Covalent Surface Functionalization of Bovine Serum Albumin to Magnesium Surface to Provide Robust Corrosion Inhibition and Enhance In Vitro Osteo-Inductivity
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
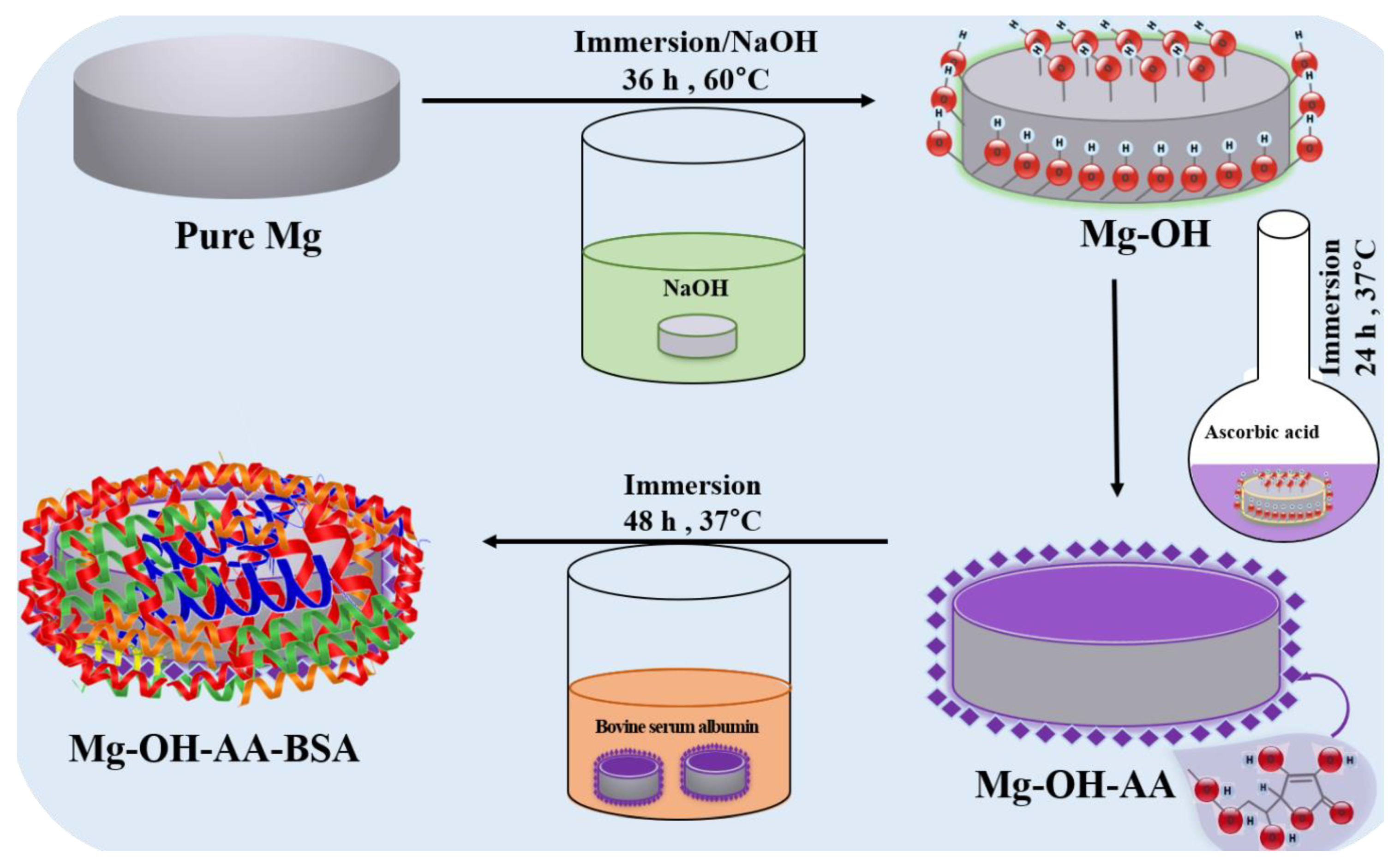
2.2. Sample Preparation
2.3. Materials Characterization
2.3.1. Physical Characterization
2.3.2. Electrochemical Measurement
2.3.3. Hydrogen Evolution Test
2.3.4. In Vitro Cells Interaction
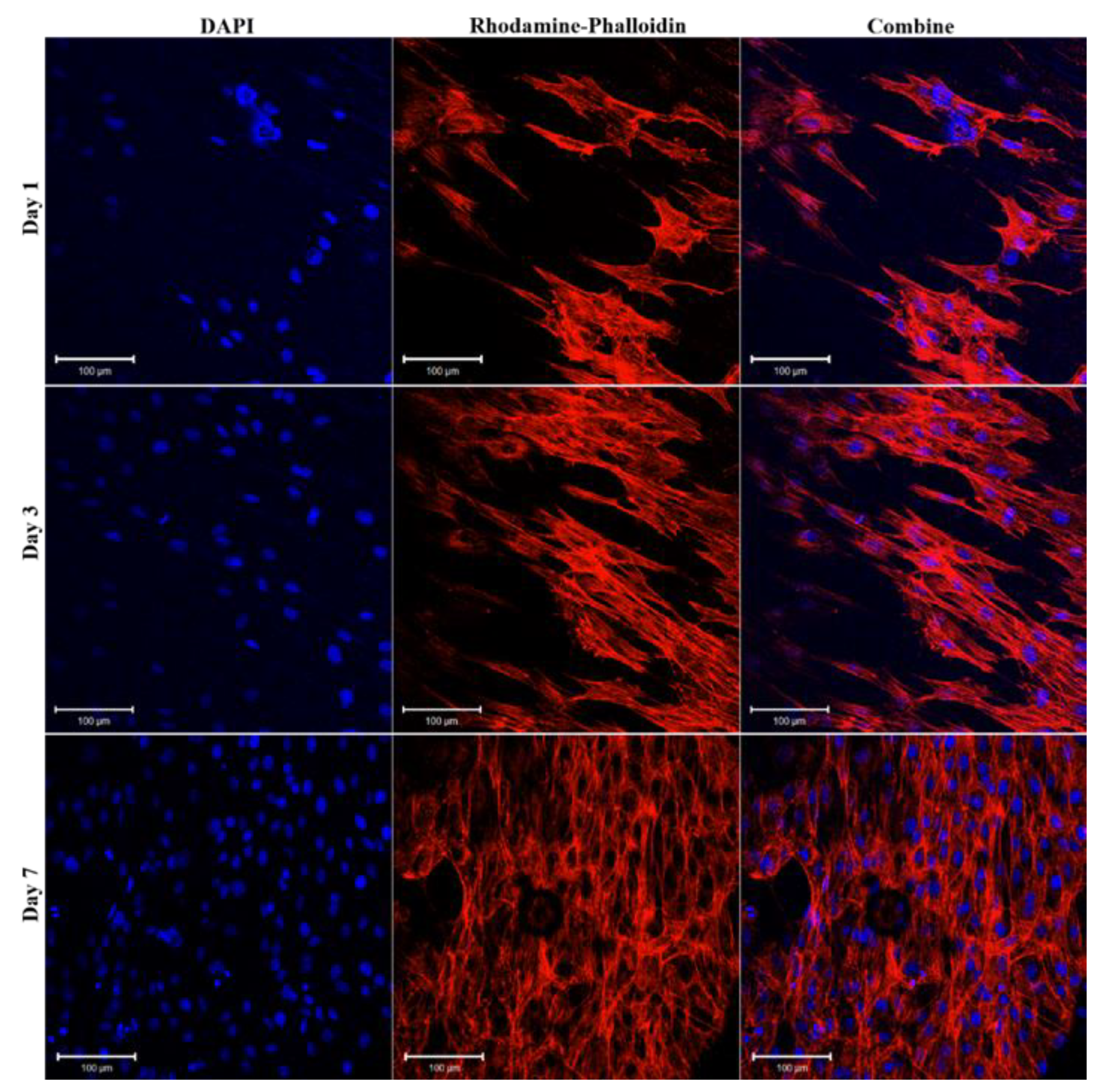
2.3.5. Cell Morphology Observation
2.3.6. ALP Activity
2.3.7. Type I Collagen Analysis
2.3.8. Statistical Analysis
3. Results and Discussion
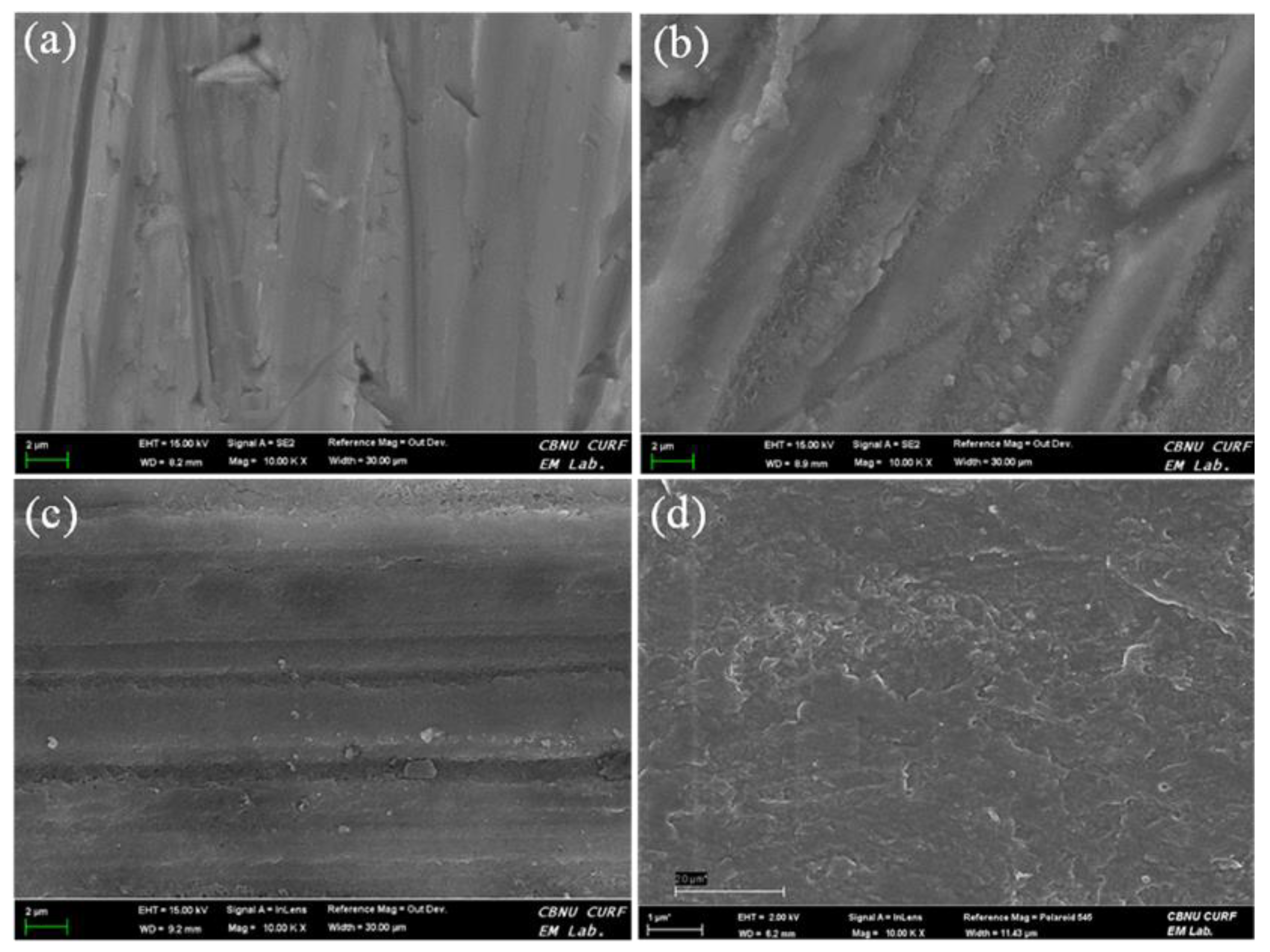
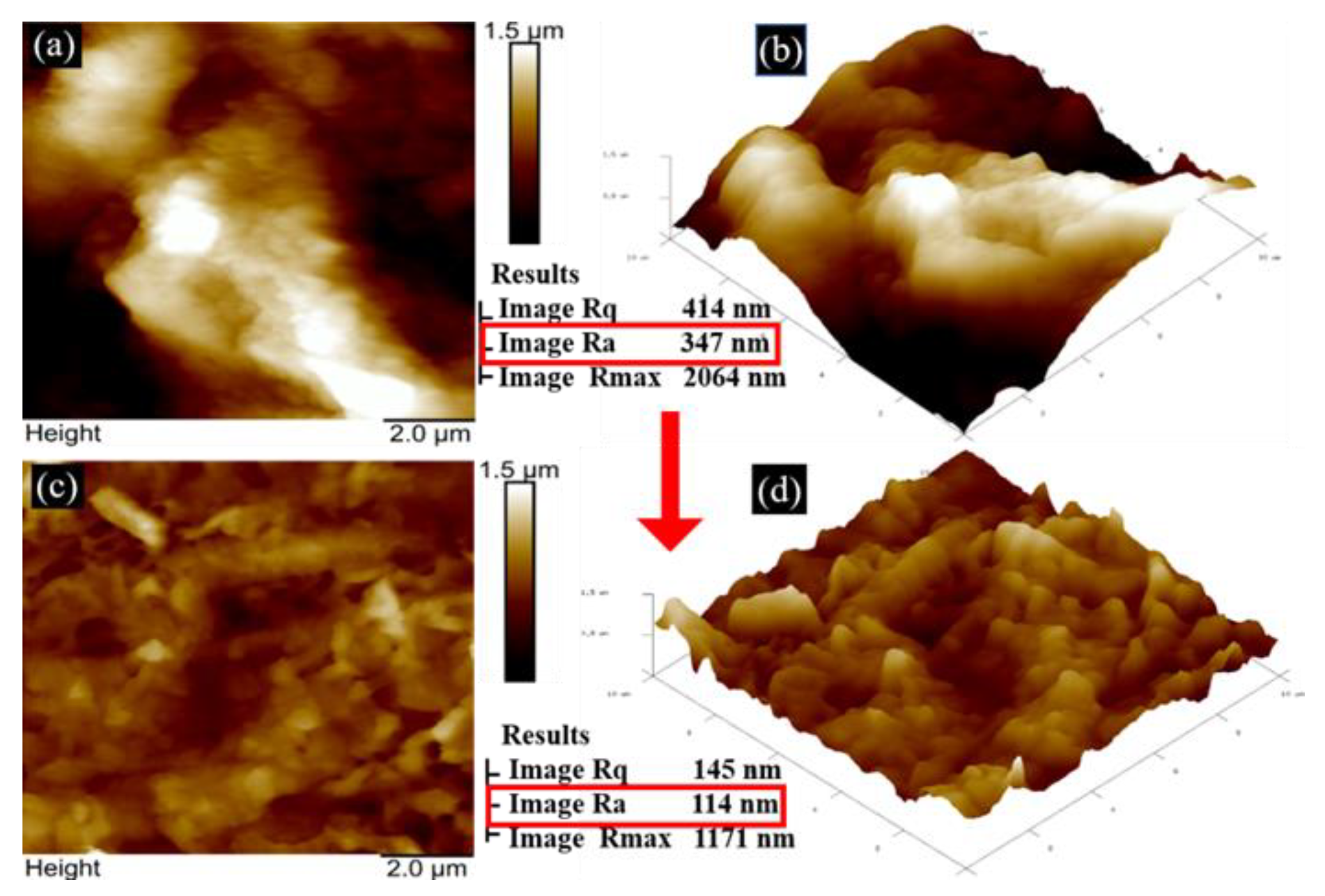
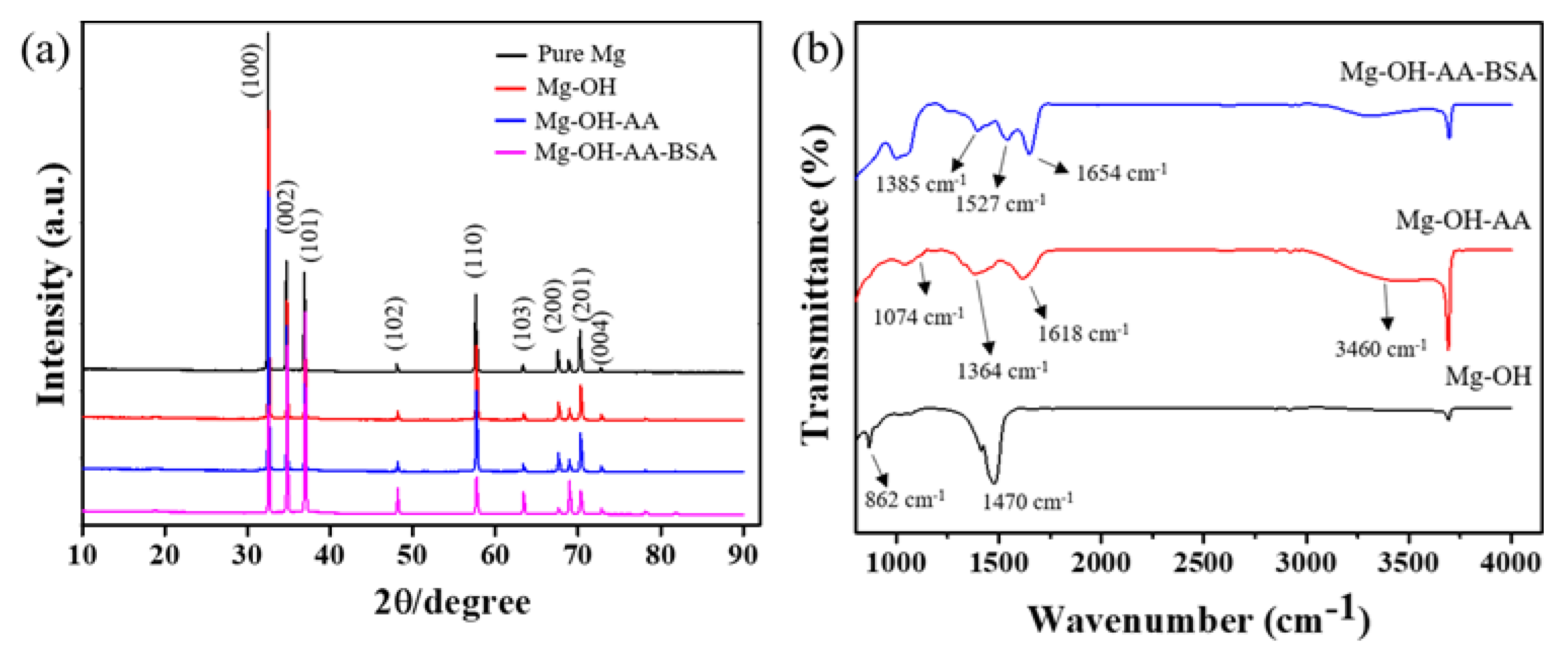
3.1. Surface Analysis
3.2. Electrochemical Study
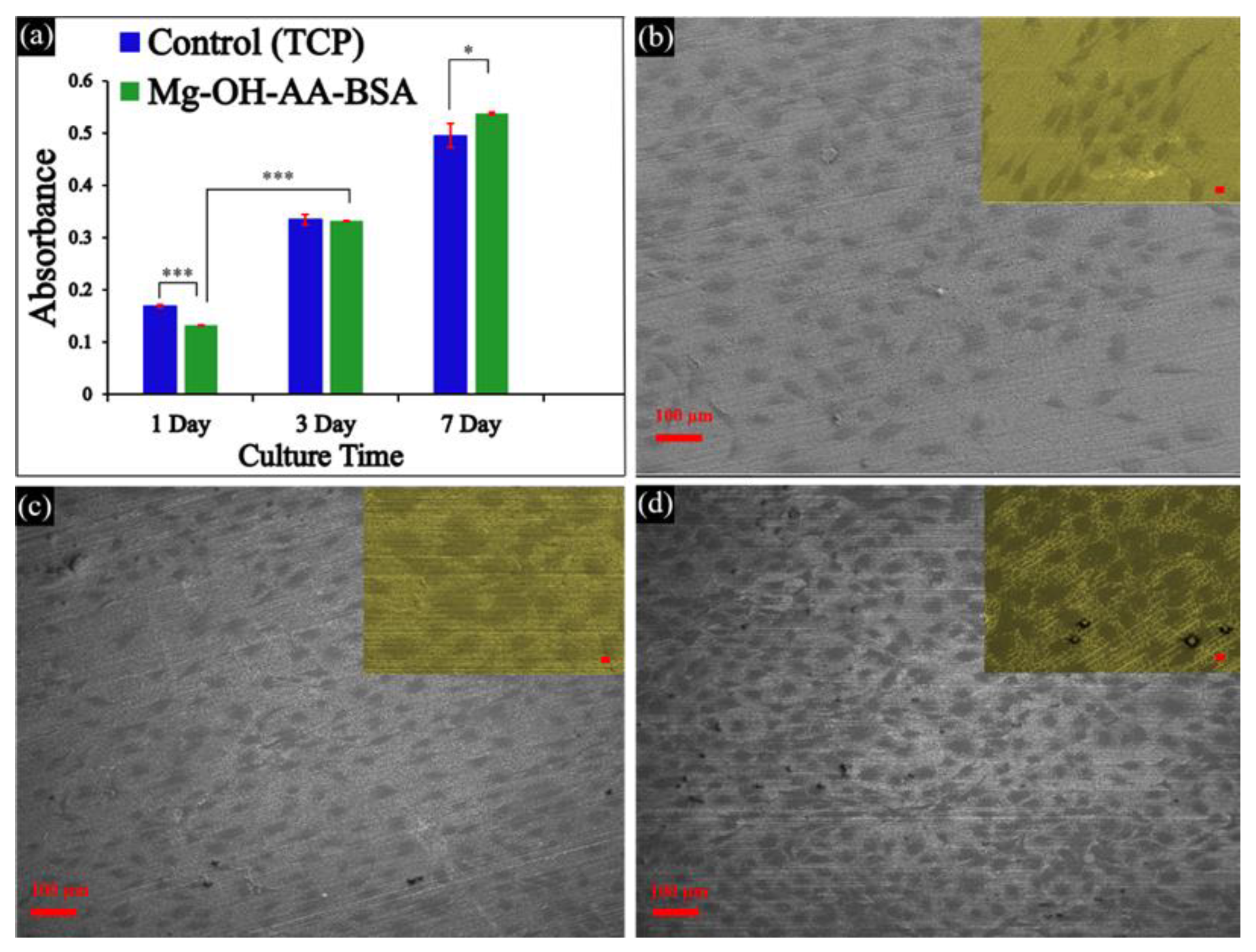
3.3. In vitro Cell Viability
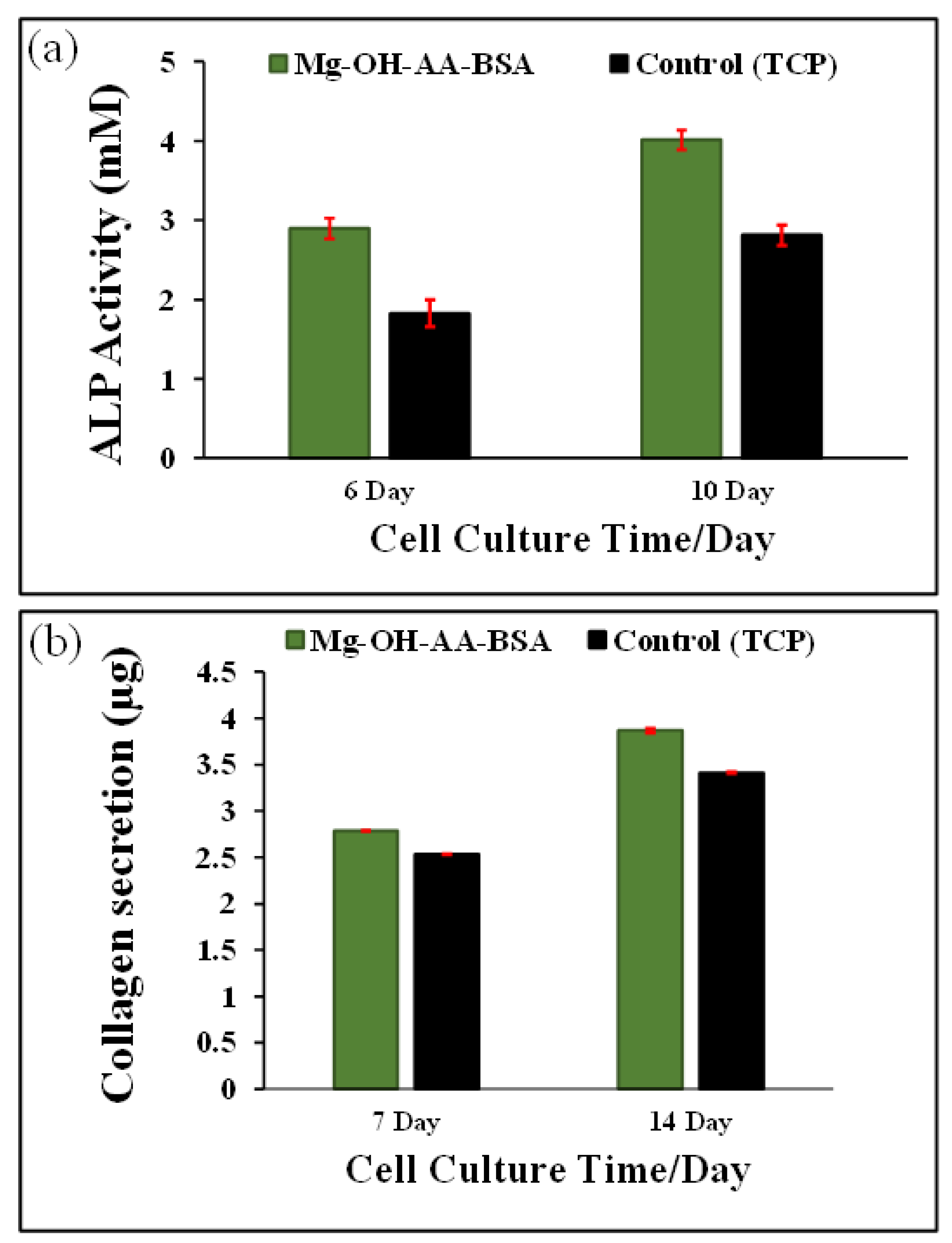
3.4. Analysis of ALP Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vapniarsky, N.; Huwe, L.W.; Arzi, B.; Houghton, M.K.; Wong, M.E.; Wilson, J.W.; Hatcher, D.C.; Hu, J.C.; Athanasiou, K.A. Tissue engineering toward temporomandibular joint disc regeneration. Sci. Transl. Med. 2018, 10, 1802. [Google Scholar] [CrossRef]
- Wong, H.M.; Zhao, Y.; Tam, V.; Wu, S.; Chu, P.K.; Zheng, Y.; To, M.K.; Leung, F.K.; Luk, K.D.; Cheung, K.M.; et al. In vivo stimulation of bone formation by aluminum and oxygen plasma surface-modified magnesium implants. Biomaterials 2013, 34, 9863–9876. [Google Scholar] [CrossRef] [PubMed]
