Evaluation of Eleview® Bioadhesive Properties and Cushion-Forming Ability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Eleview®, Cosmo Technologies Ltd, Dublin, Ireland
- Exrtacellular Matrix: Oasis Wound Matrix, Smith & Nephew, Inc., Fort Worth, TX, USA
- Eleview® without poloxamer 188. This formulation was prepared at Pharmaceutical Development department of Cosmo SpA, Lainate (MI), Italy
- Poloxamer 10% solution. This solution was prepared at Pharmaceutical Development department of Cosmo SpA, Lainate (MI) Italy. Poloxamer 188 was provided by BASF, Geismar, LA, USA
- Normal saline (0.9% NaCl), Eurospital, Trieste, Italy
- Frozen cleaned porcine stomach, Area Qualità S.r.l., Borghetto di Borbera (AL), Italy
- MucoUp®, Seikagaku Corporation, Tokyo, Japan
2.2. Bioadhesive Test
- Eleview®
- Eleview without poloxamer
- Poloxamer solution
- Eleview®
- Normal saline
- MucoUp®
2.3. Cushion-Forming Ability Test
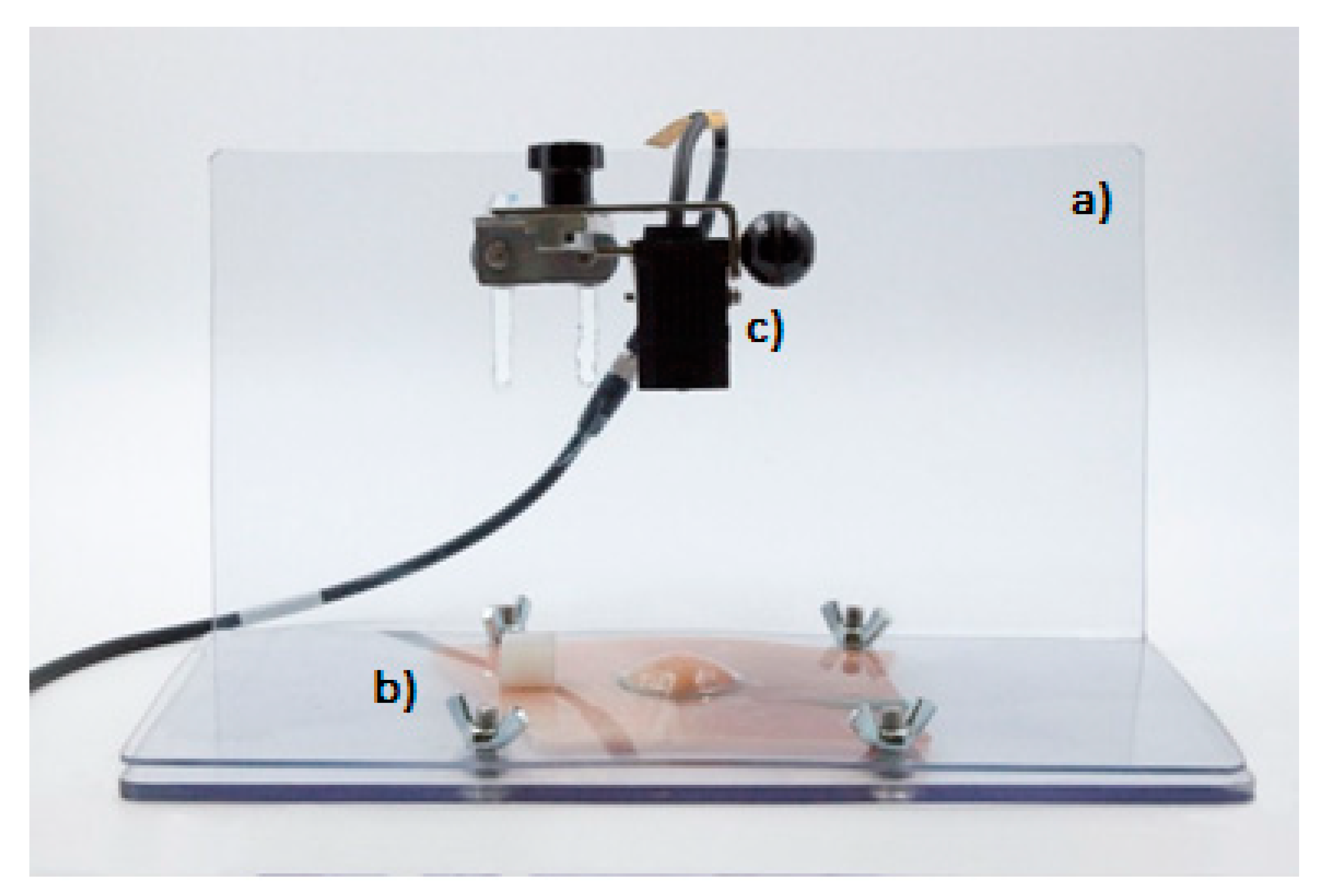
- Plexiglass support base (dimensions: b 27 × 15 cm2; height 15 cm) (Figure 3a)
- Mobile perforated plexiglass part (dimensions: 27 × 15 cm2; hole Ø 3 cm) (Figure 3b)
- Multifunctional analog laser sensor Mod IL-065 (Figure 3c). The instrument was used to detect cushion height variations (mm) through a multifunctional analog laser sensor (Mod IL-065) equipped with a graphic interface (software: Datasink100), which allowed to capture the cushion height every minute, then to record and to elaborate the data collected.
