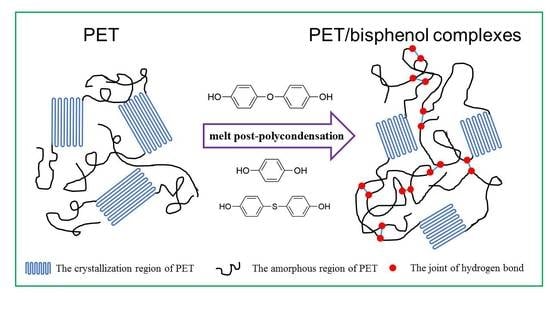
Crystallization and Thermal Behaviors of Poly(ethylene terephthalate)/Bisphenols Complexes through Melt Post-Polycondensation
Abstract
:1. Introduction
2. Materials and Methods
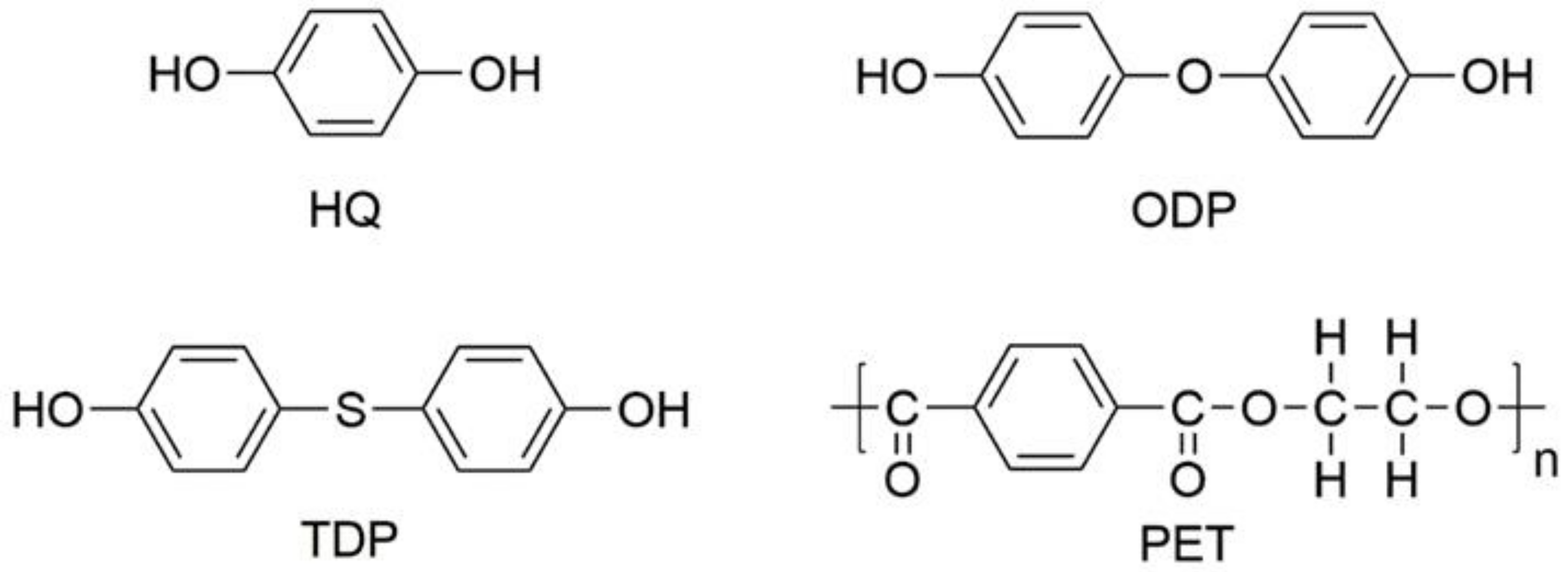
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
3.1. Melt Post-Polycondensation
3.2. Nonisothermal Crystallization and Melting Behaviors
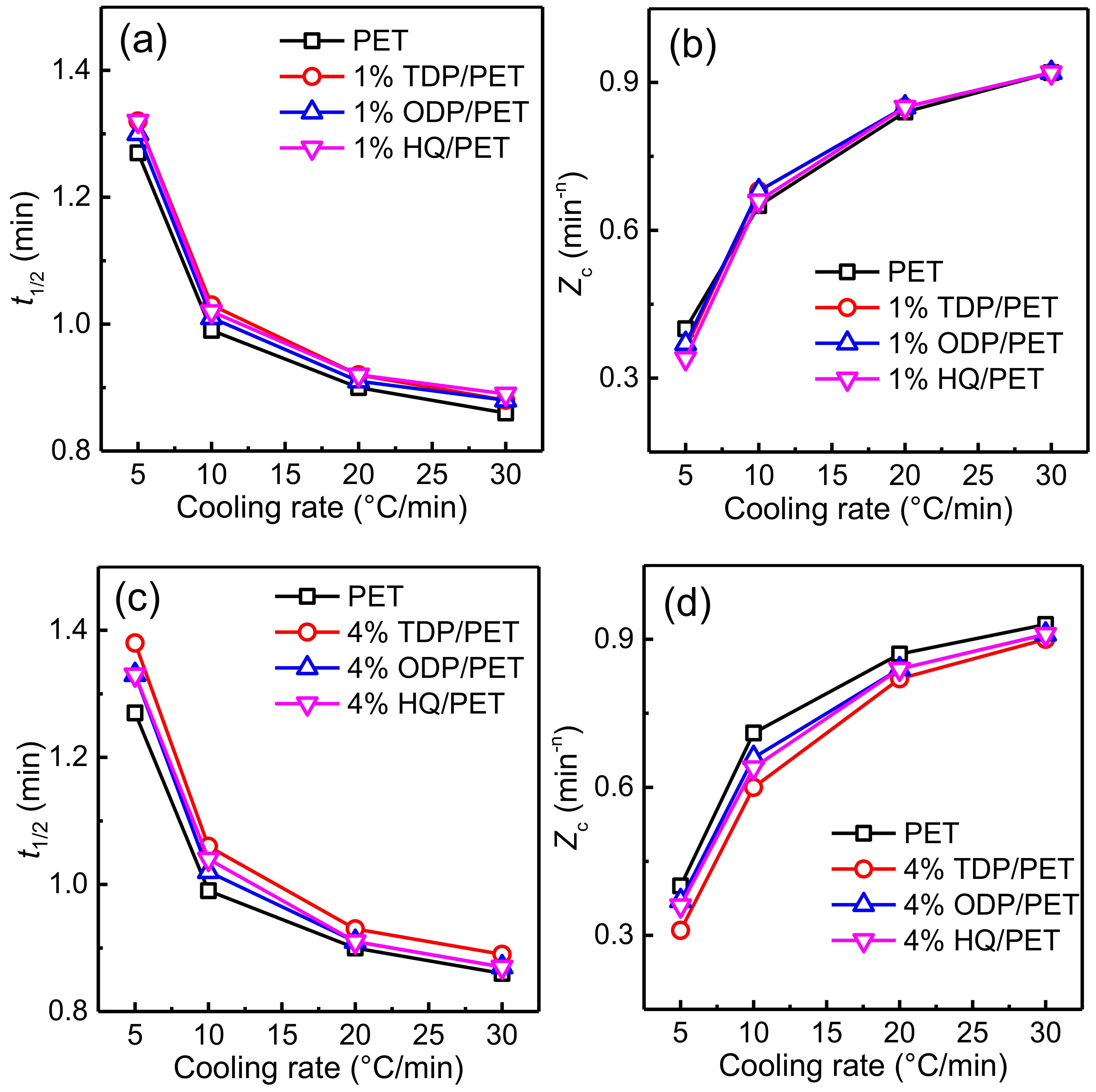
3.3. Effects of Cooling Rates
3.4. Crystalline Structure and Crystallinity
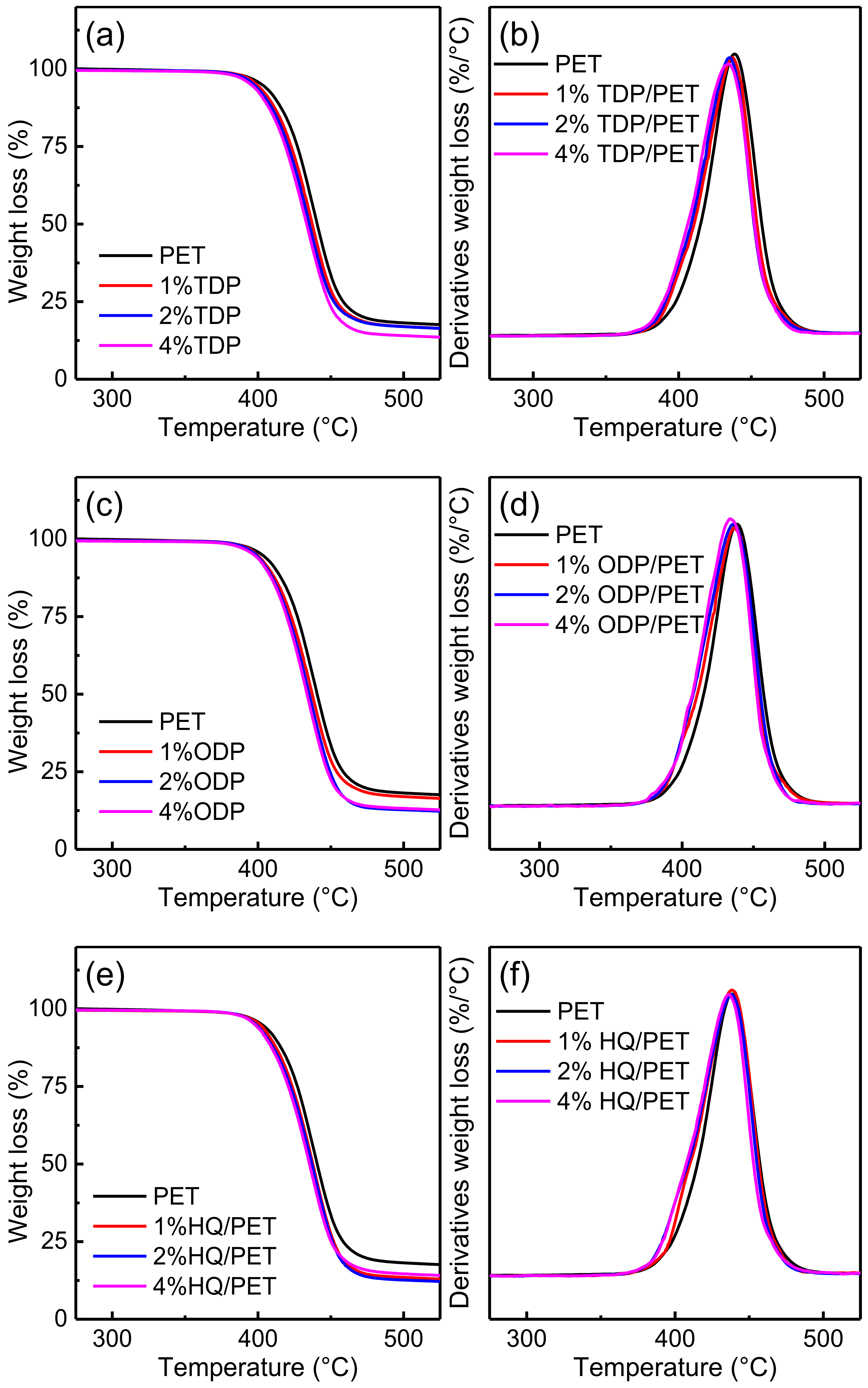
3.5. Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benvenuta-Tapia, J.; Vivaldo-Lima, E.; Guerrero-Santos, R. Effect of copolymers synthesized by nitroxide-mediated polymerization as chain extenders of postconsumer poly(ethylene terephthalate) waste. Polym. Eng. Sci. 2019, 59, 2255–2264. [Google Scholar] [CrossRef]
- Jafari, S.M.A.; Khajavi, R.; Goodarzi, V.; Kalaee, M.R.; Khonakdar, H.A. Nonisothermal crystallization kinetic studies on melt processed poly(ethylene terephthalate)/polylactic acid blends containing graphene oxide and exfoliated graphite nanoplatelets. J. Appl. Polym. Sci. 2019, 136, 47569. [Google Scholar] [CrossRef]
- Antoniadis, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Chrissafis, K. Non-isothermal crystallization kinetic of poly(ethylene terephthalate)/fumed silica (PET/SiO2) prepared by in situ polymerization. Thermochim. Acta 2010, 510, 103–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Li, H.; Du, Z. Effect of cyclotrimerization of bisphenol-A dicyanate monomer on poly(ethylene terephthalate) chain extension. Polym. Eng. Sci. 2011, 51, 1791–1796. [Google Scholar] [CrossRef]
- Bao, J.; Guo, G.; Lu, W.; Zhang, X.; Mao, H.; Dong, X.; Chen, S.; Lu, W.; Chen, W. Thermally induced physical gelation and phase transition of stereocomplexable poly(lactic acid)/poly(ethylene glycol) copolymers: Effects of hydrophilic homopolymers. Polymer 2020, 208, 122965. [Google Scholar] [CrossRef]
