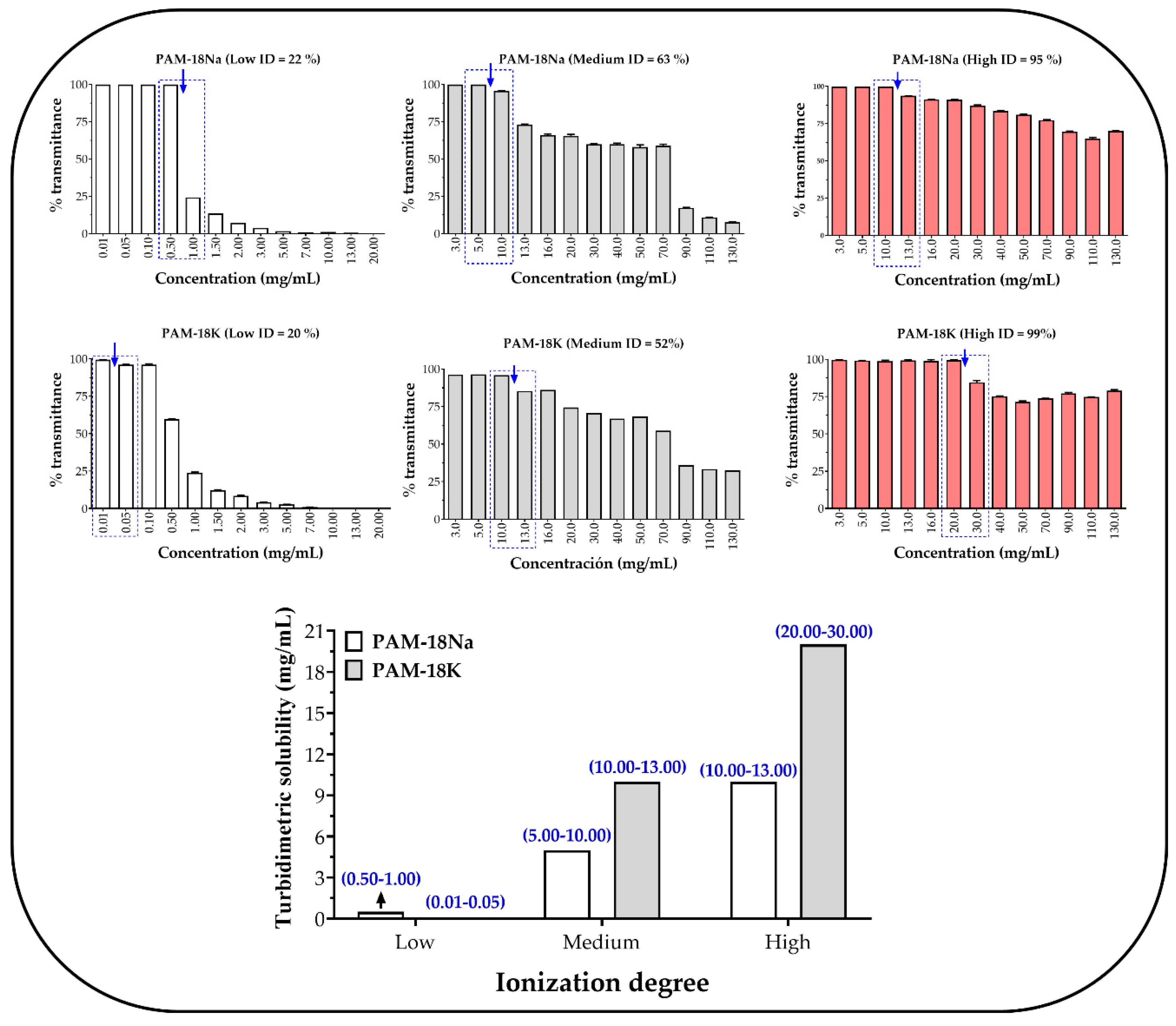
2.2. Turbidimetric Solubility Test
2.3. Change of Polyectrolyte Physicochemical Variables over Time
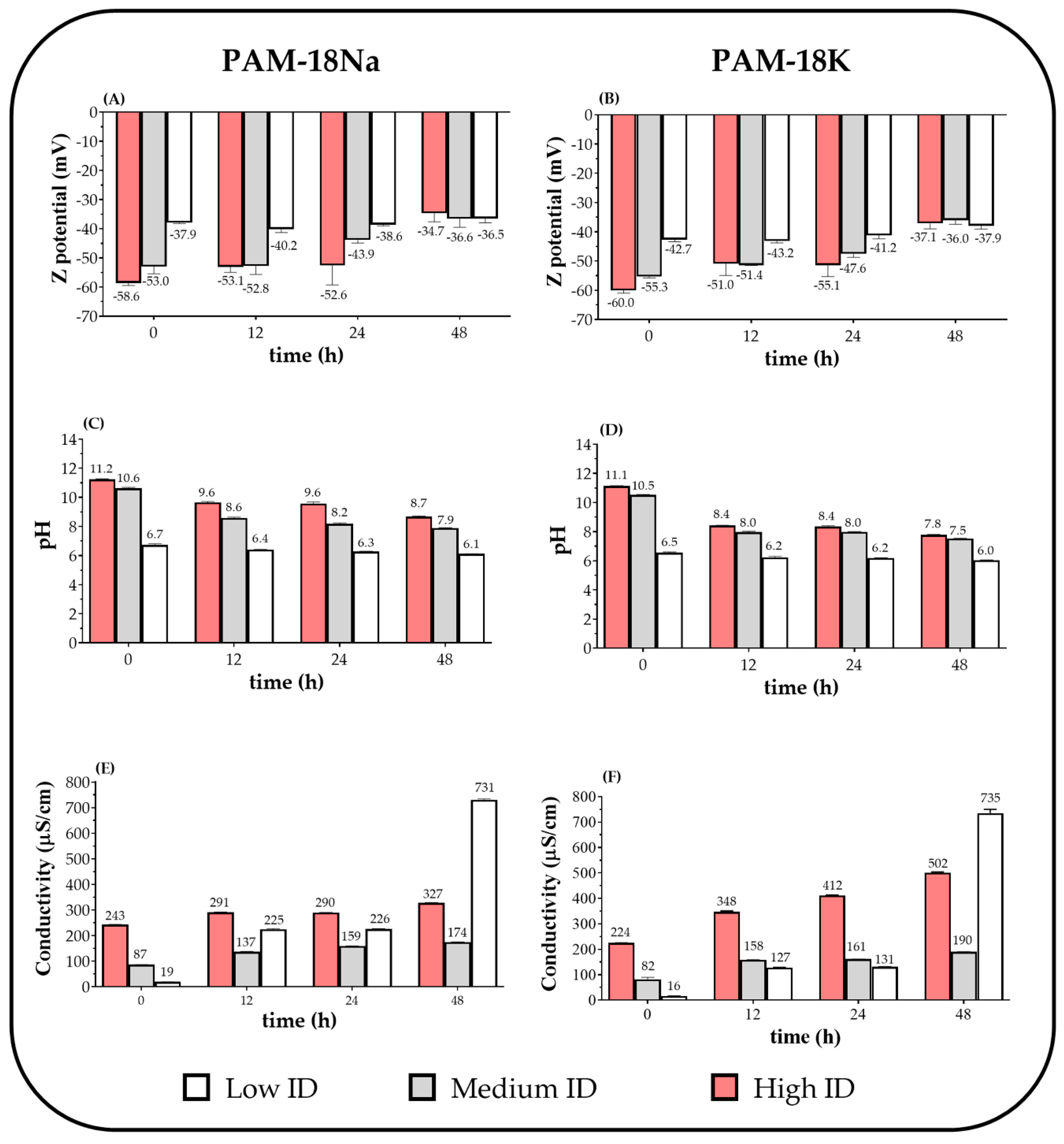
2.3.1. Zeta Potential, Electrical Conductivity, and pH
2.3.2. Particle Size and Polydispersity
2.3.3. Surface Tension
2.4. Graphs and Statistical Analysis
3. Results and Discussion
3.1. Turbidimetric Solubility Test
3.2. Change of Polyectrolyte Physicochemical Variables over Time
3.2.1. Zeta Potential, pH, and Electrical Conductivity Measurements
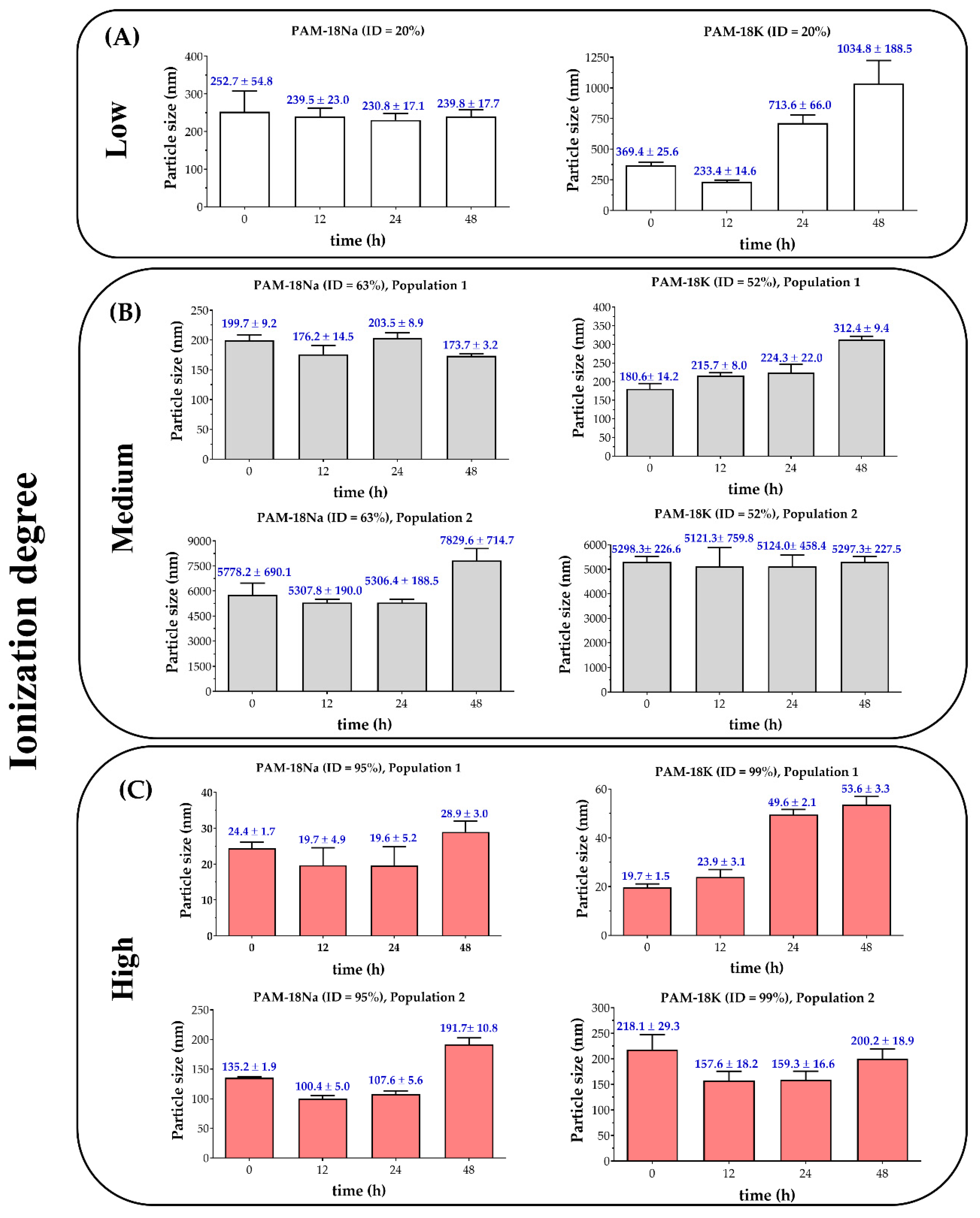
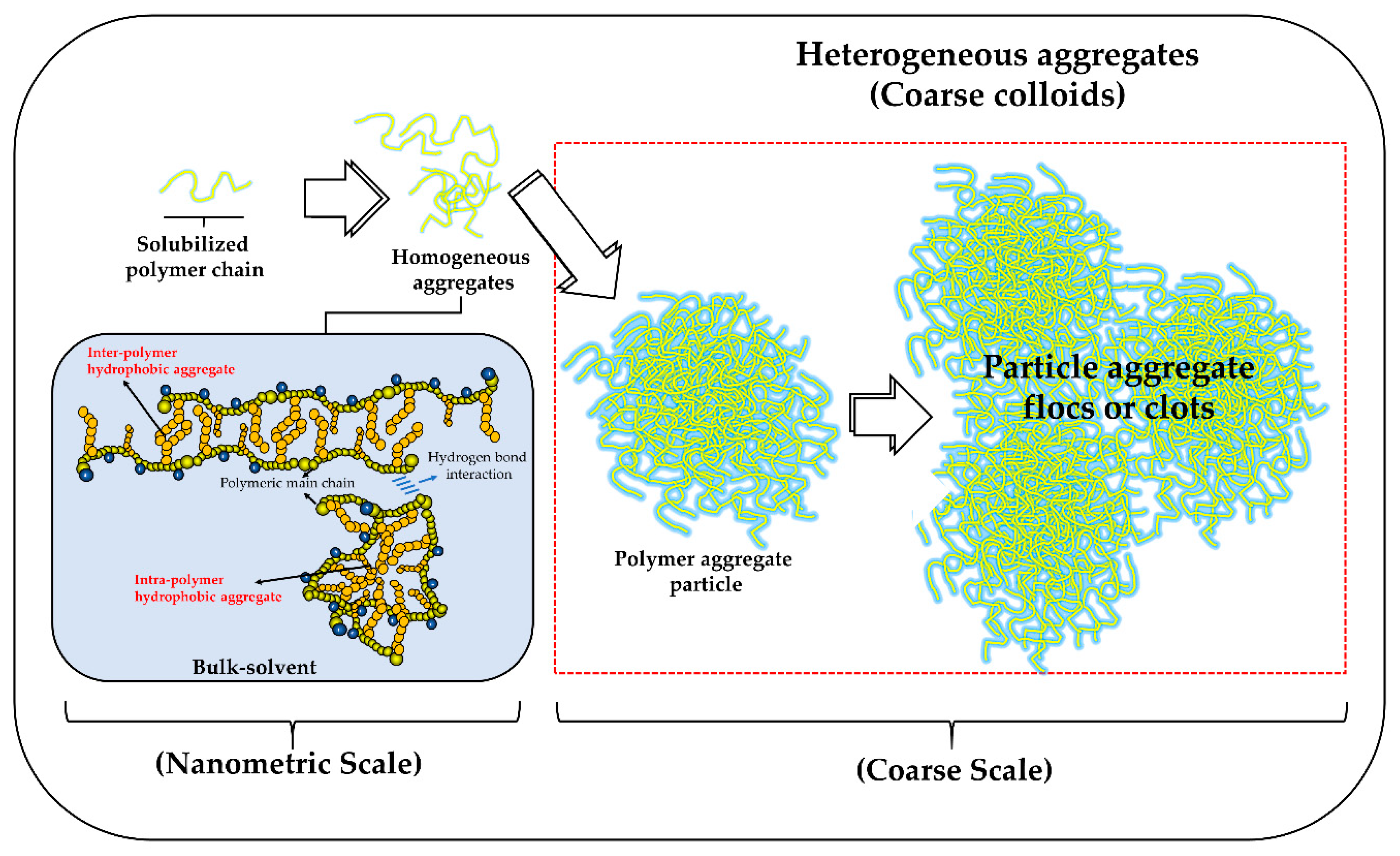
3.2.2. Particle Size and Polydispersity
3.3. Surface Tension Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexandridis, P. Amphiphilic copolymers and their applications. Curr. Opin. Colloid Interface Sci. 1996, 1, 490–501. [Google Scholar] [CrossRef]
- Kötz, J.; Kosmella, S.; Beitz, T. Self-assembled polyelectrolyte systems. Prog. Polym. Sci. 2001, 26, 1199–1232. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.L.; Virtanen, J.; Tenhu, H. Hydrophobically modified responsive polyelectrolytes. Langmuir 1999, 15, 4259–4265. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Hydrophobically modified polyelectrolytes in dilute salt-free solutions. Macromolecules 2000, 33, 8097–8105. [Google Scholar] [CrossRef]
