Self-Healing and Mechanical Properties of Thermoplastic Polyurethane/Eugenol-Based Phenoxy Resin Blends via Exchange Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TPU/Phenoxy Blends
2.2.1. Synthesis of EE
2.2.2. Synthesis of Phenoxy Resin from EE
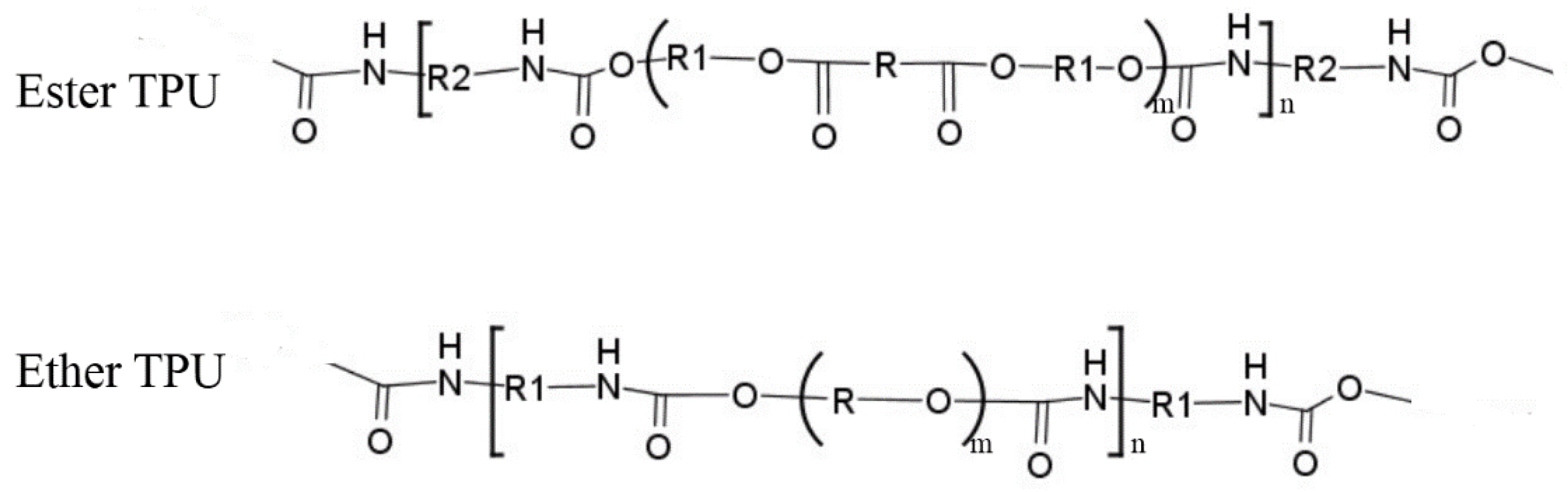
2.2.3. Preparation of TPU/Phenoxy Resin Blends
2.3. Characterization
3. Results and Discussion
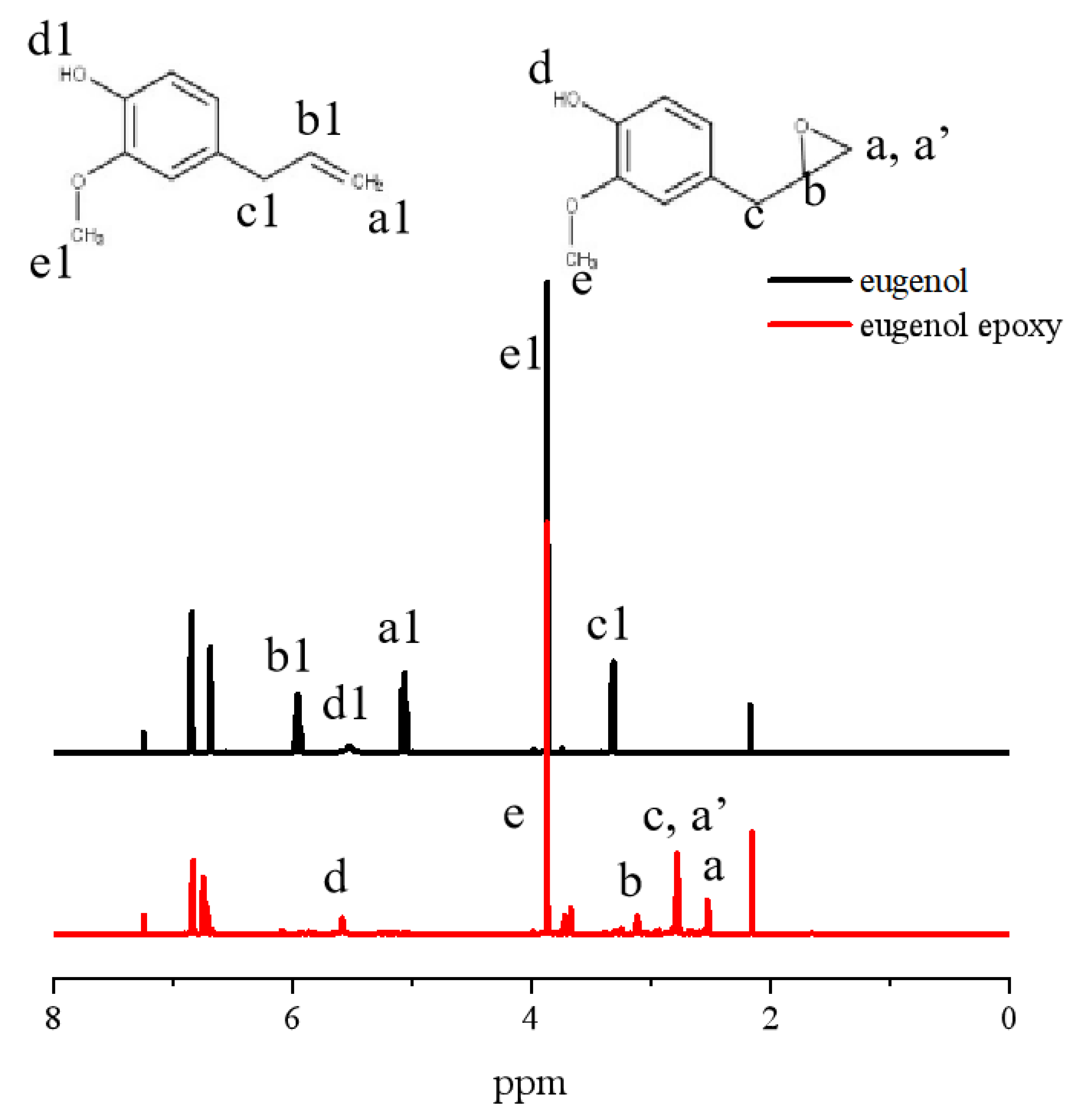
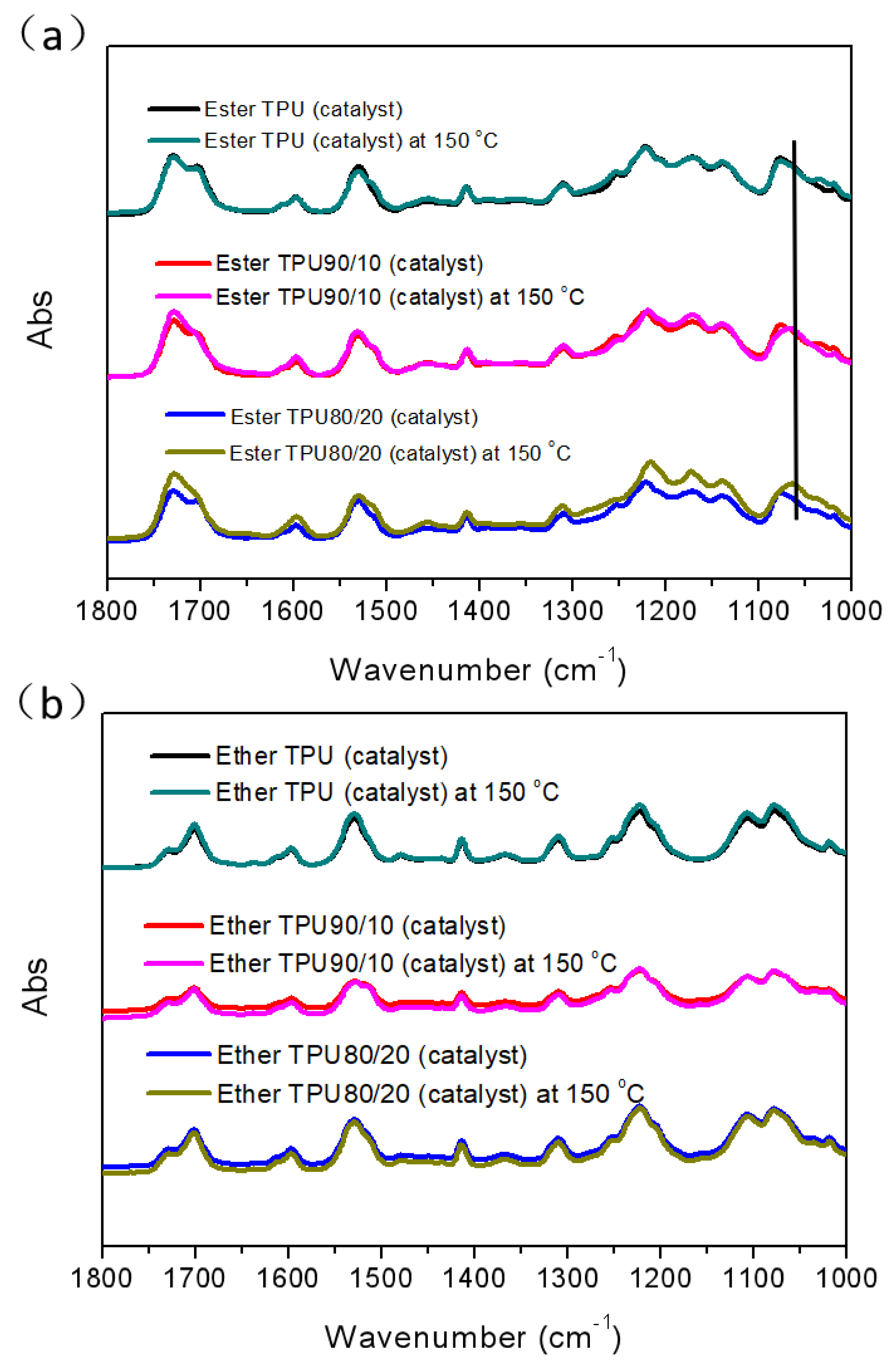
3.1. Synthesis and Characterization of Phenoxy Resin
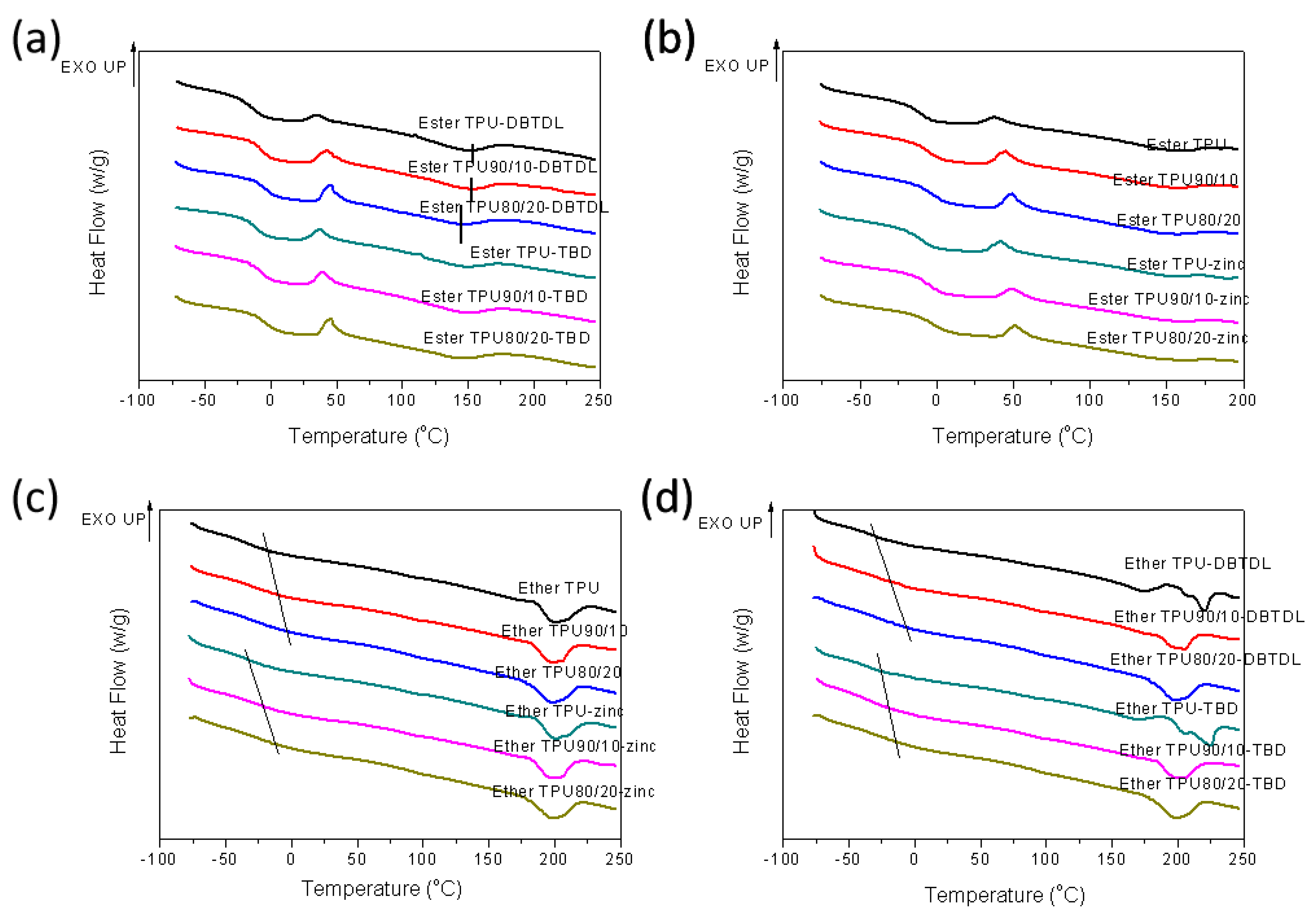
3.2. Thermal Properties of Blends
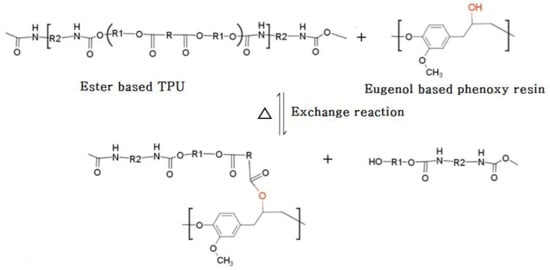
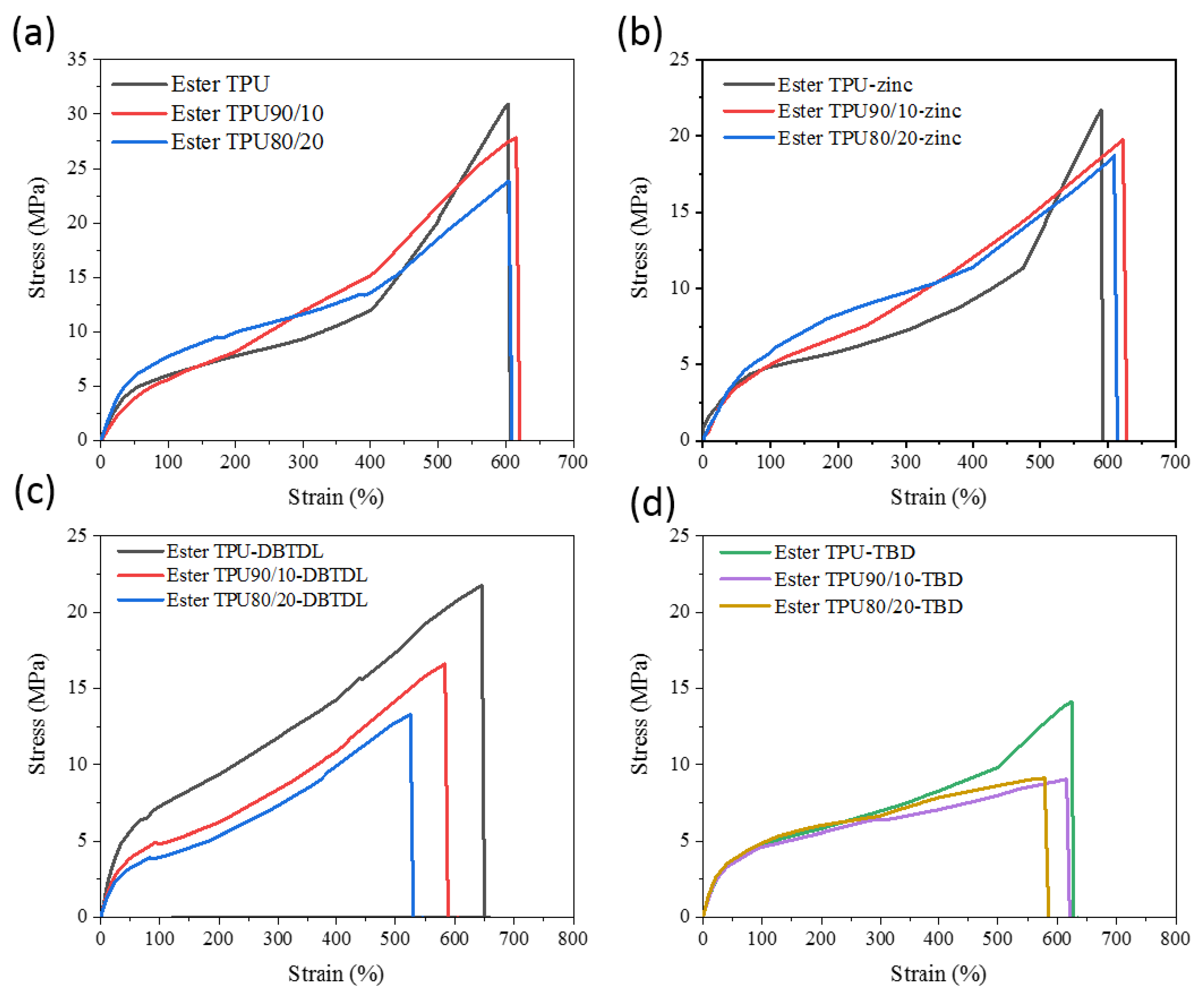
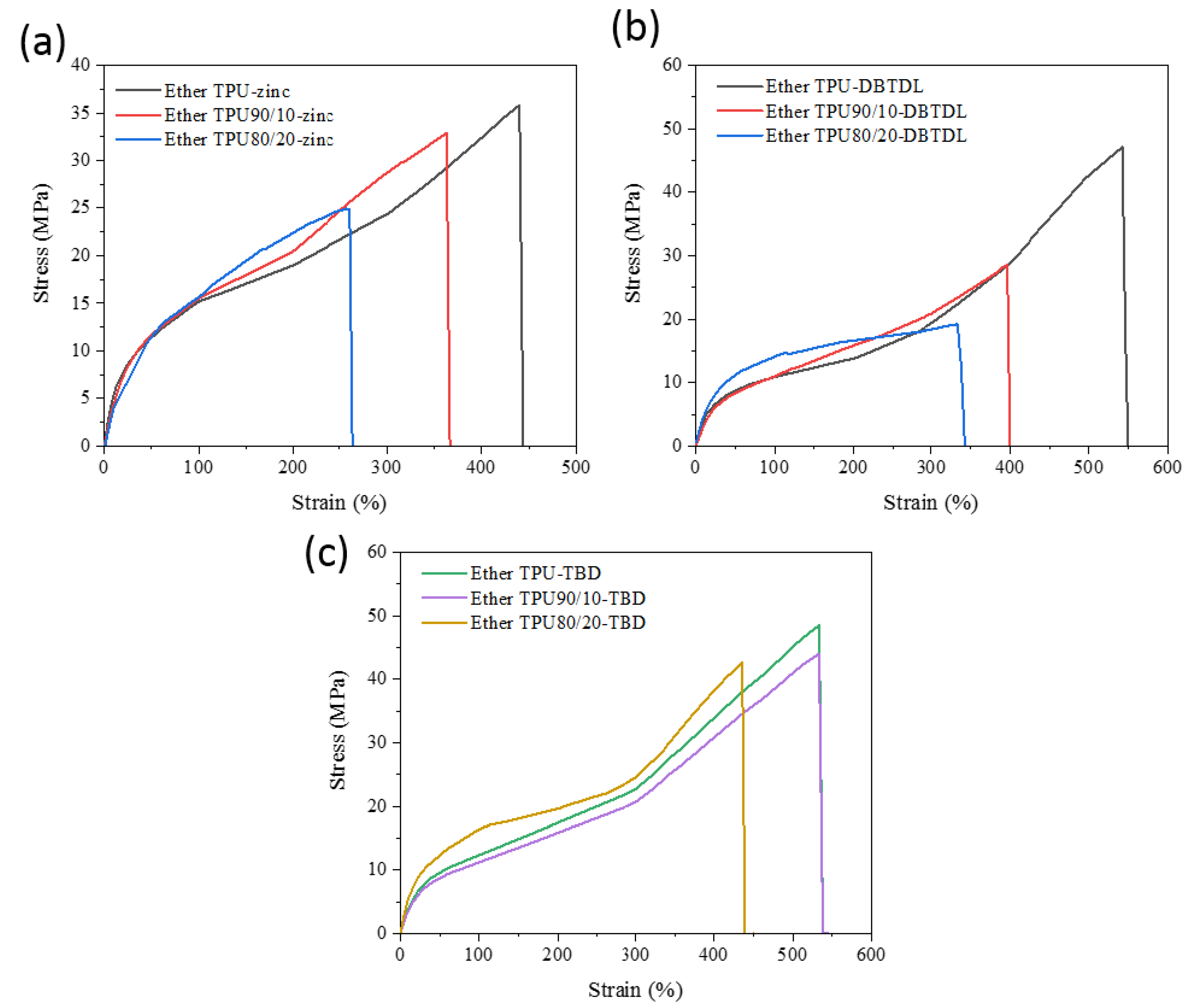
3.3. Mechanical Properties and Exchange Reaction of the Blends
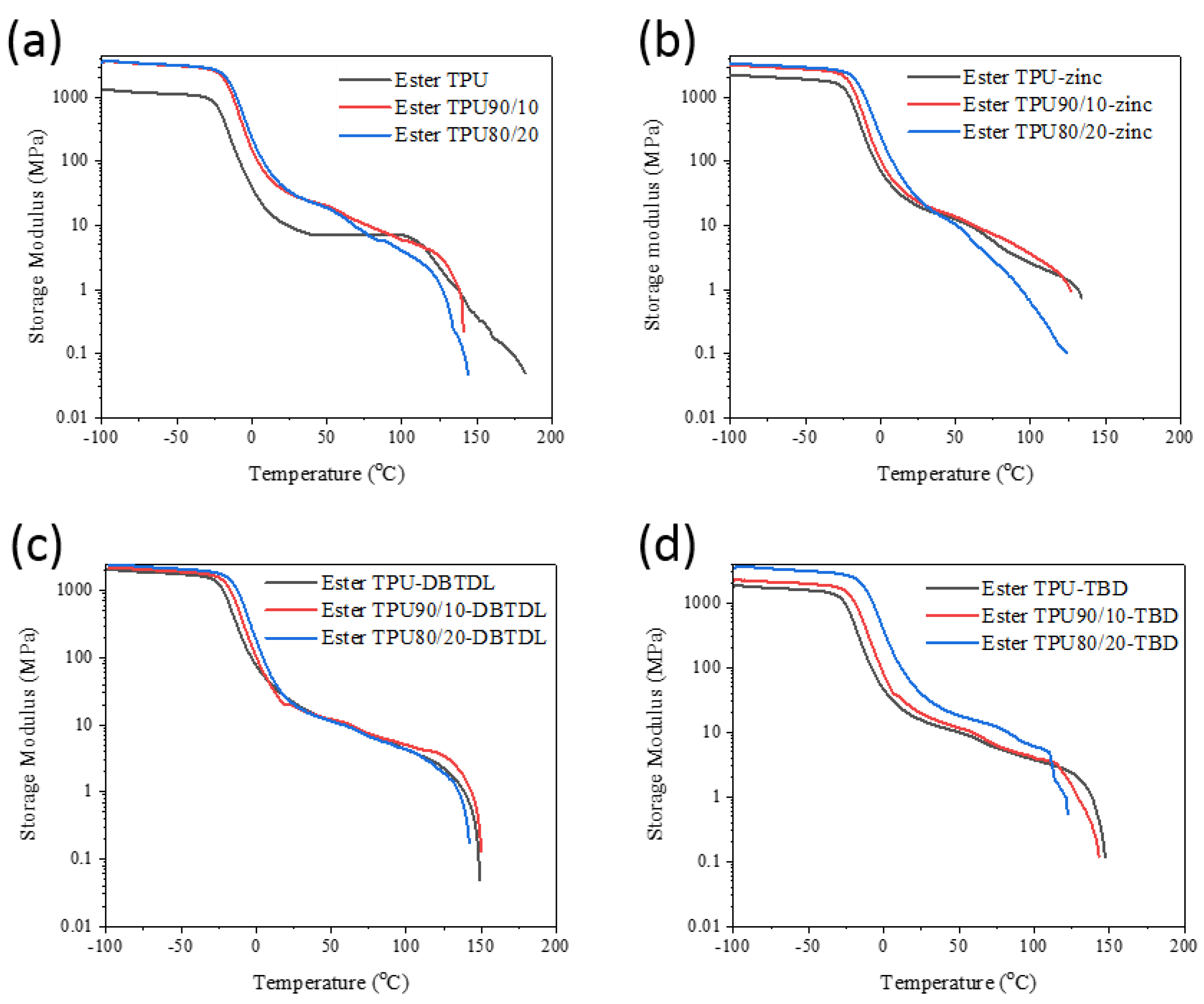
3.4. Dynamic Mechanical Properties of the Blends
3.5. Self-Healing and Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Su, C.C.; Wang, S.C.; Chen, W.J.; Lee, L.T. Phase compatibilization through chemical exchange reactions in blends of copolyesters with poly (hydroxyether of bisphenol-A) upon annealing. Ind. Eng. Chem. Res. 2013, 52, 12587–12595. [Google Scholar] [CrossRef]
- Long, J.; Michael, P.W.; Zhang, J.W. Study of biodegradable polyactide/poly (butylene adipate-co-terephethalate) blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar]
- Krache, R.; Debbah, I. Some mechanical and thermal prpperties of PC/ABS blends. Mater. Sci. Appl. 2011, 2, 404–410. [Google Scholar]
- Farmahini-Farahani, M.; Jafari, S.H.; Khonakdar, H.A.; Komber, H.; Yacaril, A.; Tarameshloul, M. Investigation of exchange reactions and rheological response of reactive blends of poly (trimethylene terephthalate) and phenoxy resin. Polym. Int. 2008, 57, 612–617. [Google Scholar] [CrossRef]