- Bao, J.; Dong, X.; Chen, S.; Lu, W.; Zhang, X.; Chen, W. Fractionated crystallization and fractionated melting behaviors of poly(ethylene glycol) induced by poly(lactide) stereocomplex in their block copolymers and blends. Polymer 2020, 190, 122189. [Google Scholar] [CrossRef]
- Tong, Z.; Zhou, J.; Wang, R.-Y.; Xu, J.-T. Interplay of microphase separation, crystallization and liquid crystalline ordering in crystalline/liquid crystalline block copolymers. Polymer 2017, 130, 1–9. [Google Scholar] [CrossRef]
- Ma, J.; Yu, L.; Chen, S.; Chen, W.; Wang, Y.; Guang, S.; Zhang, X.; Lu, W.; Wang, Y.; Bao, J. Structure–Property Evolution of Poly(ethylene terephthalate) Fibers in Industrialized Process under Complex Coupling of Stress and Temperature Field. Macromolecules 2018, 52, 565–574. [Google Scholar] [CrossRef]
- Gaonkar, A.A.; Murudkar, V.V.; Deshpande, V.D. Comparison of crystallization kinetics of polyethylene terephthalate (PET) and reorganized PET. Thermochim. Acta 2020, 683, 178472. [Google Scholar] [CrossRef]
- Liu, Y.; Wirasaputra, A.; Jiang, Z.; Liu, S.; Zhao, J.; Fu, Y. Fabrication of improved overall properties of poly (ethylene terephthalate) by simultaneous chain extension and crystallization promotion. J. Therm. Anal. Calorim. 2018, 133, 1447–1454. [Google Scholar] [CrossRef]
- Hassan, M.K.; Cakmak, M. Strain-Induced Crystallization during Relaxation Following Biaxial Stretching of PET Films: A Real-Time Mechano-Optical Study. Macromolecules 2015, 48, 4657–4668. [Google Scholar] [CrossRef]
- Zekriardehani, S.; Jabarin, S.A.; Gidley, D.R.; Coleman, M.R. Effect of Chain Dynamics, Crystallinity, and Free Volume on the Barrier Properties of Poly(ethylene terephthalate) Biaxially Oriented Films. Macromolecules 2017, 50, 2845–2855. [Google Scholar] [CrossRef]