- Ghimici, L.; Dranca, I.; Dragan, S.; Lupascu, T.; Maftuleac, A. Hydrophobically modified cationic polyelectrolytes. Eur. Polym. J. 2001, 37, 227–231. [Google Scholar] [CrossRef]
- Zhang, L.M. New water-soluble cellulosic polymers: A review. Macromol. Mater. Eng. 2001, 286, 267–275. [Google Scholar] [CrossRef]
- Bromberg, L. Hydrophobically Modified Polyelectrolytes And Polyelectrolyte Block Copolymers. In Handbook of Surfaces and Interfaces of Materials; Elsevier: Amsterdan, The Netherlands, 2001; pp. 369–404. [Google Scholar]
- Olea, A.F. Hydrophobic Polyelectrolytes. In Ionic Interactions in Natural and Synthetic Macromolecules; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 915–922. ISBN 9780470529270. [Google Scholar]
- Martínez, F.; Uribe, E.; Olea, A.F. Copolymerization of maleic anhydride with styrene and α-olefins. Molecular and thermal characterization. J. Macromol. Sci. Pure Appl. Chem. 2005, 47, 1063–1072. [Google Scholar]
- Salamanca, C.H.; Urbano, B.; Olea, A.F. Potential drug delivery system: Study of the association of a model nitroimidazole drug with aggregates of amphiphilic polymers on aqueous solution. Brazilian J. Pharm. Sci. 2011, 47, 725–731. [Google Scholar] [CrossRef]
