In-Situ Rheological Studies of Cationic Lignin Polymerization in an Acidic Aqueous System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Lignin-graft-poly METAC (LM)
2.3. Rheology Settings
2.3.1. Flow Test
2.3.2. Reaction Product
2.4. Structural Characterization of LM
2.4.1. Fourier Transform Infrared (FT-IR)
2.4.2. Water Solubility Analysis
2.4.3. Hydroxyl Group Content Analysis for LM-1 and LM-2
2.4.4. Unreacted Lignin and Monomer for LM-1 and LM-2
2.4.5. Elemental Analysis
2.4.6. Charge Density
2.4.7. Molecular Weight Analysis
2.4.8. Particle Size Analysis
2.4.9. Radius of Gyration (Rg)
3. Results and Discussions
3.1. Polymerization Mechanism
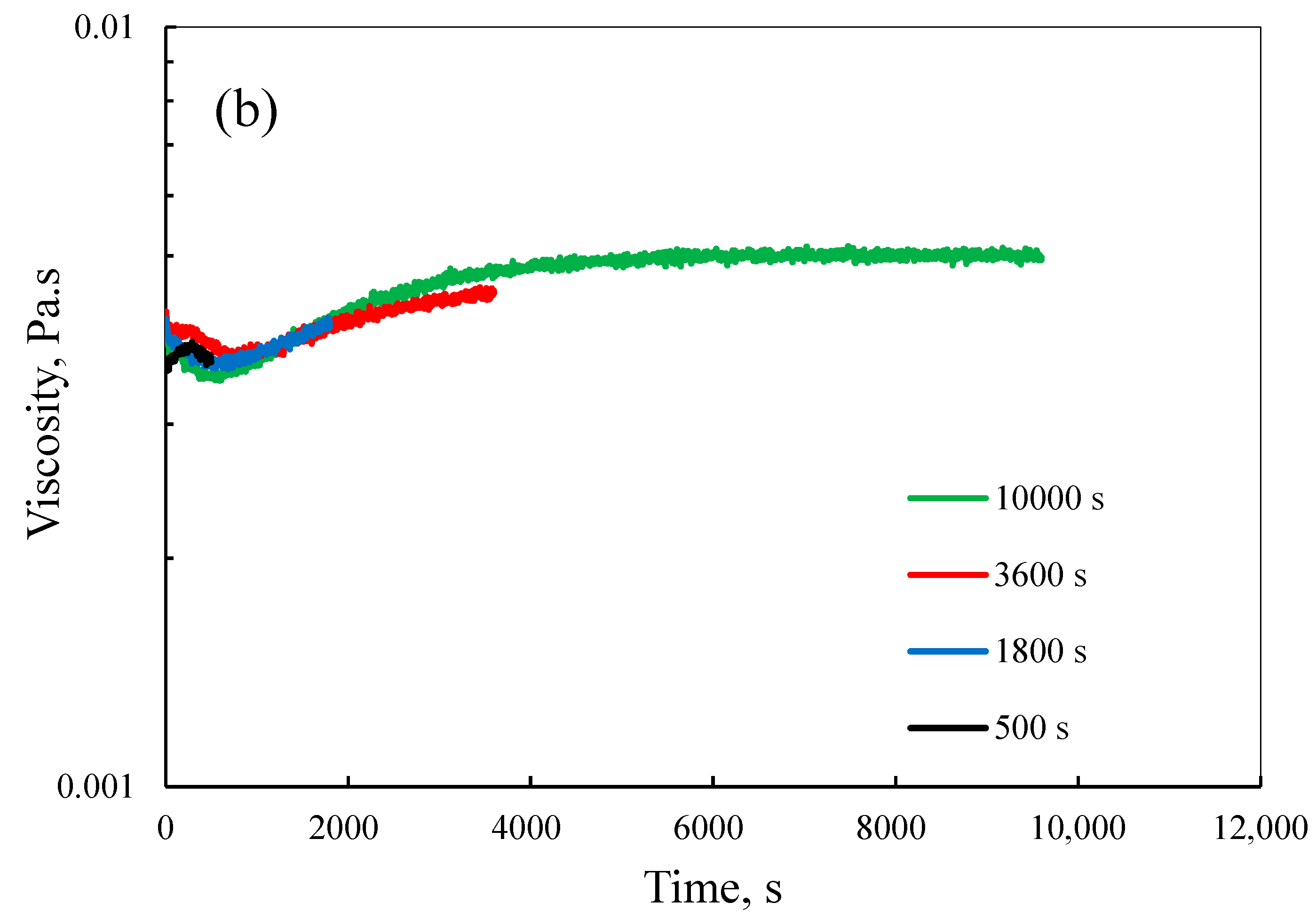
3.2. Effect of Process Conditions on Viscosity Evolution during Polymerization
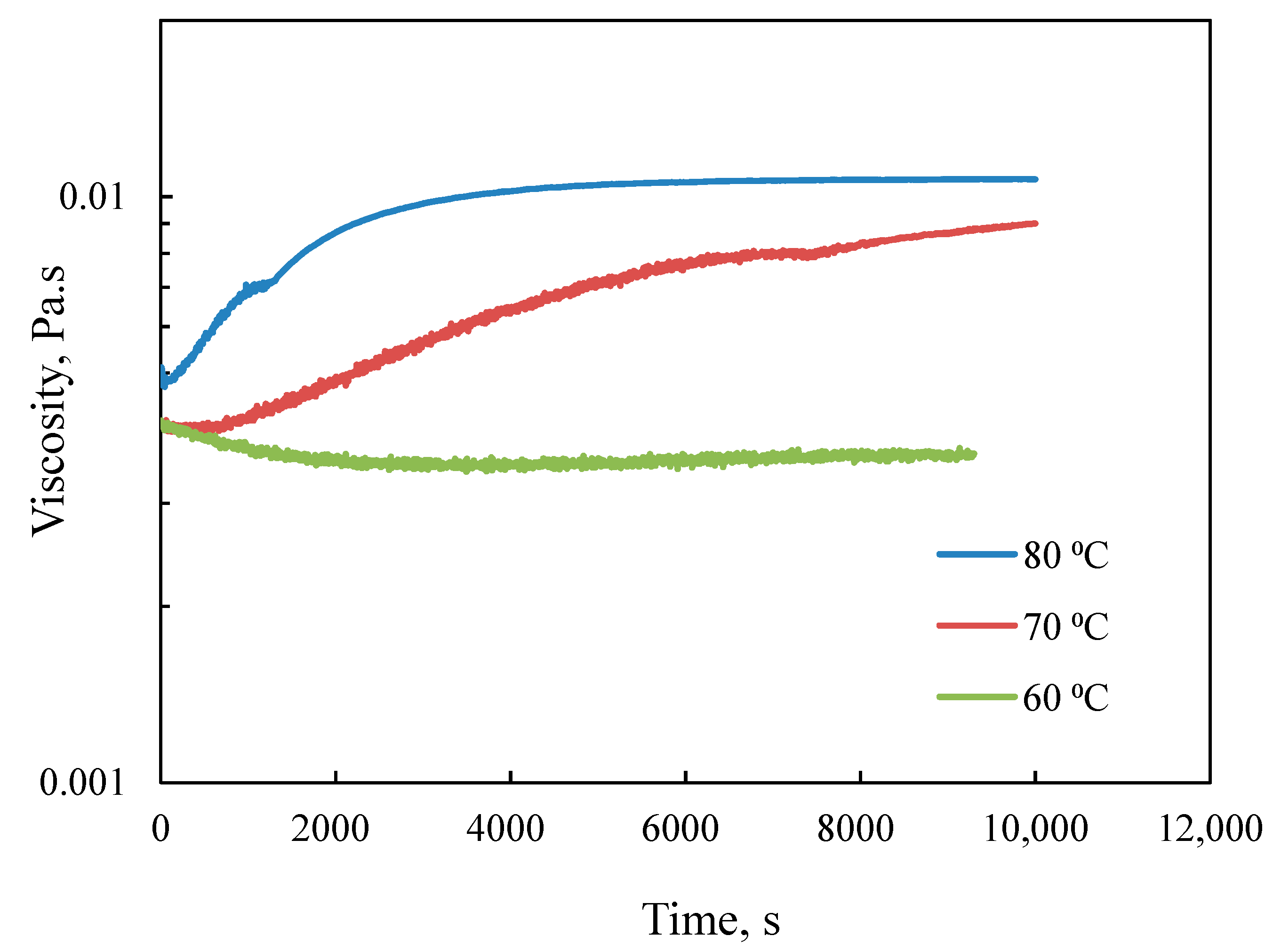
3.2.1. Effect of Temperature
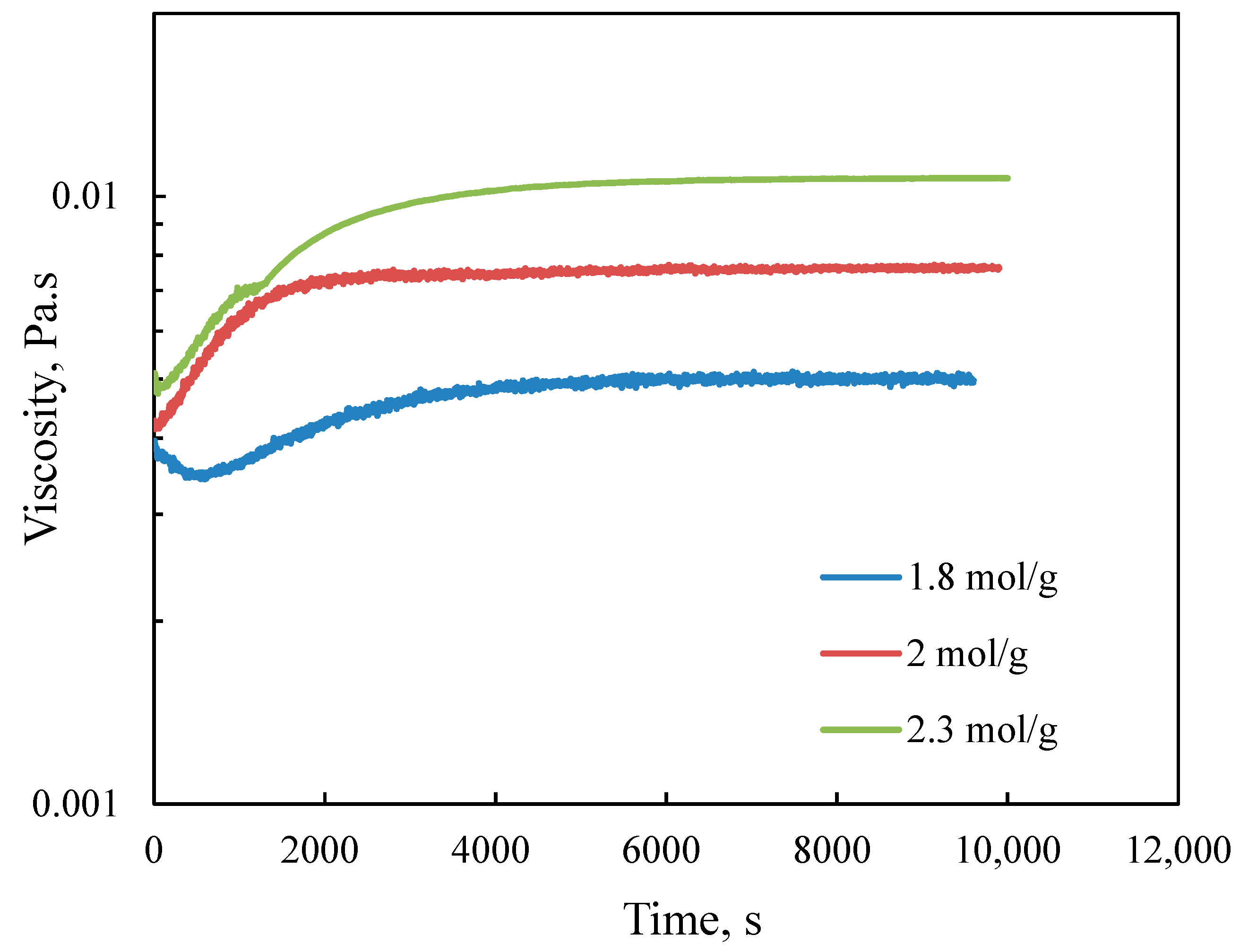
3.2.2. Effect of Molar Ratio
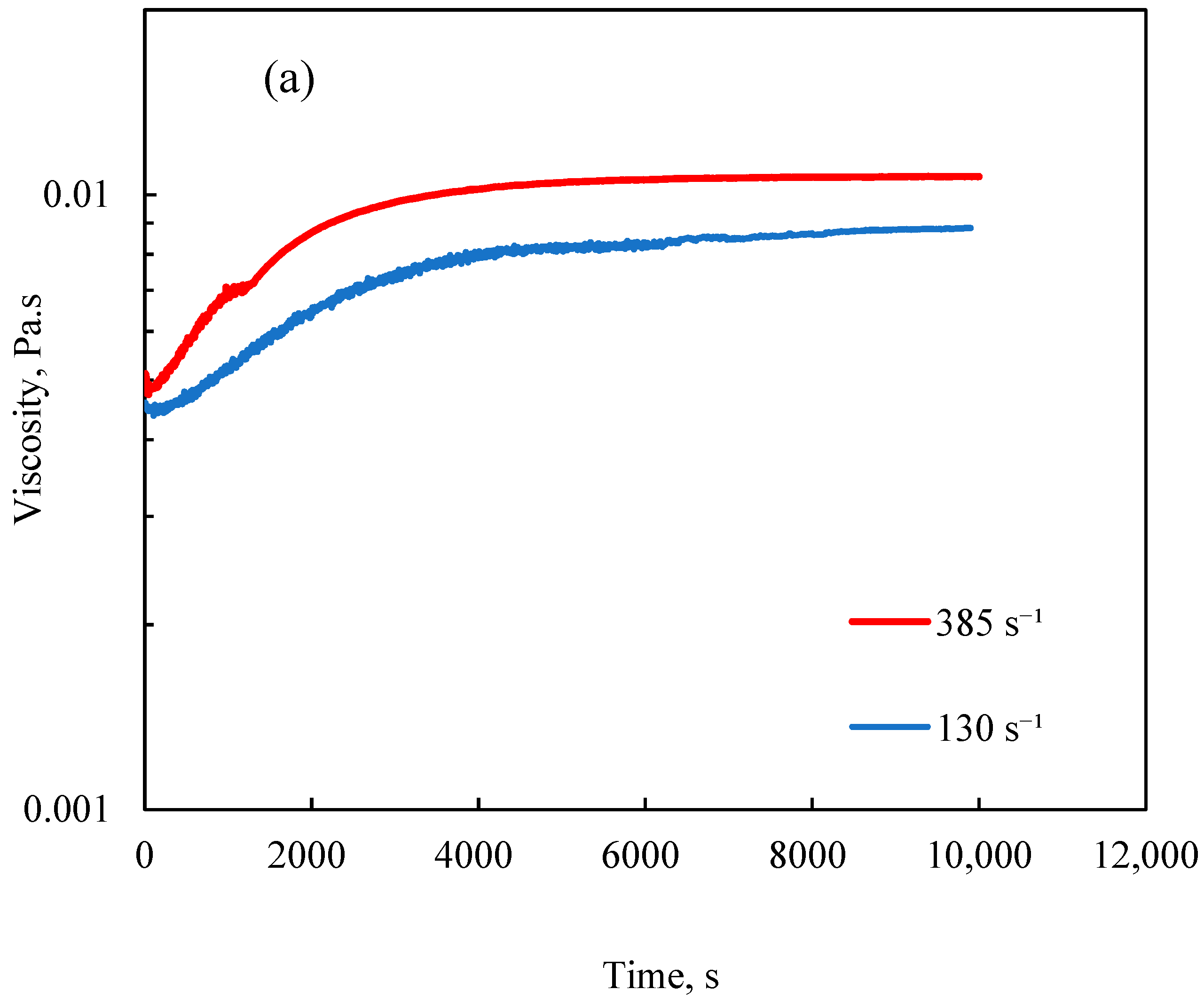
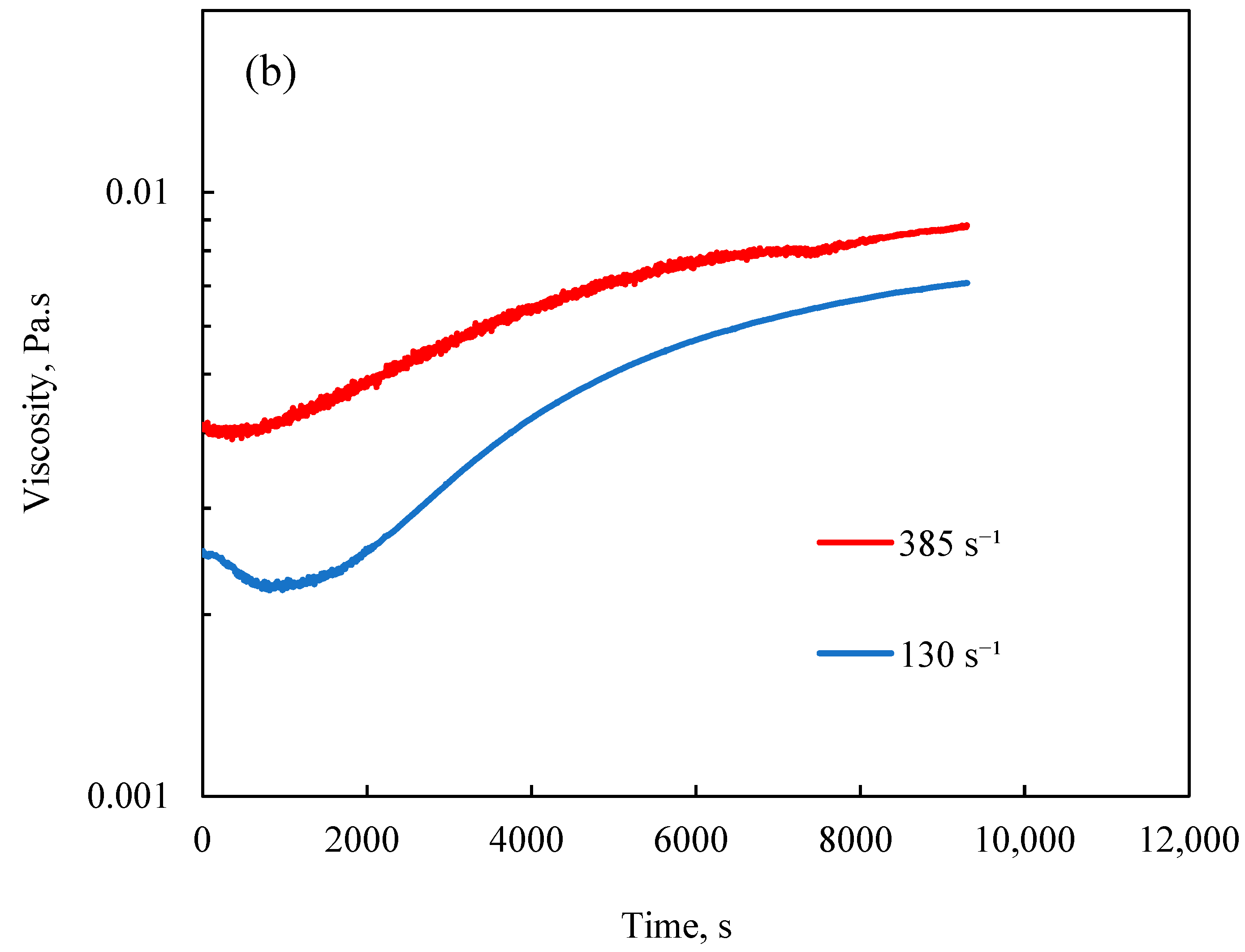
3.2.3. Effect of Shear Rate
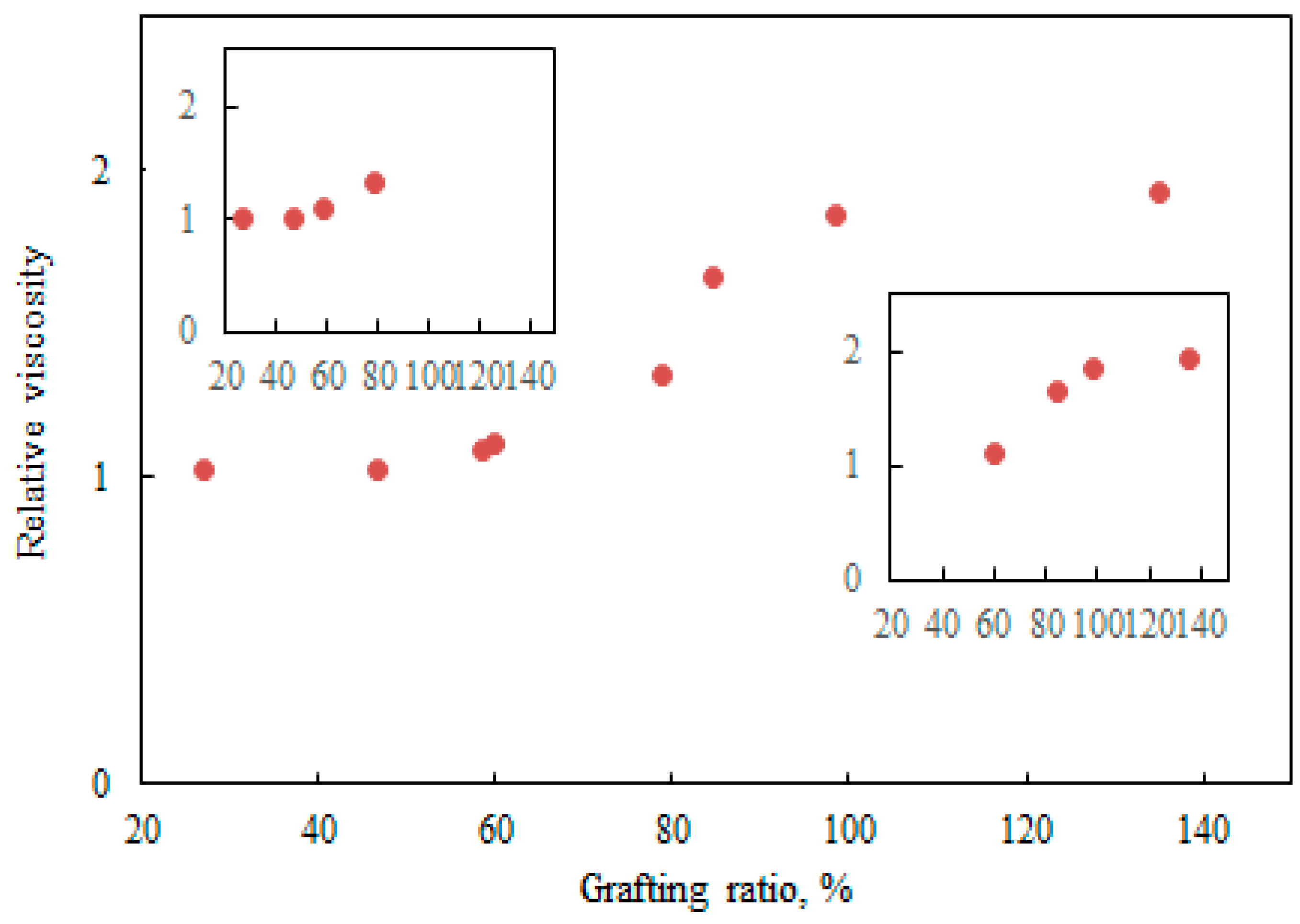
3.3. Characteristics of Cationic Polymers
3.4. Insights into LM Characteristics during Polymerization
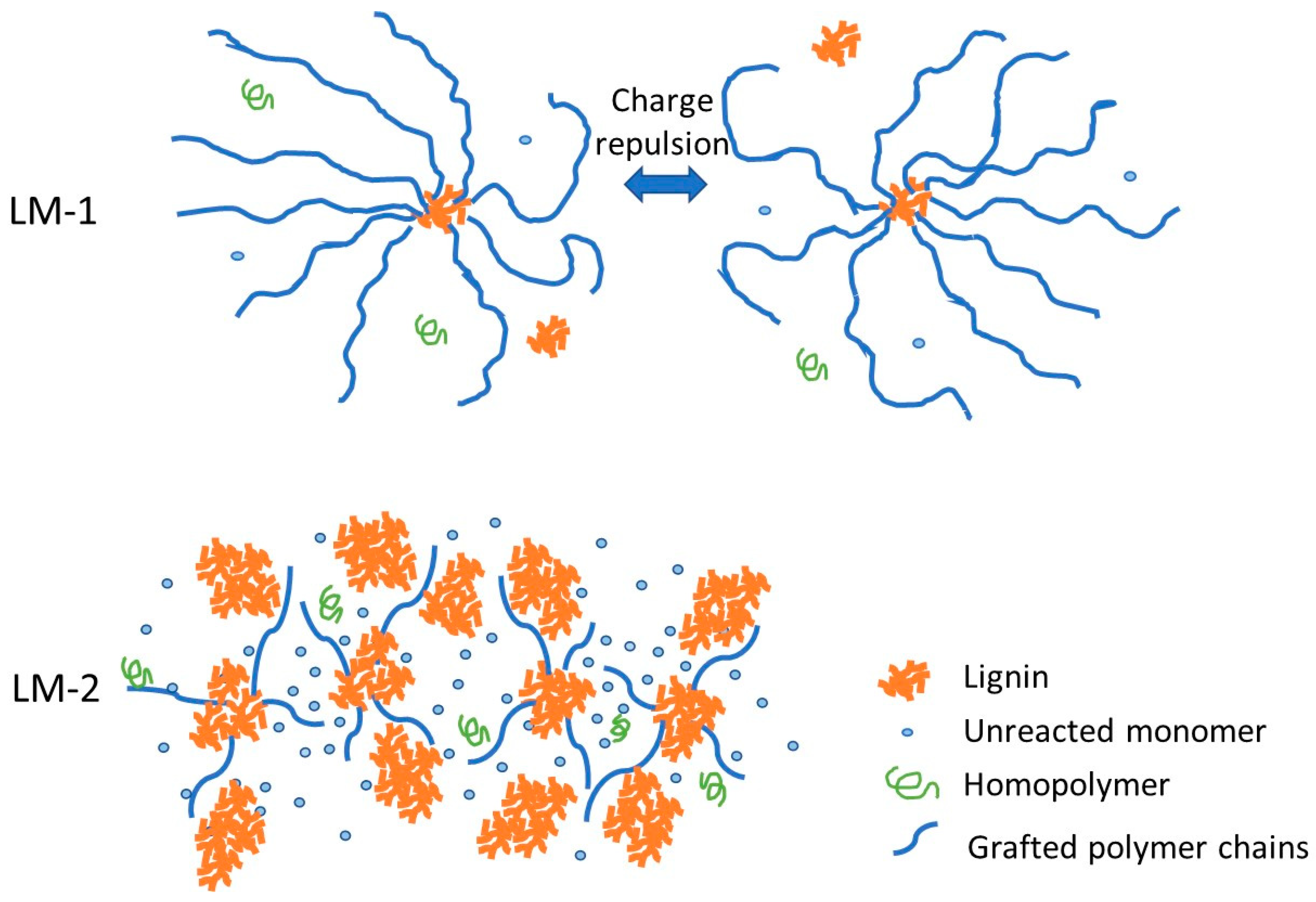
3.5. Microstructure of Reaction Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability
Conflicts of Interest
References
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K.H. Catalytic oxidation of lignin in solvent systems for production of renewable chemicals: A Review. Polymers 2017, 9, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luckeneder, P.; Gavino, J.; Kuchernig, R.; Petutschnigg, A.; Tondi, G. Sustainable