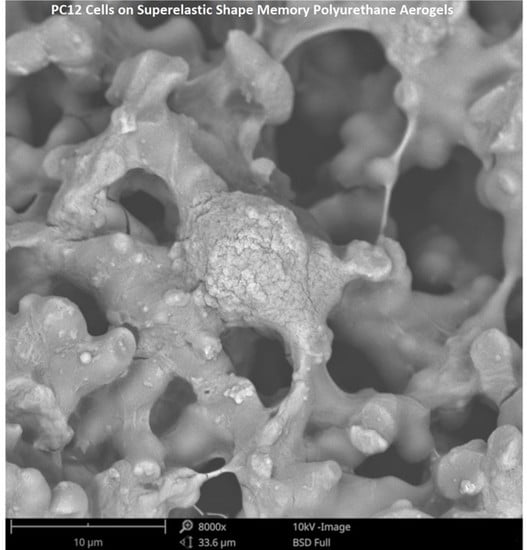
Nerve Response to Superelastic Shape Memory Polyurethane Aerogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Superelastic Shape Memory Polyurethane Aerogels (SSMPA)
2.2. Compression of SSMPA
2.3. Cell Culture
2.4. Scanning Electron Microscopy
2.5. Cell Response Measurement
2.6. Pore Diameter Measurement
2.7. Surface Roughness (Sa) Measurement
2.8. Stiffness Measurement
2.9. Statistical Analysis
3. Results
3.1. Effect of Radial Compression on the SSMPA Properties
3.1.1. Pore Diameter
3.1.2. Surface Roughness (Sa)
3.1.3. Young’s Modulus
3.1.4. Comparison of Pore Diameter and Surface Roughness
3.2. Impact of SSMPA Properties on Cell Shape and Neurite Extension
3.2.1. Neurite Length
3.2.2. Number of Neurites per Cell Body
3.2.3. Cell Body Area
3.3. Nerve Response to Sa
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Van Helvert, S.; Storm, C.; Friedl, P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 2018, 20, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Yao, D.; Jiang, J.; Guo, X.; Gao, Y.; Li, Q.; Shen, C. Effects of aligned and random fibers with different diameter on cell behaviors. Colloids Surf. B Biointerfaces 2018, 171, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, L.; Sun, M.; Zhang, Y.; Chen, L.; Rong, Y.; Li, Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Eroshenko, N.; Ramachandran, R.; Yadavalli, V.K.; Rao, R.R. Effect