- Chen, H.; Pyda, M.; Cebe, P. Non-isothermal crystallization of PET/PLA blends. Thermochim. Acta 2009, 492, 61–66. [Google Scholar] [CrossRef]
- Topkanlo, H.A.; Ahmadi, Z.; Taromi, F.A. An in-depth study on crystallization kinetics of PET/PLA blends. Iran. Polym. J. 2018, 27, 13–22. [Google Scholar] [CrossRef]
- Li, Z.-M.; Yang, W.; Li, L.-B.; Xie, B.-H.; Huang, R.; Yang, M.-B. Morphology and nonisothermal crystallization of in situ microfibrillar poly(ethylene terephthalate)/polypropylene blend fabricated through slit-extrusion, hot-stretch quenching. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 374–385. [Google Scholar] [CrossRef]
- Yan, Y.; Gooneie, A.; Ye, H.; Deng, L.; Qiu, Z.; Reifler, F.A.; Hufenus, R. Morphology and Crystallization of Biobased Polyamide 56 Blended with Polyethylene Terephthalate. Macromol. Mater. Eng. 2018, 303, 1800214. [Google Scholar] [CrossRef]
- Anoop Anand, K.; Agarwal, U.S.; Joseph, R. Carbon nanotubes induced crystallization of poly(ethylene terephthalate). Polymer 2006, 47, 3976–3980. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, H. Preparation and properties of deep dye fibers from poly(ethylene terephthalate)/SiO2 nanocomposites byin situ polymerization. J. Appl. Polym. Sci. 2007, 105, 2363–2369. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, H.; Gong, X.; Liu, J.; Huang, L.; Wang, W.; Wang, Y.; Zhao, Z.; Belfiore, L.A.; et al. Fluorescent SiO2@Tb3+(PET-TEG)3Phen Hybrids as Nucleating Additive for Enhancement of Crystallinity of PET. Polymers 2020, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Wang, Y.; Wang, J.; Wang, S.; Zhuang, H.; Liu, J.; Huang, L.; Wang, Y.; Wang, W.; Belfiore, L.A.; et al. Preparation of Hybrid Nanoparticle Nucleating Agents and Their Effects on the Crystallization Behavior of Poly(ethylene terephthalate). Materials 2018, 11, 587. [Google Scholar] [CrossRef] [Green Version]
