Plasma-Polymer-Fluorocarbon Thin Film Coated Nanostructured-Polyethylene Terephthalate Surface with Highly Durable Superhydrophobic and Antireflective Properties
Abstract
1. Introduction
2. Materials and Methods
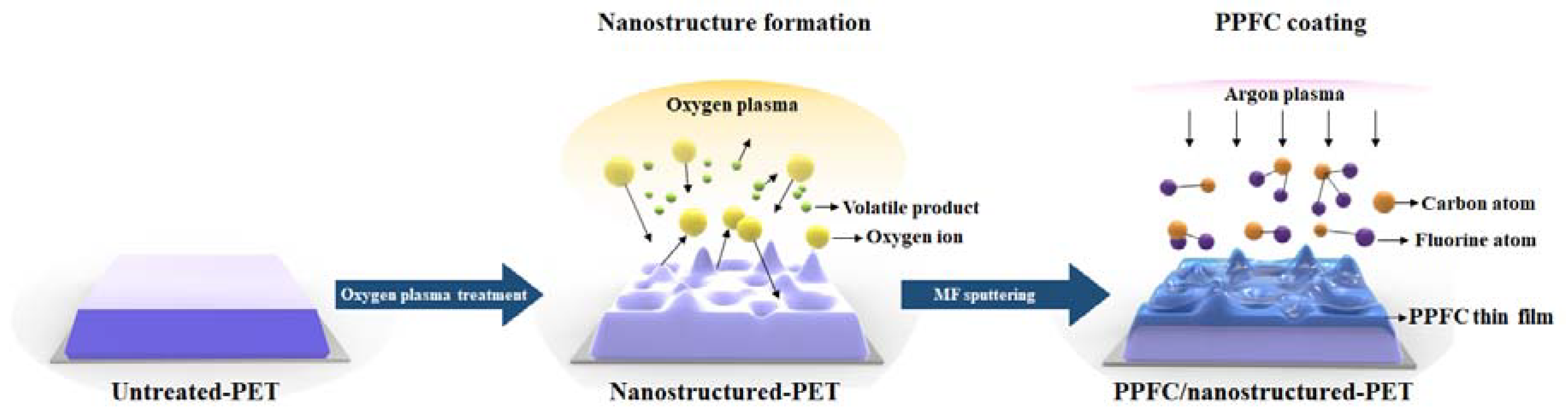
2.1. Preparation of Nanostructured-PET Films
2.2. Preparation of PPFC/Nanostructured-PET Films
2.3. Characterizations
3. Results and Discussion
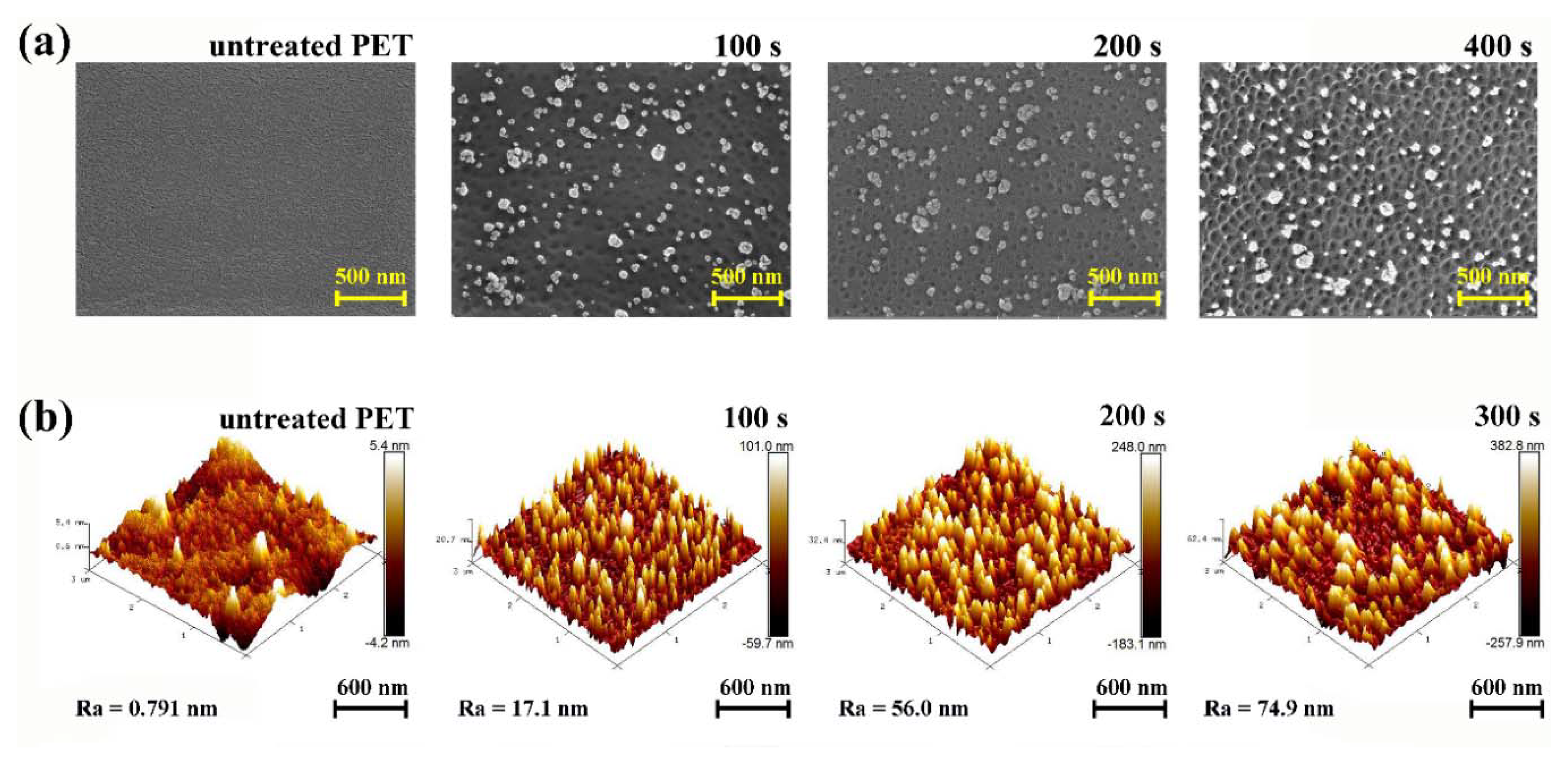
3.1. Formation of the Nanostructured-Polymer Surface
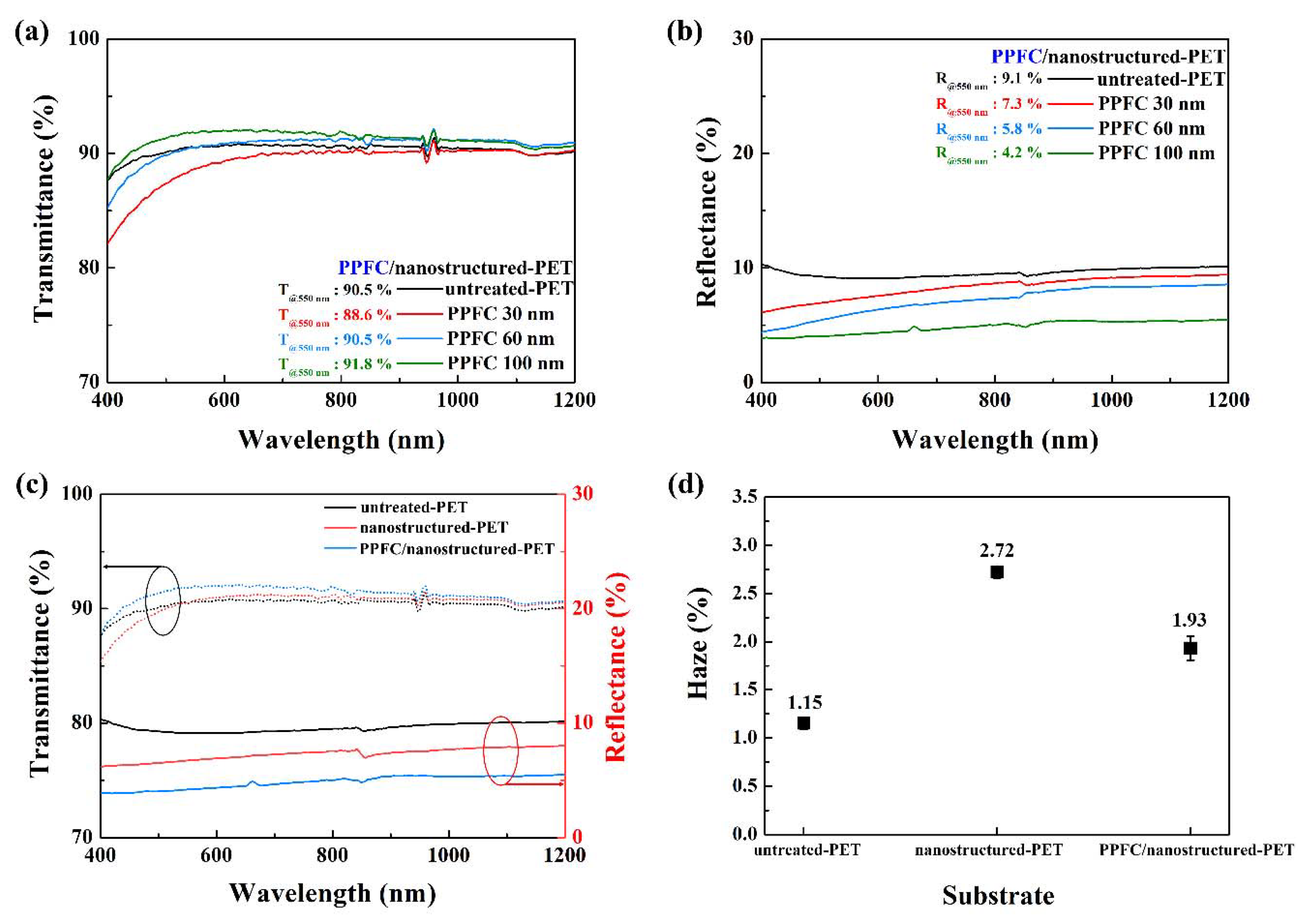
3.2. Antireflective Properties of PPFC/Nanostructured-PET Films
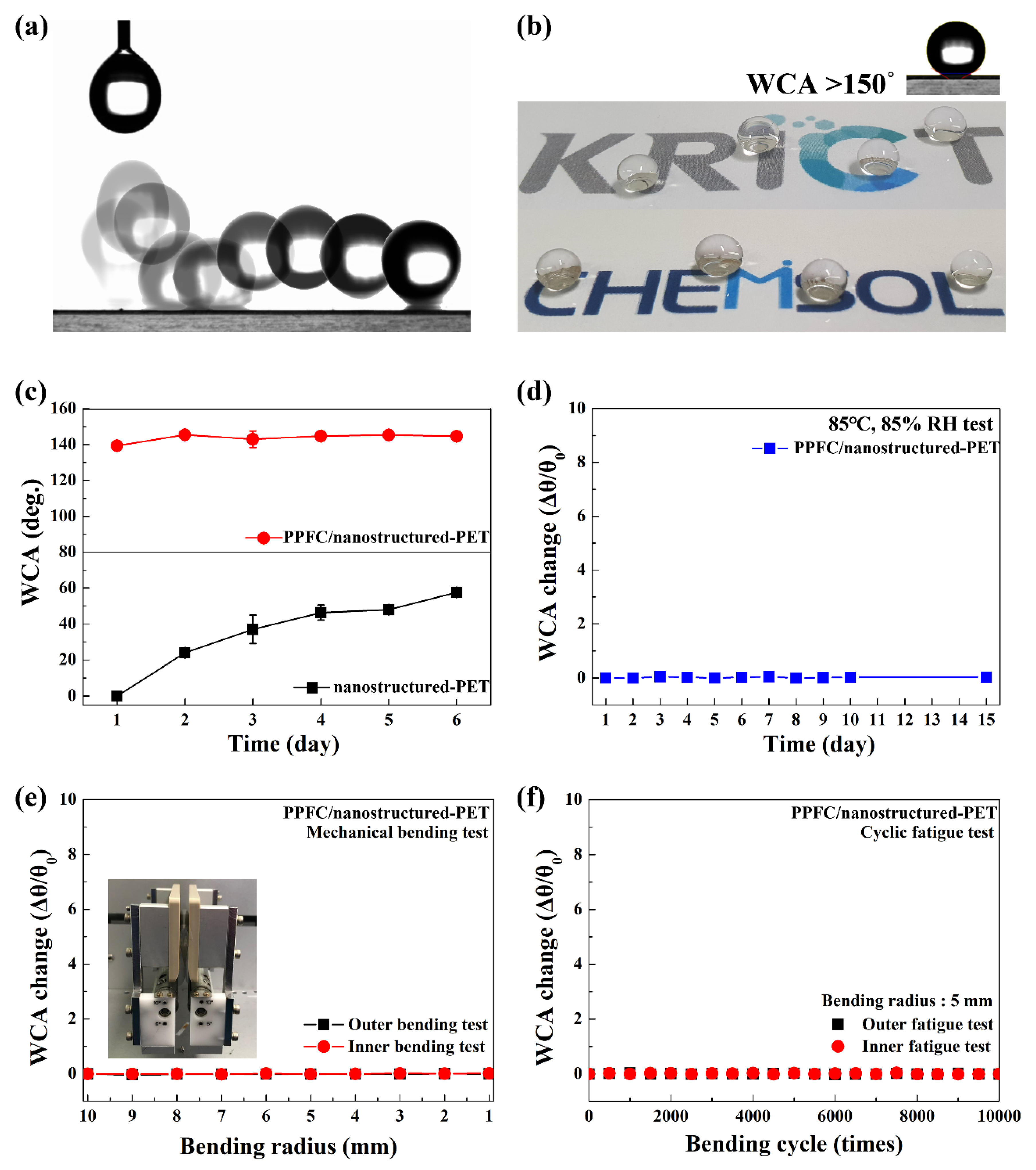
3.3. Superhydrophobic Surface and Durable Properties of PPFC/Nanostructured-PET Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abourayana, H.M.; Dowling, D.P. Plasma processing for tailoring the surface properties of polymers. In Surface Energy; InTech: Rijeka, Croatia, 2015; pp. 123–152. [Google Scholar]
- Mobarakeh, L.F.; Jafari, R.; Farzaneh, M. Robust icephobic, and anticorrosive plasma polymer coating. Cold Reg. Sci. Technol. 2018, 151, 89–93. [Google Scholar] [CrossRef]
- Kamegawa, T.; Irikawa, K.; Yamashita, H. Multifunctional surface designed by nanocomposite coating of polytetrafluoroethylene and TiO2 photocatalyst: Self-cleaning and superhydrophobicity. Sci. Rep. 2017, 7, 13628. [Google Scholar] [CrossRef]
- Steiner, C.; Fichtner, J.; Fahlteich, J. Nanostructuring of polymer surfaces by magnetron plasma treatment. Surf. Coat. Technol. 2018, 336, 72–79. [Google Scholar] [CrossRef]
