Fabrication of Highly Porous Polymeric Nanocomposite for the Removal of Radioactive U(VI) and Eu(III) Ions from Aqueous Solution
Abstract
1. Introduction
2. Experimental
2.1. Materials
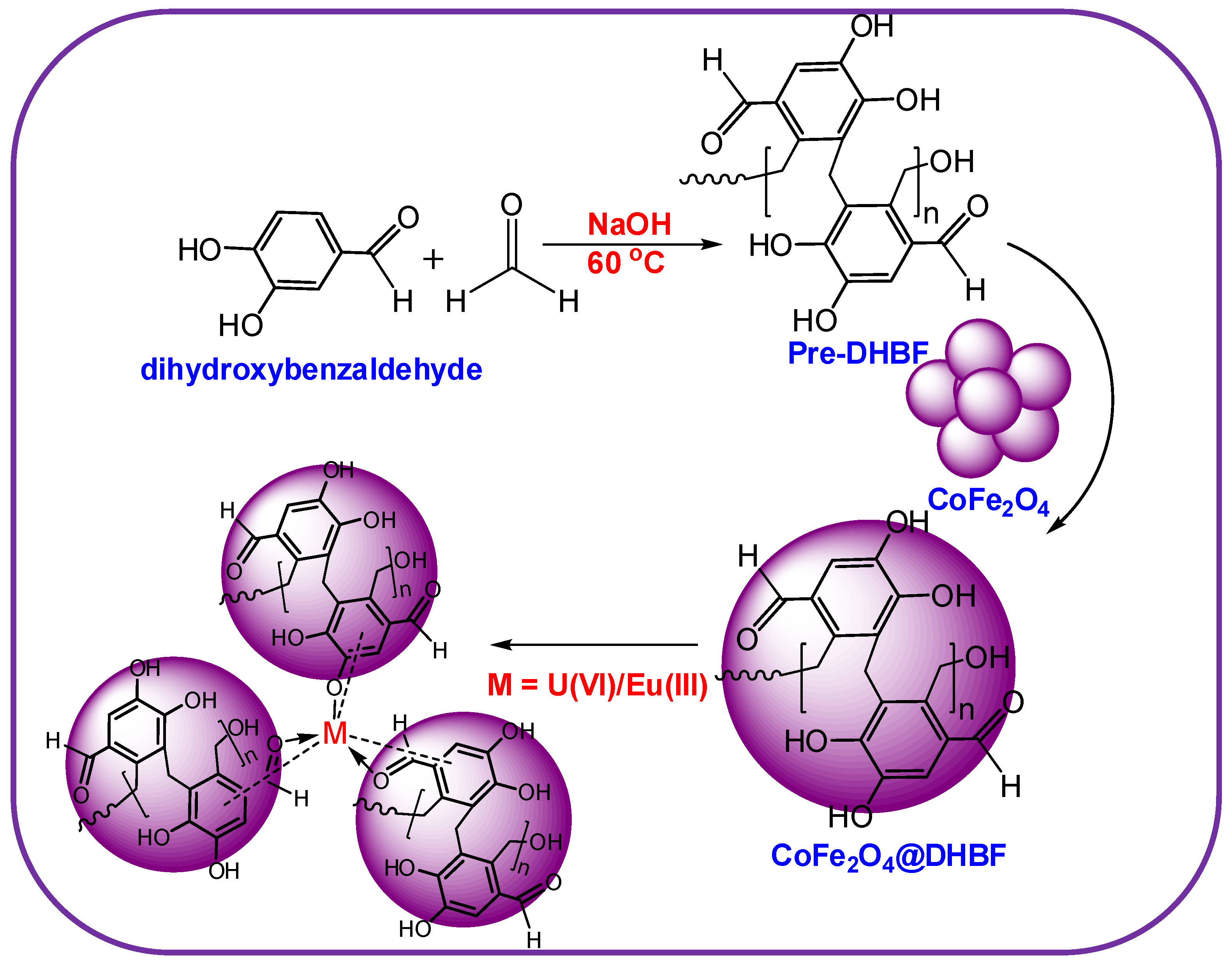
2.2. Fabrication of the Nanocomposite
3. Results and Discussion
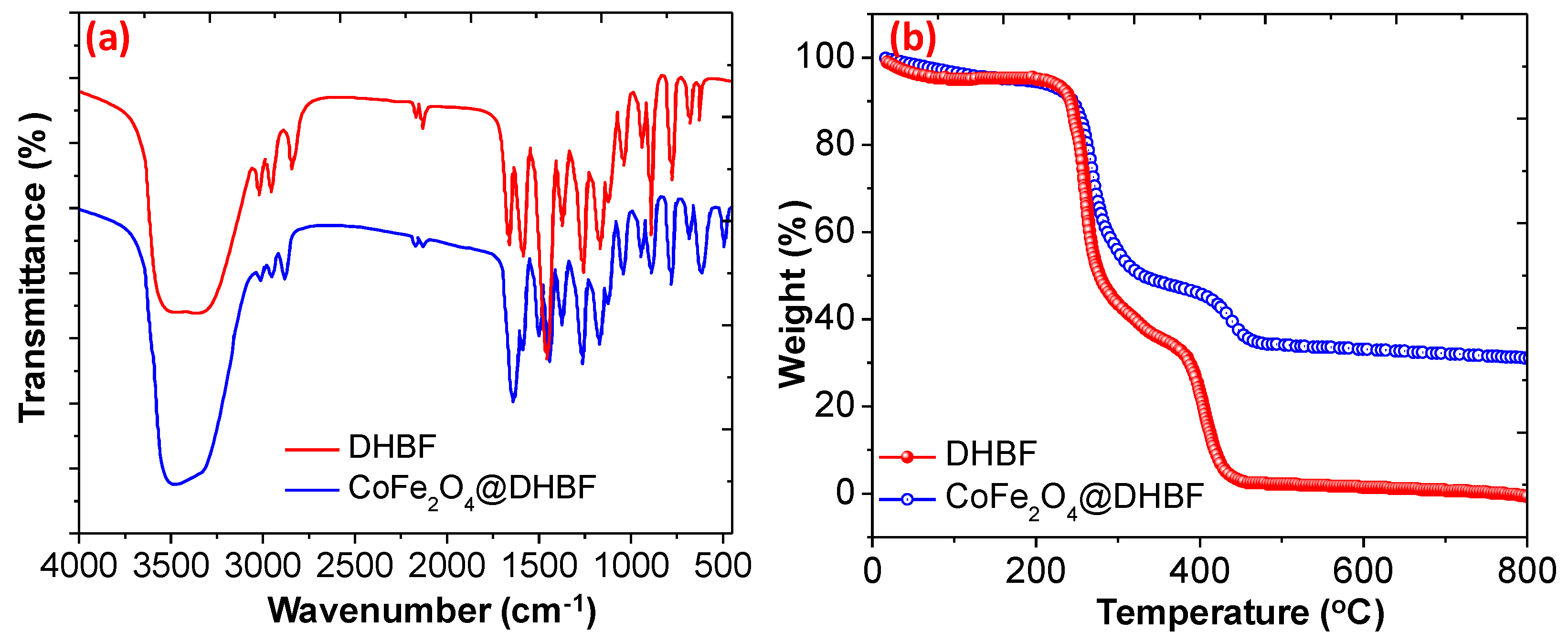
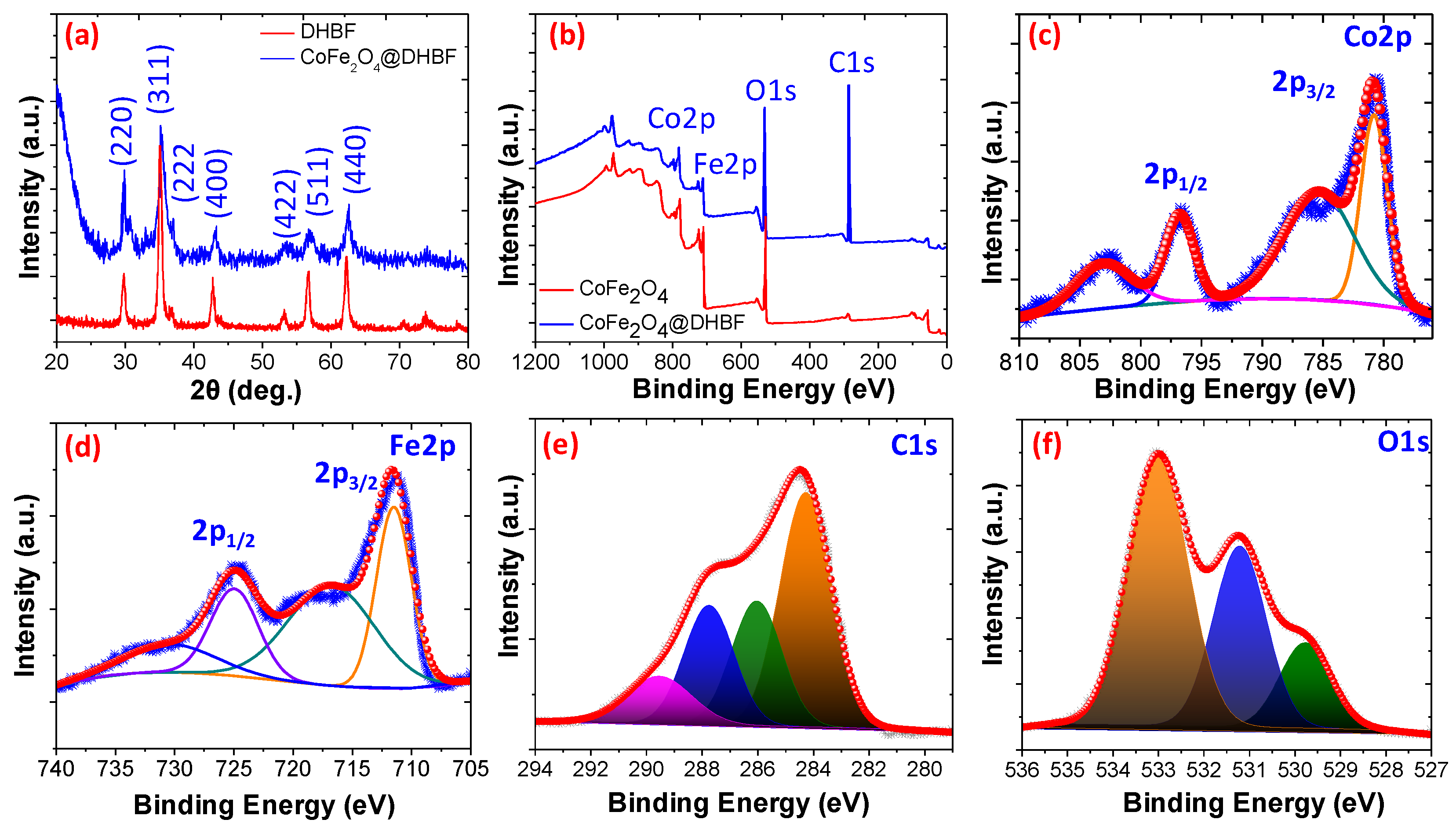
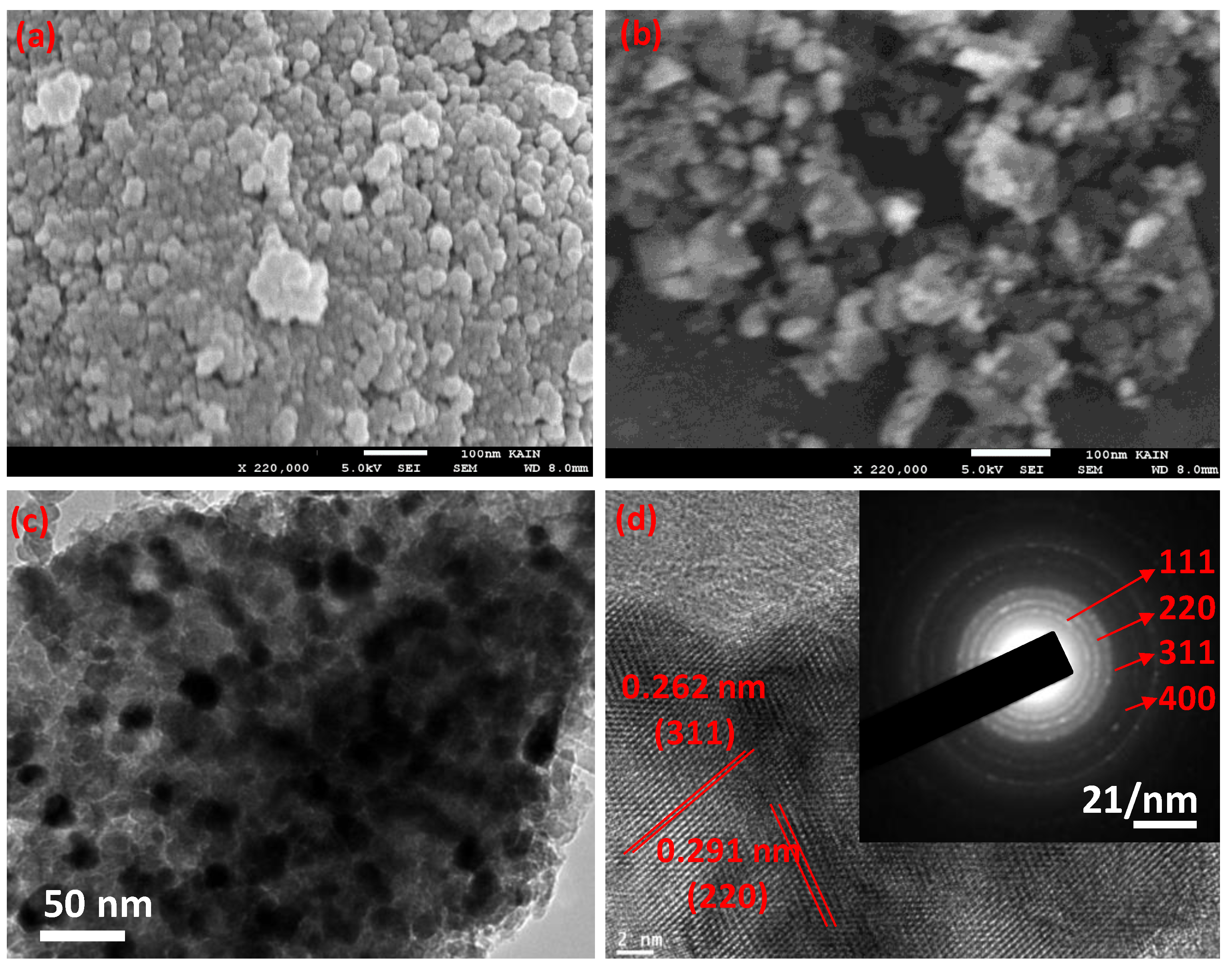
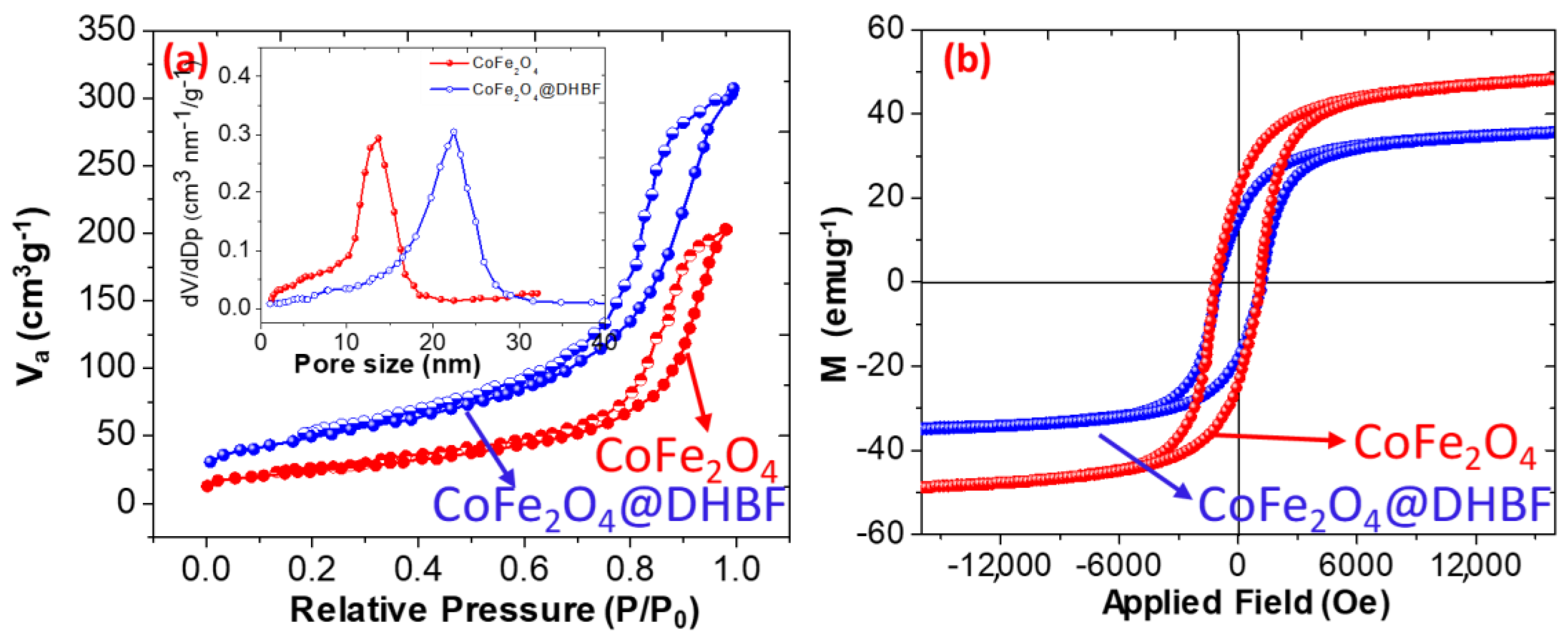
3.1. Characterization of the Nanocomposite
3.2. Batch Adsorption of the Radioactive Ions
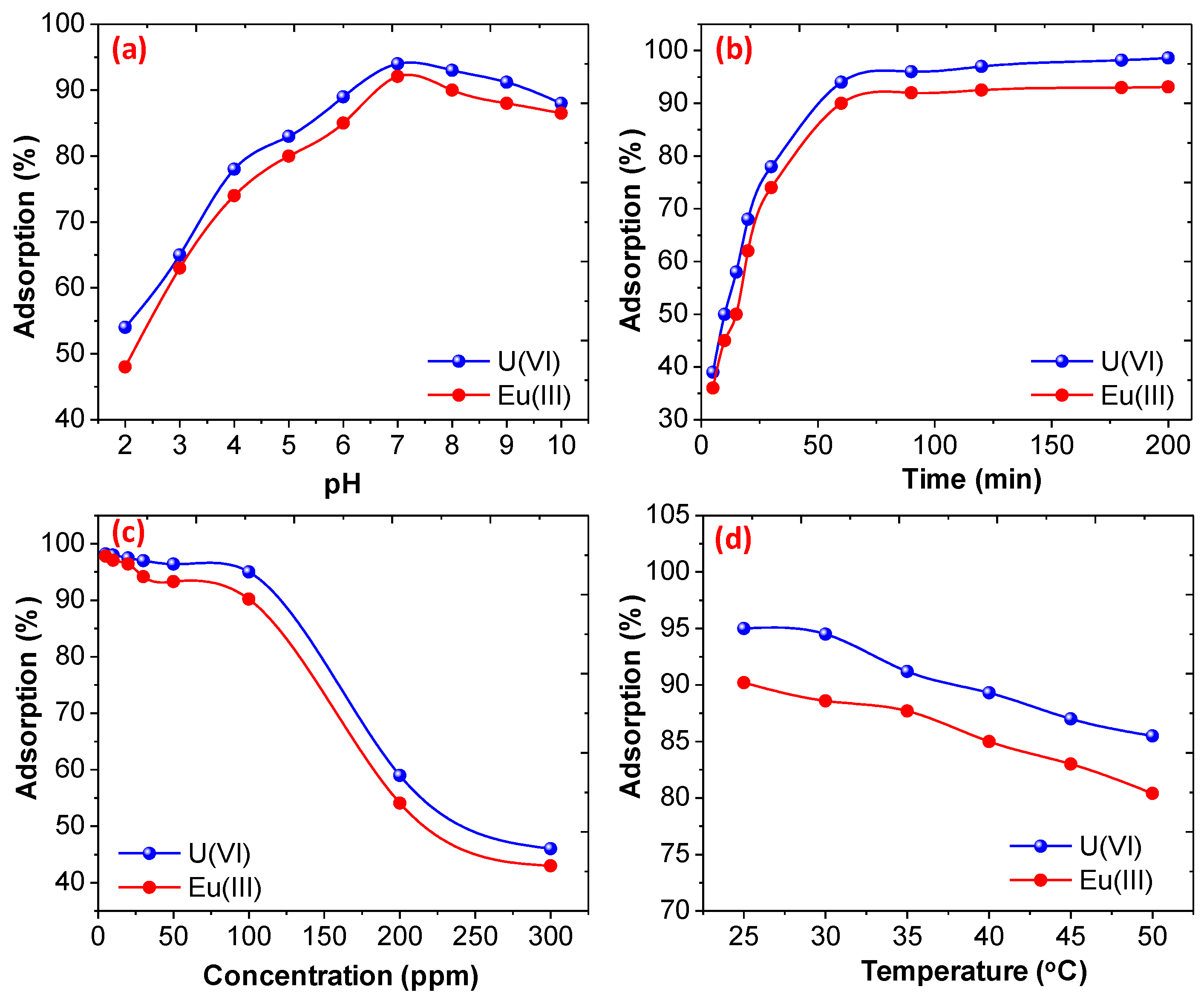
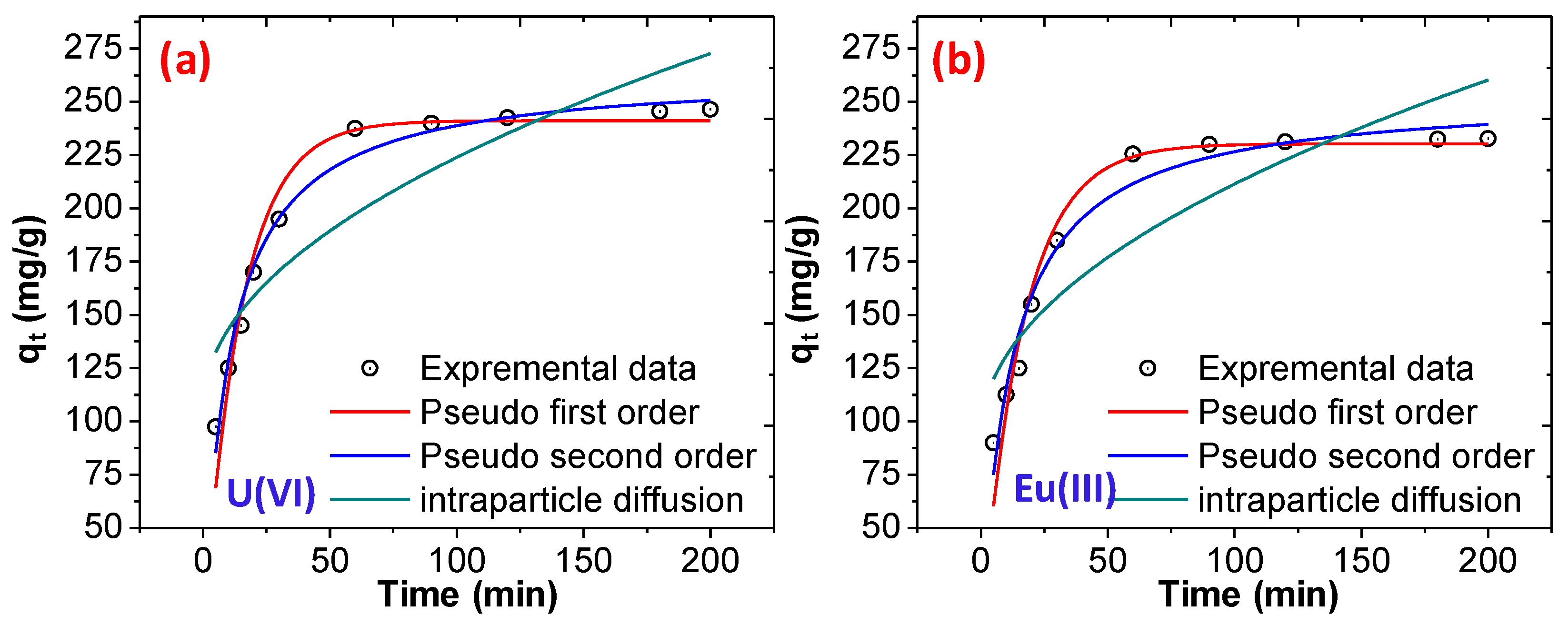
3.2.1. Effect of pH, Initial Concentration, Initial Contact Time
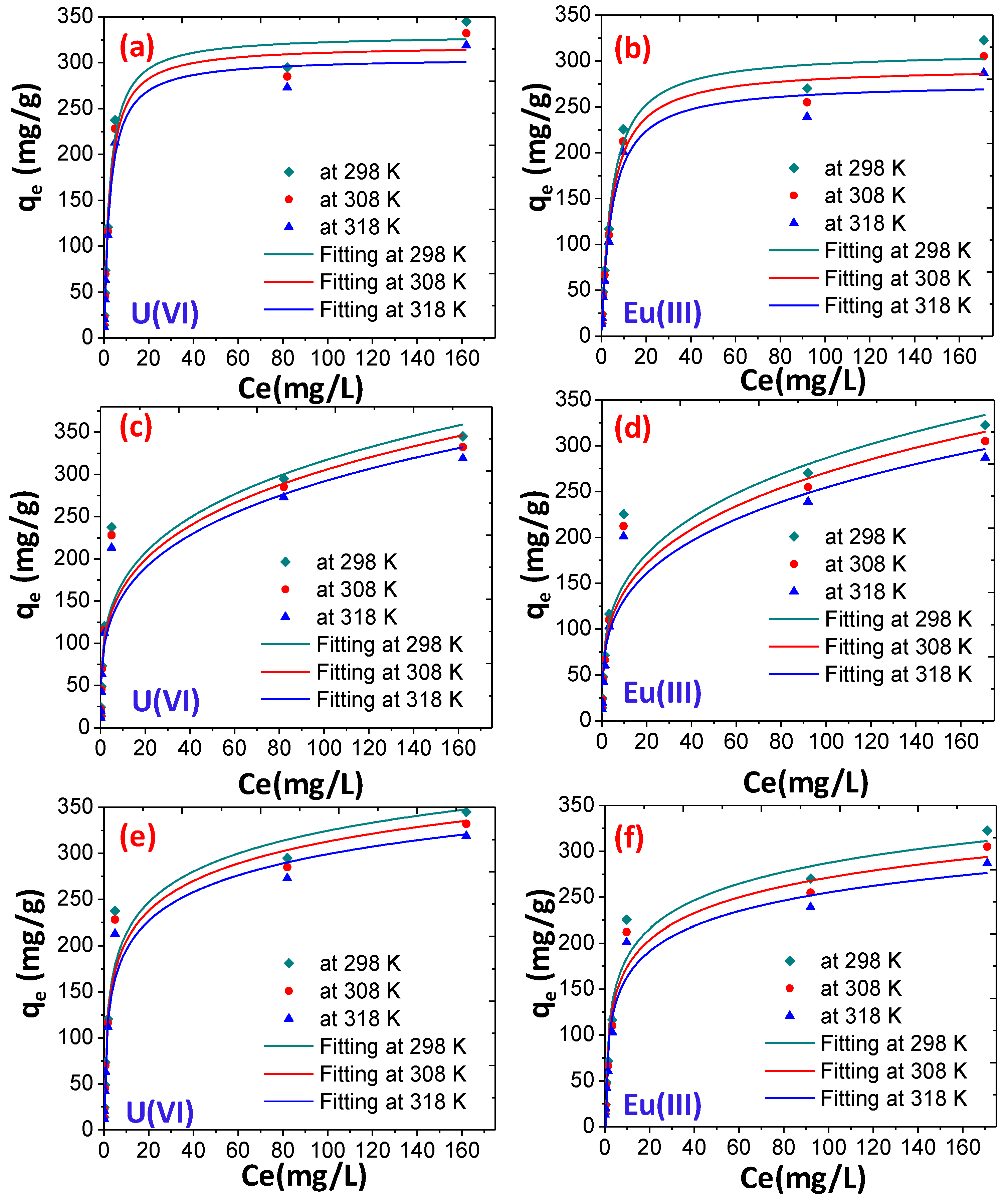
3.2.2. Adsorption Isotherms
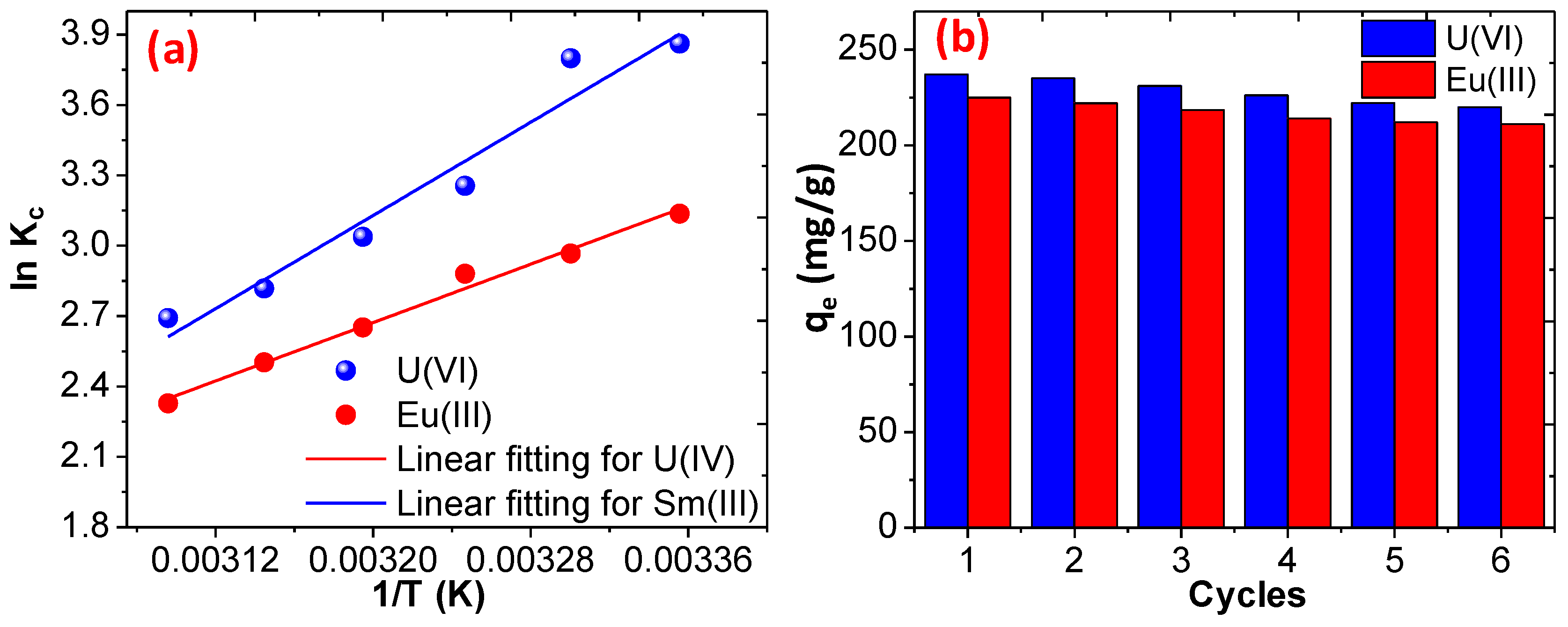
3.3. Reusability and Regeneration Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel Metal-Organic Framework (MOF) Based Composite Material for the Sequestration of U(VI) and Th(IV) Metal Ions from Aqueous Environment. ACS Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef] [PubMed]
- Asic, A.; Kurtovic-Kozaric, A.; Besic, L.; Mehinovic, L.; Hasic, A.; Kozaric, M.; Hukic, M.; Marjanovic, D. Chemical toxicity and radioactivity of depleted uranium: The evidence from in vivo and in vitro studies. Environ. Res. 2017, 156, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.Q.; Yuan, L.Y.; Zhu, L.; Liu, Z.R.; Chu, S.Q.; Zheng, L.R.; Zhang, J.; Chai, Z.F.; Shi, W.Q. Introduction of amino groups into acid-resistant MOFs for enhanced U(vi) sorption. J. Mater. Chem. A 2015, 3, 525–534. [Google Scholar] [CrossRef]
- Chen, L.; Yu, X.; Zhao, Z. Effect of humic acid, pH and ionic strength on the sorption of Eu(III) on red earth and its solid component. J. Radioanal. Nucl. Chem. 2007, 274, 187–193. [Google Scholar] [CrossRef]
- Dai, S.; Wang, N.; Qi, C.; Wang, X.; Ma, Y.; Yang, L.; Liu, X.; Huang, Q.; Nie, C.; Hu, B.; et al. Preparation of core-shell structure Fe3O4@C@MnO2 nanoparticles for efficient elimination of U(VI) and Eu(III) ions. Sci. Total Environ. 2019, 685, 986–996. [Google Scholar] [CrossRef]
- Guo, W.; Nie, C.; Wang, L.; Li, Z.; Zhu, L.; Zhu, L.; Zhu, Z.; Shi, W.; Yuan, L. Easily prepared and stable functionalized magnetic ordered mesoporous silica for efficient uranium extraction. Sci. China Chem. 2016, 59, 629–636. [Google Scholar] [CrossRef]
- Huang, S.; Pang, H.; Li, L.; Jiang, S.; Wen, T.; Zhuang, L.; Hu, B.; Wang, X. Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from MIL-53. Chem. Eng. J. 2018, 353, 157–166. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Zheng, L.; Zhou, L.; Chai, Z.; Wang, X.; Shi, W. Interaction mechanism of uranium(VI) with three-dimensional graphene oxide-chitosan composite: Insights from batch experiments, IR, XPS, and EXAFS spectroscopy. Chem. Eng. J. 2017, 328, 1066–1074. [Google Scholar] [CrossRef]