- Chen, G.X.; Kim, H.S.; Kim, E.S.; Yoon, J.S. Compatibilization-like effect of reactive organoclay on the poly (l-lactide)/poly (butylene succinate) blends. Polymer 2005, 46, 11829–11836. [Google Scholar] [CrossRef]
- Sadhan, J.; Nisha, P.; Dhawal, D. Compatibilization of PBT-PPE blends using low molecular weight epoxy. Polymer 2001, 42, 8681–8693. [Google Scholar]
- Naoki, Y.; Osamu, A.; Katsunori, S.; Katsuyuki, A.; Shigeaki, T. Morphologies and properties of cured epoxy/phenoxy containing carboxyl groups blends as adhesives for flexible printed circuits (FPCs). J. Adhes. Soc. Jpn. 2011, 47, 478–484. [Google Scholar]
- Yokoyama, N.; Nonaka, Y.; Kurata, T.; Sakai, S.; Takahashi, S.; Kasemura, T. Morphologies and properties of cured epoxy/brominated-pheoxy blends. J. Appl. Polym. Sci. 2007, 104, 1702–1713. [Google Scholar] [CrossRef]
- Yang, C.F.; Wang, H.C.; Su, C.C. Enhancing the compatibility of poly(1,4-butylene adipate) and phenoxy resin in blends. Materials 2017, 10, 692. [Google Scholar] [CrossRef]
- Dixit, V.; Nagpal, A.K.; Singhal, R. Synthesis and characterization of phenoxy modified epoxy blends. Malays. Polym. J. 2010, 5, 69–83. [Google Scholar]
- Eguiazabal, J.I.; Nazabal, J. Influence of interchange reactions in the nature and properties of miscible 50/50 poly(butylene terephthalaye)/phenoxy blends. J. Mat. Sci. 1990, 30, 1522–1528. [Google Scholar] [CrossRef]
- Eguiazabal, J.I.; Iruin, J.J. Miscibility and thermal decomposition in phenoxy/poly(ethylene terephthalate) and phenoxy/poly(butylene terephthalate) blends. Mater. Chem. Phys. 1987, 18, 147–154. [Google Scholar] [CrossRef]
