Temperature and pH-Dependent Response of Poly(Acrylic Acid) and Poly(Acrylic Acid-co-Methyl Acrylate) in Highly Concentrated Potassium Chloride Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures
2.2.1. Synthesis of Linear Poly(Acrylic Acid-co-Methyl Acrylate) (P(AA-co-MA)) via Free Radical Polymerization
2.2.2. Synthesis of Star-Shaped Poly(Acrylic Acid) (PAA) and P(AA-co-MA) via Atom Transfer Radical Polymerization (ATRP) (Example S1, Scheme 1)
2.3. Instrumentation
3. Results and Discussion
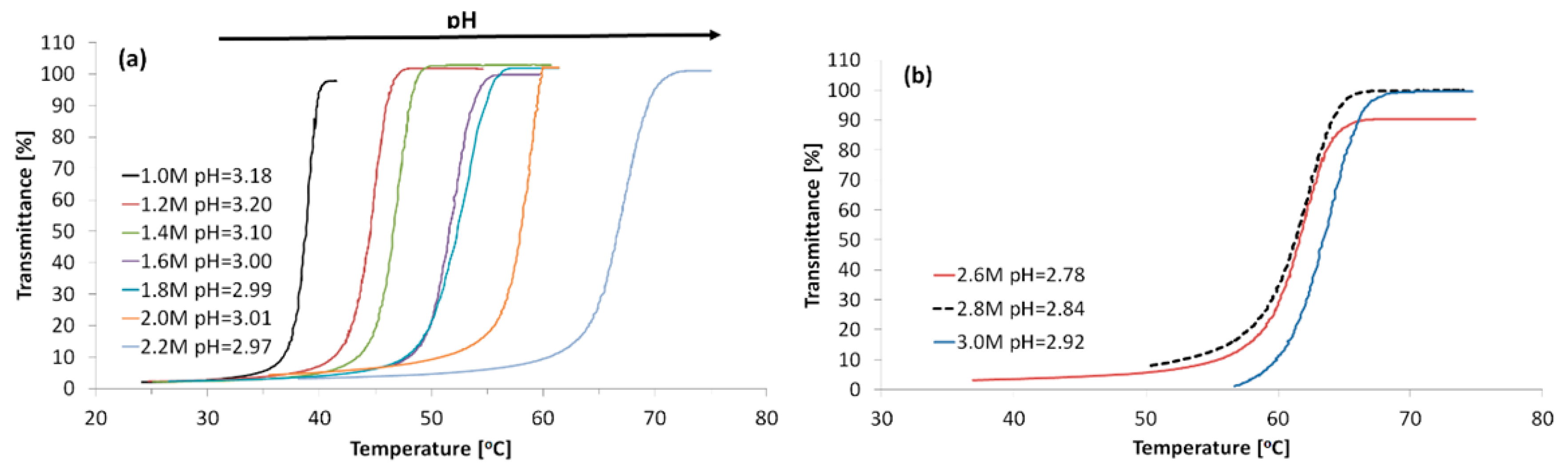