- Huang, Z.W.; Li, Z.J.; Wu, Q.Y.; Zheng, L.R.; Zhou, L.M.; Chai, Z.F.; Wang, X.L.; Shi, W.Q. Simultaneous elimination of cationic uranium(vi) and anionic rhenium(vii) by graphene oxide-poly(ethyleneimine) macrostructures: A batch, XPS, EXAFS, and DFT combined study. Environ. Sci. Nano 2018, 5, 2077–2087. [Google Scholar] [CrossRef]
- Dao, T.-H.; Vu, T.-Q.-M.; Nguyen, N.-T.; Pham, T.-T.; Nguyen, T.-L.; Yusa, S.-I.; Pham, T.-D. Adsorption Characteristics of Synthesized Polyelectrolytes onto Alumina Nanoparticles and their Application in Antibiotic Removal. Langmuir 2020, 36, 13001–13011. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Dao, T.H.; Truong, T.T.; Nguyen, T.M.T.; Pham, T.D. Adsorption characteristic of ciprofloxacin antibiotic onto synthesized alpha alumina nanoparticles with surface modification by polyanion. J. Mol. Liq. 2020, 309, 113150. [Google Scholar] [CrossRef]
- Pham, T.D.; Bui, T.T.; Trang Truong, T.T.; Hoang, T.H.; Le, T.S.; Duong, V.D.; Yamaguchi, A.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of beta-lactam cefixime onto nanosilica fabricated from rice HUSK with surface modification by polyelectrolyte. J. Mol. Liq. 2020, 298, 111981. [Google Scholar] [CrossRef]
- Dao, T.H.; Nguyen, N.T.; Nguyen, M.N.; Ngo, C.L.; Luong, N.H.; Le, D.B.; Pham, T.D. Adsorption behavior of polyelectrolyte onto alumina and application in ciprofloxacin removal. Polymers 2020, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Mathews, T.; Beaugelin-Seiller, K.; Garnier-Laplace, J.; Gilbin, R.; Adam, C.; Della-Vedova, C. A probabilistic assessment of the chemical and radiological risks of chronic exposure to uranium in freshwater ecosystems. Environ. Sci. Technol. 2009, 43, 6684–6690. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Regil, E.; Ortíz-Oliveros, H.B.; Fernández-Valverde, S.M.; Granados-Correa, F. Eu (III) sorption from an aqueous solution onto SrTiO3 and surface complex behavior. Chem. Eng. J. 2014, 254, 349–356. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Al-Shahrani, T.; Al-hokbany, N.; Alshehri, S.M. Preparation of chitosan based magnetic nanocomposite for tetracycline adsorption: Kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2020, 147, 258–267. [Google Scholar] [CrossRef]