- Goh, S.H.; Lee, S.Y.; Siow, K.S.; Hong, J. Miscibility of some phenoxy/polymethacrylate blends. Polym. Bulletin 1993, 30, 691–696. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.C.; Su, C.C.; Yang, C.F. Chemical interaction-induced evolution of phase compatibilization in blends of poly (hydroxyl ether of bisphenol-A)/poly (1, 4-butylene terephthalate). Materials 2018, 11, 1667. [Google Scholar] [CrossRef]
- Cho, S.J.; Cho, J.H.; Lee, K.H. Phase behavior and its effects on crystallization in a poly(trimethylene terephthalate)/phenoxy resin blend. Polymers 2016, 8, 21. [Google Scholar] [CrossRef]
- Bonab, V.S.; Manas-Zloczower, I. Revisiting thermoplastic polyurethane, from composition to morphology and properties. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1553–1564. [Google Scholar] [CrossRef]
- Emi, G.B.; Andela, P.; Ivan, S. Mirela, L. Blends of thermoplastic polyurethane and polypropylene. Thermal and morphological behavior. J. Appl. Polym. Sci. 2010, 117, 1378–1384. [Google Scholar]
- Zhou, R.; Gao, W.Q.; Xia, L.C.; Wu, H.; Guo, S.Y. Structural engineering of polyurethane coatings for high performance applications. J. Mater. Sci. 2018, 53, 9350–9362. [Google Scholar]
- Chattopadhyay, D.K.; Raju, K.V.S.N. The study of damping property and mechanism of thermoplastic polyurethane/phenolic resin through a combined experiment and molecular dynamics simulation. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Jeong, H.M.; Ahn, B.K.; Kim, B.K. Miscibility and shape memory effect of thermoplastic polyurethane blends with phenoxy resin. Euro. Polym. J. 2001, 37, 2245–2252. [Google Scholar] [CrossRef]