phenolic fractions as basis for furfuryl alcohol-based co-polymers and their use as wood adhesives. Polymers 2016, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, G. Preparation and characterization of high surface area activated carbon fibers from lignin. Polymers 2016, 8, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharehkhani, S.; Zhang, Y.; Fatehi, P. Lignin-derived platform molecules through TEMPO catalytic oxidation strategies, Prog. Energy. Combust. Sci. 2019, 72, 59–89. [Google Scholar] [CrossRef]
- Gharehkhani, S.; Ghavidel, N.; Fatehi, P. Kraft lignin–tannic acid as a green stabilizer for oil/water emulsion. ACS Sustain. Chem. Eng. 2018, 7, 2370–2379. [Google Scholar] [CrossRef]
- Hasan, A.; Fatehi, P. Cationic kraft lignin-acrylamide copolymer as a flocculant for clay suspensions: (2) Charge density effect. Sep. Purif. Technol. 2019, 210, 963–972. [Google Scholar] [CrossRef]
- Gupta, C.; Sverdlove, M.J.; Washburn, N.R. Molecular architecture requirements for polymer-grafted lignin superplasticizers. Soft. Matter. 2015, 11, 2691–2699. [Google Scholar] [CrossRef] [Green Version]
- Mai, C.; Majcherczyk, A.; Hüttermann, A. Chemo-enzymatic synthesis and characterization of graft copolymers from lignin and acrylic compounds. Enzyme. Microb. Technol. 2000, 27, 167–175. [Google Scholar] [CrossRef]