- Tserepi, A.; Gogolides, E.; Bourkoula, A.; Kanioura, A.; Kokkoris, G.P.; Petrou, S.; Kakabakos, S.E. Plasma nanotextured polymeric surfaces for controlling cell attachment and proliferation: A short review. Plasma Chem. Plasma Process. 2016, 36, 107–120. [Google Scholar] [CrossRef]
- Junkar, I.; Vesel, A.; Cvelbar, U.; Mozetic, M.; Strnad, S. Influence of oxygen and nitrogen plasma treatment on polyethylene terephthalate (PET) polymers. Vacuum 2010, 84, 83–85. [Google Scholar] [CrossRef]
- Al-Maliki, H.; Zsidai, L.; Samyn, P.; Szakál, Z.; Keresztes, R.; Kalácska, G. Effects of atmospheric plasma treatment on adhesion and tribology of aromatic thermoplastic polymers. Poly. Eng. Sci. 2018, 58, E93–E103. [Google Scholar] [CrossRef]
- Sanchis, R.; Fenollar, O.; García, D.; Sánchez, L.; Balart, R. Improved adhesion of LDPE films to polyolefin foams for automotive industry using low-pressure plasma. Int. J. Adhes. Adhes. 2008, 27, 445–451. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Park, C.H. Influence of micro and nano-scale roughness on hydrophobicity of a plasma-treated woven fabric. Text. Res. J. 2017, 87, 193–207. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Wang, Y.-J. Optimization of plasma surface modification parameter for fabricating a hot embossing mold with high surface finish. Int. J. Adv. Manuf. Technol. 2017, 91, 3363–3369. [Google Scholar] [CrossRef]
- Park, J.B.; Choi, J.Y.; Lee, S.H.; Song, Y.S.; Yeom, G.Y. Polymer surface texturing for direct inkjet patterning by atmospheric pressure plasma treatment. Soft Matter 2012, 87, 5020–5026. [Google Scholar] [CrossRef]