- Xu, K.M.; Zhang, F.S.; Zhang, X.L.; Hu, Q.M.; Wu, H.; Guo, S.Y. Molecular insights into hydrogen bonds in polyurethane/hindered phenol hybrids: Evolution and relationship with damping properties. J. Mater. Chem. 2014, 2, 8545–8556. [Google Scholar] [CrossRef]
- Palanivelu, K.; Balakrishnan, S.; Rengasamy, E. Thermoplastic polyurethane toughened polyacetal blends. Polymer 2009, 19, 75–83. [Google Scholar] [CrossRef]
- Arostegui, A.; Nazabal, J. Stiffer and supr-tough poly (butylene terephthalate) based blends by modification with phenoxy and maleated poly (ethylene-octene) copolymers. Polymer 2003, 44, 239–249. [Google Scholar] [CrossRef]
- Pramod, K.; Ilyas, U.K.; Singh, M.; Kamal, Y.T.; Ahmad, S.; Ansari, S.H.; Ali, J. High-performance thin-layer chromatographic analysis of eugenol in developed nanoemulsion gel and nanoparticles: Validation of a stability-indicating method. Act. Chromatogr. 2015, 27, 571–582. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, L.; Gao, Z.; Kessler, M.R. Synthesis and characterization of methacrylated eugenol as a sustainable reactive diluent for a maleinated acrylated epoxidized soybean oil resin. ACS Sustain. Chem. Eng. 2017, 5, 8876–8883. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.L.; Samir, A.A.; Langlois, A.; Renard, E. Antibacterial and antioxidant photoinitiated epoxy co-networks of resorcinol and eugenol derivatives. Mat. Com. 2017, 12, 19–28. [Google Scholar] [CrossRef]
- Sun, L.; Singh, S.; Joo, M.; Simmones, B.A.; Auer, M. Non-invasive imaging of cellulose microfibril orientation within plant cell walls by polarized Raman microspectroscopy. Biotechnol. Bioeng. 2016, 113, 82–90. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, R.R. Organic thermal stabilizers for rigid poly (vinyl chloride). Part 8, Eugenol (4-allyl-2-methoxy-phenol). Polym. Degrad. Stab. 2007, 92, 587–595. [Google Scholar] [CrossRef]