- Chile, L.E.; Kaser, S.J.; Hatzikiriakos, S.G.; Mehrkhodavandi, P. Synthesis and thermorheological analysis of biobased lignin-graft-poly (lactide) copolymers and their blends. ACS Sustain. Chem. Eng. 2018, 6, 1650–1661. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, X.; Xie, J.; Feng, B.; Han, Q. Synthesis of a novel tunable lignin-based star copolymer and its flocculation performance in the treatment of kaolin suspension. Sep. Purif. Technol. 2019, 210, 355–363. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kadla, J.F. Preparation of a thermoresponsive lignin-based biomaterial through atom transfer radical polymerization. Biomacromolecules. 2010, 11, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kong, F.; Gao, W.; Fatehi, P. Novel process for generating cationic lignin based flocculant. Ind. Eng. Chem. Res. 2018, 57, 6595–6608. [Google Scholar] [CrossRef]
- Sifri, R.J.; Padilla-Vélez, O.; Coates, G.W.; Fors, B.P. Controlling the shape of molecular weight distributions in coordination polymerization and its ımpact on physical properties. JACS 2020, 142, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Demoz, A.; Mikula, R.J. Role of mixing energy in the flocculation of mature fine tailings. J. Environ. Eng. 2011, 138, 129–136. [Google Scholar] [CrossRef]
- Carreau, P.J.; Chhabra, R.P.; Cheng, J. Effect of rheological properties on power consumption with helical ribbon agitators. AIChE. J. 1993, 39, 1421–1430. [Google Scholar] [CrossRef]
- Cassagnau, P.; Gimenez, J.; Bounor-Legaré, V.; Michel, A. New rheological developments for reactive processing of poly (ε-caprolactone). C. R. Chim. 2006, 9, 1351–1362. [Google Scholar] [CrossRef]