- Aslankoohi, N.; Mondal, D.; Rizkalla, A.S.; Mequanint, K. Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers 2019, 11, 1437. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Lin, C.Y.; Saito, E.; Lin, C.Y.; Schek, R.D.; Taboas, J.M.; Williams, J.M.; Partee, B.; Flanagan, C.L.; Diggs, A.; et al. Engineering craniofacial scaffolds. Orthod. Craniofac. Res. 2005, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, K.; Chen, Y.; Wang, Y.; Shi, F.; Nie, Y.; Liu, T. Design and Biophysical Characterization of Poly (l-Lactic) Acid Microcarriers with and without Modification of Chitosan and Nanohydroxyapatite. Polymers 2018, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef]
- Shah, N.J.; Hyder, M.N.; Moskowitz, J.S.; Quadir, M.A.; Morton, S.W.; Seeherman, H.J.; Padera, R.F.; Spector, M.; Hammond, P.T. Surface-Mediated Bone Tissue Morphogenesis from Tunable Nanolayered Implant Coatings. Sci. Transl. Med. 2013, 5, 191ra83. [Google Scholar] [CrossRef]
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering Complex Tissues. Sci. Transl. Med. 2012, 4, 160rv12. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Mousa, H.M.; Tiwari, A.P.; Ko, S.W.; Park, C.H.; Kim, C.S. Development of polyamide-6,6/chitosan electrospun hybrid nanofibrous scaffolds for tissue engineering application. Carbohydr. Polym. 2016, 148, 107–114. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, B.K.; Lee, J.; Joong, O.K.; Kim, B.; Park, C.H.; Kim, C.S. A conducting neural interface of polyurethane/silk-functionalized multiwall carbon nanotubes with enhanced mechanical strength for neuroregeneration. Mater. Sci. Eng. C 2019, 102, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.S.; Watson, B.M.; Kasper, F.K.; Mikos, A.G. Building Bridges: Leveraging Interdisciplinary Collaborations in the Development of Biomaterials to Meet Clinical Needs. Adv. Mater. 2012, 24, 4995–5013. [Google Scholar] [CrossRef] [PubMed]
- Vidal, G.; Blanchi, T.; Mieszawska, A.J.; Calabrese, R.; Rossi, C.; Vigneron, P.; Duval, J.-L.; Kaplan, D.L.; Egles, C. Enhanced cellular adhesion on titanium by silk functionalized with titanium binding and RGD peptides. Acta Biomater. 2013, 9, 4935–4943. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160. [Google Scholar] [CrossRef]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef]
- Wang, C.; Yi, Z.; Sheng, Y.; Tian, L.; Qin, L.; Ngai, T.; Lin, W. Development of a novel biodegradable and anti-bacterial polyurethane coating for biomedical magnesium rods. Mater. Sci. Eng. C 2019, 99, 344–356. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials 2014, 35, 8553–8565. [Google Scholar] [CrossRef]
- Ma, J.; Thompson, M.; Zhao, N.; Zhu, D. Similarities and differences in coatings for magnesium-based stents and orthopaedic implants. J. Orthop. Transl. 2014, 2, 118–130. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Adachi, S.; Abe, Y.; Oki, S.; Nakata, K.; Kamita, M. Corrosion Protection of Mg-Li Alloy by Plasma Thermal Spraying of Aluminum. Plasma Process. Polym. 2007, 4, S593–S596. [Google Scholar] [CrossRef]