- Ahamad, T.; Ruksana; Chaudhary, A.A.; Naushad, M.; Alshehri, S.M. Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. Int. J. Biol. Macromol. 2019, 134, 180–188. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Alqadami, A.A.; Khan, A. Synthesis and characterization of egg-albumen-formaldehyde based magnetic polymeric resin (MPR): Highly efficient adsorbent for Cd(II) ion removal from aqueous medium. J. Mol. Liq. 2019, 286, 110951. [Google Scholar] [CrossRef]
- Naushad, M.; Alqadami, A.A.; Ahamad, T. Removal of Cd(II) ion from aqueous environment using triaminotriethoxysilane grafted oxidized activated carbon synthesized via activation and subsequent silanization. Environ. Technol. Innov. 2020, 18, 100686. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Wang, L.; Fan, Q.; Pan, D.; Li, J.; Chi, F.; Xie, Y.; Yu, S.; Xiao, C.; et al. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci. China Chem. 2019, 62, 933–967. [Google Scholar] [CrossRef]
- Xie, Y.; Helvenston, E.M.; Shuller-Nickles, L.C.; Powell, B.A. Surface Complexation Modeling of Eu(III) and U(VI) Interactions with Graphene Oxide. Environ. Sci. Technol. 2016, 50, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Ghalami, Z.; Ghoulipour, V.; Khanchi, A. Highly efficient capturing and adsorption of cesium and strontium ions from aqueous solution by porous organic cage: A combined experimental and theoretical study. Appl. Surf. Sci. 2019, 471, 726–732. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Ueta, E.; Nakamura, T.; Kawasaki, N. Biomass potential of virgin and calcined tapioca (cassava starch) for the removal of Sr(II) and Cs(I) from aqueous solutions. Chem. Pharm. Bull. 2018, 66, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Bisla, V.; Rattan, G.; Singhal, S.; Kaushik, A. Green and novel adsorbent from rice straw extracted cellulose for efficient adsorption of Hg (II) ions in an aqueous medium. Int. J. Biol. Macromol. 2020, 161, 194–203. [Google Scholar] [CrossRef]
- Shi, X.; Qiao, Y.; An, X.; Tian, Y.; Zhou, H. High-capacity adsorption of Cr(VI) by lignin-based composite: Characterization, performance and mechanism. Int. J. Biol. Macromol. 2020, 159, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Barillet, S.; Adam-Guillermin, C.; Palluel, O.; Porcher, J.M.; Devaux, A. Uranium bioaccumulation and biological disorders induced in zebrafish (Danio rerio) after a depleted uranium waterborne exposure. Environ. Pollut. 2011, 159, 495–502. [Google Scholar] [CrossRef]
- Cai, Y.; Yuan, F.; Wang, X.; Sun, Z.; Chen, Y.; Liu, Z.; Wang, X.; Yang, S.; Wang, S. Synthesis of core–shell structured Fe3O4@carboxymethyl cellulose magnetic composite for highly efficient removal of Eu(III). Cellulose 2017, 24, 175–190. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, H.; Chen, S.; Long, F.; Huang, B.; Yang, B.; Pan, X. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem. Eng. J. 2019, 356, 69–80. [Google Scholar] [CrossRef]