- Tong, Z.; Zhuo, W.; Zhou, J.; Huang, R.; Jiang, G. Crystallization behavior and enhanced toughness of poly(ethylene terephthalate) composite with noncovalent modified graphene functionalized by pyrene-terminated molecules: A comparative study. J. Mater. Sci. 2017, 52, 10567–10580. [Google Scholar] [CrossRef]
- Heeley, E.L.; Hughes, D.J.; Crabb, E.M.; Bowen, J.; Bikondoa, O.; Mayoral, B.; Leung, S.; McNally, T. The formation of a nanohybrid shish-kebab (NHSK) structure in melt-processed composites of poly(ethylene terephthalate) (PET) and multi-walled carbon nanotubes (MWCNTs). Polymer 2017, 117, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Zhang, D.; Wu, M.; Ruan, S.; Castro, J.M.; Lee, L.J.; Chen, F. Impacts of Carbonaceous Particulates on Extrudate Semicrystalline Polyethylene Terephthalate Foams: Nonisothermal Crystallization, Rheology, and Infrared Attenuation Studies. Ind. Eng. Chem. Res. 2020, 59, 15586–15597. [Google Scholar] [CrossRef]
- Lee, A.S.; Jeon, H.; Choi, S.-S.; Park, J.; Hwang, S.Y.; Jegal, J.; Oh, D.X.; Kim, B.C.; Hwang, S.S. Crystallization derivation of amine functionalized T12 polyhedral oligomeric silsesquioxane-conjugated poly(ethylene terephthalate). Compos. Sci. Technol. 2017, 146, 42–48. [Google Scholar] [CrossRef]
- Sirin, H.; Turan, D.; Ozkoc, G.; Gurdag, S. POSS reinforced PET based composite fibers: “Effect of POSS type and loading level”. Compos. Part B Eng. 2013, 53, 395–403. [Google Scholar] [CrossRef]
- Lorenzo, M.L.D.; Errico, M.E.; Avella, M. Thermal and morphological characterization of poly(ethylene terephthalate)/calcium carbonate nanocomposites. J. Mater. Sci. 2002, 37, 2351–2358. [Google Scholar] [CrossRef]
- Li, W.; Kong, X.; Zhou, E.; Ma, D. Isothermal crystallization kinetics of poly(ethylene terephthalate)–poly(ethylene oxide) segmented copolymer with two crystallizing blocks. Polymer 2005, 46, 11655–11663. [Google Scholar] [CrossRef]