- Julmi, S.; Krüger, A.K.; Waselau, A.C.; Meyer-Lindenberg, A.; Wriggers, P.; Klose, C.; Maier, H.J. Processing and coating of open-pored absorbable magnesium-based bone implants. Mater. Sci. Eng. C 2019, 98, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Nastassja, L.; Vicki, C.; Rebekah, D. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar]
- Li, J.; Li, J.J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- González-García, D.M.; Marcos-Fernández, Á.; Rodríguez-Lorenzo, L.M.; Jiménez-Gallegos, R.; Vargas-Becerril, N.; Téllez-Jurado, L. Synthesis and in Vitro Cytocompatibility of Segmented Poly(Ester-Urethane)s and Poly(Ester-Urea-Urethane)s for Bone Tissue Engineering. Polymers 2018, 10, 991. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Shrestha, S.; Shrestha, B.K.; Park, C.H.; Kim, C.S. A controlled surface geometry of polyaniline doped titania nanotubes biointerface for accelerating MC3T3-E1 cells growth in bone tissue engineering. Chem. Eng. J. 2018, 350, 57–68. [Google Scholar] [CrossRef]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg-Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Shrestha, S.; Baral, E.R.; Lee, J.Y.; Kim, B.S.; Park, C.H.; Pant, H.R. π-Conjugated polyaniline-assisted flexible titania nanotubes with controlled surface morphology as regenerative medicine in nerve cell growth. Chem. Eng. J. 2019, 360, 701–713. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, B.K.; Kim, J.I.; Ko, S.W.; Park, C.H.; Kim, C.S. Electrodeless coating polypyrrole on chitosan grafted polyurethane with functionalized multiwall carbon nanotubes electrospun scaffold for nerve tissue engineering. Carbon 2018, 136, 430–443. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Shrestha, S.; Tiwari, A.P.; Kim, J.; Ko, S.W.; Kim, H.; Park, C.H.; Kim, C.S. Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater. Des. 2017, 133, 69–81. [Google Scholar] [CrossRef]
- Viyannalage, L.; Lee, V.; Dennis, R.V.; Kapoor, D.; Haines, C.D.; Banerjee, S. From Grignard’s reagents to well-defined Mg nanostructures: Distinctive electrochemical and solution reduction routes. Chem. Commun. 2012, 48, 5169–5171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Andrews, L. Infrared Spectra of Magnesium Hydride Molecules, Complexes, and Solid Magnesium Dihydride. J. Phys. Chem. A 2004, 108, 11511–11520. [Google Scholar] [CrossRef]
- Gribov, E.N.; Bertarion, S.; Scarano, D.; Lamberti, C.; Spoto, G.; Zecchina, A. Vibrational and Thermodynamic Properties of H2 Adsorbed on MgO in the 300−20 K Interval. J. Phys. Chem. B 2004, 108, 16174–16186. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.Y. Synthesis of polyaniline nanotubes using the self-assembly behavior of vitamin C: A mechanistic study and application in electrochemical supercapacitors. J. Mater. Chem. A 2014, 2, 2830–2838. [Google Scholar] [CrossRef]
- Krimm, S.; Bandekar, J. Vibrational Spectroscopy and Conformation of Peptides, Polypeptides, and Proteins, in Advances in Protein Chemistry; Anfinsen, C.B., Edsall, J.T., Richards, F.M., Eds.; Academic Press: Pittsburgh, PA, USA, 1986; pp. 181–364. [Google Scholar]