- Cioffi, M.; Ganzeveld, K.J.; Hoffmann, A.C.; Janssen, L.P.B.M. A rheokinetic study of bulk free radical polymerization performed with a helical barrel rheometer. Polym. Eng. Sci. 2004, 44, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Ivankovic, M.; Incarnato, L.; Kenny, J.M.; Nicolais, L. Curing kinetics and chemorheology of epoxy/anhydride system. J. Appl. Polym. Sci. 2003, 90, 3012–3019. [Google Scholar] [CrossRef]
- Hoffmann, A.C.; Cioffi, M.; Janssen, L.P.B.M. Rheokinetics and the influence of shear rate on the Trommsdorff (gel) effect during free radical polymerization. Polym. Eng. Sci. 2001, 41, 595–602. [Google Scholar]
- Gimenez, J.; Cassagnau, P.; Michel, A. Bulk polymerization of ε-caprolactone: Rheological predictive laws. J. Rheol. 2000, 44, 527–547. [Google Scholar] [CrossRef]
- Bae, J.E.; Choi, J.S.; Cho, K.S. An empirical model for the viscosity of reactive polymeric fluids. Macromol. Res. 2018, 26, 484–492. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Rheokinetic analysis on the formation of metallo-polyurethanes based on hydroxyl-terminated polybutadiene. Eur. Polym. J. 2014, 50, 117–126. [Google Scholar] [CrossRef]
- Vulpe, R.; Le Cerf, D.; Dulong, V.; Popa, M.; Peptu, C.; Verestiuc, L.; Picton, L. Rheological study of in-situ crosslinkable hydrogels based on hyaluronanic acid. collagen and sericin. Mater. Sci. Eng. C. 2016, 69, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yu, L.; Simon, G.P.; Shen, S.; Xie, F.; Liu, H.; Chen, L.; Zhong, L. Rheokinetics of graft copolymerization of acrylamide in concentrated starch and rheological behaviors and microstructures of reaction products. Carbohydr. Polym. 