- Jones, M.C.; Leroux, J.C. Polymeric micelles—A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Borisov, O.V.; Halperin, A. Micelles of Polysoaps. Langmuir 1995, 11, 2911–2919. [Google Scholar] [CrossRef]
- Borisov, O.V.; Halperin, A. Micelles of polysoaps: the role of bridging interactions. Macromolecules 1996, 29, 2612–2617. [Google Scholar]
- Borisov, O.V.; Halperin, A. Self-assembly of polysoaps. Curr. Opin. Colloid Interface Sci. 1998, 4, 415–421. [Google Scholar] [CrossRef]
- Olea, A.F.; Barraza, R.G.; Fuentes, I.; Acevedo, B.; Martinez, F. Solubilization of phenols by intramolecular micelles formed by copolymers of maleic acid and olefins. Macromolecules 2002, 35, 1049–1053. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental Effects on Vibronic Band Intensities in Pyrene Monomer Fluorescence and Their Application in Studies of Micellar Systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Chu, D.Y.; Thomas, J.K. Photophysical and Photochemical Studies on a Polymeric Intramolecular Micellar System, PA-18K2. Macromolecules 1987, 20, 2133–2138. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barraza, R.G.; Acevedo, B.; Olea, A.F. Hydrophobically modified polyelectrolytes as potential drugs reservoirs of N-alkyl-nitroimidazoles. J. Chil. Chem. Soc. 2007, 52, 1115–1119. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Yarce, C.J.; Zapata, C.A.; Giraldo, J.A. Relationship between the polymeric ionization degree and powder and surface properties in materials derived from poly(maleic anhydride-alt-octadecene). Molecules 2018, 23, 320. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 23, 3–25. [Google Scholar] [CrossRef]
- Crisp, M.T.; Tucker, C.J.; Rogers, T.L.; Williams, R.O.; Johnston, K.P. Turbidimetric measurement and prediction of dissolution rates of poorly soluble drug nanocrystals. J. Control. Release 2007, 117, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Kahl, H.; Wadewitz, T.; Winkelmann, J. Surface tension of pure liquids and binary liquid mixtures. J. Chem. Eng. Data 2003, 48, 580–586. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Wolf, B.A. Solubility of polymers. Pure Appl. Chem. 1985, 28, 323–336. [Google Scholar] [CrossRef]
- Van Der Vegt, N.F.A.; Haldrup, K.; Roke, S.; Zheng, J.; Lund, M.; Bakker, H.J. Water-Mediated Ion Pairing: Occurrence and Relevance. Chem. Rev. 2016, 116, 7626–7641. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Hashemi, S.A.; Sammes, P.G.; Meldrum, I. A model for the swelling of superabsorbent polymers. Polymer (Guildf) 1998, 39, 6697–6704. [Google Scholar] [CrossRef]
- Toomey, R.; Freidank, D.; Rühe, J. Swelling behavior of thin, surface-attached polymer networks. Macromolecules 2004, 37, 882–887. [Google Scholar] [CrossRef]
- Matsuyama, A.; Tanaka, F. Theory of solvation-induced reentrant phase separation in polymer solutions. Phys. Rev. Lett. 1990, 65, 341. [Google Scholar] [CrossRef]
- Nakamura, I.; Balsara, N.P.; Wang, Z.G. Thermodynamics of ion-containing polymer blends and block copolymers. Phys. Rev. Lett. 2011, 107, 198301. [Google Scholar] [CrossRef]
- Panayiotou, C. Polymer-polymer miscibility and partial solvation parameters. Polymer (Guildf) 2013, 54, 1621–1638. [Google Scholar] [CrossRef]
- Estudio de propiedades fisicoquímicas de polilelectrólitos aniónicos en solución como potenciales reservorios de sustratos farmacológicos (Study of physicochemical properties of anionic polyelectrolytes in solution as potential reservoirs of pharmacological substrates) Home Page. Available online: http://www.tesis.uchile.cl/tesis/uchile/2007/salamanca_c/sources/salamanca_c.pdf (accessed on 23 April 2020).