- Chen, H.; Shao, D.; Li, J.; Wang, X. The uptake of radionuclides from aqueous solution by poly(amidoxime) modified reduced graphene oxide. Chem. Eng. J. 2014, 254, 623–634. [Google Scholar] [CrossRef]
- Chen, Z.; Xue, Z.; Chen, L.; Geng, Z.; Yang, R.; Chen, L.; Wang, Z. One-pot template-free synthesis of water-dispersive Fe3O4@C nanoparticles for adsorption of bovine serum albumin. New J. Chem. 2013, 37, 3731–3736. [Google Scholar] [CrossRef]
- Carvalho, T.; Pereira, A.d.S.; Bonomo, R.C.F.; Franco, M.; Finotelli, P.V.; Amaral, P.F.F. Simple physical adsorption technique to immobilize Yarrowia lipolytica lipase purified by different methods on magnetic nanoparticles: Adsorption isotherms and thermodynamic approach. Int. J. Biol. Macromol. 2020, 160, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.M.N.; Pereira, C.R.; Foletto, E.L.; Dotto, G.L. Alternative synthesis for ZnFe2O4/chitosan magnetic particles to remove diclofenac from water by adsorption. Int. J. Biol. Macromol. 2019, 131, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Lian, Z.; Xian, H.; Jiang, R.; Wang, N.; Weng, Y.; Peng, X.; Wang, S.; Ouyang, X.K. Adsorption of Pb(II) from aqueous solution by polyacrylic acid grafted magnetic chitosan nanocomposite. Int. J. Biol. Macromol. 2020, 154, 1537–1547. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Iakovleva, E.; Sillanpää, M. Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 2020, 164, 3621–3631. [Google Scholar] [CrossRef]
- Yu, S.; Cui, J.; Wang, J.; Zhong, C.; Wang, X.; Wang, N. Facile fabrication of Cu(II) coordinated chitosan-based magnetic material for effective adsorption of reactive brilliant red from aqueous solution. Int. J. Biol. Macromol. 2020, 149, 562–571. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Peng, Y.; Feng, L.; Li, X.; Zhao, C.; Sarfaraz, K. Novel cationic polymer modified magnetic chitosan beads for efficient adsorption of heavy metals and dyes over a wide pH range. Int. J. Biol. Macromol. 2020, 156, 289–301. [Google Scholar] [CrossRef]
- Gabal, M.A.; Kosa, S.; Almutairi, T.S. Cr-substitution effect on the structural and magnetic properties of nano-sized NiFe2O4 prepared via novel chitosan route. J. Magn. Magn. Mater. 2014, 356, 37–41. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zou, S.; Lu, C.; Bai, H.; Mu, H.; Duan, J. Removal and adsorption mechanism of tetracycline and cefotaxime contaminants in water by NiFe2O4-COF-chitosan-terephthalaldehyde nanocomposites film. Chem. Eng. J. 2020, 382, 123008. [Google Scholar] [CrossRef]