- Thaw the frozen porcine stomach and keep it at 37 °C in a thermal blanket;
- Remove the packaging, open the stomach using a bistoury, and clean the mucosa using disposable paper towels;
- Cut a 10 × 10 cm2 portion of the stomach using a scalpel and place it on the plexiglass base; and
- Fix the stomach portion with the mobile perforate plexiglass part (hole diameter: 3 cm), tightening the appropriate screws.
2.4. Statistical Analysis
3. Results and Discussion
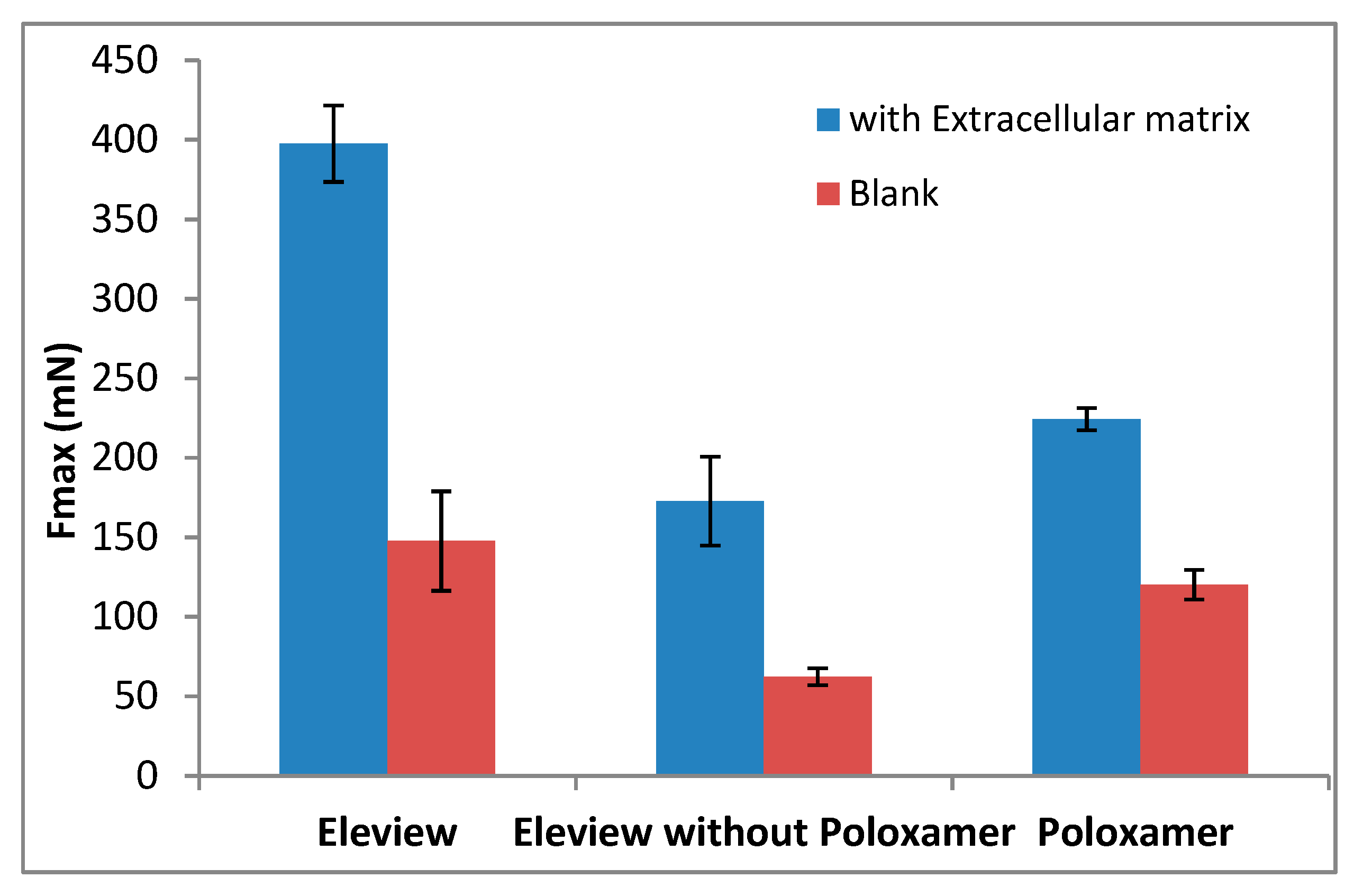
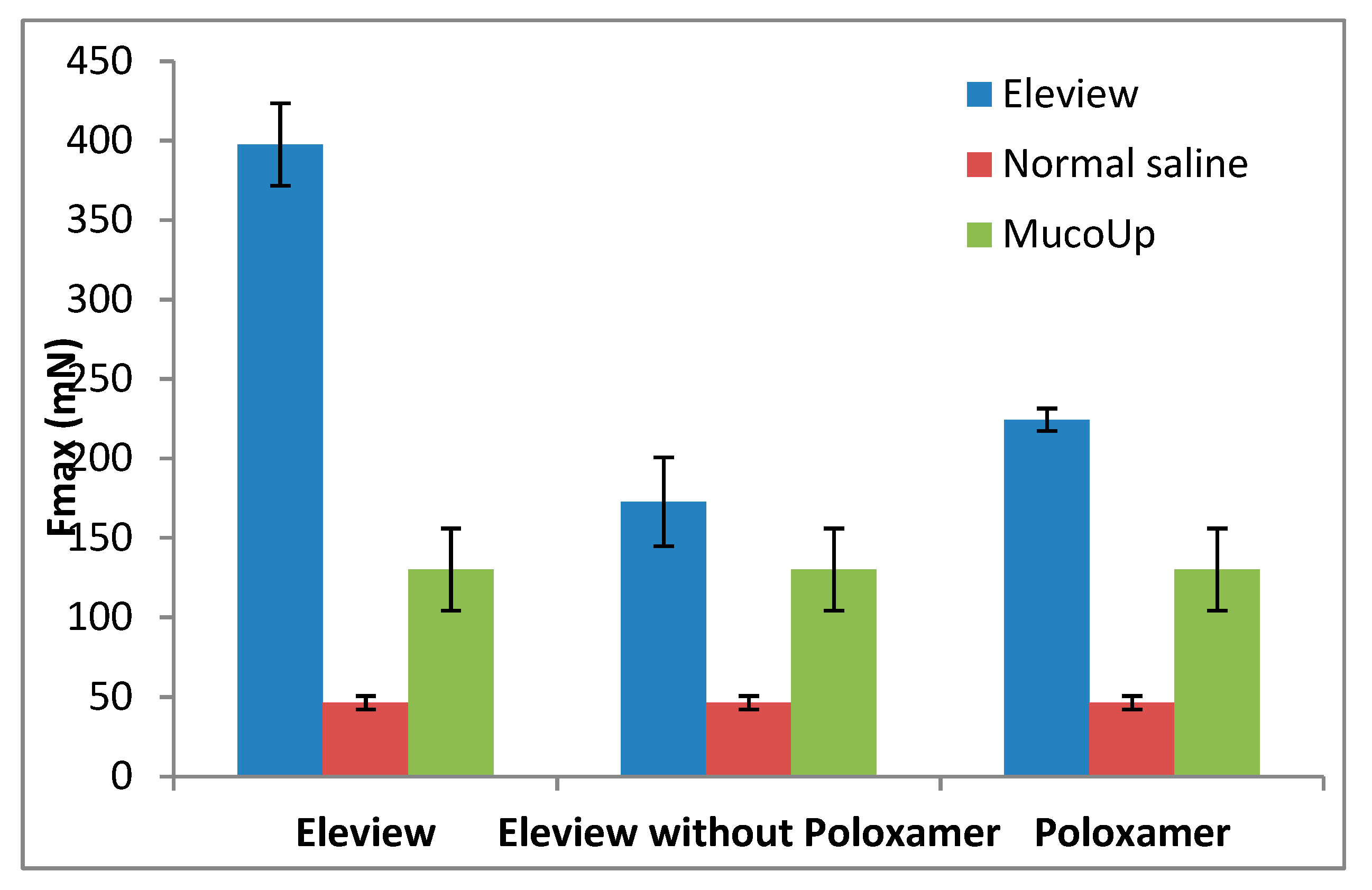
3.1. Bioadhesive Test
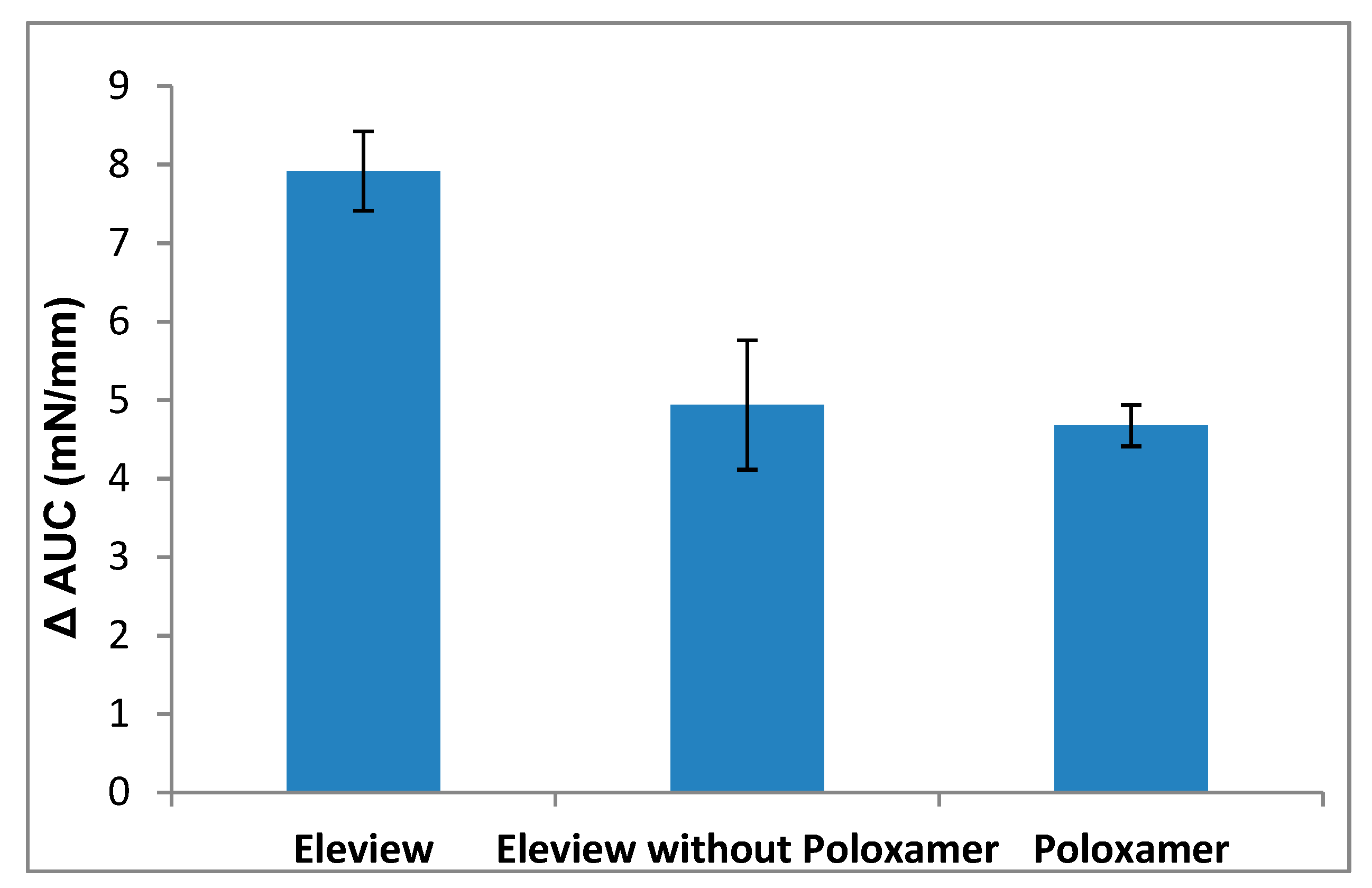
3.1.1. Adhesion Evaluation of Eleview®, Eleview® without Poloxamer, and Poloxamer
3.1.2. Bioadhesion Evaluation of Eleview® vs. Normal Saline and MucoUp®
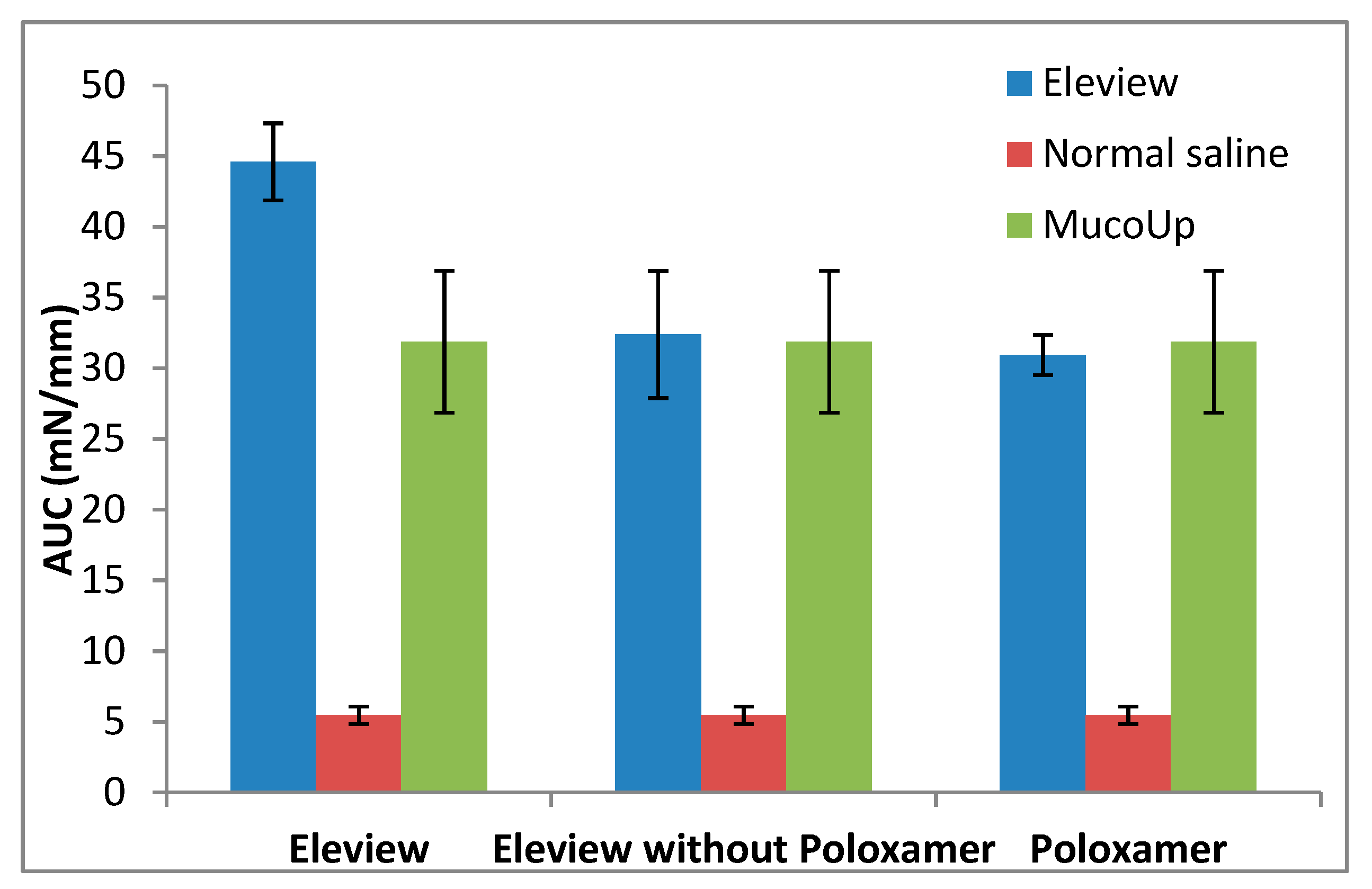
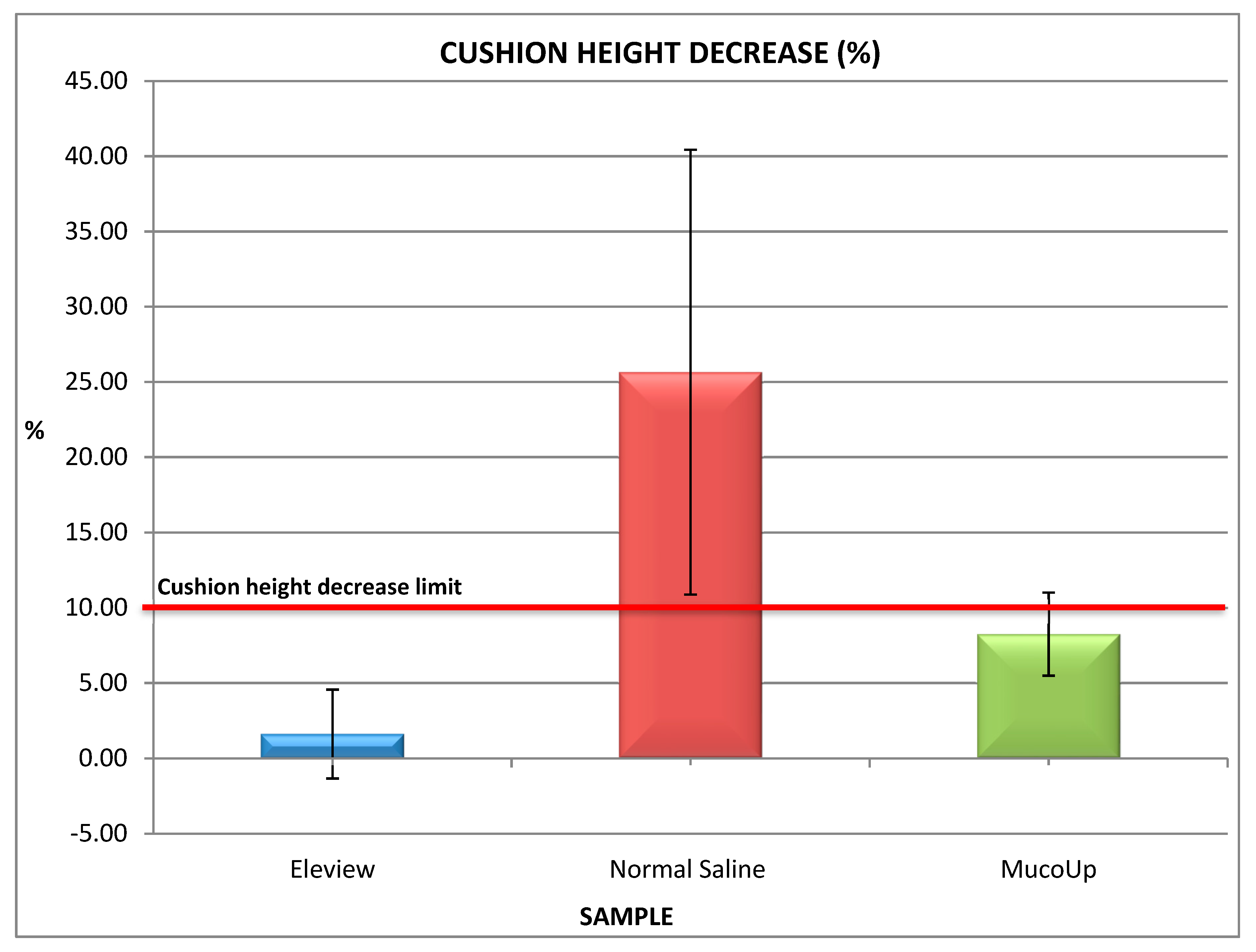
3.2. Cushion-Forming Ability
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Girotra, M.; Triadafilopoulos, G.; Friedland, S. Utility and performance characteristics of a novel submucosal injection agent (EleviewTM) for endoscopic mucosal resection and endoscopic submucosal dissection. Transl. Gastroenterol. Hepatol. 2018, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- ASGE Technology Committee. Endoscopic mucosal resection. Gastrointest. Endosc. 2015, 82, 215–226. [Google Scholar] [CrossRef] [PubMed]