of substrate stiffness on early human embryonic stem cell differentiation. J. Biol. Eng. 2013, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. USA 2012, 109, 10334–10339. [Google Scholar] [CrossRef]
- Su, W.-T.; Liao, Y.-F.; Wu, T.-W.; Wang, B.-J.; Shih, Y.-Y. Microgrooved patterns enhanced PC12 cell growth, orientation, neurite elongation, and neuritogenesis. J. Biomed. Mater. Res. Part A 2012, 101, 185–194. [Google Scholar] [CrossRef]
- Britland, S.; Perridge, C.; Denyer, M.; Morgan, H.; Curtis, A.; Wilkinson, C. Morphogenetic guidance cues can interact synergistically and hierarchically in steering nerve cell growth. Exp. Biol. Online 2013, 1, 1–15. [Google Scholar] [CrossRef]
- Li, W.; Tang, Q.Y.; Jadhav, A.D.; Narang, A.; Qian, W.X.; Shi, P.; Pang, S.W. Large-scale Topographical Screen for Investigation of Physical Neural-Guidance Cues. Sci. Rep. 2015, 5, 8644. [Google Scholar] [CrossRef]
- Hanson, J.N.; Motala, M.J.; Heien, M.L.; Gillette, M.; Sweedler, J.V.; Nuzzo, R.G. Textural guidance cues for controlling process outgrowth of mammalian neurons. Lab Chip 2009, 9, 122–131. [Google Scholar] [CrossRef]
