Biorefinery Approach for Aerogels
Abstract
:1. Introduction
2. Green Chemistry and Sustainability in the Manufacture of Bio-Based Aerogels
3. Case Studies
3.1. Aerogels from Lignocellulose
3.1.1. Aerogels from Cellulose
- It is a linear polymer as shown in Figure 3.
- Due to the numerous intra- and intermolecular hydrogen bonds, cellulose can be organised in crystals or be less ordered (amorphous). Crystal forms, allomorphs, also can be different. The majority of cellulose is organised in allomorph called “cellulose I” existing in native celluloses (plants, wood, bacteria). Next is “cellulose II” which is cellulose precipitated (or coagulated, or regenerated) from a solution or obtained by a treatment (swelling) in strong alkali (mercerisation). A detailed review on cellulose solvents can be found in [65]. Other cellulose allomorphs, cellulose III and IV, are obtained under special treatments.
- Cellulose macromolecules can be organized in “nanocellulose” which can be in the form of: (a) flexible nanofibers (cellulose nanofibers, CNF) and (b) crystals or whiskers (cellulose nanocrystals, CNC). Bacterial cellulose is also one of the types of nanocellulose.
- Microcrystalline cellulose (MCC) consists of highly crystalline cellulose I particles of few tens of microns in length and low aspect ratio. MCC often serves as a starting matter of cellulose II based aerogels as it is high purity low molecular weight cellulose which is rather easy to dissolve.
- Natural fibers extracted from wood or plants are often called “cellulose fibers” despite that they contain hemicelluloses, lignin and other natural components (waxes, pectin and inorganic molecules). The composition of natural fibers strongly depends on the type of plant or wood from which they are extracted and on the extraction steps (for example, delignification). “Cellulose fibers” can also be called “pulp” or “pulp fibers”; in the latter cases this concerns fibers extracted from wood.
- Finally, “cellulosic polymers” may be used to name cellulose ethers and esters. Chemical modification of cellulose leads to completely different polymer properties. For example, contrary to cellulose polymer, cellulose ethers can be water-soluble (for example, carboxymethyl cellulose) and cellulose esters can be thermoplastic (for example, cellulose acetate).
Cellulose I Based Aerogels
CNF-Aerogels
CNC-Aerogels
BC-Aerogels
Nanocellulose Aerogels Application Perspectives
Cellulose II Aerogels (via Dissolution-Coagulation Route)
Cellulose II Aerogels Application Perspectives
3.1.2. Lignin Aerogels
3.1.3. Hemicelluloses Aerogels
- Pentoses (C5H8O4)n (l-arabinose, d-xylose)
- Hexoses (C6H10O5)n (d-galactose, d-glucose, d-mannose)
- Uronic acids (d-glucuronic acid, d-galacturonic acid)
3.1.4. Aerogels from Entire Lignocellulose
3.1.5. Functionalization as a Tool to Enhance the Properties of Lignocellulose Porous Materials for Environmental Applications
3.2. Aerogels from Marine Polysaccharides
3.2.1. Aerogels from Alginate
3.2.2. Aerogels from Carrageenan
3.3. Aerogels from Chitosan
3.4. Aerogels from Pectin
3.5. Aerogels from Starch
3.6. Aerogels from Proteins
3.7. Aerogels from Organic Acids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nat. Cell Biol. 1931, 127, 741. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded-Aerogels. J. Phys. Chem. 1932, 36, 52–64. [Google Scholar] [CrossRef]
- Teichner, S.J.; Nicolaon, G.A. Method of Preparing Inorganic Aerogels. U.S. Patent 3,672,833, 27 June 1972. [Google Scholar]
- Smith, D.M.; Maskara, A.; Boes, U. Aerogel-based thermal insulation. J. Non-Cryst. Solids 1998, 225, 254–259. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.C.; Meador, M.A.B.; McCorkle, L.; Mueller, C.; Wilmoth, N. Synthesis and Properties of Step-Growth Polyamide Aerogels Cross-linked with Triacid Chlorides. Chem. Mater. 2014, 26, 4163–4171. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Alemán, C.R.; Hanson, K.; Ramirez, N.; Vivod, S.L.; Wilmoth, N.; McCorkle, L. Polyimide Aerogels with Amide Cross-Links: A Low Cost Alternative for Mechanically Strong Polymer Aerogels. ACS Appl. Mater. Interfaces 2015, 7, 1240–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigacci, A.; Marechal, J.; Repoux, M.; Moreno, M.; Achard, P. Preparation of polyurethane-based aerogels and xerogels for thermal superinsulation. J. Non-Cryst. Solids 2004, 350, 372–378. [Google Scholar] [CrossRef]