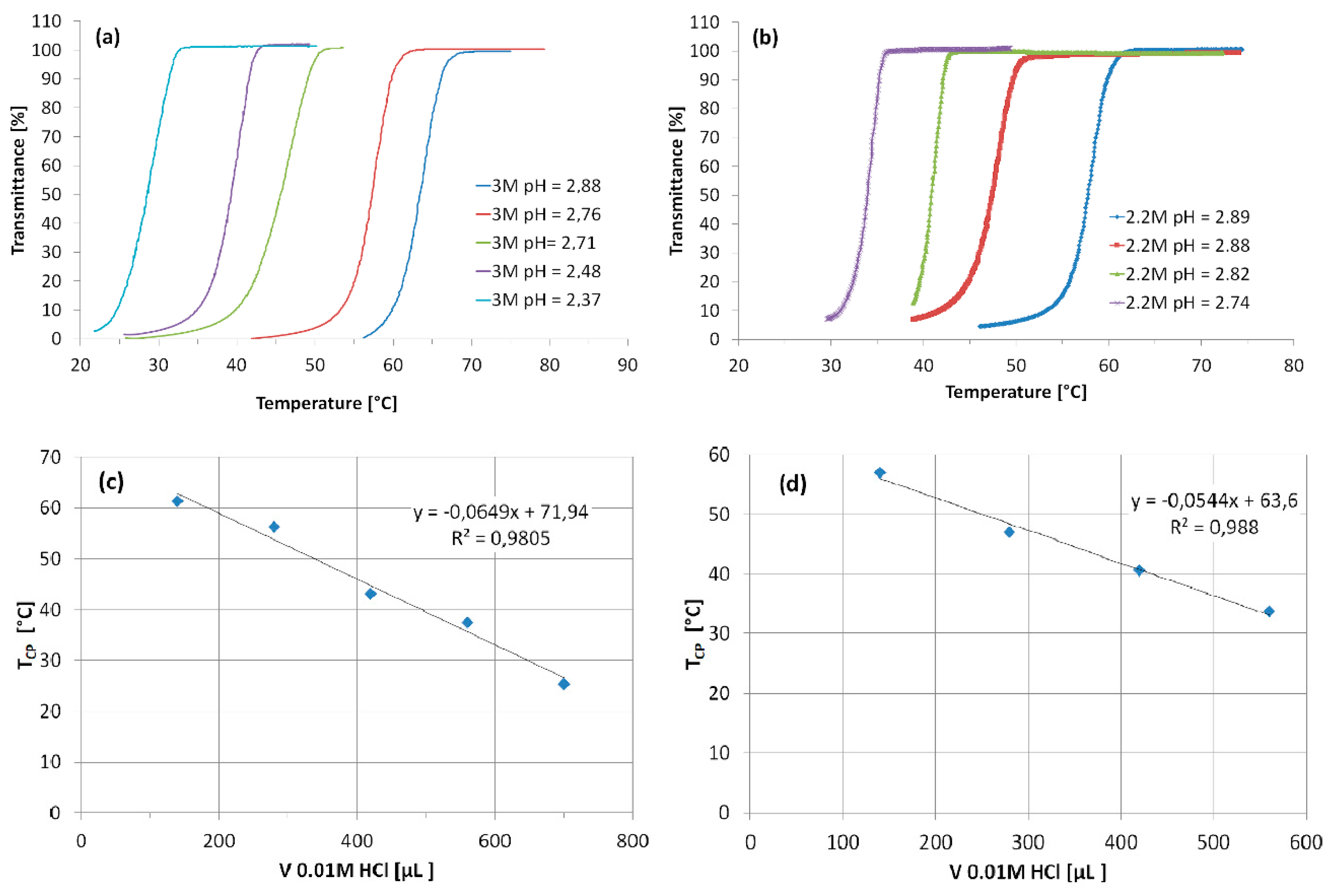
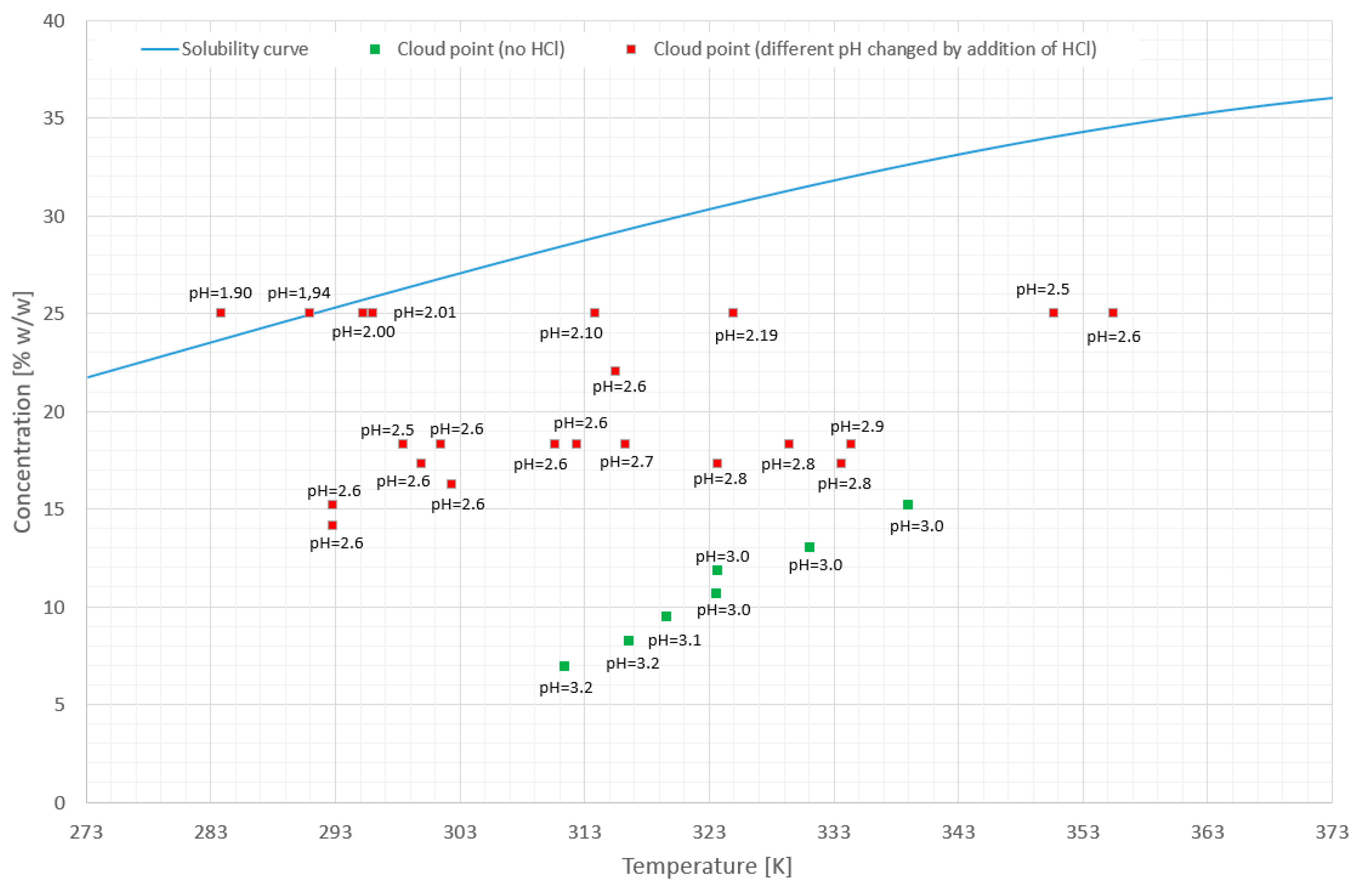
3.1. Phase Transition of High Molecular Weight PAA in Aqueous KCl Solutions
3.2. Particle Size Distribution of High Molecular Weight PAA
3.3. Scanning Electron Microscopy (SEM) Analysis of High Molecular Weight PAA
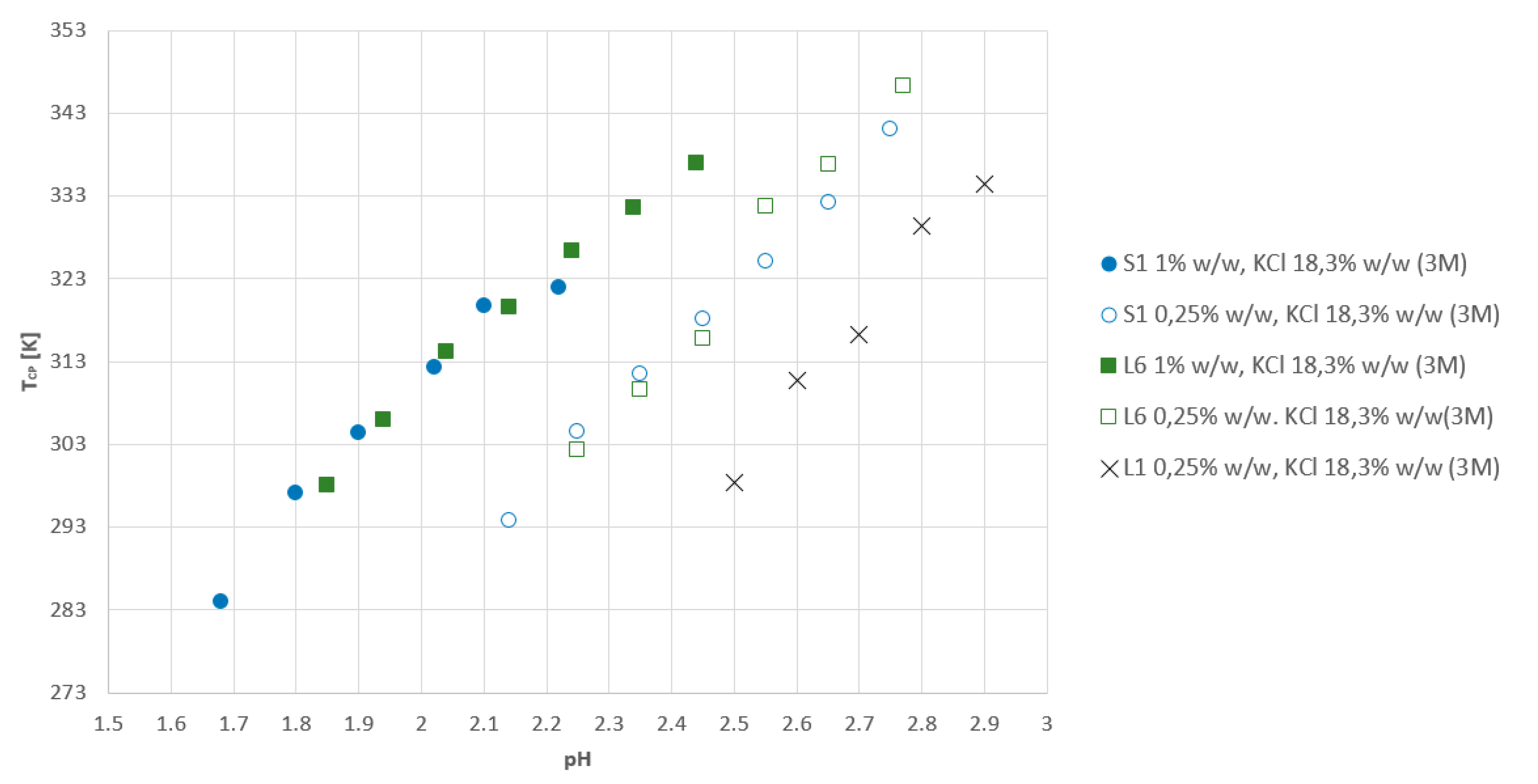
3.4. Influence of Molecular Weight and Composition of Polymers on TCP in KCl Solutions
3.5. Synthesis and Characterization of Well-Defined AA/MA Copolymers with Different Topology
4. Conclusions
- (1)
- The TCP of PAA strongly depends on pH and salt concentration.
- (2)
- The TCP of PAA decreased upon the incorporation of hydrophobic repeating units, such as MA.
- (3)
- A comparison of linear and star-shaped AA/MA copolymers with nearly the same composition showed that the star-shaped topology decreased the TCP to 284 K.
- (4)
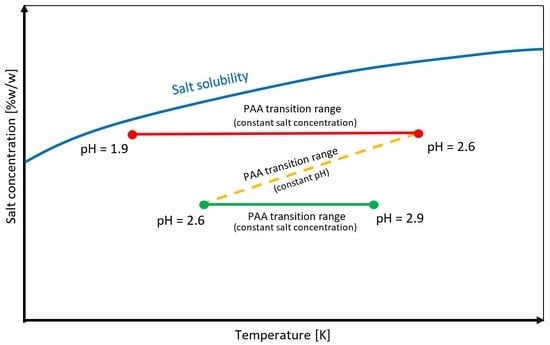
- It is possible to design process parameters to allow the phase transition to occur in the metastable zone of the salt solution.