- Gao, R.; Liu, Q.; Wang, J.; Zhang, X.; Yang, W.; Liu, J.; Liu, L. Fabrication of fibrous szaibelyite with hierarchical structure superhydrophobic coating on AZ31 magnesium alloy for corrosion protection. Chem. Eng. J. 2014, 241, 352–359. [Google Scholar] [CrossRef]
- Liu, W.; Kong, Y.; Tu, P.; Lu, J.; Liu, C.; Liu, W.; Han, J.; Liu, J. Physical-chemical stability and in vitro digestibility of hybrid nanoparticles based on the layer-by-layer assembly of lactoferrin and BSA on liposomes. Food Funct. 2017, 8, 1688–1697. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Chen, M.; Zhang, W.; Mao, J.; Zhao, Y.; Maitz, M.F.; Huang, N.; Wan, G. Sandwiched polydopamine (PDA) layer for titanium dioxide (TiO2) coating on magnesium to enhance corrosion protection. Corros. Sci. 2015, 96, 67–73. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Chen, M.; Zhao, S.; Mao, J.; Qu, A.; Li, W.; Zhao, Y.; Huang, N.; Wan, G. Strengthened corrosion control of poly (lactic acid) (PLA) and poly (ε-caprolactone) (PCL) polymer-coated magnesium by imbedded hydrophobic stearic acid (SA) thin layer. Corros. Sci. 2016, 112, 327–337. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Omanovic, S.; Roscoe, S.G. Electrochemical Studies of the Adsorption Behavior of Bovine Serum Albumin on Stainless Steel. Langmuir 1999, 15, 8315–8321. [Google Scholar] [CrossRef]