- Salerno, A.; Pascual, C.D. Bio-based polymers, supercritical fluids and tissue engineering. Process. Biochem. 2015, 50, 826–838. [Google Scholar] [CrossRef]
- Buwalda, S.J. Bio-based composite hydrogels for biomedical applications. Multifunct. Mater. 2020, 3, 022001. [Google Scholar] [CrossRef]
- Pantić, M.; Horvat, G.; Knez, Ž.; Novak, Z. Preparation and Characterization of Chitosan-Coated Pectin Aerogels: Curcumin Case Study. Molecules 2020, 25, 1187. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Ruíz, A.; Escobar-García, D.M.; Quintana, M.; Pozos-Guillen, A.; Flores, H. Synthesis and Characterization of a New Collagen-Alginate Aerogel for Tissue Engineering. J. Nanomater. 2019, 2019, 2875375. [Google Scholar] [CrossRef] [Green Version]
- Raman, S.; Keil, C.; Dieringer, P.; Hübner, C.; Bueno, A.; Gurikov, P.; Nissen, J.; Holtkamp, M.; Karst, U.; Haase, H.; et al. Alginate aerogels carrying calcium, zinc and silver cations for wound care: Fabrication and metal detection. J. Supercrit. Fluids 2019, 153, 104545. [Google Scholar] [CrossRef]
- Edwards, J.V.; Fontenot, K.R.; Liebner, F.W.; Condon, B.D. Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity. Sensors 2018, 18, 2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Groult, S.; Budtova, T. Thermal conductivity/structure correlations in thermal super-insulating pectin aerogels. Carbohydr. Polym. 2018, 196, 73–81. [Google Scholar] [CrossRef]
- Chtchigrovsky, M.; Lin, Y.; Ouchaou, K.; Chaumontet, M.; Robitzer, M.; Quignard, F.; Taran, F. Dramatic Effect of the Gelling Cation on the Catalytic Performances of Alginate-Supported Palladium Nanoparticles for the Suzuki–Miyaura Reaction. Chem. Mater. 2012, 24, 1505–1510. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Bernard, I.; Marin, M.A.; Ward, K.R.; McHugh, M.A. Superabsorbent alginate aerogels. J. Supercrit. Fluids 2013, 79, 202–208. [Google Scholar] [CrossRef]
- Soorbaghi, F.P.; Isanejad, M.; Salatin, S.; Ghorbani, M.; Jafari, S.; Derakhshankhah, H. Bioaerogels: Synthesis approaches, cellular uptake, and the biomedical applications. Biomed. Pharm. 2019, 111, 964–975. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-J.; Yuen, A.C.Y.; Li, A.; Lin, B.; Chen, T.B.Y.; Yang, W.; Lu, H.-D.; Yeoh, G.H. Recent progress in bio-based aerogel absorbents for oil/water separation. Cellulose 2019, 26, 6449–6476. [Google Scholar] [CrossRef]
- Illera, D.; Mesa, J.; Gomez, H.; Maury, H. Cellulose Aerogels for Thermal Insulation in Buildings: Trends and Challenges. Coatings 2018, 8, 345. [Google Scholar] [CrossRef] [Green Version]
- Cherubini, F.; Jungmeier, G.; Mandl, M.; Philips, C.; Wellisch, M.; Jrgensen, H.; Skiadas, I.; Boniface, L.; Dohy, M.; Pouet, J. IEA Bioenergy Task 42 on Biorefineries: Co-Production of Fuels, Chemicals, Power and Materials from Biomass. IEA Bioenergy Task. 2007, pp. 1–37. Available online: https://www.ieabioenergy.com/wp-content/uploads/2013/10/Task-42-Booklet.pdf (accessed on 24 November 2020).
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Clark, J.H.; Deswarte, F.E.I. The Biorefinery Concept-An Integrated Approach. Introd. Chem. Biomass 2008, 1–20. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Env. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; the “Gold Book” XML on-line corrected version: http://goldbook.iupac.org, 2006, created by Nic, M., Jirat, J., Kosata, B.; updates compiled by Jenkins, A.; Last update 2014-02-24; version: 2.3.3; Blackwell Scientific Publications: Oxford, UK, 2014; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]
- Pierre, A.C. History of Aerogels. In Aerogels Handbook; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2011; pp. 3–18. [Google Scholar]
- Available online: https://www.Epa.Gov/Greenchemistry/Basics-Green-Chemistry (accessed on 20 October 2020).