Author Contributions
Funding
Conflicts of Interest
References
- Lemanowicz, M.; Gierczycki, A.; Kuźnik, W. Review of stimuli-responsive polymers application as stabilization agents in solid-liquid dispersion systems. Polimery 2016, 61, 92–97. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Neugebauer, D. Synthesis and characterization of D-(−)-salicine-based star copolymers containing pendant carboxyl groups with fluorophore dyes. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2399–2411. [Google Scholar] [CrossRef]
- Strandman, S.; Zhu, X.X. Thermo-responsive block copolymers with multiple phase transition temperatures in aqueous solutions. Prog. Polym. Sci. 2015, 42, 154–176. [Google Scholar] [CrossRef]
- Weiss, J.; Böttcher, C.; Laschewsky, A. Self-assembly of double thermoresponsive block copolymers end-capped with complementary trimethylsilyl groups. Soft Matter 2011, 7, 483–492. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y.; Kuboshima, Y.; Yamamoto, K.; Aoyagi, T. Synthesis and characterization of double thermo-responsive block copolymer consisting N-isopropylacrylamide by atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6142–6150. [Google Scholar] [CrossRef]
- Neugebauer, D.; Odrobińska, J.; Bielas, R.; Mielańczyk, A. Design of systems based on 4-armed star-shaped polyacids for indomethacin delivery. New J. Chem. 2016, 40, 10002–10011. [Google Scholar] [CrossRef]
- Maji, T.; Banerjee, S.; Biswas, Y.; Mandal, T.K. Dual-Stimuli-Responsive l -Serine-Based Zwitterionic UCST-Type Polymer with Tunable Thermosensitivity. Macromolecules 2015, 48, 4957–4966. [Google Scholar] [CrossRef]