- Qin, H.; Zhao, Y.; An, Z.; Cheng, M.; Wang, Q.; Cheng, T.; Wang, Q.; Wang, J.; Jiang, Y.; Zhang, X.; et al. Enhanced antibacterial properties, biocompatibility, and corrosion resistance of degradable Mg-Nd-Zn-Zr alloy. Biomaterials 2015, 53, 211–220. [Google Scholar] [CrossRef] [PubMed]
- MacDonell, R.; Hamrick, M.W.; Isales, C.M. Protein/amino-acid modulation of bone cell function. BoneKEy Rep. 2016, 5, 827. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, L.; Zhou, B.; Wu, H.; Zheng, L.; Zhao, J. Effect of apatite formation of biphasic calcium phosphate ceramic (BCP) on osteoblastogenesis using simulated body fluid (SBF) with or without bovine serum albumin (BSA). Mater. Sci. Eng. C 2017, 70, 955–961. [Google Scholar] [CrossRef]
- Yohay, D.A.; Zhang, J.; Thrailkill, K.M.; Arthur, J.M.; Quarles, L.D. Role of serum in the developmental expression of alkaline phosphatase in MC3T3-E1 osteoblasts. J. Cell. Physiol. 1994, 158, 467–475. [Google Scholar] [CrossRef]
- Raic, A.; Friedrich, F.; Kratzer, D.; Bieback, K.; Lahann, J.; Lee-Thedieck, C. Potential of electrospun cationic BSA fibers to guide osteogenic MSC differentiation via surface charge and fibrous topography. Sci. Rep. 2019, 9, 20003. [Google Scholar] [CrossRef]

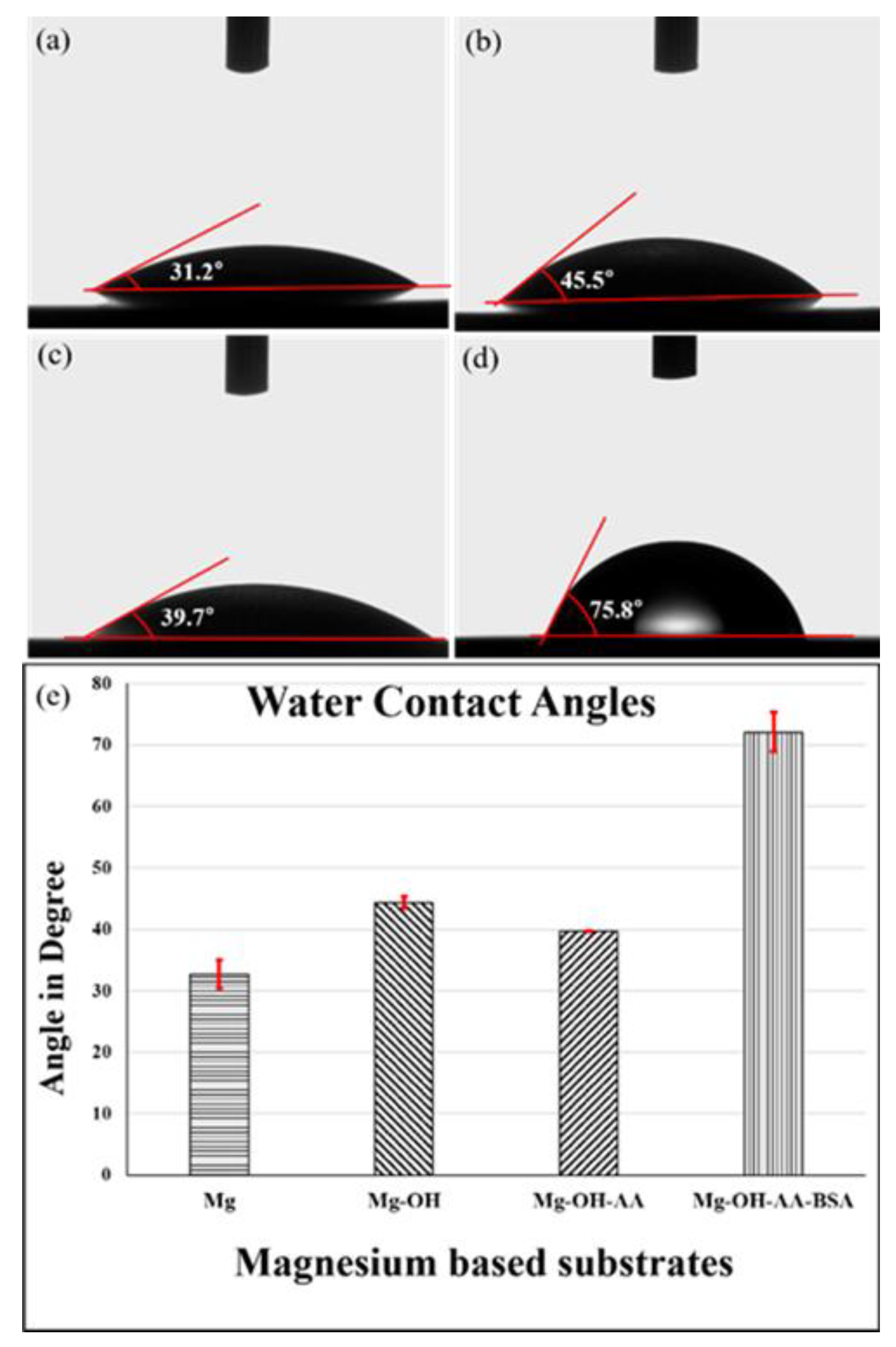
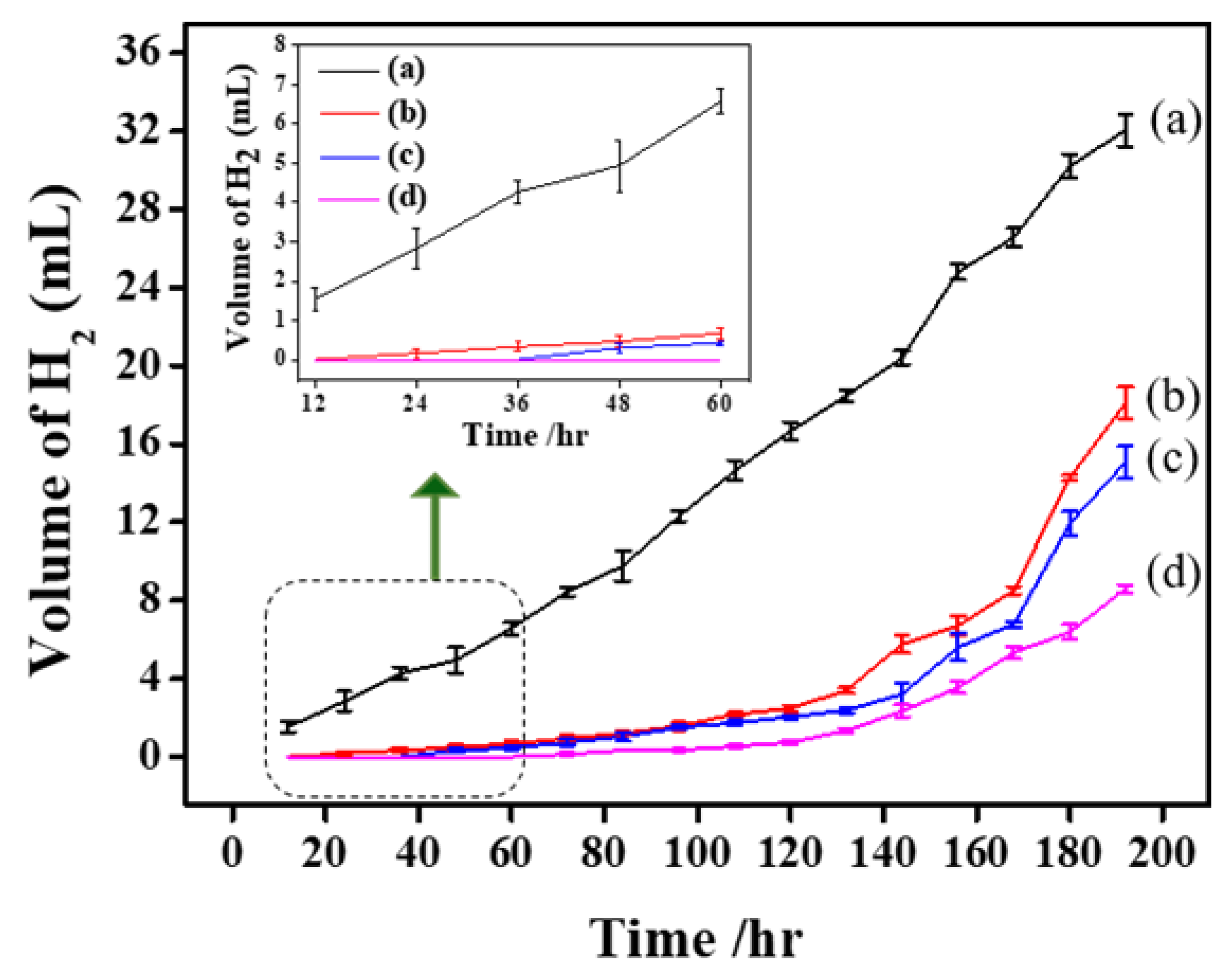
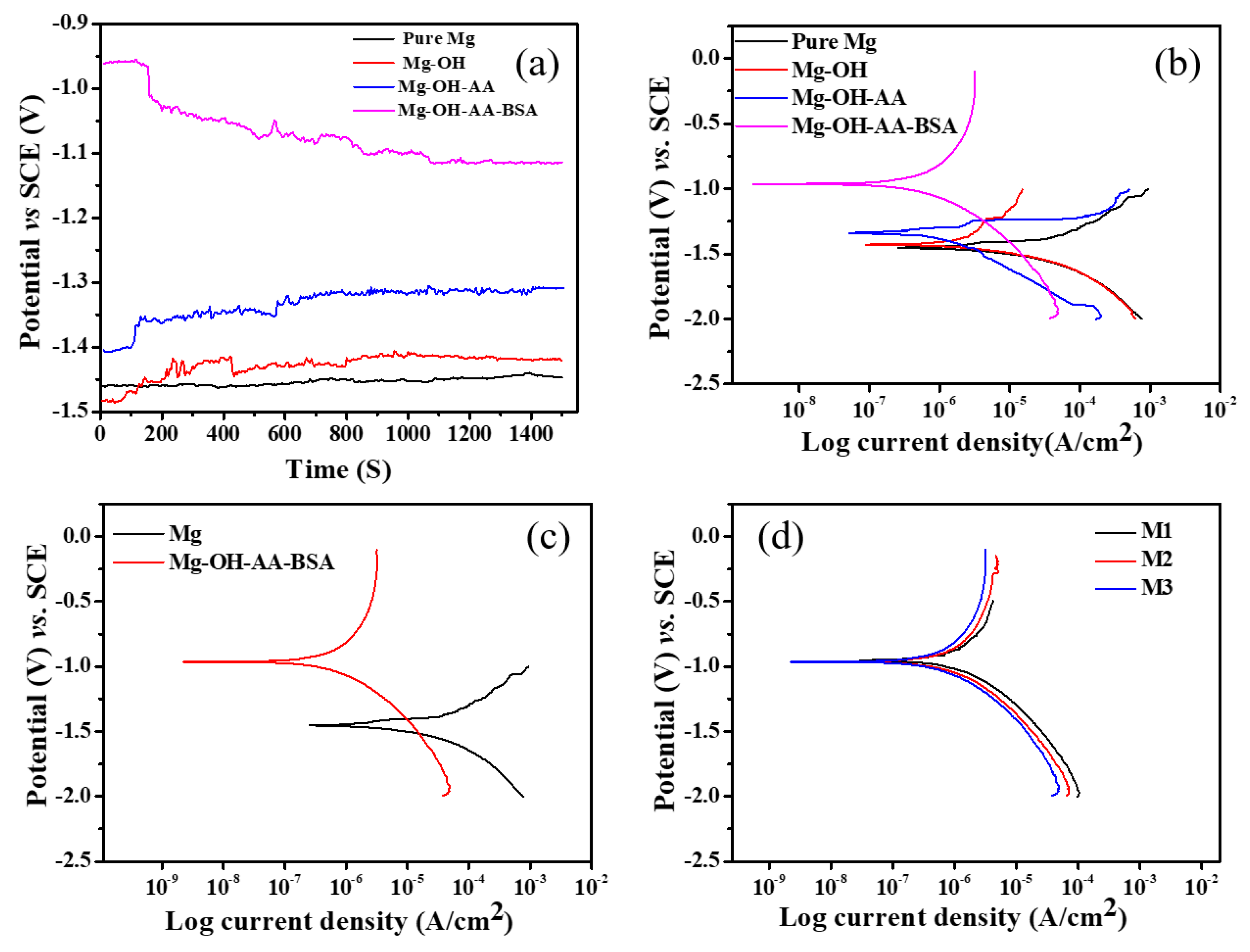
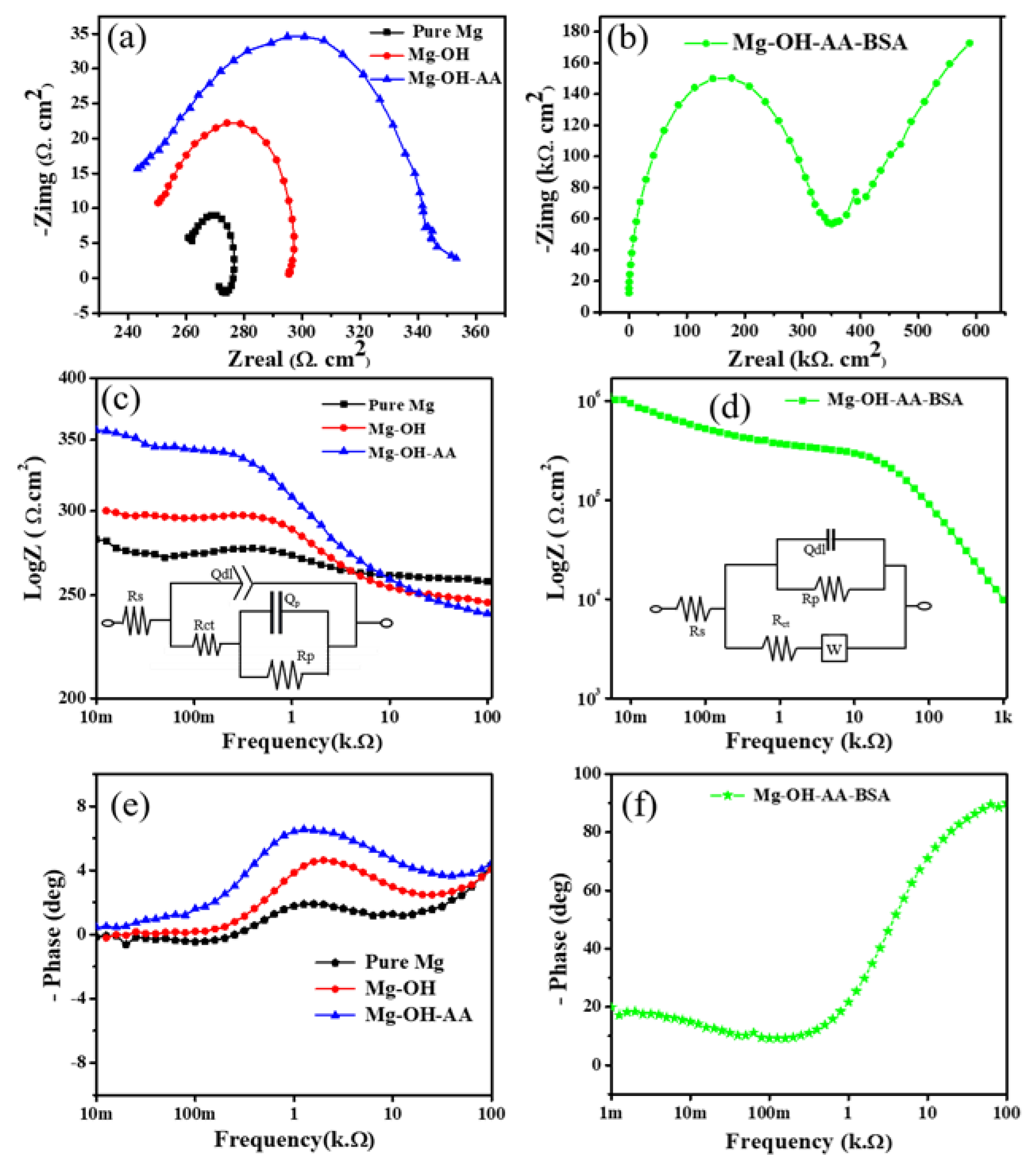
| Samples | Polarization Parameters | Nyquist Plots | ||||||
|---|---|---|---|---|---|---|---|---|
| Ecorr | Icorr | −βc | Rs | Rct | Rp | Qdl | Qp | |
| Vs.(SCE) | (μA.cm2) | (mV/dec) | (kΩ. cm2) | (K Ω. cm2) | (K Ω. cm2) | (µF.cm−2) | (µF.cm−2) | |