- Harvey, B.G.; Sahagun, C.M.; Guenthner, A.J.; Groshens, T.J.; Cambrea, L.R.; Reams, J.T.; Mabry, J.M. A high-performance renewable thermosetting resin derived from eugenol. ChemSusChem 2014, 7, 1964–1969. [Google Scholar] [CrossRef]
- Deng, J.P.; Yang, B.; Chen, C.; Liang, J.Y. Renewable eugenol-based polymeric oil-absorbent microspheres: Preparation and oil absorption ability. ACS Sustain. Chem. Eng. 2015, 3, 599–605. [Google Scholar] [CrossRef]
- Liu, K.W.; Madbouly, S.A.; Kessler, M.R. Biorenewable thermosetting copolymer based on soybean oil and eugenol. Eur. Polym. J. 2015, 69, 16–28. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis of eugenol-based polybenzoxazine-POSS nanocomposites for low dielectric applications. Polym. Compos. 2015, 36, 1973–1982. [Google Scholar] [CrossRef]
- Miao, J.T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased heat resistant epoxy resin with extremely high biomass content from 2,5-furandicarboxylic acid and eugenol. ACS Sustain. Chem. Eng. 2017, 5, 7003–7011. [Google Scholar] [CrossRef]
- Francois, C.; Pourchet, S.; Bonia, G.; Fontaine, S.; Gaillard, Y.; Placet, V.; Galkin, M.V.; Orebom, A.; Samec, J.; Plasseraud, L. Diglycidylether of iso-eugenol: A suitable lignin derived synthon for epoxy thrmoset applications. RSC Adv. 2016, 6, 68732–68738. [Google Scholar] [CrossRef]
- Van Bogart, J.W.C.; Gibson, P.E.; Cooper, S.L. Structure property relationship in polycaprolactone-polyurethanes. J. Polym. Sci. Polym. Phys. 1983, 21, 65–95. [Google Scholar] [CrossRef]