- Kelley, E.G.; Albert, J.N.L.; Sullivan, M.O.; Epps, T.H. Stimuli-responsive copolymer solution and surface assemblies for biomedical applications. Chem. Soc. Rev. 2013, 42, 7057–7071. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, M.; Trewyn, B.G. Polymer-based stimuli-responsive nanosystems for biomedical applications. Biotechnol. J. 2013, 8, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. First example of a universal and cost-effective approach: Polymers with tunable upper critical solution temperature in water and electrolyte solution. Macromolecules 2012, 45, 3910–3918. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Kanazawa, H.; Yamamoto, K.; Matsushima, Y.; Takai, N.; Kikuchi, A.; Sakurai, Y.; Okano, T. Temperature-responsive chromatography using poly(N-isopropylacrylamide)-modified silica. Anal. Chem. 1996, 68, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Okano, T.; Hsu, R.; Kim, S.W. Thermo-sensitive polymers as on-off switches for drug release. Die Makromol. Chem. Rapid Commun. 1987, 8, 481–485. [Google Scholar] [CrossRef]
- Sanford, A.A.; Weissman, J.M.; Sunkara, H.B. Thermally Switchable Optical Devices. U.S. Patent US 6,165,389, 26 December 2000. [Google Scholar]
- Chen, J.P.; Huffman, A.S. Polymer-protein conjugates. II. Affinity precipitation separation of human immunogammaglobulin by a poly(N-isopropylacrylamide)-protein A conjugate. Biomaterials 1990, 11, 631–634. [Google Scholar] [CrossRef]
- Burdukova, E.; Li, H.; Bradshaw, D.J.; Franks, G.V. Poly (N-isopropylacrylamide) (PNIPAM) as a flotation collector: Effect of temperature and molecular weight. Miner. Eng. 2010, 23, 921–927. [Google Scholar] [CrossRef]
- Lemanowicz, M.; Gierczycki, A.; Kuźnik, W.; Sancewicz, R.; Imiela, P. Determination of Lower Critical Solution Temperature of thermosensitive flocculants. Miner. Eng. 2014, 69, 170–176. [Google Scholar] [CrossRef]
- Zhao, C.; Dolmans, L.; Zhu, X.X. Thermoresponsive Behavior of Poly(acrylic acid-co-acrylonitrile) with a UCST. Macromolecules 2019, 52, 4441–4446. [Google Scholar] [CrossRef]