- Tan, G.; Lee, J.-H.; Lan, Y.-H.; Wei, M.-K.; Peng, L.-H.; Cheng, I.-C.; Wu, S.-T. Broadband antireflection film with moth-eye-like structure for flexible display applications. Optica 2017, 4, 678–683. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Han, Y. Polymer thin films for antireflection coatings. J. Mater. Chem. C 2013, 1, 2266–2285. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Superhydrophobic, passive microvalves with controllable opening threshold: Exploiting plasma nanotextured microfluidics for a programmable flow switchboard. Microfluid. Nanofluid. 2014, 17, 489–498. [Google Scholar] [CrossRef]
- Hasebe, T.; Nagashima, S.; Kamijo, A.; Moon, M.-W.; Kashiwagi, Y.; Hotta, A.; Lee, K.-R.; Takahashi, K.; Yamagami, T.; Suzuki, T. Hydrophobicity and non-thrombogenicity of nanoscale dual rough surface coated with fluorine-incorporated diamond-like carbon films: Biomimetic surface for blood-contacting medical devices. Diam. Relat. Mater. 2013, 38, 14–18. [Google Scholar] [CrossRef]
- Park, S.J.; Ko, T.-J.; Yoon, J.; Moon, M.-W.; Oh, K.H.; Han, J.H. Copper circuit patterning on polymer using selective surface modification and electroless plating. Appl. Surf. Sci. 2017, 396, 1678–1684. [Google Scholar] [CrossRef]
- Ko, T.-J.; Oh, K.H.; Moon, M.-W. Plasma-induced hetero-nanostructures on a polymer with selective metal co-deposition. Adv. Mater. Interfaces 2015, 2, 1400431. [Google Scholar] [CrossRef]