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ran, Y.; Xi, J.; Wang, J. Polymeric hybrid aerogels and their biomedical applications. Soft Matter 2020, 16, 9160–9175. [Google Scholar] [CrossRef]
- Rudaz, C.; Courson, R.; Bonnet, L.; Calas-Etienne, S.; Sallée, H.; Budtova, T. Aeropectin: Fully Biomass-Based Mechanically Strong and Thermal Superinsulating Aerogel. Biomacromolecules 2014, 15, 2188–2195. [Google Scholar] [CrossRef]
- Saelices, C.J.; Seantier, B.; Cathala, B.; Grohens, Y. Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef]
- Plappert, S.F.; Nedelec, J.-M.; Rennhofer, H.; Lichtenegger, H.C.; Liebner, F.W. Strain Hardening and Pore Size Harmonization by Uniaxial Densification: A Facile Approach toward Superinsulating Aerogels from Nematic Nanofibrillated 2,3-Dicarboxyl Cellulose. Chem. Mater. 2017, 29, 6630–6641. [Google Scholar] [CrossRef]
- Druel, L.; Bardl, R.; Vorwerg, W.; Budtova, T. Starch Aerogels: A Member of the Family of Thermal Superinsulating Materials. Biomacromolecules 2017, 18, 4232–4239. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, R.; Gurikov, P.; Meissner, I.; Smirnova, I. Preparation of Biopolymer Aerogels Using Green Solvents. J. Vis. Exp. 2016, 113, e54116. [Google Scholar] [CrossRef] [Green Version]
- Bendahou, D.; Bendahou, A.; Seantier, B.; Lebeau, B.; Grohens, Y.; Kaddami, H. Structure-Thermal Conductivity Tentative Correlation for Hybrid Aerogels Based on Nanofibrillated Cellulose-Mesoporous Silica Nanocomposite. J. Renew. Mater. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Bendahou, D.; Bendahou, A.; Seantier, B.; Grohens, Y.; Kaddami, H. Nano-fibrillated cellulose-zeolites based new hybrid composites aerogels with super thermal insulating properties. Ind. Crop. Prod. 2015, 65, 374–382. [Google Scholar] [CrossRef]
- Gavillon, R.; Budtova, T. Aerocellulose: New Highly Porous Cellulose Prepared from Cellulose–NaOH Aqueous Solutions. Biomacromolecules 2008, 9, 269–277. [Google Scholar] [CrossRef]
- Pircher, N.; Carbajal, L.; Schimper, C.; Bacher, M.; Rennhofer, H.; Nedelec, J.-M.; Lichtenegger, H.C.; Rosenau, T.; Liebner, F. Impact of selected solvent systems on the pore and solid structure of cellulose aerogels. Cellulose 2016, 23, 1949–1966. [Google Scholar] [CrossRef] [Green Version]
- Plappert, S.F.; Nedelec, J.-M.; Rennhofer, H.; Lichtenegger, H.; Bernstorff, S.; Liebner, F.W. Self-Assembly of Cellulose in Super-Cooled Ionic Liquid under the Impact of Decelerated Antisolvent Infusion: An Approach toward Anisotropic Gels and Aerogels. Biomacromolecules 2018, 19, 4411–4422. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Formation of nanoporous aerogels from wheat starch. Carbohydr. Polym. 2016, 147, 125–132. [Google Scholar] [CrossRef]
- García-González, C.; Uy, J.; Alnaief, M.; Smirnova, I. Preparation of tailor-made starch-based aerogel microspheres by the emulsion-gelation method. Carbohydr. Polym. 2012, 88, 1378–1386. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Dusemund, B.; Kos Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S. Safety and Efficacy of Microcrystalline Cellulose for all Animal Species. Efsa J. 2020, 18, e06209. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saito, T.; Isogai, A. Aerogels with 3D Ordered Nanofiber Skeletons of Liquid-Crystalline Nanocellulose Derivatives as Tough and Transparent Insulators. Angew. Chem. Int. Ed. 2014, 53, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Parsons, G.N.; Khan, S.A.; Rojas, O.J. Synthesis of organic aerogels with tailorable morphology and strength by controlled solvent swelling following Hansen solubility. Sci. Rep. 2018, 8, 2106. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Zakaria, S.; Chia, C.H.; Chen, R.S.; Ellis, A.V.; Kaco, H. Highly porous regenerated cellulose hydrogel and aerogel prepared from hydrothermal synthesized cellulose carbamate. PLoS ONE 2017, 12, e0173743. [Google Scholar] [CrossRef] [PubMed]