- Käfer, F.; Liu, F.; Stahlschmidt, U.; Jérôme, V.; Freitag, R.; Karg, M.; Agarwal, S. LCST and UCST in One: Double Thermoresponsive Behavior of Block Copolymers of Poly(ethylene glycol) and Poly(acrylamide-co-acrylonitrile). Langmuir 2015, 31, 8940–8946. [Google Scholar] [CrossRef]
- Lee, H.N.; Rosen, B.M.; Fenyvesi, G.; Sunkara, H.B. UCST and LCST phase behavior of poly(trimethylene ether) glycol in water. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4311–4315. [Google Scholar] [CrossRef]
- Cho, Y.; Zhang, Y.; Christensen, T.; Sagle, L.B.; Chilkoti, A.; Cremer, P.S. Effects of hofmeister anions on the phase transition temperature of elastin-like polypeptides. J. Phys. Chem. B 2008, 112, 13765–13771. [Google Scholar] [CrossRef] [PubMed]
- Deyerle, B.A.; Zhang, Y. Effects of hofmeister anions on the aggregation behavior of PEO-PPO-PEO triblock copolymers. Langmuir 2011, 27, 9203–9210. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.; Lo Nostro, P.; Ninham, B.W. The present state of affairs with Hofmeister effects. Curr. Opin. Colloid Interface Sci. 2004, 9, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Lüpfert, C. Influence of anions and cations on the dipole potential of phosphatidylcholine vesicles: A basis for the Hofmeister effect. Biophys. J. 1999, 76, 2614–2624. [Google Scholar] [CrossRef]
- Baldwin, R.L. How Hofmeister ion interactions affect protein stability. Biophys. J. 1996, 71, 2056–2063. [Google Scholar] [CrossRef]
- Conway, B.E.; Ayranci, E. Effective ionic radii and hydration volumes for evaluation of solution properties and ionic adsorption. J. Solut. Chem. 1999, 28, 163–192. [Google Scholar] [CrossRef]
- Karlström, G. On the effective interaction between an ion and a hydrophobic particle in polar solvents. A step towards an understanding of the Hofmeister effect? Phys. Chem. Chem. Phys. 2003, 5, 3238–3246. [Google Scholar] [CrossRef]