- Nishat, N.; Ahmad, S.; Tansir Ahamad, R. Synthesis and characterization of antibacterial polychelates of urea-formaldehyde resin with Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), and Zn(II) metal ions. J. Appl. Polym. Sci. 2006, 100, 928–936. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.-Y.; Fu, Y.-Q.; Zong, E.-M.; Jiang, S.-T.; Li, J.-B.; Zhu, J.-Q.; Zhu, Y.-Y. Magnetic NiFe2O4/MWCNTs functionalized cellulose bioadsorbent with enhanced adsorption property and rapid separation. Carbohydr. Polym. 2020, 252, 117158. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, W.; Chen, W.; Wang, J.; Yang, Q.; Zhu, W.; Wang, J. One-pot synthesis of NiFe2O4 integrated with EDTA-derived carbon dots for enhanced removal of tetracycline. Chem. Eng. J. 2017, 310, 187–196. [Google Scholar] [CrossRef]
- Deng, Y.; Zou, X.; Liu, Z.; Wang, J.; Wang, Z.; Tang, J. Co7Fe3/CoFe2O4@C Lamellar composites derived from Co–Fe LDH/PVA as an effective heterogeneous activator of peroxymonosulfate. J. Alloys Compd. 2021, 854, 157244. [Google Scholar] [CrossRef]
- Gan, L.; Zhong, Q.; Geng, A.; Wang, L.; Song, C.; Han, S.; Cui, J.; Xu, L. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Sci. Total Environ. 2019, 694, 133705. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, T.; Nishat, N. New antimicrobial epoxy-resin-bearing Schiff-base metal complexes. J. Appl. Polym. Sci. 2008, 107, 2280–2288. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Aldalbahi, A.; Al-Hajji, A.B.; Chaudhary, A.A.; in het Panhuis, M.; Alhokbany, N.; Ahamad, T. Development of carboxymethyl cellulose-based hydrogel and nanosilver composite as antimicrobial agents for UTI pathogens. Carbohydr. Polym. 2016, 138, 229–236. [Google Scholar] [CrossRef]
- Nishat, N.; Hasnain, S.; Ahmad, T.; Parveen, A. Synthesis, characterization, and biological evaluation of new polyester containing Schiff base metal complexes. J. Therm. Anal. Calorim. 2011, 105, 969–979. [Google Scholar] [CrossRef]
- Ikram, S.; Jacob, J.; Mahmood, K.; Mehboob, K.; Maheen, M.; Ali, A.; Amin, N.; Hussain, S.; Ashraf, F.; Ilyas, S.Z. A Kinetic study of Tb3+ and Dy3+ co-substituted CoFe2O4 spinel ferrites using temperature dependent XRD, XPS and SQUID measurements. Ceram. Int. 2020, 46, 15943–15948. [Google Scholar] [CrossRef]
- Kulkarni, P.; Balkrishna, R.G.; Ghosh, D.; Rawat, R.S.; Medwal, R.; Chowdari, B.V.R.; Karim, Z.; Reddy, M.V. Molten salt synthesis of CoFe2O4 and its energy storage properties. Mater. Chem. Phys. 2021, 257, 123747. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Zheng, Y.; Jing, T.; Tian, J.; Zheng, H.; Wang, N.; Nan, J.; Ma, J. Free-radical and surface electron transfer dominated bisphenol A degradation in system of ozone and peroxydisulfate co-activated by CoFe2O4-biochar. Appl. Surf. Sci. 2020, 147887, in press. [Google Scholar]