- Simón-Herrero, C.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Hydroxyethyl cellulose/alumina-based aerogels as lightweight insulating materials with high mechanical strength. J. Mater. Sci. 2018, 53, 1556–1567. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; He, X.; Zhang, X.; Zhang, W.; Zhang, X. Polyethylenimine-Grafted Cellulose Nanofibril Aerogels as Versatile Vehicles for Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef]
- Guan, Y.; Rao, J.; Wu, Y.; Gao, H.; Liu, S.; Chen, G.; Peng, F. Hemicelluloses-based magnetic aerogel as an efficient adsorbent for Congo red. Int. J. Biol. Macromol. 2020, 155, 369–375. [Google Scholar] [CrossRef]
- Yang, H.; Sheikhi, A.; Van De Ven, T.G.M. Reusable Green Aerogels from Cross-Linked Hairy Nanocrystalline Cellulose and Modified Chitosan for Dye Removal. Langmuir 2016, 32, 11771–11779. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Fierro, V. Lignin–phenol–formaldehyde aerogels and cryogels. Microporous Mesoporous Mater. 2013, 168, 19–29. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Meng, Y.; Cheng, Y.; Lu, J.; Wang, H. Novel graphene oxide/aminated lignin aerogels for enhanced adsorption of malachite green in wastewater. Colloids Surf. A Phys. Eng. Asp. 2020, 603, 125281. [Google Scholar] [CrossRef]
- Harper, B.J.; Clendaniel, A.; Sinche, F.; Way, D.; Hughes, M.; Schardt, J.; Simonsen, J.; Stefaniak, A.B.; Harper, S.L. Impacts of chemical modification on the toxicity of diverse nanocellulose materials to developing zebrafish. Cellulose 2016, 23, 1763–1775. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, A.; Otuechere, C.A.; Adebayo, O.L.; Ajisodun, I. Synthesis and toxicity profiling of sebacic acid-modified cellulose from unexploited watermelon exocarp. Polym. Bull. 2020, 1–25. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.; Zhang, X.; Meng, X.; Tong, G.; Deng, Y. Temperature-sensitive poly-NIPAm modified cellulose nanofibril cryogel microspheres for controlled drug release. Cellulose 2016, 23, 415–425. [Google Scholar] [CrossRef]
- Tripathi, A.; Parsons, G.N.; Rojas, O.J.; Khan, S.A. Featherlight, Mechanically Robust Cellulose Ester Aerogels for Environmental Remediation. ACS Omega 2017, 2, 4297–4305. [Google Scholar] [CrossRef] [PubMed]
- Mißfeldt, F.; Gurikov, P.; Lölsberg, W.; Weinrich, D.; Lied, F.; Fricke, M.; Smirnova, I. Continuous Supercritical Drying of Aerogel Particles: Proof of Concept. Ind. Eng. Chem. Res. 2020, 59, 11284–11295. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Song, A.; Huang, Y.; Liu, B.; Cao, H.; Zhong, X.; Lin, Y.; Wang, M.; Li, X.; Zhong, W. Gel polymer electrolyte based on polyethylene glycol composite lignocellulose matrix with higher comprehensive performances. Electrochim. Acta 2017, 247, 505–515. [Google Scholar] [CrossRef]
- French, A.D. Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 2017, 24, 4605–4609. [Google Scholar] [CrossRef]
- Liebert, T. Cellulose Solvents—Remarkable History, Bright Future. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 3–54. [Google Scholar]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2012. [Google Scholar]
- Saito, T.; Kuramae, R.; Wohlert, J.; Berglund, L.A.; Isogai, A. An Ultrastrong Nanofibrillar Biomaterial: The Strength of Single Cellulose Nanofibrils Revealed via Sonication-Induced Fragmentation. Biomacromolecules 2013, 14, 248–253. [Google Scholar] [CrossRef]
- Miyashiro, D.; Hamano, R.; Umemura, K. A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterial 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, L.; Noël, M.; Aitomäki, Y.; Öman, T.; Oksman, K. Production potential of cellulose nanofibers from industrial residues: Efficiency and nanofiber characteristics. Ind. Crop. Prod. 2016, 92, 84–92. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Tarrés, Q.; Delgado-Aguilar, M.; González, I.; Mutjé, P.; Rodríguez, A.; Espinosa, E.; Rodríguez, A. Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 2016, 23, 837–852. [Google Scholar] [CrossRef]
- Rol, F.; Saini, S.; Meyer, V.; Petit-Conil, M.; Bras, J. Production of cationic nanofibrils of cellulose by twin-screw extrusion. Ind. Crop. Prod. 2019, 137, 81–88. [Google Scholar] [CrossRef]