- Donald, H.; Jenkins, B.; Marcus, Y. Viscosity S-Coefficients of Ions in Solution. Chem. Rev. 1995, 95, 2695–2724. [Google Scholar]
- Hey, M.J.; Clough, J.M.; Taylor, D.J. Ion effects on macromolecules in aqueous solution. Nature 1976, 262, 807–809. [Google Scholar] [CrossRef]
- Gregory, J. Processing of Solid–Liquid Suspensions. In Processing of Solid-Liquid Suspensions; Shamlou, P.A., Ed.; Elsevier: Oxford, UK, 1993; pp. 59–92. ISBN 9780750611343. [Google Scholar]
- Bogacz, W.; Gierczycki, A.; Kuźnik, W.; Lemanowicz, M. Application of Stimuli Sensitive Polymers in Conducting the Crystallization Process. Polish patent P.411522, 9 March 2015. [Google Scholar]
- Jones, J.A.; Novo, N.; Flagler, K.; Pagnucco, C.D.; Carew, S.; Cheong, C.; Kong, X.Z.; Burke, N.A.D.; Stöver, H.D.H. Thermoresponsive copolymers of methacrylic acid and poly(ethylene glycol) methyl ether methacrylate. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6095–6104. [Google Scholar] [CrossRef]
- Shibanuma, T.; Aoki, T.; Sanui, K.; Ogata, N.; Kikuchi, A.; Sakurai, Y.; Okano, T. Thermosensitive Phase-Separation Behavior of Poly(acrylic acid)-graft-poly(N,N-dimethylacrylamide) Aqueous Solution. Macromolecules 2000, 33, 444–450. [Google Scholar] [CrossRef]
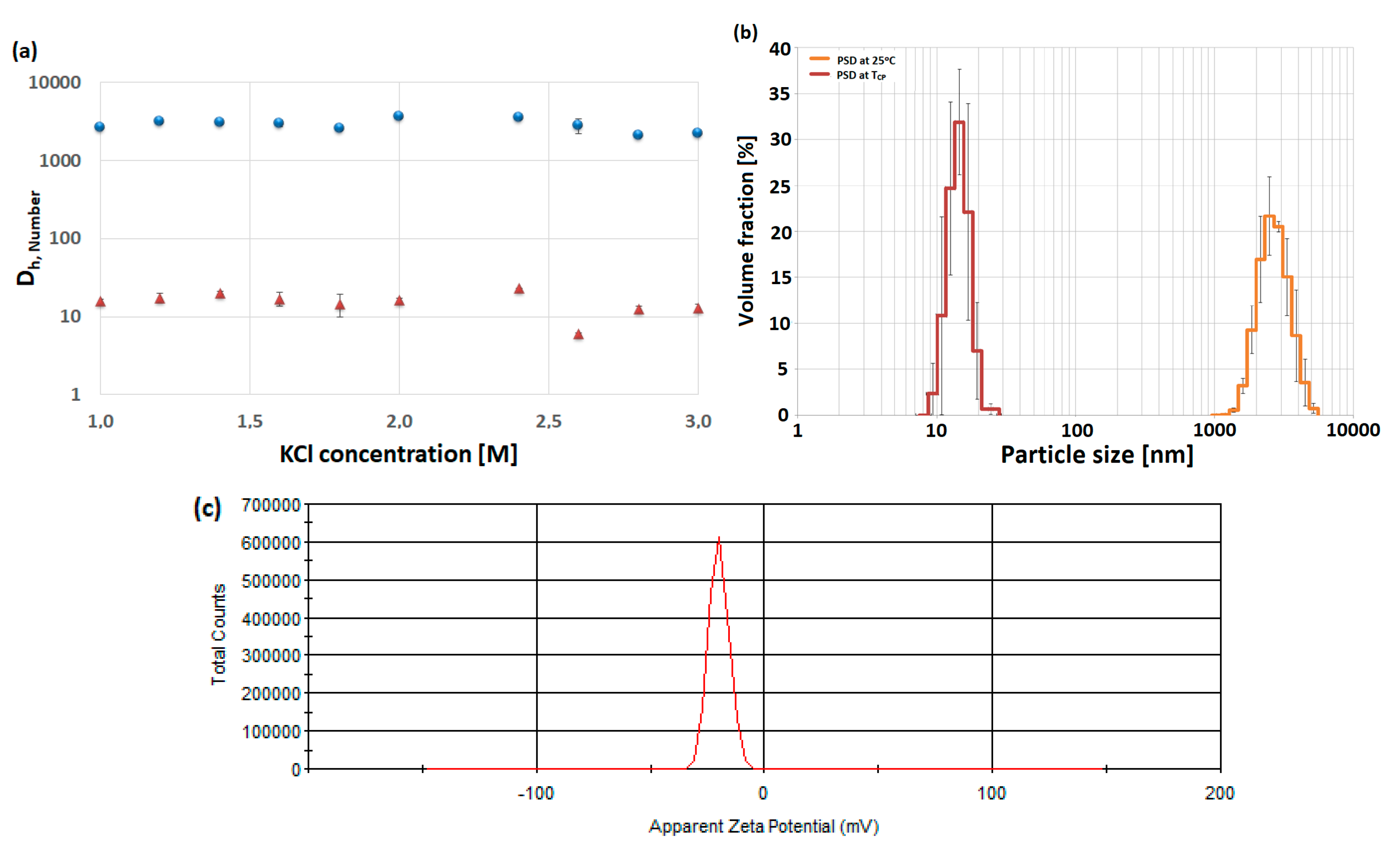
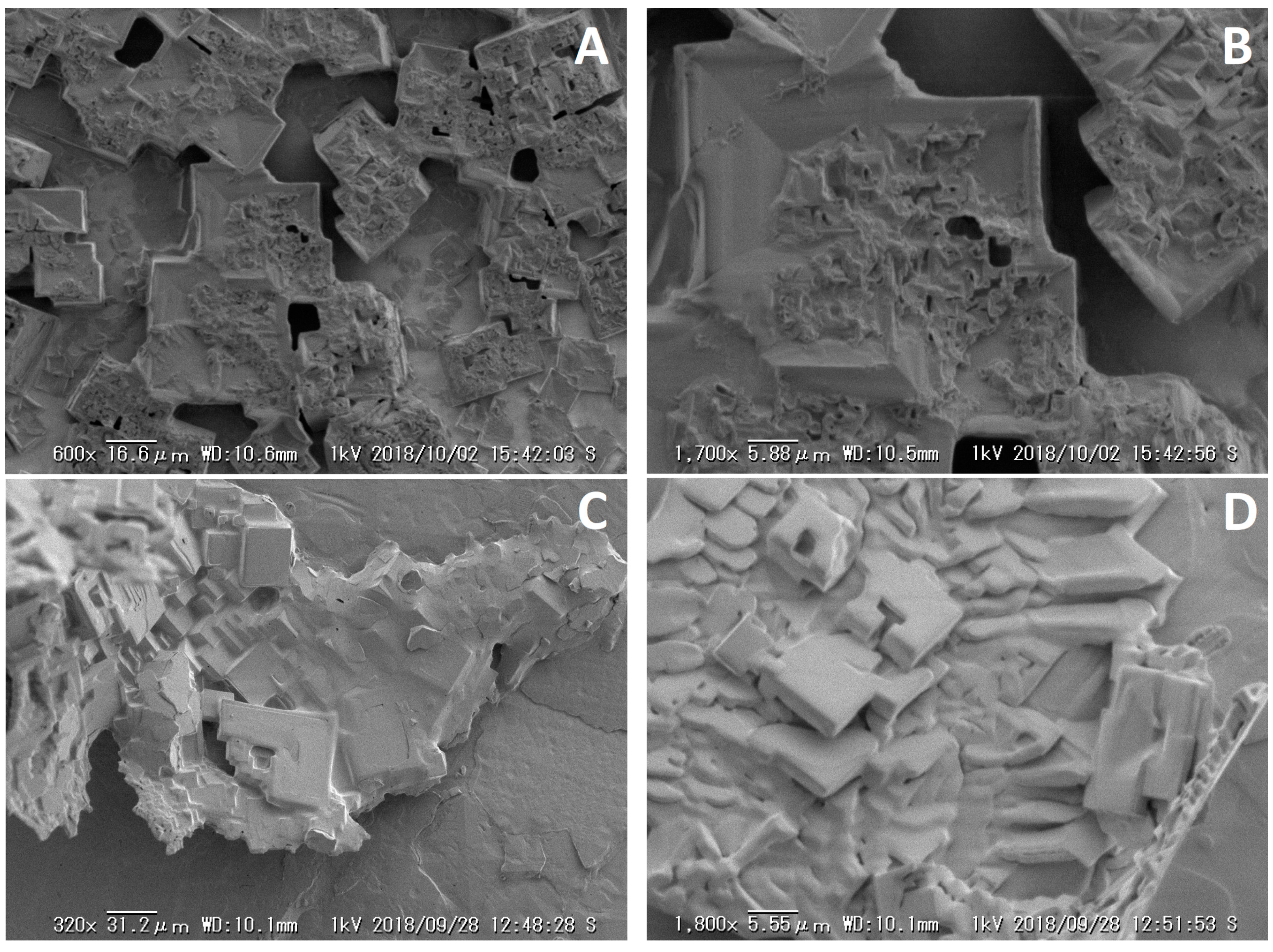
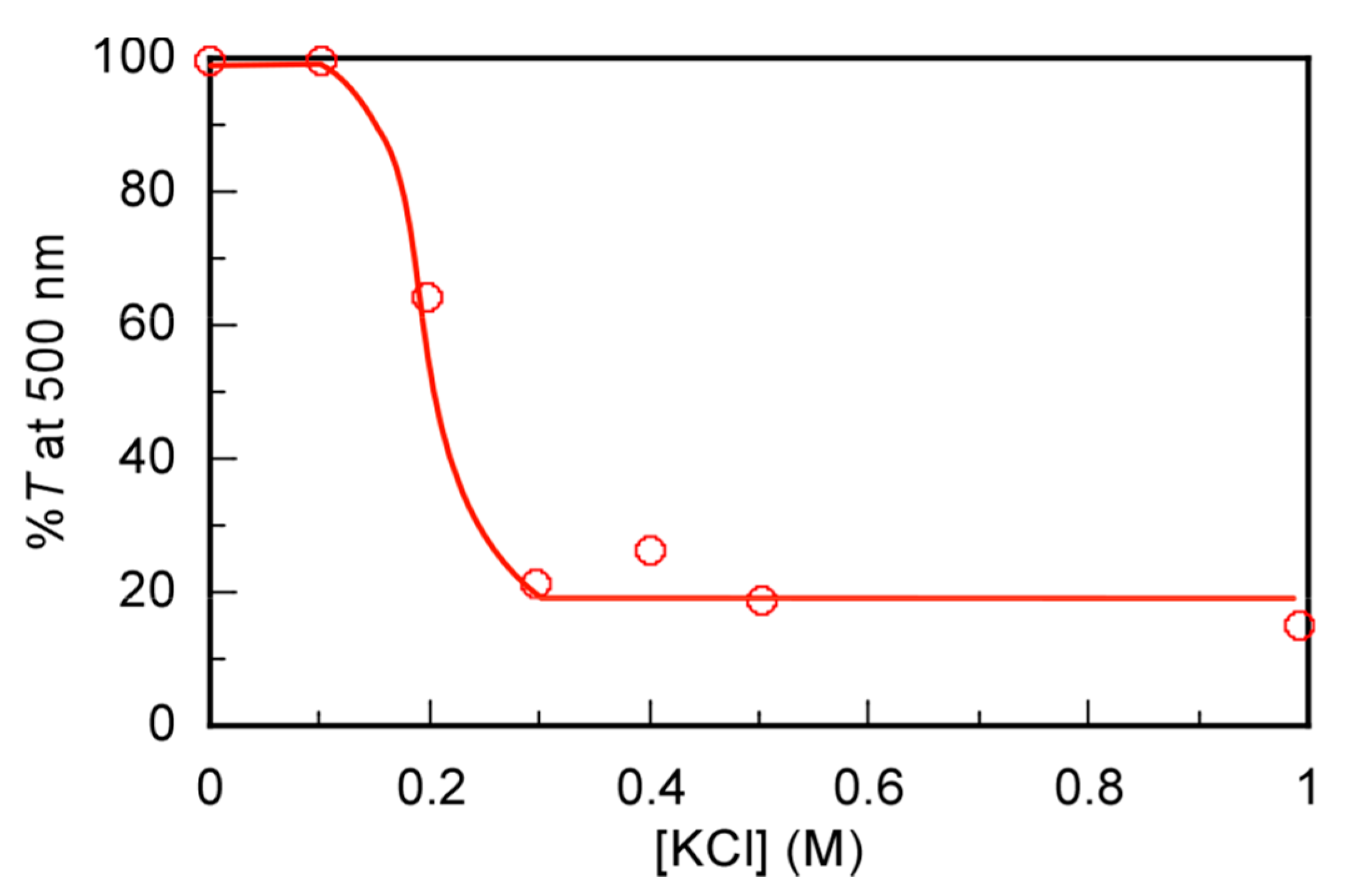
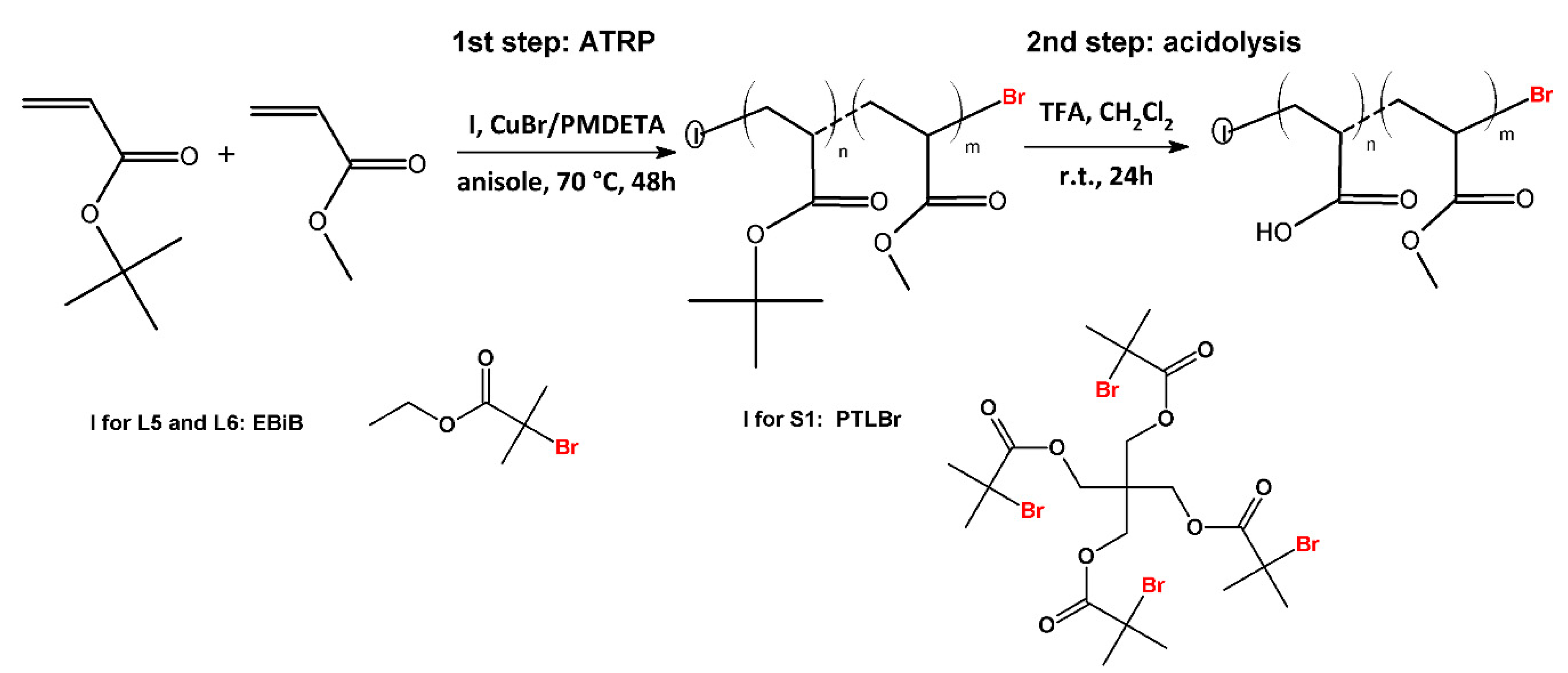
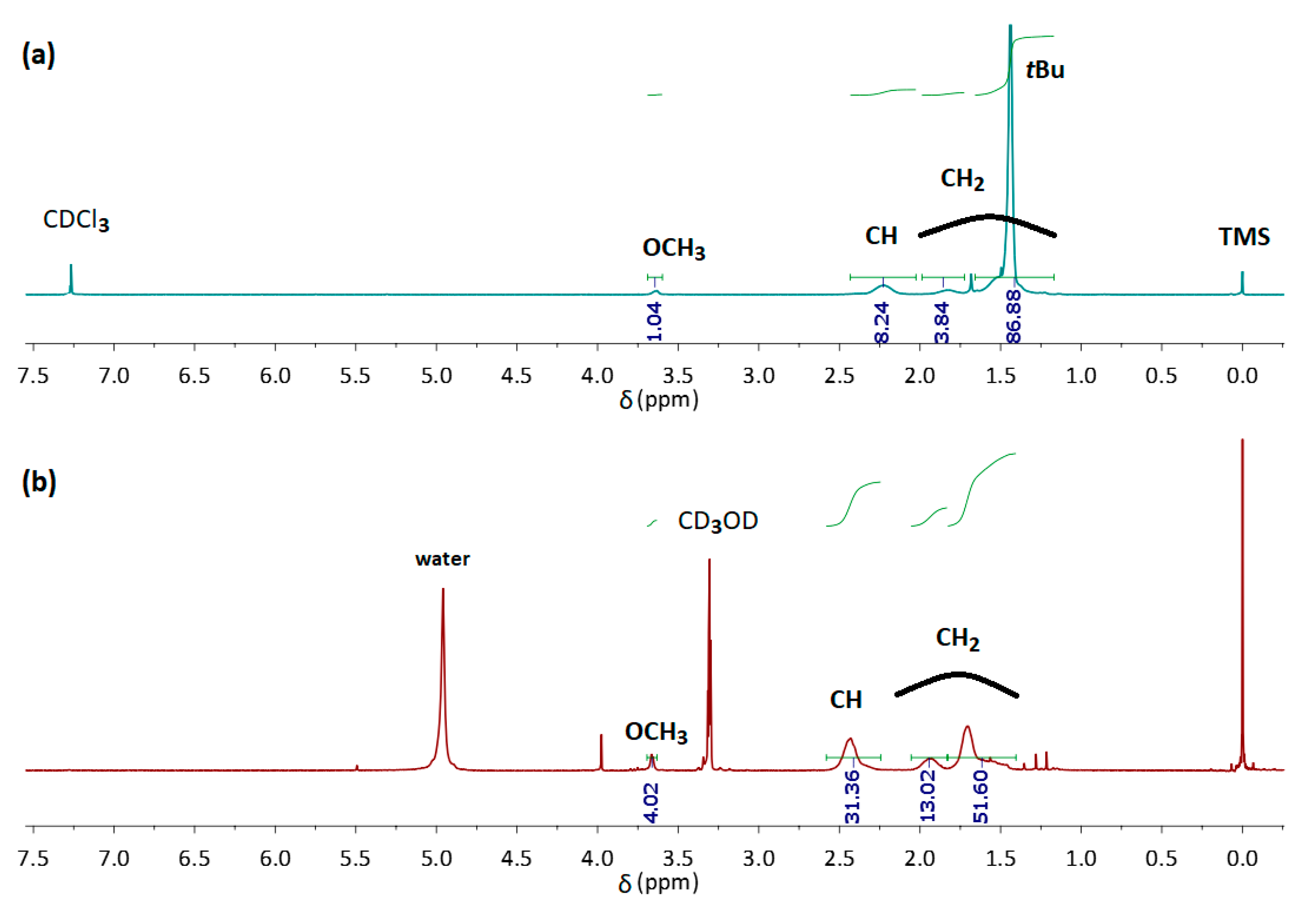
| Name | DPAA/DPMA | Mn,theo [g/mol] | Mn,SEC a [g/mol] | Đa | TCPb | pH |
|---|---|---|---|---|---|---|
| L1 * | - | - | 65,600 | 13.3 | 56.3 | 2.8 |
| L2 | 326/- | 25,000 | 23,500 | 2.4 | 49.5 | 2.8 |
| L3 | 99/- | 7100 | 7100 | 1.4 | 40.5 | 2.8 |