- Phan, L.T.; Yoon, S.M.; Moon, M.-W. Plasma-based nanostructuring of polymers: A review. Polymers 2017, 9, 417. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.; Lee, J.H.; Lee, S.-J. Moisture barrier films containing plasma polymer fluorocarbon/inorganic multilayers fabricated via continuous roll-to-roll sputtering. Plasma Process. Polym. 2018, 15, 1700221. [Google Scholar] [CrossRef]
- Cho, E.; Kim, S.H.; Kim, M.; Park, J.-S.; Lee, S.-J. Super-hydrophobic and antimicrobial properties of Ag-PPFC nanocomposite thin films fabricated using a ternary carbon nanotube-Ag-PTFE composite sputtering target. Surf. Coat. Technol. 2019, 370, 18–23. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, C.H.; Choi, W.J.; Lee, T.G.; Cho, S.K.; Yang, Y.S.; Lee, J.H.; Lee, S.-J. Fluorocarbon thin films fabricated using carbon nanotube/ polytetrafluoroethylene composite polymer targets via mid-frequency sputtering. Sci. Rep. 2017, 7, 1451. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.; Lee, J.H.; Lee, S.-J. Self-cleaning transparent heat mirror with a plasma polymer fluorocarbon thin film fabricated by a continuous roll-to-roll sputtering process. ACS Appl. Mater. Interfaces 2018, 10, 10454–10460. [Google Scholar] [CrossRef]
- Kim, M.; Kang, T.-W.; Kim, S.H.; Jung, E.H.; Park, H.H.; Seo, J.; Lee, S.-J. Antireflective, self-cleaning and protective film by continuous sputtering of a plasma polymer on inorganic multilayer for perovskite solar cells application. Sol. Energy Mater. Sol. Cells 2019, 191, 55–61. [Google Scholar] [CrossRef]