- Yin, W.; Venkitachalam, S.M.; Jarrett, E.; Staggs, S.; Leventis, N.; Lu, H.; Rubenstein, D.A. Biocompatibility of surfactant-templated polyurea-nanoencapsulated macroporous silica aerogels with plasma platelets and endothelial cells. J. Biomed. Mater. Res. Part A 2009, 92, 1431–1439. [Google Scholar] [CrossRef]
- Sabri, F.; Boughter, J.D., Jr.; Gerth, D.; Skalli, O.; Phung, T.-C.N.; Tamula, G.-R.M.; Leventis, N. Histological Evaluation of the Biocompatibility of Polyurea Crosslinked Silica Aerogel Implants in a Rat Model: A Pilot Study. PLoS ONE 2012, 7, e50686. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.J.; Skalli, O.; Sabri, F. Investigation of surface topography and stiffness on adhesion and neurites extension of PC12 cells on crosslinked silica aerogel substrates. PLoS ONE 2017, 12, e0185978. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.R.; Lynch, K.J.; Chandrasekaran, S.; Skalli, O.; Worsley, M.; Sabri, F. PC-12 cells adhesion and differentiation on carbon aerogel scaffolds. MRS Commun. 2018, 8, 1426–1432. [Google Scholar] [CrossRef]
- Sabri, F.; Sebelik, M.E.; Meacham, R.; Boughter, J.D.; Challis, M.J.; Leventis, N. In Vivo Ultrasonic Detection of Polyurea Crosslinked Silica Aerogel Implants. PLoS ONE 2013, 8, e66348. [Google Scholar] [CrossRef]