- Li, X.; Sun, Y.; Zong, Y.; Wei, Y.; Liu, X.; Li, X.; Peng, Y.; Zheng, X. Size-effect induced cation redistribution on the magnetic properties of well-dispersed CoFe2O4 nanocrystals. J. Alloys Compd. 2020, 841, 155710. [Google Scholar] [CrossRef]
- Tang, J.; Wang, K.; Lu, Y.; Liang, N.; Qin, X.; Tian, G.; Zhang, D.; Feng, S.; Yue, H. Mesoporous core–shell structure NiFe2O4@polypyrrole micro-rod with efficient electromagnetic wave absorption in C, X, Ku wavebands. J. Magn. Magn. Mater. 2020, 514, 167268. [Google Scholar] [CrossRef]
- Devi, R.; Gogoi, S.; Dutta, H.S.; Bordoloi, M.; Sanghi, S.K.; Khan, R. Au/NiFe2O4 nanoparticle-decorated graphene oxide nanosheets for electrochemical immunosensing of amyloid beta peptide. Nanoscale Adv. 2020, 2, 239–248. [Google Scholar] [CrossRef]
- Mansour, S.F.; Imam, N.G.; Goda, S.; Abdo, M.A. Constructive coupling between BiFeO3 and CoFe2O4; promising magnetic and dielectric properties. J. Mater. Res. Technol. 2020, 9, 1434–1446. [Google Scholar] [CrossRef]
- Praveena, M.G.; Kumar, A.S.; Kala, M.S.; Bhowmik, R.N.; Nair, S.S.; Thomas, S.; Anantharaman, M.R. Interface assisted strain-induced magnetoelectric coupling in core-shell nanostructures of CoFe2O4 @ZnO. J. Magn. Magn. Mater. 2020, 513, 167252. [Google Scholar] [CrossRef]
- Qasim, M.; Asghar, K.; Das, D. Preparation and characterization of CoFe2O4 and CoFe2O4@Albumen nanoparticles for biomedical applications. Ceram. Int. 2019, 45, 24971–24981. [Google Scholar] [CrossRef]
- Mahdikhah, V.; Ataie, A.; Babaei, A.; Sheibani, S.; Ow-Yang, C.W.; Abkenar, S.K. CoFe2O4/Fe magnetic nanocomposite: Exchange coupling behavior and microwave absorbing property. Ceram. Int. 2020, 46, 17903–17916. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Al-Maswari, B.M.; Ahmed, J.; Alothman, Z.A.; Alshehri, S.M.; Alqadami, A.A. Synthesis of a recyclable mesoporous nanocomposite for efficient removal of toxic Hg2+ from aqueous medium. J. Ind. Eng. Chem. 2017, 53, 268–275. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. Fabrication of magnetic polymeric resin for the removal of toxic metals from aqueous medium: Kinetics and adsorption mechanisms. J. Water Process Eng. 2020, 36, 101284. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ahamad, T. Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: Kinetics, isotherm and mechanism. J. Environ. Manag. 2018, 223, 29–36. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; AlOthman, Z.A.; Al-Muhtaseb, A.a.H. Green and eco-friendly nanocomposite for the removal of toxic Hg(II) metal ion from aqueous environment: Adsorption kinetics & isotherm modelling. J. Mol. Liq. 2019, 279, 1–8. [Google Scholar]