| L4 | 96/8 | - | 45,300 | 7.0 | 26.2 | 3.1 |
| Name | tBuA/MA | AA/MA | ||||||
|---|---|---|---|---|---|---|---|---|
| DPtBuA/DPMA | Mn,theo [g/mol] | Mn,SEC [g/mol] | Đ | DPAA/DPMA | Mn,theo [g/mol] | Mn,SEC [g/mol] | Đ | |
| L5 | 96/2 | 12,500 | 13,500 | 1.18 | 96/2 | 7100 | n.d. | n.d. |
| L6 | 184/3 | 23,800 | 38,800 | 1.16 | 184/3 | 13,500 | n.d. | n.d. |
| S1 | 188/4 | 24,440 | 67,100 | 1.14 | 188/4 | 13,900 | n.d. | n.d. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinek, A.; Kupczak, M.; Mielańczyk, A.; Lemanowicz, M.; Yusa, S.-i.; Neugebauer, D.; Gierczycki, A. Temperature and pH-Dependent Response of Poly(Acrylic Acid) and Poly(Acrylic Acid-co-Methyl Acrylate) in Highly Concentrated Potassium Chloride Aqueous Solutions. Polymers 2020, 12, 486. https://doi.org/10.3390/polym12020486
Sinek A, Kupczak M, Mielańczyk A, Lemanowicz M, Yusa S-i, Neugebauer D, Gierczycki A. Temperature and pH-Dependent Response of Poly(Acrylic Acid) and Poly(Acrylic Acid-co-Methyl Acrylate) in Highly Concentrated Potassium Chloride Aqueous Solutions. Polymers. 2020; 12(2):486. https://doi.org/10.3390/polym12020486
Chicago/Turabian StyleSinek, Aleksander, Maria Kupczak, Anna Mielańczyk, Marcin Lemanowicz, Shin-ichi Yusa, Dorota Neugebauer, and Andrzej Gierczycki. 2020. "Temperature and pH-Dependent Response of Poly(Acrylic Acid) and Poly(Acrylic Acid-co-Methyl Acrylate) in Highly Concentrated Potassium Chloride Aqueous Solutions" Polymers 12, no. 2: 486. https://doi.org/10.3390/polym12020486
APA StyleSinek, A., Kupczak, M., Mielańczyk, A., Lemanowicz, M., Yusa, S.-i., Neugebauer, D., & Gierczycki, A. (2020). Temperature and pH-Dependent Response of Poly(Acrylic Acid) and Poly(Acrylic Acid-co-Methyl Acrylate) in Highly Concentrated Potassium Chloride Aqueous Solutions. Polymers, 12(2), 486. https://doi.org/10.3390/polym12020486