- Allison, S.W.; Baker, E.S.; Lynch, K.J.; Sabri, F. In vivo X-Ray excited optical luminescence from phosphor-doped aerogel and Sylgard 184 composites. Radiat. Phys. Chem. 2017, 135, 88–93. [Google Scholar] [CrossRef]
- Allison, S.W.; Baker, E.S.; Lynch, K.J.; Sabri, F. In Vivo X-Ray Imaging of Phosphor-Doped PDMS and Phosphor-Doped Aerogel Biomaterials. Int. J. Polym. Mater. 2015, 64, 823–830. [Google Scholar] [CrossRef]
- Sabri, F.; Lynch, K.J.; Allison, S.; Allison, S.W. Polymer-Encapsulated Phosphor Particles for In Vivo Phosphor Luminescence Applications. Int. J. Polym. Mater. 2015, 64, 690–694. [Google Scholar] [CrossRef]
- W Yin, W.; Rubenstein, D.A. Biomedical Applications of Aerogels. In Aerogels Handbook; Advances in Sol-Gel Derived Materials and Technologies; Springer: New York, NY, USA, 2011; pp. 683–694. [Google Scholar]
- Hadley, J.; Hirschman, J.; Morshed, B.I.; Sabri, F. RF Coupling of Interdigitated Electrode Array on Aerogels for in vivo Nerve Guidance Applications. MRS Adv. 2019, 4, 1237–1244. [Google Scholar] [CrossRef]
- Sabri, F.; Gerth, D.; Tamula, G.-R.M.; Phung, T.-C.N.; Lynch, K.J.; Boughter, J.D., Jr. Novel Technique for Repair of Severed Peripheral Nerves in Rats Using Polyurea Crosslinked Silica Aerogel Scaffold. J. Investig. Surg. 2014, 27, 294–303. [Google Scholar] [CrossRef]