- Flores, I.; Etxeberria, A.; Irusta, L.; Calafel, I.; Vega, J.F.; Martínez-Salazar, J.; Sardon, H.; Müller, A.J. PET-ran-PLA Partially Degradable Random Copolymers Prepared by Organocatalysis: Effect of Poly(l-lactic acid) Incorporation on Crystallization and Morphology. ACS Sustain. Chem. Eng. 2019, 7, 8647–8659. [Google Scholar] [CrossRef]
- Flores, I.; Basterretxea, A.; Etxeberria, A.; González, A.; Ocando, C.; Vega, J.F.; Martínez-Salazar, J.; Sardon, H.; Müller, A.J. Organocatalyzed Polymerization of PET-mb-poly(oxyhexane) Copolymers and Their Self-Assembly into Double Crystalline Superstructures. Macromolecules 2019, 52, 6834–6848. [Google Scholar] [CrossRef]
- Wei, G.; Wang, L.; Chen, G.; Gu, L. Synthesis and characterization of poly(ethylene-co-trimethylene terephthalate)s. J. Appl. Polym. Sci. 2006, 100, 1511–1521. [Google Scholar] [CrossRef]
- Lewis, C.L.; Spruiell, J.E. Crystallization of 2-methyl-1,3-propanediol substituted poly(ethylene terephthalate). I. Thermal behavior and isothermal crystallization. J. Appl. Polym. Sci. 2006, 100, 2592–2603. [Google Scholar] [CrossRef]
- Kiyotsukuri, T.; Masuda, T.; Tsutsumi, N. Preparation and properties of poly(ethylene terephthalate) copolymers with 2,2-dialkyl-1,3-propanediols. Polymer 1994, 35, 1274–1279. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, X.; Huang, L.; Li, F.; Liu, S.; Yu, J. Poly(ethylene terephthalate) copolyesters and fibers modified with NPG and SIPE for improved hydrophilicity and dyeability. J. Text. Inst. 2017, 108, 1949–1956. [Google Scholar] [CrossRef]
- Zhao, H.B.; Wang, Y.Z. Design and Synthesis of PET-Based Copolyesters with Flame-Retardant and Antidripping Performance. Macromol. Rapid Commun. 2017, 38, 1700451. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Luo, F.; Bai, H.; Si, P.; Lei, X.; Ding, S.; Ji, L. A study on mediating the crystallization behavior of PBT through intermolecular hydrogen-bonding. RSC Adv. 2016, 6, 17510–17518. [Google Scholar] [CrossRef]