- Ishak, Z.I.; Sairi, N.A.; Allias, Y.; Aroua, M.K.T.; Yusoff, R. Production of glycerol carbonate from glycerol with aid of ionic liquid as catalyst. Chem. Eng. J. 2016, 297, 128–138. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, C.R.; Lee, D.S. Large Improvement in the Mechanical Properties of Polyurethane Nanocomposites Based on a Highly Concentrated Graphite Nanoplate/Polyol. Nanomaterials 2019, 9, 389. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Hillmyer, M.A.; Dichtel, W.R. Structural effects on the reprocessability and stress relaxation of crosslinked polyhydroxyurethanes. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, S.R.; Lee, D.S. Sorbitol as a chain extender of polyurethane prepolymers to prepare self-healable and robust polyhydroxyurethane elastomers. Molecules 2018, 23, 2515. [Google Scholar] [CrossRef]





| Sample Codes | Blends | Catalysts | |||
|---|---|---|---|---|---|
| TPU | Phenoxy Resin | Zinc Acetate | DBTDL | TBD | |
| (g) | (g) | (wt%) | (wt%) | (wt%) | |
| Ester TPU | 100 | 0 | |||
| Ester TPU90/10 | 90 | 10 | |||
| Ester TPU80/20 | 80 | 20 | |||
| Ester TPU-zinc | 100 | 0 | 0.1 | ||
| Ester TPU90/10-zinc | 90 | 10 | 0.1 | ||
| Ester TPU80/20-zinc | 80 | 20 | 0.1 | ||
| Ether TPU-zinc | 100 | 0 | 0.1 | ||
| Ether TPU90/10-zinc | 90 | 10 | 0.1 | ||
| Ether TPU80/20-zinc | 80 | 20 | 0.1 | ||
| Ester TPU-DBTDL | 100 | 0 | 0.1 | ||
| Ester TPU90/10-DBTDL | 90 | 10 | 0.1 | ||
| Ester TPU80/20-DBTDL | 80 | 20 | 0.1 | ||
| Ether TPU-DBTDL | 100 | 0 | 0.1 | ||
| Ether TPU90/10-DBTDL | 90 | 10 | 0.1 | ||
| Ether TPU80/20-DBTDL | 80 | 20 | 0.1 | ||
| Ester TPU-TBD | 100 | 0 | 0.1 | ||
| Ester TPU90/10-TBD | 90 | 10 | 0.1 | ||
| Ester TPU80/20-TBD | 80 | 20 | 0.1 | ||
| Ether TPU-TBD | 100 | 0 | 0.1 | ||
| Ether TPU90/10-TBD | 90 | 10 | 0.1 | ||
| Ether TPU80/20-TBD | 80 | 20 | 0.1 | ||