- Naushad, M.; Ahamad, T.; Sharma, G.; Al-Muhtaseb, A.a.H.; Albadarin, A.B.; Alam, M.M.; Alothman, Z.A.; Alshehri, S.M.; Ghfar, A.A. Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem. Eng. J. 2016, 300, 306–316. [Google Scholar] [CrossRef]
- Ahamad, T.; Ruksana; Naushad, M.; Al-Maswari, B.M.; Alshehri, S.M. Fabrication of highly porous adsorbent derived from bio-based polymer metal complex for the remediation of water pollutants. J. Clean. Prod. 2019, 208, 1317–1326. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; Abdullah Alothman, Z.; Alshehri, S.M.; Ghfar, A.A. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism. J. Clean. Prod. 2017, 156, 426–436. [Google Scholar] [CrossRef]
- Goyal, N.; Gao, P.; Wang, Z.; Cheng, S.; Ok, Y.S.; Li, G.; Liu, L. Nanostructured chitosan/molecular sieve-4A an emergent material for the synergistic adsorption of radioactive major pollutants cesium and strontium. J. Hazard. Mater. 2020, 392, 122494. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, X.; Wang, J. Biosorption of Sr2+ and Cs+ onto Undaria pinnatifida: Isothermal titration calorimetry and molecular dynamics simulation. J. Mol. Liq. 2020, 319, 114146. [Google Scholar] [CrossRef]
- Asgari, E.; Sheikhmohammadi, A.; Yeganeh, J. Application of the Fe3O4-chitosan nano-adsorbent for the adsorption of metronidazole from wastewater: Optimization, kinetic, thermodynamic and equilibrium studies. Int. J. Biol. Macromol. 2020, 164, 694–706. [Google Scholar] [CrossRef]
- Mohammadabadi, S.I.; Javanbakht, V. Lignin extraction from barley straw using ultrasound-assisted treatment method for a lignin-based biocomposite preparation with remarkable adsorption capacity for heavy metal. Int. J. Biol. Macromol. 2020, 164, 1133–1148. [Google Scholar] [CrossRef]
| Metal Ions | Isotherm Models | Parameters | Temperature (°C) | ||
|---|---|---|---|---|---|
| 25 | 35 | 45 | |||
| U(VI) | Langmuir model | qm (mg·g−1) | 330.63 | 319.01 | 3.5.81 |
| KL (L·mg−1) | 0.3940 | 0.3879 | 0.3730 | ||
| R2 | 0.9920 | 0.9929 | 0.9952 | ||
| Freundlich model | Kf (mg1−1/n·L1/n·g−1) | 94.24 | 90.03 | 89.46 | |
| n | 3.80 | 3.77 | 3.71 | ||
| R2 | 0.8670 | 0.8669 | 0.8750 | ||
| Temkinmodel | Kt (L/g) | 8.31 | 8.10 | 7.76 | |
| Bt | 48.27 | 46.71 | 44.95 | ||
| R2 | 0.9579 | 0.9487 | 0.9643 | ||
| Eu (III) | Langmuir model | qm (mg·g−1) | 310.70 | 293.72 | 276.40 |