- Espinosa, E.; Rol, F.; Bras, J.; Rodríguez, A. Production of lignocellulose nanofibers from wheat straw by different fibrillation methods. Comparison of its viability in cardboard recycling process. J. Clean. Prod. 2019, 239, 118083. [Google Scholar] [CrossRef]
- Moriana, R.; Vilaplana, F.; Ek, M. Cellulose Nanocrystals from Forest Residues as Reinforcing Agents for Composites: A Study from Macro- to Nano-Dimensions. Carbohydr. Polym. 2016, 139, 139–149. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dasan, Y.K.; Khan, I.; Soleimani, H.; Usmani, A. 9—Application of nanocrystalline cellulose: Processing and biomedical applications. In Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Abdul Khalil, H.P.S., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 215–240. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Castro, C.; Cleenwerck, I.; Trček, J.; Zuluaga, R.; De Vos, P.; Caro, G.; Aguirre, R.; Putaux, J.; Ganan, P. Gluconacetobacter Medellinensis Sp. Nov., Cellulose-and Non-Cellulose-Producing Acetic Acid Bacteria Isolated from Vinegar. Int. J. Syst. Evol. Microbiol. 2013, 63, 1119–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jozala, A.F.; De Lencastre-Novaes, L.C.; Lopes, A.M.; Santos-Ebinuma, V.D.C.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanskul, S.; Amornthatree, K.; Jaturonlak, N. A new cellulose-producing bacterium, Rhodococcus sp. MI 2: Screening and optimization of culture conditions. Carbohydr. Polym. 2013, 92, 421–428. [Google Scholar] [CrossRef] [PubMed]
- MohammadKazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Arcot, L.R.; Gröschel, A.H.; Linder, M.B.; Rojas, O.J.; Ikkala, O. Self-Assembly of Native Cellulose Nanostructures. Handb. Nanocellulose Cellul. Nanocomposites 2017, 123–174. [Google Scholar] [CrossRef]
- Martoïa, F.; Cochereau, T.; Dumont, P.; Orgéas, L.; Terrien, M.; Belgacem, M. Cellulose nanofibril foams: Links between ice-templating conditions, microstructures and mechanical properties. Mater. Des. 2016, 104, 376–391. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Nemoto, J.; Saito, T.; Isogai, A. Simple Freeze-Drying Procedure for Producing Nanocellulose Aerogel-Containing, High-Performance Air Filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef] [PubMed]
- Saelices, C.J.; Seantier, B.; Grohens, Y.; Capron, I. Thermal Superinsulating Materials Made from Nanofibrillated Cellulose-Stabilized Pickering Emulsions. ACS Appl. Mater. Interfaces 2018, 10, 16193–16202. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Buchtová, N.; Pradille, C.; Bouvard, J.-L.; Budtova, T. Mechanical properties of cellulose aerogels and cryogels. Soft Matter 2019, 15, 7901–7908. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative Study of Aerogels Obtained from Differently Prepared Nanocellulose Fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Heath, L.; Thielemans, W. Cellulose nanowhisker aerogels. Green Chem. 2010, 12, 1448–1453. [Google Scholar] [CrossRef]
- Bakaic, E.; Smeets, N.M.B.; Hoare, T. Injectable hydrogels based on poly(ethylene glycol) and derivatives as functional biomaterials. RSC Adv. 2015, 5, 35469–35486. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Q.; Huang, F.; Pu, Y.; Pan, S.; Ragauskas, A.J. Preparation and characteristics of cellulose nanowhisker reinforced acrylic foams synthesized by freeze-casting. RSC Adv. 2014, 4, 12148. [Google Scholar] [CrossRef]
- Müller, A.; Zink, M.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. Bacterial nanocellulose with a shape-memory effect as potential drug delivery system. RSC Adv. 2014, 4, 57173–57184. [Google Scholar] [CrossRef] [Green Version]
- Liebner, F.W.; Haimer, E.; Wendland, M.; Neouze, M.-A.; Schlufter, K.; Miethe, P.; Heinze, T.; Potthast, A.; Rosenau, T. Aerogels from Unaltered Bacterial Cellulose: Application of scCO2 Drying for the Preparation of Shaped, Ultra-Lightweight Cellulosic Aerogels. Macromol. Biosci. 2010, 10, 349–352. [Google Scholar] [CrossRef]