| Sample Codes | Tgs (°C) | Tm (°C) | Tc (°C) | Tensile Strength (MPa) | Elongation at Break (%) | Storage Modulus at 20 °C (MPa) |
|---|---|---|---|---|---|---|
| Ester TPU | −18 | - | 36 | 30.9 ± 1% | 600 ± 1% | 11.2 |
| Ester TPU90/10 | −12 | 155 | 44 | 27.8 ± 1.2% | 615 ± 0.9% | 37.2 |
| Ester TPU80/20 | −11 | 151 | 49 | 23.5 ± 1.1% | 600 ± 0.8% | 41.9 |
| Ester TPU-zinc | −20 | 150 | 35 | 16 ± 1% | 616 ± 0.8% | 23.5 |
| Ester TPU90/10-zinc | −12 | 145 | 38 | 19.7 ± 1.1% | 615 ± 1% | 28 |
| Ester TPU80/20-zinc | −11 | 143 | 47 | 18.7 ± 1.2% | 619 ± 1% | 35.7 |
| Ester TPU-DBTDL | −16 | 148 | 35 | 21.7 ± 1.5% | 645 ± 1.3% | 17.3 |
| Ester TPU90/10-DBTDL | −11 | 145 | 42 | 16.6 ± 1.6% | 583 ± 1.3% | 22.8 |
| Ester TPU80/20-DBTDL | −7 | 141 | 46 | 13.2 ± 1.5% | 524 ± 1.3% | 52.1 |
| Ester TPU-TBD | −17 | 147 | 37 | 14.1 ± 1.5% | 620 ± 1.2% | 25.6 |
| Ester TPU90/10-TBD | −13 | 144 | 40 | 9.2 ± 1.3% | 615 ± 1% | 20.3 |
| Ester TPU80/20-TBD | −10 | 139 | 45 | 9 ± 1.4% | 578 ± 1% | 25.3 |
| Ether TPU-zinc | −25 | 200 | - | 35 ± 0.9% | 440 ± 1% | 54.4 |
| Ether TPU90/10-zinc | −22 | 197 | - | 32 ± 1% | 360 ± 1.3% | 133.8 |
| Ether TPU80/20-zinc | −16 | 194 | - | 24 ± 1% | 260 ± 1.5% | 151.8 |
| Ether TPU-DBTDL | −25 | 221 | - | 47 ± 1.2% | 542 ± 1.6% | 30.4 |
| Ether TPU90/10-DBTDL | −18 | 200 | - | 28 ± 1% | 396 ± 0.8% | 120 |
| Ether TPU80/20-DBTDL | −9 | 195 | - | 12 ± 1.1% | 338 ± 1.3% | 150.6 |
| Ether TPU-TBD | −27 | 220 | - | 48.4 ± 1% | 533 ± 1.2% | 107.1 |
| Ether TPU90/10-TBD | −21 | 201 | - | 44 ± 1.2% | 520 ± 1.4% | 118.5 |
| Ether TPU80/20-TBD | −16 | 198 | - | 42 ± 1% | 435 ± 1.2% | 154.3 |
| Sample | After Heat Treatment (without cut) | After Cut and Heal Test | Healing Efficiency (%) | ||