- ASGE Technology Committee. Endoscopic submucosal dissection. Gastrointest. Endosc. 2015, 81, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, F. Removing a large polyp with the new lifting agent Eleview. Gastroenterology & Endoscopy News, Retroflexions, 14 July 2017; 1–7. [Google Scholar]
- Spadaccinia, M.; Hassan, C.; Maselli, R.; D’Amico, F.; Lamonaca, L.; Craviotto, V.; Repici, A. Efficacy and safety of SIC-8000 (Eleview®) for submucosal injection for endoscopic mucosal resection and endoscopic submucosal dissection in an in vivo porcine model. Dig. Liver Dis. 2018, 50, 260–266. [Google Scholar] [CrossRef]
- Cosmo Technologies Ltd. Eleview [Instructions for Use]; Cosmo Technologies Ltd.: Dublin, Ireland, 2018. [Google Scholar]
- Repici, A.; Wallace, M.; Sharma, P.; Bhandari, P.; Lollo, G.; Maselli, R.; Cesare Hassan, C.; Rex, D.K. A novel submucosal injection solution for endoscopic resection of large colorectal lesions: A randomized, double-blind trial. Gastrointest. Endosc. 2018, 88, 527–535. [Google Scholar] [CrossRef]
- Johnson, D.A.; Wallace, M.B.; Kaul, V.; Kaltenbach, T. Incomplete Resection Rates in Polyps Smaller Than 2 Centimeters. In Clinical Roundtable Monograph, Gastroenterology & Hepatology August 2018. In Proceedings of the from a Live Clinical Roundtable Held during Digestive Disease Week, Washington, DC, USA, 2–5 June 2018. [Google Scholar]