- Lee, S.; Byeon, E.; Jung, S.; Kim, D.-G. Heterogeneity of hard skin layer in wrinkled PDMS surface fabricated by Ar ion-beam irradiation. Sci. Rep. 2018, 8, 14063. [Google Scholar] [CrossRef]
- Lee, S.; Byun, E.-Y.; Kim, J.-K.; Kim, D.-G. Ar and O2 linear ion beam PET treatments using an anode layer ion source. Curr. Appl. Phys. 2014, 14, S180–S182. [Google Scholar] [CrossRef]
- Raut, H.K.; Ganesh, V.A.; Nair, A.S.; Ramakrishna, S. Anti-reflective coatings: A critical, in-depth review. Energy Environ. Sci. 2011, 4, 3779–3804. [Google Scholar] [CrossRef]
- Li, Z.; Song, C.; Li, Q.; Xiang, X.; Yang, H.; Wang, X.; Gao, J. Hybrid nanostructured antireflection coating by self-assembled nanosphere lithography. Coatings 2019, 9, 453. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Foletti, S.; Palasantzas, G.; Arikawa, K. Light on the moth-eye corneal nipple array of butterflies. Proc. Biol. Sci. 2006, 273, 661–667. [Google Scholar] [CrossRef]
- Ji, S.; Song, K.; Nguyen, T.B.; Kim, N.; Lim, H. Optimal moth eye nanostructure array on transparent glass towards broadband antireflection. ACS Appl. Mater. Interfaces 2013, 5, 10731–10737. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Z. Nanostructured self-cleaning coating with antireflection properties. In Self-Cleaning Coatings Structure, Fabrication and Application; Royal Society of Chemistry: London, UK, 2016; pp. 166–192. [Google Scholar]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Vassallo, E.; Cremona, A.; Ghezzi, F.; Ricci, D. Characterization by optical emission spectroscopy of an oxygen plasma used for improving PET wettability. Vaccum 2010, 84, 902–906. [Google Scholar] [CrossRef]