2018, 192, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.; Fatehi, P. Water soluble kraft lignin–acrylic acid copolymer: synthesis and characterization. Green. Chem. 2015, 17, 4355. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Guo, J.; Li, F.; Fan, W.; Liao, Y.; Guan, Q. Characterization and evaluation of dewatering properties of PADB, a highly efficient cationic flocculant. Ind. Eng. Chem. Res. 2014, 53, 2572–2582. [Google Scholar] [CrossRef]
- Pal, S.; Mal, D.; Singh, R.P. Cationic starch: an effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Wang, S.; Kong, F.; Fatehi, P.; Hou, Q. Cationic High Molecular Weight Lignin Polymer: A Flocculant for the Removal of Anionic Azo-Dyes from Simulated Wastewater. Molecules 2018, 23, 2005. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process. Saf. Environ. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Q.; Kong, F.; Fatehi, P. Production of cationic xylan–METAC copolymer as a flocculant for textile industry. Carbohydr. Polym 2015, 124, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C. Molecular weight dependence of the relative viscosity of solutions of polymers at the critical concentration. Eur. Polym. J. 1977, 13, 739–741. [Google Scholar] [CrossRef]
- Hayahara, T.; Takao, S. Relationship between polymer concentration and molecular weight in the viscosity behavior of concentrated solution. Kolloid-Z. Z. Polym. 1968, 225, 106–111. [Google Scholar] [CrossRef]
- Li, M.; Jin, E.; Chen, S. Effects of graft modification on apparent viscosity, adhesion, and film properties of sesbania gum for warp sizing. J. Text. Inst. 2020, 111, 309–317. [Google Scholar] [CrossRef]