- Sapci, I.; Gorgun, E. Endoscopic mucosal resection and endoscopic submucosal dissection: Initial experience with a new injectable solution. In Proceedings of the SAGES 2017 Annual Meeting, Houston, TX, USA, 22–25 March 2017. [Google Scholar]
- Kantsevoy, S.; Aihara, H. Endoscopic Submucosal Dissection: Specialized GI Resection Technique. Gastroenterology & Endoscopy News, 13 June 2018. [Google Scholar]
- Injectable solution bests saline in trial to lift tough polyp. Gastroenterology & Endoscopy News, 14 July 2017; 1–6.
- Douglas, K.; Rex, M.D. Advances in Endoscopic Mucosal Resection Using Eleview, Gastroenterology and Endoscopy News, September 2017. Available online: https://eleviewus.com/wp-content/uploads/2018/04/4510_1-ELE17172-Rex-Tech-Today-BB1712_4MSC.pdf (accessed on 3 February 2020).
- Rex, D.K.; Wallace, M.; Hassan, C. A randomized, double-blind trial of a new injectable solution (SIC 8000) for endoscopic resection of large sessile polyps of the colon: An interim report. Gastrointest. Endosc. 2017, 85, AB101. [Google Scholar] [CrossRef]
- Patel, H.R.; Patel, R.P.; Patel, M.M. Poloxamers: A pharmaceutical excipients with therapeutic behaviors. Int. J. PharmTech Res. 2009, 1, 299–303. [Google Scholar]