- Bai, S.; Han, H.; Huang, X.; Xu, W.; Kaplan, D.L.; Zhu, H.; Lu, Q. Silk scaffolds with tunable mechanical capability for cell differentiation. Acta Biomater. 2015, 20, 22–31. [Google Scholar] [CrossRef]
- Sabri, F.; Cole, J.A.; Scarbrough, M.C.; Leventis, N. Investigation of Polyurea-Crosslinked Silica Aerogels as a Neuronal Scaffold: A Pilot Study. PLoS ONE 2012, 7, e33242. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.J.; Skalli, O.; Sabri, F. Growing Neural PC-12 Cell on Crosslinked Silica Aerogels Increases Neurite Extension in the Presence of an Electric Field. J. Funct. Biomater. 2018, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.R.; Peng, C.; Skalli, O.; Sabri, F. Tunable neuronal scaffold biomaterials through plasmonic photo-patterning of aerogels. MRS Commun. 2019, 9, 1249–1255. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef]
- Malakooti, S.; Zhao, E.; Tsao, N.; Bian, N.; Soni, R.U.; Doulah, A.S.U.; Sotiriou-Leventis, C.; Leventis, N.; Lu, H. Synthesis of aerogel foams through a pressurized sol-gel method. Polymer 2020, 208, 122925. [Google Scholar] [CrossRef]
- Donthula, S.; Mandal, C.; Schisler, J.; Leventis, T.; Meador, M.A.B.; Sotiriou-Leventis, C.; Leventis, N. Nanostructure-Dependent Marcus-Type Correlation of the Shape. ACS Appl. Mater. Interfaces 2018, 10, 23321–23334. [Google Scholar] [CrossRef]