- Xu, J.; Bietz, J.A.; Felker, F.C.; Carriere, C.J.; Wirtz, D. Rheological properties of vital wheat gluten suspensions. Cereal. Chem. 2001, 78, 181–185. [Google Scholar] [CrossRef]
- Jia, Y.; Zheng, M.; Xu, Q.; Zhong, C. Rheological behaviors of Pickering emulsions stabilized by TEMPO-oxidized bacterial cellulose. Carbohydr. Polym. 2019, 215, 263–271. [Google Scholar] [CrossRef]
- Ghoshal, G.; Shivhare, U.S.; Banerjee, U.C. Rheological properties and microstructure of xylanase containing whole wheat bread dough. J. Food Sci. Technol. 2017, 54, 1928–1937. [Google Scholar] [CrossRef]
- Soares, B.G.; Riany, N.; Silva, A.A.; Barra, G.M.; Livi, S. Dual-role of phosphonium–based ionic liquid in epoxy/MWCNT systems: Electric, rheological behavior and electromagnetic interference shielding effectiveness. Eur. Polym. J. 2016, 84, 77–88. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Nakka, J.S.; Jansen, K.M.B.; Ernst, L.J. Effect of chain flexibility in the network structure on the viscoelasticity of epoxy thermosets. J. Polym. Res. 2011, 18, 1879–1888. [Google Scholar] [CrossRef] [Green Version]
- Everaers, R.; Sukumaran, S.K.; Grest, G.S.; Svaneborg, C.; Sivasubramanian, A.; Kremer, K. Rheology and microscopic topology of entangled polymeric liquids. Science 2004, 303, 823–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Heuzey, M.C.; Bégin, A.; Carreau, P.J. Viscoelastic properties of chitosan solutions: Effect of concentration and ionic strength. J. Food. Eng. 2006, 74, 500–515. [Google Scholar] [CrossRef]