- Luo, F.-L.; Luo, F.-H.; Xing, Q.; Zhang, X.-Q.; Jiao, H.-Q.; Yao, M.; Luo, C.-T.; Wang, D.-J. Hydrogen-bonding induced change of crystallization behavior of poly(butylene succinate) in its mixtures with bisphenol A. Chin. J. Polym. Sci. 2013, 31, 1685–1696. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Inoue, Y. Thermal and infrared spectroscopic studies on hydrogen-bonding interactions between poly-(ε-caprolactone) and some dihydric phenols. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2108–2117. [Google Scholar] [CrossRef]
- He, Y.; Asakawa, N.; Li, J.; Inoue, Y. Effects of low molecular weight compounds with hydroxyl groups on properties of poly(L-lactic acid). J. Appl. Polym. Sci. 2001, 82, 640–649. [Google Scholar] [CrossRef]
- He, Y.; Asakawa, N.; Inoue, Y. Blends of poly(3-hydroxybutyrate)/4,4′-thiodiphenol and poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/ 4,4′-thiodiphenol: Specific interaction and properties. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2891–2900. [Google Scholar] [CrossRef]
- Si, P.; Luo, F. Hydrogen bonding interaction and crystallization behavior of poly (butylene succinate-co-butylene adipate)/thiodiphenol complexes. Polym. Adv. Technol. 2016, 27, 1413–1421. [Google Scholar] [CrossRef]
- Raimo, M.; Lotti, E. Rebuilding growth mechanisms through visual observations. ChemTexts 2016, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Mai, K. Non-isothermal crystallization and melting behavior of compatibilized polypropylene/recycled poly(ethylene terephthalate) blends. Eur. Polym. J. 2007, 43, 3538–3549. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Qiu, Z.; Fujinami, S.; Komura, M.; Nakajima, K.; Ikehara, T.; Nishi, T. Nonisothermal Crystallization Kinetics of Poly(butylene succinate) and Poly(ethylene succinate). Polym. J. 2004, 36, 642–646. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Yang, W. Nonisothermal crystallization kinetics of biodegradable poly(butylene succinate)/poly(vinyl phenol) blend. J. Appl. Polym. Sci. 2007, 104, 972–978. [Google Scholar] [CrossRef]