- Giesbers, M.; Kleijn, J.M.; Cohen Stuart, M.A. The electrical double layer on gold probed by electrokinetic and surface force measurements. J. Colloid Interface Sci. 2002, 248, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Blees, M.H. Foundations of Colloid Science. Colloids Surf. A Physicochem. Eng. Asp. 2002, 210, 125. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential - What they are and what they are not ? J. Control. Release 2016, 235, 337–351. [Google Scholar]
- Yin, L. Dynamic Light Scattering. In Nanotechnology Research Methods for Foods and Bioproducts; Wiley-Blackwell: Oxford, UK, 2012; ISBN 9780813817316. [Google Scholar]
- Sakho, E.H.M.; Allahyari, E.; Oluwafemi, O.S.; Thomas, S.; Kalarikkal, N. Dynamic Light Scattering (DLS). In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier: Amsterdan, The Netherlands, 2017; ISBN 9780323461450. [Google Scholar]
- Ivanov, I.B.; Danov, K.D.; Kralchevsky, P.A. Flocculation and coalescence of micron-size emulsion droplets. Colloids Surf. A 1999, 152, 161–182. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Katoh, Y.; Ohshima, H. Colloidal stability of aqueous polymeric dispersions: Effect of pH and salt concentration. Colloids Surf. B Biointerfaces 2005, 42, 53–58. [Google Scholar] [CrossRef]
- Churaev, N.V. Surface forces in wetting films. Adv. Colloid Interface Sci. 2003, 65, 263–274. [Google Scholar] [CrossRef]
- Yang, K.; Lin, Y.; Lu, X.; Neimark, A.V. Solvation forces between molecularly rough surfaces. J. Colloid Interface Sci. 2011, 362, 382–388. [Google Scholar] [CrossRef]
- Shvartzman-Cohen, R.; Florent, M.; Goldfarb, D.; Szleifer, I.; Yerushalmi-Rozen, R. Aggregation and self-assembly of amphiphilic block copolymers in aqueous dispersions of carbon nanotubes. Langmuir 2008, 24, 4625–4632. [Google Scholar] [CrossRef]
- Wershaw, R.L. Molecular aggregation of humic substances. Soil Sci. 1999, 164, 803–813. [Google Scholar] [CrossRef]
- Savariar, E.N.; Aathimanikandan, S.V.; Thayumanavan, S. Supramolecular assemblies from amphiphilic homopolymers: Testing the scope. J. Am. Chem. Soc. 2006, 128, 16224–16230. [Google Scholar] [CrossRef]
- Zhu, Z.; Kang, W.; Sarsenbekuly, B.; Yang, H.; Dai, C.; Yang, R.; Fan, H. Preparation and solution performance for the amphiphilic polymers with different hydrophobic groups. J. Appl. Polym. Sci. 2017, 134, 20. [Google Scholar] [CrossRef]
- Zana, R. Micellization of amphiphiles: Selected aspects. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123, 27–35. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, I.; Palacio, M.M.; Yarce, C.J.; Oñate-Garzón, J.; Salamanca, C.H. Relationship between the Ionization Degree and the Inter-Polymeric Aggregation of the Poly(maleic acid-alt-octadecene) Salts Regarding Time. Polymers 2020, 12, 1036. https://doi.org/10.3390/polym12051036
Reyes I, Palacio MM, Yarce CJ, Oñate-Garzón J, Salamanca CH. Relationship between the Ionization Degree and the Inter-Polymeric Aggregation of the Poly(maleic acid-alt-octadecene) Salts Regarding Time. Polymers. 2020; 12(5):1036. https://doi.org/10.3390/polym12051036
Chicago/Turabian StyleReyes, Isabella, Maria M. Palacio, Cristhian J. Yarce, Jose Oñate-Garzón, and Constain H. Salamanca. 2020. "Relationship between the Ionization Degree and the Inter-Polymeric Aggregation of the Poly(maleic acid-alt-octadecene) Salts Regarding Time" Polymers 12, no. 5: 1036. https://doi.org/10.3390/polym12051036
APA StyleReyes, I., Palacio, M. M., Yarce, C. J., Oñate-Garzón, J., & Salamanca, C. H. (2020). Relationship between the Ionization Degree and the Inter-Polymeric Aggregation of the Poly(maleic acid-alt-octadecene) Salts Regarding Time. Polymers, 12(5), 1036. https://doi.org/10.3390/polym12051036