- Malakooti, S.; Rostami, S.; Churu, H.G.; Luo, H.; Clark, J.; Casarez, F.; Rettenmaier, O.; Daryadel, S.; Minary-Jolandan, M.; Sotiriou-Leventis, C.; et al. Scalable, hydrophobic and highly-stretchable poly(isocyanurate–urethane) aerogels. RSC Adv. 2018, 8, 21214–21223. [Google Scholar] [CrossRef]
- Donthula, S.; Mandal, C.; Leventis, T.; Schisler, J.; Saeed, A.M.; Sotiriou-Leventis, C.; Leventis, N. Shape Memory Superelastic Poly(isocyanurate-urethane) Aerogels (PIR-PUR) for Deployable Panels and Biomimetic Applications. Chem. Mater. 2017, 29, 4461–4477. [Google Scholar] [CrossRef]
- Bang, A.; Buback, C.; Sotiriou-Leventis, C.; Leventis, N. Flexible Aerogels from Hyperbranched Polyurethanes: Probing the Role of Molecular Rigidity with Poly(Urethane Acrylates) Versus Poly(Urethane Norbornenes). Chem. Mater. 2014, 26, 6979–6993. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; McCarver, P.M.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Fractal Multiscale Nanoporous Polyurethanes: Flexible to Extremely Rigid Aerogels from Multifunctional Small Molecules. Chem. Mater. 2013, 25, 3205–3224. [Google Scholar] [CrossRef]
- Brunetti, V.; Maiorano, G.; Rizzello, L.; Sorce, B.; Sabella, S.; Cingolani, R.; Pompa, P.P. Neurons sense nanoscale roughness with nanometer sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 6264–6269. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.R.; Puhl, D.L.; Ziemba, A.M.; Johnson, C.D.L.; Doedee, J.; Bao, J.; Gilbert, R.J. Exploring the effects of electrospun fiber surface nanotopography on neurite outgrowth and branching in neuron cultures. PLoS ONE 2019, 14, e0211731. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, C.P.; Dolatshahi-Pirouz, A.; Foss, M.; Chevallier, J.; Fink, T.; Zachar, V.; Besenbacher, F.; Yoshida, K. Nanoscale topography reduces fibroblast growth, focal adhesion size and migration-related gene expression on platinum surfaces. Colloids Surf. B Biointerfaces 2011, 85, 189–197. [Google Scholar] [CrossRef] [PubMed]
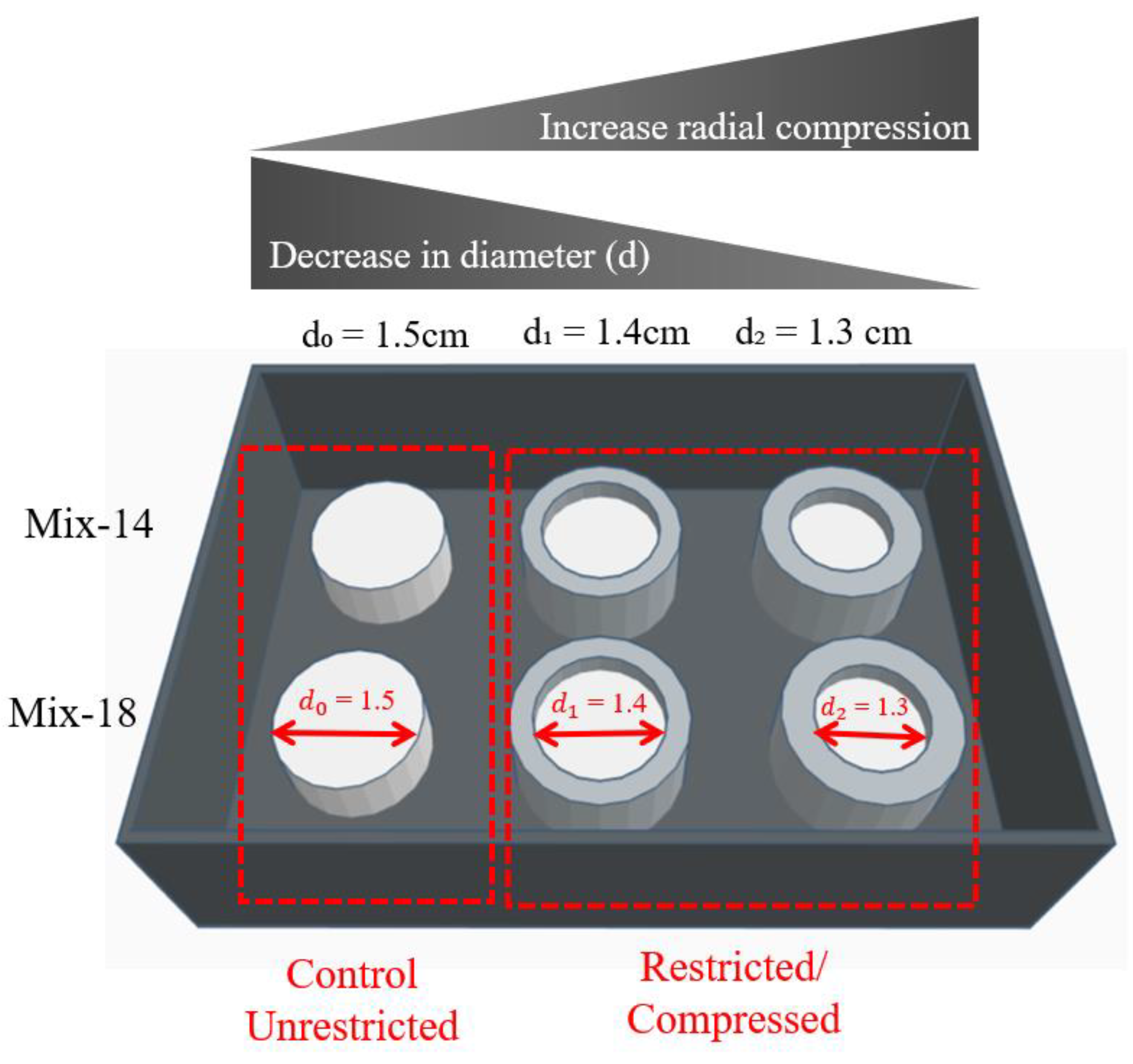
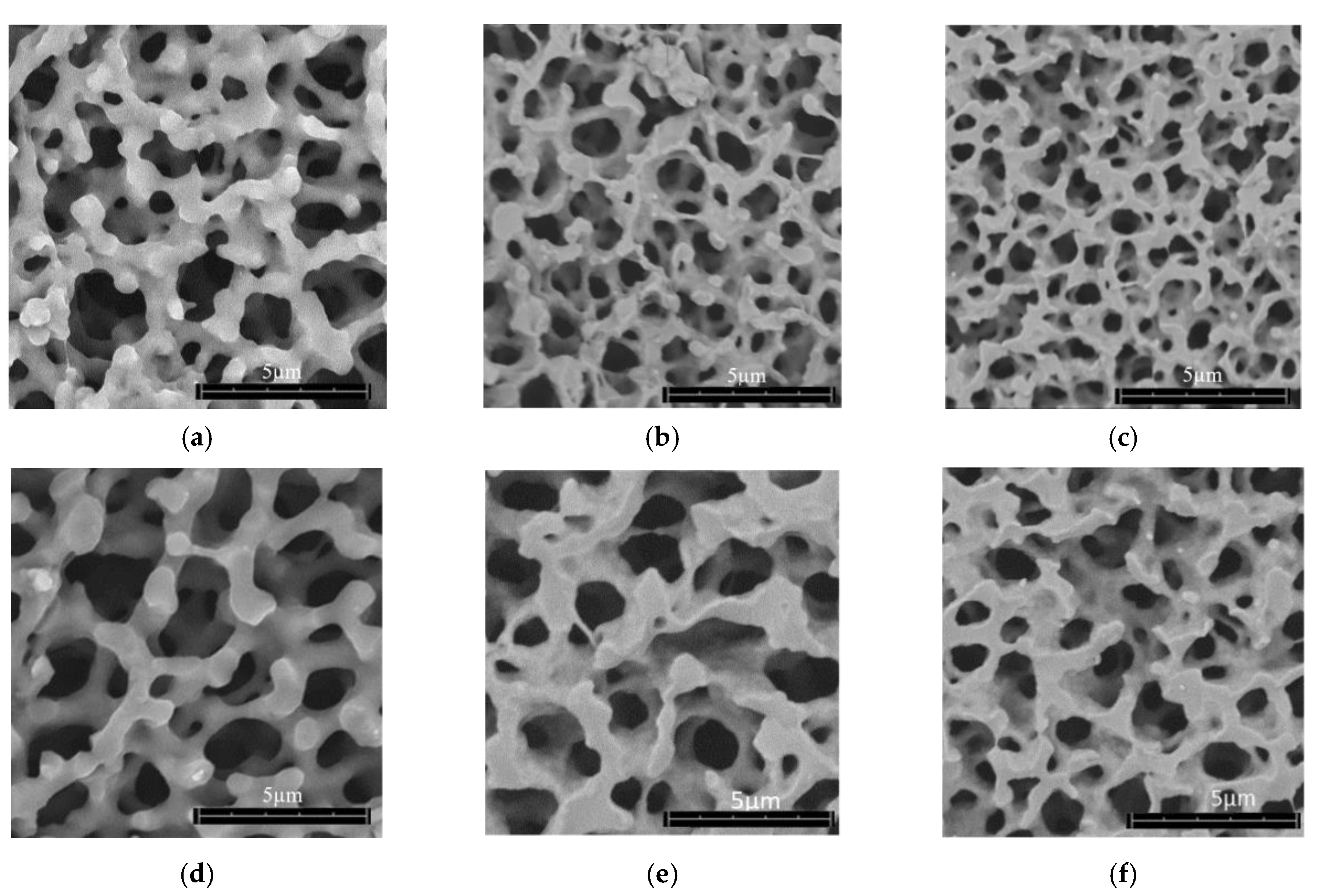
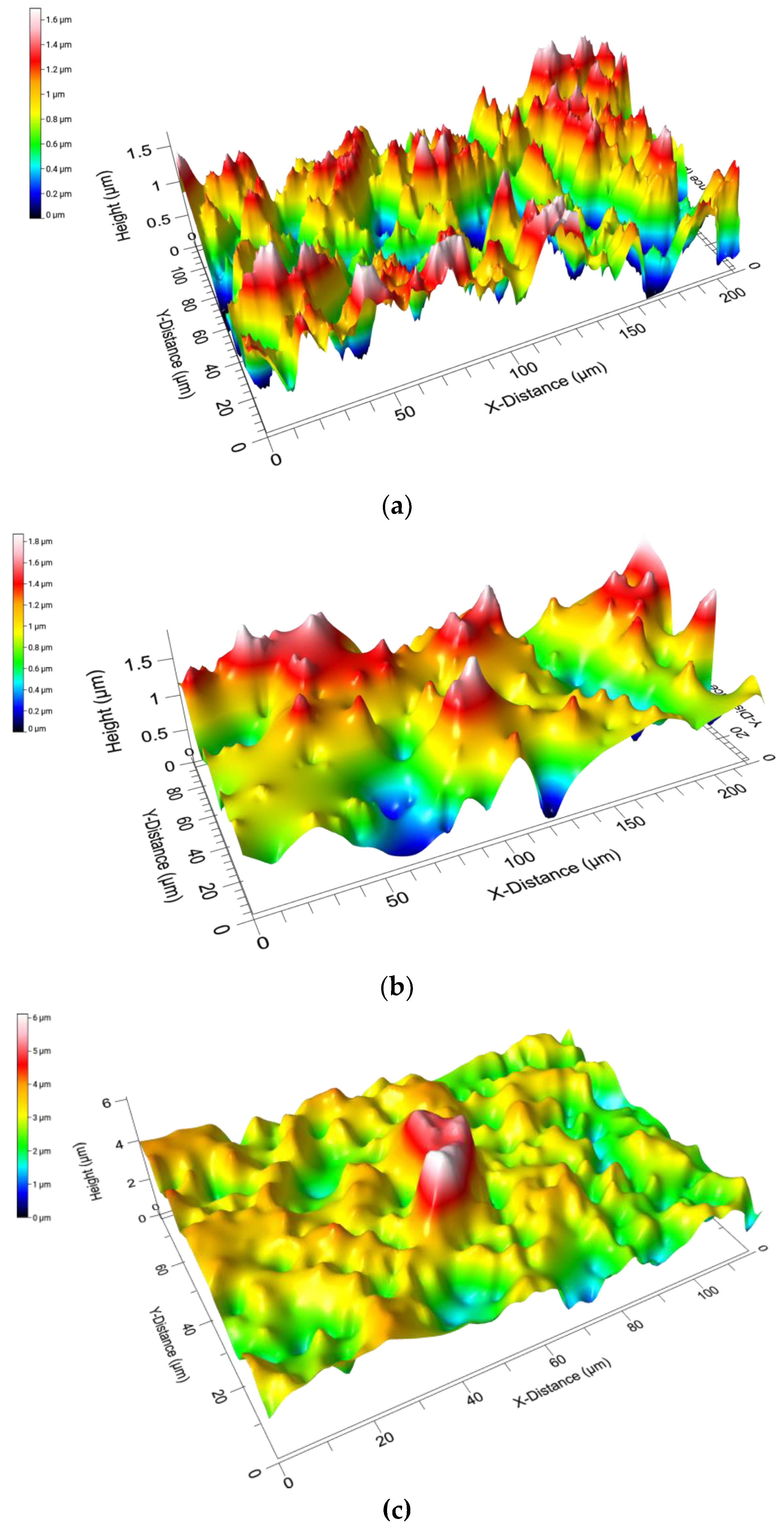
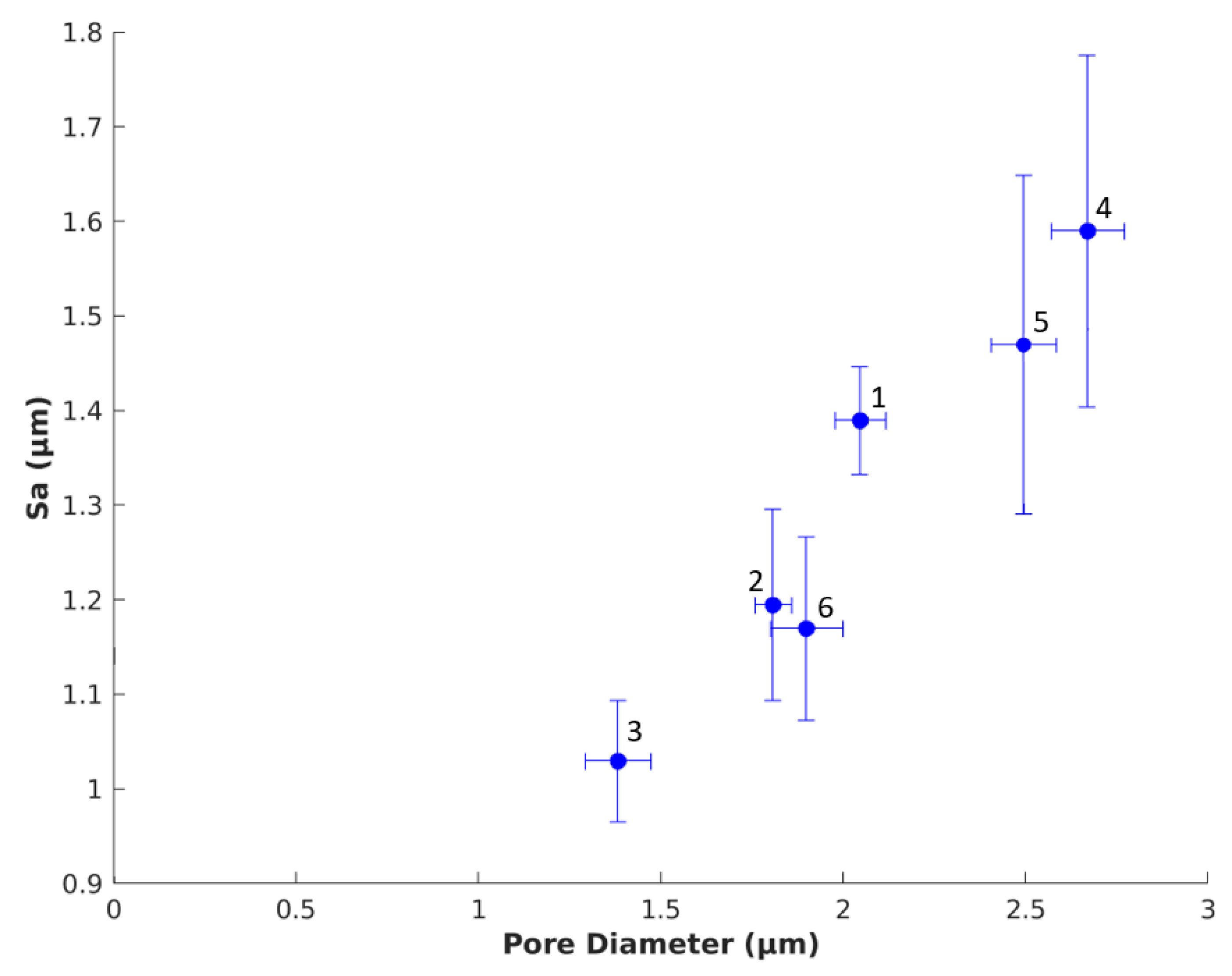



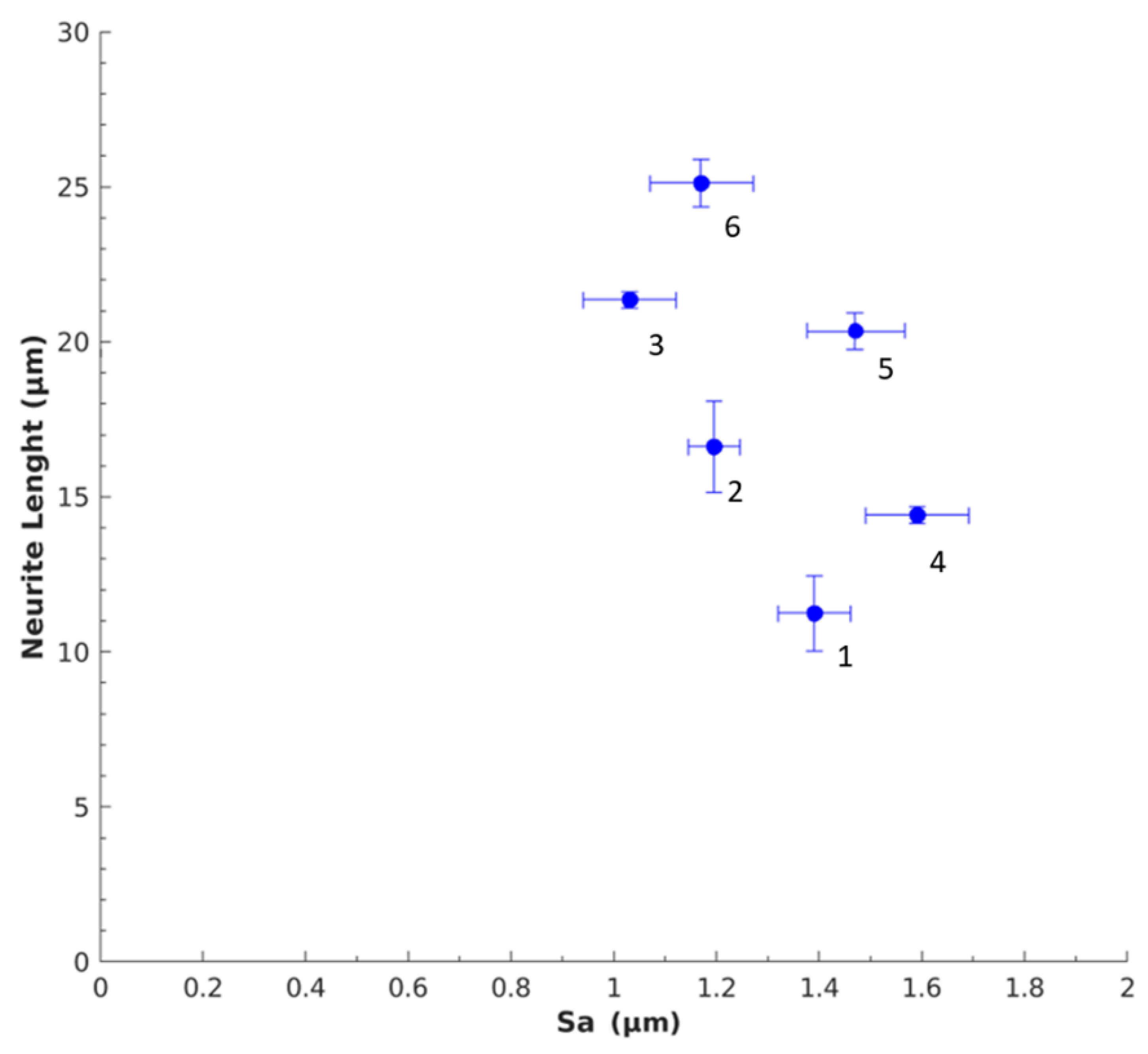
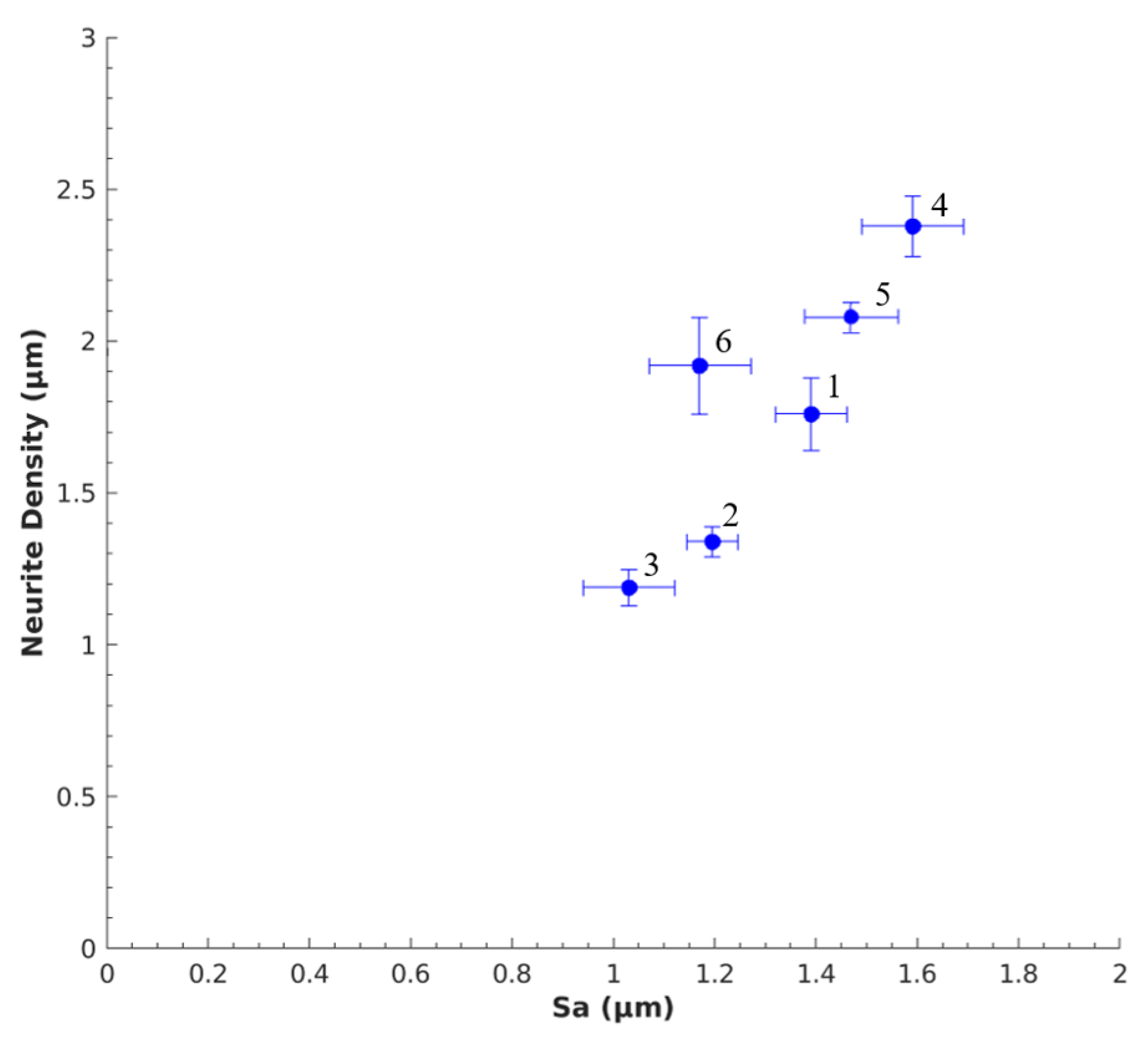
| Aerogel Type | Degree of Radial Compression | Pore Diameter (µm) | Label |
|---|---|---|---|
| Mix-14 | d0 = 1.5 | 2.05 ± 0.16 | 1 |
| d1 = 1.4 | 1.81 ± 0.10 | 2 | |
| d2 = 1.3 | 1.38 ± 0.06 | 3 | |
| Mix-18 | d0 = 1.5 | 2.67 ± 0.19 | 4 |
| d1 = 1.4 | 2.50 ± 0.18 | 5 | |
| d2 = 1.3 | 1.90 ± 0.10 | 6 |
| Aerogel Type | Degree of Radial Compression | Sa (µm) | Label |
|---|---|---|---|