- Haimer, E.; Wendland, M.; Schlufter, K.; Frankenfeld, K.; Miethe, P.; Potthast, A.; Rosenau, T.; Liebner, F.W. Loading of Bacterial Cellulose Aerogels with Bioactive Compounds by Antisolvent Precipitation with Supercritical Carbon Dioxide. Macromol. Symp. 2010, 294, 64–74. [Google Scholar] [CrossRef]
- Pereira, A.L.S.; Feitosa, J.P.A.; Morais, J.P.S.; Rosa, M.D.F. Bacterial cellulose aerogels: Influence of oxidation and silanization on mechanical and absorption properties. Carbohydr. Polym. 2020, 250, 116927. [Google Scholar] [CrossRef]
- Köse, K.; Mavlan, M.; Youngblood, J.P. Applications and impact of nanocellulose based adsorbents. Cellulose 2020, 27, 2967–2990. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, T.; Jia, X.; Zhao, J.; Li, Q.; Ao, C.; Deng, X.; Zhang, X.; Zhang, W.; Lu, C. Honeycomb-structured carbon aerogels from nanocellulose and skin secretion of Andrias davidianus for highly compressible binder-free supercapacitors. Carbohydr. Polym. 2020, 245, 116554. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Zou, L.; Wang, F.; Wang, X.; Zhang, Y.; Yao, X. Nanocellulose-derived highly porous carbon aerogels for supercapacitors. Carbon 2016, 99, 203–211. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, Y.; Van Bochove, B.; Mäkilä, E.; Seppälä, J.; Xu, W.; Willför, S.; Xu, C. Robust shape-retaining nanocellulose-based aerogels decorated with silver nanoparticles for fast continuous catalytic discoloration of organic dyes. Sep. Purif. Technol. 2020, 242, 116523. [Google Scholar] [CrossRef]
- Ferreira, F.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- De Oliveira, J.P.; Bruni, G.P.; Fabra, M.J.; Zavareze, E.D.R.; López-Rubio, A.; Martínez-Sanz, M. Development of food packaging bioactive aerogels through the valorization of Gelidium sesquipedale seaweed. Food Hydrocoll. 2019, 89, 337–350. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Tan, T.H.; Lee, H.V.; Yehya Dabdawb, W.A.; Hamid, S.B.B.O.A.A. Chapter 5—A review of nanocellulose in the drug-delivery system. In Materials for Biomedical Engineering; Holban, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–164. [Google Scholar]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef]
- Shawkataly, A.K.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymer 2020, 12, 1759. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Z.; Chen, B.; Zhang, W.; Sharma, S.; Gu, W.; Deng, Y. Solid-state flexible polyaniline/silver cellulose nanofibrils aerogel supercapacitors. J. Power Sources 2014, 246, 283–289. [Google Scholar] [CrossRef]
- Yang, X.; Shi, K.; Zhitomirsky, I.; Cranston, E.D. Cellulose Nanocrystal Aerogels as Universal 3D Lightweight Substrates for Supercapacitor Materials. Adv. Mater. 2015, 27, 6104–6109. [Google Scholar] [CrossRef]
- Wu, Z.; Li, C.; Liang, H.-W.; Chen, J.-F.; Yu, S. Ultralight, Flexible, and Fire-Resistant Carbon Nanofiber Aerogels from Bacterial Cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef]
- Kuhn, J.; Ebert, H.-P.; Arduini-Schuster, M.; Büttner, D.; Fricke, J. Thermal transport in polystyrene and polyurethane foam insulations. Int. J. Heat Mass Transf. 1992, 35, 1795–1801. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Sèbe, G.; Wu, R.; Virtudazo, R.V.R.; Tingaut, P.; Koebel, M.M. Multiscale Assembly of Superinsulating Silica Aerogels Within Silylated Nanocellulosic Scaffolds: Improved Mechanical Properties Promoted by Nanoscale Chemical Compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- He, X.; Cheng, L.; Wang, Y.; Zhao, J.; Zhang, W.; Lu, C. Aerogels from quaternary ammonium-functionalized cellulose nanofibers for rapid removal of Cr(VI) from water. Carbohydr. Polym. 2014, 111, 683–687. [Google Scholar] [CrossRef]
- Sajab, M.S.; Chia, C.H.; Chan, C.H.; Zakaria, S.; Kaco, H.; Chook, S.W.; Chin, S.X.; Noor, A.M. Bifunctional graphene oxide–cellulose nanofibril aerogel loaded with Fe(iii) for the removal of cationic dye via simultaneous adsorption and Fenton oxidation. RSC Adv. 2016, 6, 19819–19825. [Google Scholar] [CrossRef]