- Cohen, N.; Dotan, A.; Dodiuk, H.; Kenig, S. Superhydrophobic coatings and their durability. Mater. Manuf. Process. 2016, 31, 1143–1155. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-inspired sustainable and durable superhydrophobic materials: From nature to market. J. Mater. Chem. A 2019, 7, 16643–16670. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Kim, M.; Park, J.-S.; Lee, S.-J. Plasma-Polymer-Fluorocarbon Thin Film Coated Nanostructured-Polyethylene Terephthalate Surface with Highly Durable Superhydrophobic and Antireflective Properties. Polymers 2020, 12, 1026. https://doi.org/10.3390/polym12051026
Cho E, Kim M, Park J-S, Lee S-J. Plasma-Polymer-Fluorocarbon Thin Film Coated Nanostructured-Polyethylene Terephthalate Surface with Highly Durable Superhydrophobic and Antireflective Properties. Polymers. 2020; 12(5):1026. https://doi.org/10.3390/polym12051026
Chicago/Turabian StyleCho, Eunmi, Mac Kim, Jin-Seong Park, and Sang-Jin Lee. 2020. "Plasma-Polymer-Fluorocarbon Thin Film Coated Nanostructured-Polyethylene Terephthalate Surface with Highly Durable Superhydrophobic and Antireflective Properties" Polymers 12, no. 5: 1026. https://doi.org/10.3390/polym12051026
APA StyleCho, E., Kim, M., Park, J.-S., & Lee, S.-J. (2020). Plasma-Polymer-Fluorocarbon Thin Film Coated Nanostructured-Polyethylene Terephthalate Surface with Highly Durable Superhydrophobic and Antireflective Properties. Polymers, 12(5), 1026. https://doi.org/10.3390/polym12051026