| KL (L·mg−1) | 0.2152 | 0.2132 | 0.2113 | ||
| R2 | 0.9913 | 0.9912 | 0.9901 | ||
| Freundlich model | Kf (mg1−1/n·L1/n·g−1) | 77.76 | 73.19 | 68.34 | |
| n | 3.53 | 3.52 | 3.50 | ||
| R2 | 0.8969 | 0.9010 | 0.8967 | ||
| Temkinmodel | Kt (L/g) | 6.34 | 6.32 | 6.19 | |
| Bt | 44.54 | 42.06 | 39.67 | ||
| R2 | 0.9478 | 0.9472 | 0.9439 | ||
| Metal Ions | Kinetic Models | Parameters | |
|---|---|---|---|
| U(VI) | PFO model | qe (mg·g−1) | 241.16 |
| k1 (min−1) | 0.067 | ||
| R2 | 0.9510 | ||
| PSO model | qe (mg·g−1) | 263.89 | |
| k2 (g·mg−1·min−1) | 3.61 × 10−4 | ||
| R2 | 0.9907 | ||
| Intra-particle diffusion | C | 106.15 | |
| Kdif (mg g−1 min−1/2) | 11.77 | ||
| R2 | 0.9514 | ||
| Eu(III) | PFO model | qe (mg·g−1) | 230.31 |
| k1 (min−1) | 0.060 | ||
| R2 | 0.9524 | ||
| PSO model | qe (mg·g−1) | 253.31 | |
| k2 (g·mg−1·min−1) | 3.30 × 10−4 | ||
| R2 | 0.9912 | ||
| Intra-particle diffusion | C | 93.58 | |
| Kdif (mg g−1 min−1/2) | 11.78 | ||
| R2 | 0.8795 | ||
| Temperature (K) | U(VI) | Eu(III) | ||||
|---|---|---|---|---|---|---|
| Entropy (ΔS) | Enthalpy (ΔH) | Gibbs Free Energy (ΔG) | Entropy (ΔS) | Enthalpy (ΔH) | Gibbs Free Energy (ΔG) | |
| 298 | −0.0605 | −25.87 | −7.82 | −0.107 | −41.79 | −9.64 |
| 303 | −0.0605 | −25.87 | −7.51 | −0.107 | −41.79 | −9.10 |
| 308 | −0.0605 | −25.87 | −7.21 | −0.107 | −41.79 | −8.56 |
| 313 | −0.0605 | −25.87 | −6.91 | −0.107 | −41.79 | −8.02 |
| 318 | −0.0605 | −25.87 | −6.60 | −0.107 | −41.79 | −7.48 |
| 323 | −0.0605 | −25.87 | −6.30 | −0.107 | −41.79 | −6.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahamad, T.; Naushad, M.; Ubaidullah, M.; Alshehri, S. Fabrication of Highly Porous Polymeric Nanocomposite for the Removal of Radioactive U(VI) and Eu(III) Ions from Aqueous Solution. Polymers 2020, 12, 2940. https://doi.org/10.3390/polym12122940
Ahamad T, Naushad M, Ubaidullah M, Alshehri S. Fabrication of Highly Porous Polymeric Nanocomposite for the Removal of Radioactive U(VI) and Eu(III) Ions from Aqueous Solution. Polymers. 2020; 12(12):2940. https://doi.org/10.3390/polym12122940
Chicago/Turabian StyleAhamad, Tansir, Mu. Naushad, Mohd Ubaidullah, and Saad Alshehri. 2020. "Fabrication of Highly Porous Polymeric Nanocomposite for the Removal of Radioactive U(VI) and Eu(III) Ions from Aqueous Solution" Polymers 12, no. 12: 2940. https://doi.org/10.3390/polym12122940
APA StyleAhamad, T., Naushad, M., Ubaidullah, M., & Alshehri, S. (2020). Fabrication of Highly Porous Polymeric Nanocomposite for the Removal of Radioactive U(VI) and Eu(III) Ions from Aqueous Solution. Polymers, 12(12), 2940. https://doi.org/10.3390/polym12122940