| Sample | Cooling Rate | |||
|---|---|---|---|---|
| 5 °C/Min | 10 °C/Min | 20 °C/Min | 30 °C/Min | |
| PET | 2.28 | 2.08 | 2.09 | 1.93 |
| 1% TDP/PET | 2.35 | 2.20 | 2.26 | 2.21 |
| 2% TDP/PET | 2.49 | 2.39 | 2.22 | 2.29 |
| 4% TDP/PET | 2.48 | 2.41 | 2.30 | 2.27 |
| 1% ODP/PET | 2.37 | 2.38 | 2.22 | 2.26 |
| 2% ODP/PET | 2.58 | 2.52 | 2.48 | 2.31 |
| 4% ODP/PET | 2.20 | 2.06 | 2.05 | 1.99 |
| 1% HQ/PET | 2.58 | 2.57 | 2.32 | 2.35 |
| 2% HQ/PET | 2.34 | 2.38 | 2.12 | 2.12 |
| 4% HQ/PET | 2.27 | 2.26 | 2.06 | 2.00 |
| Bisphenol Content (wt%) | / | TDP | ODP | HQ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 1 | 2 | 4 | 1 | 2 | 4 | |
| Tdmax (°C) | 438.6 | 437.2 | 435.2 | 434.2 | 436.7 | 436.0 | 434.0 | 438.3 | 438.1 | 436.7 |
| Td5% (°C) | 402.6 | 399.1 | 397.1 | 394.9 | 399.1 | 398.4 | 396.8 | 401.1 | 398.4 | 397.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xie, S.; Guang, S.; Bao, J.; Zhang, X.; Chen, W. Crystallization and Thermal Behaviors of Poly(ethylene terephthalate)/Bisphenols Complexes through Melt Post-Polycondensation. Polymers 2020, 12, 3053. https://doi.org/10.3390/polym12123053
Chen S, Xie S, Guang S, Bao J, Zhang X, Chen W. Crystallization and Thermal Behaviors of Poly(ethylene terephthalate)/Bisphenols Complexes through Melt Post-Polycondensation. Polymers. 2020; 12(12):3053. https://doi.org/10.3390/polym12123053
Chicago/Turabian StyleChen, Shichang, Shangdong Xie, Shanshan Guang, Jianna Bao, Xianming Zhang, and Wenxing Chen. 2020. "Crystallization and Thermal Behaviors of Poly(ethylene terephthalate)/Bisphenols Complexes through Melt Post-Polycondensation" Polymers 12, no. 12: 3053. https://doi.org/10.3390/polym12123053