- Kosmas, M.K. On the mean radius of gyration of a polymer chain. J. Phys. A 1981, 14, 2779. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Aseyev, V.; von Haartman, E.; Karaman, D.Ş.; Mäkilä, E.; Tenhu, H.; Rosenholm, J.; Salonen, J. Size, stability, and porosity of mesoporous nanoparticles characterized with light scattering. Nanoscale Res. Lett. 2017, 12, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, H.R.; Phillips, D.J.; Wilson, N.R.; Gibson, M.I.; Rourke, J.P. One-step grafting of polymers to graphene oxide. Polym. Chem. 2015, 6, 8270–8274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MIu, L.; Bogatyreva, N.S.; Galzitskaia, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar]
- Graessley, W.W. Effect of long branches on the flow properties of polymers. Acc. Chem. Res. 1977, 10, 332–339. [Google Scholar] [CrossRef]
- Kabanemi, K.K.; Hétu, J.F. A reptation-based model to the dynamics and rheology of linear entangled polymers reinforced with nanoscale rigid particles. J. Non-Newton. Fluid. Mech. 2010, 165, 866–878. [Google Scholar] [CrossRef] [Green Version]



| Sample | Temperature (°C) | METAC/Lignin Molar Ratio | Shear Rate (s−1) | Molecular Weight (Kg/mol) | Charge Density (meq/g) | Grafting Ratio (%) |
|---|---|---|---|---|---|---|
| LM-1 | 80 | 2.3 | 385 | 990.5 | 3.8 | 137.2 |
| LM-3 | 70 | 2.3 | 385 | 625.8 | 3.1 | 98.9 |
| LM-4 | 60 | 2.3 | 385 | - * | 1.4 | 41.5 |
| LM-5 | 80 | 2 | 385 | 823.4 | 3.4 | 103.3 |
| LM-6 | 80 | 1.8 | 385 | 512.4 | 2.2 | 79.5 |
| LM-7 | 80 | 2.3 | 130 | 798.5 | 3.2 | 101.2 |