| Pure Mg | −1.46 | 10.42 | 0.108 | 0.26 | 0.012 | 0.014 | 62.43 | 8 |
| Mg-OH | −1.43 | 1.04 | 0.148 | 0.24 | 0.051 | 0.049 | 3.41 | 5.26 |
| Mg-OH-AA | −1.34 | 0.61 | 0.135 | 0.23 | 0.11 | 0.068 | 1.62 | 2.1 |
| Mg-OH-AA-BSA | −0.96 | 0.20 | 0.274 | 11.66 | 344.52 | 283.51 | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Shrestha, S.; Shrestha, B.K.; Park, C.H.; Kim, C.S. Covalent Surface Functionalization of Bovine Serum Albumin to Magnesium Surface to Provide Robust Corrosion Inhibition and Enhance In Vitro Osteo-Inductivity. Polymers 2020, 12, 439. https://doi.org/10.3390/polym12020439
Lee SY, Shrestha S, Shrestha BK, Park CH, Kim CS. Covalent Surface Functionalization of Bovine Serum Albumin to Magnesium Surface to Provide Robust Corrosion Inhibition and Enhance In Vitro Osteo-Inductivity. Polymers. 2020; 12(2):439. https://doi.org/10.3390/polym12020439
Chicago/Turabian StyleLee, Seo Yeon, Sita Shrestha, Bishnu Kumar Shrestha, Chan Hee Park, and Cheol Sang Kim. 2020. "Covalent Surface Functionalization of Bovine Serum Albumin to Magnesium Surface to Provide Robust Corrosion Inhibition and Enhance In Vitro Osteo-Inductivity" Polymers 12, no. 2: 439. https://doi.org/10.3390/polym12020439
APA StyleLee, S. Y., Shrestha, S., Shrestha, B. K., Park, C. H., & Kim, C. S. (2020). Covalent Surface Functionalization of Bovine Serum Albumin to Magnesium Surface to Provide Robust Corrosion Inhibition and Enhance In Vitro Osteo-Inductivity. Polymers, 12(2), 439. https://doi.org/10.3390/polym12020439