- Parra-Blanco, A.; Gonzalez, N.; Gonzalez, R.; Ortiz-Fernandez-Sordo, J.; Ordieres, C. Animal models for endoscopic training: Do we really need them? Endoscopy 2013, 45, 478–484. [Google Scholar] [CrossRef]
- ASGE Technology Committee. Endoscopic simulators. Gastrointest. Endosc. 2011, 73, 861–867. [Google Scholar] [CrossRef]
- Parra-Blanco, A.; Arnau, M.R.; Nicolas-Perez, D.; Gimeno-Garcia, A.Z.; Gonzalez, N.; Diaz-Acosta, J.A.; Jimenez, A.; Quintero, E. Endoscopic submucosal dissection training with pig models in a Western country. World J. Gastroenterol. 2010, 16, 2895–2900. [Google Scholar] [CrossRef]
- Nelson, D.B.; Bosco, J.J.; Curtis, W.D.; Faigel, D.O.; Kelsey, P.B.; Leung, J.W.; Mills, M.R.; Smith, P.; Tarnasky, P.R.; VanDam, J. Endoscopy simulators. Gastrointest. Endosc. 1999, 50, 935–937. [Google Scholar]
- Gonzales, N.; Parra-Blanco, A.; Villa-Gomez, M.; Gamba, A.; Taullard, A.; Silveira, A.; Sanguinetti, A.; Olano, C.; Cohen, H. Gastric endoscopic submucosal dissection: From animal model to patient. World J. Gastroenterol. 2013, 19, 8326–8334. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Sequeiros, E.; de Miguel, D.B.; Foruny Olcina, J.R.; Gonzalez Martin, J.A.; Garcia, M.; Juzgado Lucas, D.; Garrido, E.; Gonzalez, C.; Parra Blanco, A.; Arnau, M.R.; et al. Training model for teaching endoscopic submucosal dissection of gastric tumors. Rev. Española Enferm. Dig. 2009, 101, 546–552. [Google Scholar] [CrossRef]