| LM-8 | 70 | 2.3 | 130 | 524.1 | 2.9 | 84.3 |
| Molar Ratio of 1.8 | Molar Ratio of 2.3 | |||||
|---|---|---|---|---|---|---|
| Time s | Molecular Weight (kg/mol) | Grafting Ratio (%) | Charge Density (meq/g) | Molecular Weight (Kg/mol) | Grafting Ratio (%) | Charge Density (meq/g) |
| 500 | - * | 27.4 | 1.0 | - * | 59.8 | 1.8 |
| 1800 | - * | 46.7 | 1.1 | - * | 84.5 | 2.0 |
| 3600 | - * | 58.5 | 1.6 | 520.7 | 98.5 | 2.5 |
| 10,000 | 512.4 | 79.5 | 2.2 | 990.5 | 137.2 | 3.8 |
| Sample | Unreacted Lignin (%) | Unreacted METAC (%) | Phenolic Hydroxyl Content (mmol/g) | Solubility (%) |
|---|---|---|---|---|
| LM-1 | 0.8 | 10.2 | 1.45 | 97% |
| LM-2 | 6.5 | 48.5 | 1.67 | 70% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharehkhani, S.; Gao, W.; Fatehi, P. In-Situ Rheological Studies of Cationic Lignin Polymerization in an Acidic Aqueous System. Polymers 2020, 12, 2982. https://doi.org/10.3390/polym12122982
Gharehkhani S, Gao W, Fatehi P. In-Situ Rheological Studies of Cationic Lignin Polymerization in an Acidic Aqueous System. Polymers. 2020; 12(12):2982. https://doi.org/10.3390/polym12122982
Chicago/Turabian StyleGharehkhani, Samira, Weijue Gao, and Pedram Fatehi. 2020. "In-Situ Rheological Studies of Cationic Lignin Polymerization in an Acidic Aqueous System" Polymers 12, no. 12: 2982. https://doi.org/10.3390/polym12122982
APA StyleGharehkhani, S., Gao, W., & Fatehi, P. (2020). In-Situ Rheological Studies of Cationic Lignin Polymerization in an Acidic Aqueous System. Polymers, 12(12), 2982. https://doi.org/10.3390/polym12122982