- Ookuma, S.; Igarashi, K.; Hara, M.; Aso, K.; Yoshidome, H.; Nakayama, H.; Suzuki, K.; Nakajima, K. Porous Ion-Exchanged Fine Cellulose Particles, Method for Production Thereof, and Affinity Carrier. U.S. Patent 5,196,527, 23 March 1993. [Google Scholar]
- Pinnow, M.; Fanter, C.; Kunze, J.; Fink, H.-P. Characterization of Highly Porous Materials from Cellulose Carbamate. Macromol. Symp. 2008, 262, 129–139. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2015, 23, 5–55. [Google Scholar] [CrossRef] [Green Version]
- Innerlohinger, J.; Weber, H.K.; Kraft, G. Aerocellulose: Aerogels and Aerogel-like Materials made from Cellulose. Macromol. Symp. 2006, 244, 126–135. [Google Scholar] [CrossRef]
- Sescousse, R.; Budtova, T. Influence of processing parameters on regeneration kinetics and morphology of porous cellulose from cellulose–NaOH–water solutions. Cellulose 2009, 16, 417–426. [Google Scholar] [CrossRef]
- Schestakow, M.; Karadagli, I.; Ratke, L. Cellulose aerogels prepared from an aqueous zinc chloride salt hydrate melt. Carbohydr. Polym. 2016, 137, 642–649. [Google Scholar] [CrossRef]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Rege, A.; Schestakow, M.; Karadagli, I.; Ratke, L.; Itskov, M. Micro-mechanical modelling of cellulose aerogels from molten salt hydrates. Soft Matter 2016, 12, 7079–7088. [Google Scholar] [CrossRef] [PubMed]
- Sescousse, R.; Gavillon, R.; Budtova, T. Aerocellulose from cellulose–ionic liquid solutions: Preparation, properties and comparison with cellulose–NaOH and cellulose–NMMO routes. Carbohydr. Polym. 2011, 83, 1766–1774. [Google Scholar] [CrossRef]
- Rudaz, C. Cellulose and Pectin Aerogels: Towards their Nano-Structuration. Ph.D. Thesis, Mines ParisTech, Sophia Antipolis, France, 2013. [Google Scholar]
- Demilecamps, A.; Alves, M.; Rigacci, A.; Reichenauer, G.; Budtova, T. Nanostructured interpenetrated organic-inorganic aerogels with thermal superinsulating properties. J. Non-Cryst. Solids 2016, 452, 259–265. [Google Scholar] [CrossRef]
- Liebner, F.; Pircher, N.; Schimper, C.; Haimer, E.; Rosenau, T. Aerogels: Cellulose-Based. Encycl. Biomed. Polym. Polym. Biomater. 2016, 37–75. [Google Scholar] [CrossRef]
- Pircher, N.; Fischhuber, D.; Carbajal, L.; Strauß, C.; Nedelec, J.-M.; Kasper, C.; Rosenau, T.; Liebner, F.W. Preparation and Reinforcement of Dual-Porous Biocompatible Cellulose Scaffolds for Tissue Engineering. Macromol. Mater. Eng. 2015, 300, 911–924. [Google Scholar] [CrossRef] [Green Version]
- Liebner, F.W.; Dunareanu, R.; Opietnik, M.; Haimer, E.; Wendland, M.; Werner, C.; Maitz, M.F.; Seib, F.P.; Neouze, M.-A.; Potthast, A.; et al. Shaped hemocompatible aerogels from cellulose phosphates: Preparation and properties. Holzforschung 2012, 66, 317–321. [Google Scholar] [CrossRef]
- Hu, Y.; Zhuo, H.; Zhong, L.; Tong, X.; Peng, X.; Wang, S.; Sun, R. 3D hierarchical porous N-doped carbon aerogel from renewable cellulose: An attractive carbon for high-performance supercapacitor electrodes and CO2 adsorption. RSC Adv. 2016, 6, 15788–15795. [Google Scholar] [CrossRef]
- Yang, X.; Fei, B.; Ma, J.; Liu, X.; Yang, S.; Tian, G.; Jiang, Z. Porous nanoplatelets wrapped carbon aerogels by pyrolysis of regenerated bamboo cellulose aerogels as supercapacitor electrodes. Carbohydr. Polym. 2018, 180, 385–392. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crop. Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, G.; Feng, X.; Wang, M.; Song, T.; Liu, D.; Lu, F.; Qi, H. In Situ MnO X/N-Doped Carbon Aerogels from Cellulose as Monolithic and Highly Efficient Catalysts for the Upgrading of Bioderived Aldehydes. Green Chem. 2018, 20, 3593–3603. [Google Scholar] [CrossRef]
- Rooke, J.; Sescousse, R.; Budtova, T.; Berthon-Fabry, S.; Simon, B.; Chatenet, M. Cellulose- Based Nanostructured Carbons for Energy Conversion and Storage Devices. In Green Carbon Materials: Advances and Applications; Rufford, T.E., Zhu, J., Hulicova-Jurcakova, D., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2013; pp. 89–111. [Google Scholar]