- Uraoka, T.; Saito, Y.; Yamamoto, K.; Fujii, T. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des. Dev. Ther. 2008, 2, 131–138. [Google Scholar] [CrossRef]
- Tran, R.T.; Palmer, M.; Tang, S.-J.; Abell, T.L.; Yang, J. Injectable Drug Eluting Elastomeric Polymer: A Novel Submucosal Injection Material. Gastrointest. Endosc. 2012, 75, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Fernàndez-Esparrach, G.; Shaikh, S.N.; Cohen, A.; Ryan, M.B.; Thompson, C.C. Efficacy of a reverse-phase polymer as a submucosal injection solution for EMR: A comparative study. Gastrointest. Endosc. 2009, 69, 1135–1138. [Google Scholar] [CrossRef]
- Eun, S.H.; Cho, J.Y.; Jung, I.S.; Ko, B.M.; Hong, S.J.; Ryu, C.B.; Kim, J.O.; Jin, S.Y.; Lee, J.S.; Lee, M.S.; et al. Effectiveness of Sodium Alginate as a Submucosal Injection Material for Endoscopic Mucosal Resection in Animal. Gut Liver 2007, 1, 27–32. [Google Scholar] [CrossRef]
- Akagi, T.; Yasuda, K.; Tajima, M.; Suzuki, K.; Inomata, M.; Shiraishi, N.; Sato, Y.; Kitano, S. Sodium alginate as an ideal submucosal injection material for endoscopic submucosal resection: Preliminary experimental and clinical study. Gastrointest. Endosc. 2011, 74, 1026–1032. [Google Scholar] [CrossRef]