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | Tensile Strength (MPa) | Elongation at Break (%) | ||
| Ester TPU | 30.4 | 617 | - | - | 0 |
| Ester TPU90/10 | 16.3 | 610 | 2.4 | 36 | 15 |
| Ester TPU80/20 | 14.6 | 500 | 2.6 | 74 | 18 |
| Ester TPU-zinc | 7.1 | 600 | - | - | 0 |
| Ester TPU90/10-zinc | 15.8 | 509 | 14.1 | 478 | 89 |
| Ester TPU80/20-zinc | 8 | 434 | 7.7 | 418 | 96 |
| Ester TPU-DBTDL | 6.9 | 581 | - | - | 0 |
| Ester TPU90/10-DBTDL | 12.6 | 600 | 7 | 314 | 56 |
| Ester TPU80/20-DBTDL | 8.6 | 600 | 7 | 570 | 82 |
| Ester TPU-TBD | 4 | 200 | - | - | 0 |
| Ester TPU90/10-TBD | 4.3 | 184 | 4.1 | 150 | 95 |
| Ester TPU80/20-TBD | 3.9 | 210 | 3.9 | 165 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.-Y.; Shin, S.-R.; Lee, S.-H.; Lee, D.-S. Self-Healing and Mechanical Properties of Thermoplastic Polyurethane/Eugenol-Based Phenoxy Resin Blends via Exchange Reactions. Polymers 2020, 12, 1011. https://doi.org/10.3390/polym12051011
Liang J-Y, Shin S-R, Lee S-H, Lee D-S. Self-Healing and Mechanical Properties of Thermoplastic Polyurethane/Eugenol-Based Phenoxy Resin Blends via Exchange Reactions. Polymers. 2020; 12(5):1011. https://doi.org/10.3390/polym12051011
Chicago/Turabian StyleLiang, Jing-Yu, Se-Ra Shin, Soo-Hyoung Lee, and Dai-Soo Lee. 2020. "Self-Healing and Mechanical Properties of Thermoplastic Polyurethane/Eugenol-Based Phenoxy Resin Blends via Exchange Reactions" Polymers 12, no. 5: 1011. https://doi.org/10.3390/polym12051011
APA StyleLiang, J.-Y., Shin, S.-R., Lee, S.-H., & Lee, D.-S. (2020). Self-Healing and Mechanical Properties of Thermoplastic Polyurethane/Eugenol-Based Phenoxy Resin Blends via Exchange Reactions. Polymers, 12(5), 1011. https://doi.org/10.3390/polym12051011