| Mix-14 | d0 = 1.5 | 1.39 ± 0.17 | 1 |
| d1 = 1.4 | 1.20 ± 0.25 | 2 | |
| d2 = 1.3 | 1.03 ± 0.12 | 3 | |
| Mix-18 | d0 = 1.5 | 1.59 ± 0.17 | 4 |
| d1 = 1.4 | 1.47 ± 0.23 | 5 | |
| d2 = 1.3 | 1.17 ± 0.10 | 6 |
| Aerogel Type | Degree of Radial Compression | Young’s Modulus (kPa) | Label |
|---|---|---|---|
| Mix-14 | d0 = 1.5 | 255 ± 33 | 1 |
| d1 = 1.4 | 269 ± 65 | 2 | |
| d2 = 1.3 | 298 ± 19 | 3 | |
| Mix-18 | d0 = 1.5 | 182 ± 18 | 4 |
| d1 = 1.4 | 199 ± 78 | 5 | |
| d2 = 1.3 | 203 ± 83 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sala, M.R.; Skalli, O.; Leventis, N.; Sabri, F. Nerve Response to Superelastic Shape Memory Polyurethane Aerogels. Polymers 2020, 12, 2995. https://doi.org/10.3390/polym12122995
Sala MR, Skalli O, Leventis N, Sabri F. Nerve Response to Superelastic Shape Memory Polyurethane Aerogels. Polymers. 2020; 12(12):2995. https://doi.org/10.3390/polym12122995
Chicago/Turabian StyleSala, Martina Rodriguez, Omar Skalli, Nicholas Leventis, and Firouzeh Sabri. 2020. "Nerve Response to Superelastic Shape Memory Polyurethane Aerogels" Polymers 12, no. 12: 2995. https://doi.org/10.3390/polym12122995
APA StyleSala, M. R., Skalli, O., Leventis, N., & Sabri, F. (2020). Nerve Response to Superelastic Shape Memory Polyurethane Aerogels. Polymers, 12(12), 2995. https://doi.org/10.3390/polym12122995