- Chandrasekhara, V.; Sigmon, J.C.; Surti, V.C.; Kochman, M.L. A novel gel provides durable submucosal cushion for endoscopic mucosal resection and endoscopic submucosal dissection. Surg. Endosc. 2013, 27, 3039–3042. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yube, T.; Isoda, N.; Sato, Y.; Sekine, Y.; Higashizawa, T.; Ido, K.; Kimura, K.; Kanai, N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest. Endosc. 1999, 50, 251–256. [Google Scholar] [CrossRef]
- Al-Taie, O.H.; Bauer, Y.; Dietrich, C.G.; Fischbach, W. Efficacy of submucosal injection of different solutions inclusive blood components on mucosa elevation for endoscopic resection. Clin. Exp. Gastroenterol. 2012, 5, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Sandri, G.; D’Autilia, F.; Contri, R.V.; Bonferoni, M.C.; Caramella, C.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S. Chitosan gel containing polymeric nanocapsules: A new formulation for vaginal drug delivery. Int. J. Nanomed. 2014, 9, 3151–3161. [Google Scholar]
- Vigani, B.; Faccendini, A.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Gentile, M.; Ferrari, F. Development of a Mucoadhesive and In Situ Gelling Formulation Based on _-Carrageenan for Application on Oral Mucosa and Esophagus Walls. I. A Functional In Vitro Characterization. Mar. Drugs 2019, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Amal, S.M.; El-Enin, A.; El-Feky, G.S. Formulation and Evaluation of In-situ Gelling Tenoxicam Liquid Suppositorie. J. Life Med. 2013, 1, 23–32. [Google Scholar]
- Mythri, G.; Kavitha, K.; Kumar, M.R.; Singh, S.J. Novel Mucoadhesive Polymers–A Review. J. Appl. Pharm. Sci. 2011, 1, 37–42. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannino, V.; Salandin, L.; Macelloni, C.; Longo, L.M. Evaluation of Eleview® Bioadhesive Properties and Cushion-Forming Ability. Polymers 2020, 12, 346. https://doi.org/10.3390/polym12020346
Giannino V, Salandin L, Macelloni C, Longo LM. Evaluation of Eleview® Bioadhesive Properties and Cushion-Forming Ability. Polymers. 2020; 12(2):346. https://doi.org/10.3390/polym12020346
Chicago/Turabian StyleGiannino, Valentina, Lucia Salandin, Cristina Macelloni, and Luigi Maria Longo. 2020. "Evaluation of Eleview® Bioadhesive Properties and Cushion-Forming Ability" Polymers 12, no. 2: 346. https://doi.org/10.3390/polym12020346
APA StyleGiannino, V., Salandin, L., Macelloni, C., & Longo, L. M. (2020). Evaluation of Eleview® Bioadhesive Properties and Cushion-Forming Ability. Polymers, 12(2), 346. https://doi.org/10.3390/polym12020346