- Guilminot, E.; Gavillon, R.; Chatenet, M.; Berthon-Fabry, S.; Rigacci, A.; Budtova, T. New nanostructured carbons based on porous cellulose: Elaboration, pyrolysis and use as platinum nanoparticles substrate for oxygen reduction electrocatalysis. J. Power Sources 2008, 185, 717–726. [Google Scholar] [CrossRef]
- Schoemaker, H.E.; Piontek, K. On the interaction of lignin peroxidase with lignin. Pure Appl. Chem. 1996, 68, 2089–2096. [Google Scholar] [CrossRef] [Green Version]
- Bhat, A.; Dasan, Y.; Khan, I. Extraction of Lignin from Biomass for Biodiesel Production. In Agricultural Biomass Based Potential Materials; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; pp. 155–179. [Google Scholar]
- Robinson, A.R.; Mansfield, S.D. Rapid analysis of poplar lignin monomer composition by a streamlined thioacidolysis procedure and near-infrared reflectance-based prediction modeling. Plant J. 2009, 58, 706–714. [Google Scholar] [CrossRef]
- Dos Santos Abreu, H.; Do Nascimento, A.M.; Maria, M.A. Lignin Structure and Wood Properties. Wood Fiber Sci. 1999, 31, 426–433. [Google Scholar]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Fierro, V. New tannin–lignin aerogels. Ind. Crop. Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Bhanu Rekha, V.; Ramachandralu, K.; Rasigha, T. Enhancing the Absorbency of Bagasse through Enzymatic Delignification. J. Fash. Technol. Text. Eng. 2013, 1, 2. [Google Scholar] [CrossRef]
- Brunow, G. Methods to Reveal the Structure of Lignin. Biopolym. Online 2001. [Google Scholar] [CrossRef]
- Radotić, K.; Mićić, M. Methods for Extraction and Purification of Lignin and Cellulose from Plant Tissues. In Sample Preparation Techniques for Soil, Plant, and Animal Samples; Springer: Berlin/Heidelberg, Germany, 2016; pp. 365–376. [Google Scholar]
- Saake, B.; Lehnen, R. Lignin. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Saito, T.; Brown, R.H.; Hunt, M.A.; Pickel, D.L.; Pickel, J.M.; Messman, J.M.; Baker, F.S.; Keller, M.; Naskar, A.K. Turning renewable resources into value-added polymer: Development of lignin-based thermoplastic. Green Chem. 2012, 14, 3295–3303. [Google Scholar] [CrossRef]
- Perez-Cantu, L.; Liebner, F.W.; Smirnova, I. Preparation of aerogels from wheat straw lignin by cross-linking with oligo(alkylene glycol)-α,ω-diglycidyl ethers. Microporous Mesoporous Mater. 2014, 195, 303–310. [Google Scholar] [CrossRef]
- Chen, F.; Xu, M.; Wang, L.; Li, J. Preparation and Characterization of Organic Aerogels by the Lignin-Resorcinol-Formaldehyde Copolymer. Bioresources 2011, 6, 1262–1272. [Google Scholar]
- Chen, C.; Li, F.; Zhang, Y.; Wang, B.; Fan, Y.; Wang, X.; Sun, R. Compressive, ultralight and fire-resistant lignin-modified graphene aerogels as recyclable absorbents for oil and organic solvents. Chem. Eng. J. 2018, 350, 173–180. [Google Scholar] [CrossRef]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Karaaslan, M.A.; Kadla, J.F.; Ko, F. Lignin-Based Aerogels. In Lignin in Polymer Composites; Faruk, O., Sain, M., Eds.; Elsevier: Oxford, UK; Waltham, MA, USA, 2016; pp. 67–93. [Google Scholar] [CrossRef]
- Yang, J.; An, X.; Liu, L.; Tang, S.; Cao, H.; Xu, Q.; Liu, H. Cellulose, hemicellulose, lignin, and their derivatives as multi-components of bio-based feedstocks for 3D printing. Carbohydr. Polym. 2020, 250, 116881. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.A.; Quick, A.; Taha, M.; Mignard, N.; Becquart, F.; Ayoub, A. Hemicellulose extraction and characterization for applications in paper coatings and adhesives. Ind. Crop. Prod. 2017, 107, 370–377. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; De La Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Gírio, F.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Machmudah, S.; Kanda, H.; Goto, M. Chapter 3—Hydrolysis of Biopolymers in Near-Critical and Subcritical Water. In Water Extraction of Bioactive Compounds; Dominguez González, H., González Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–107. [Google Scholar]
- Álvarez, A.; Cachero, S.; González-Sánchez, C.; Montejo-Bernardo, J.; Pizarro, C.; Bueno, J.L. Novel method for holocellulose analysis of non-woody biomass wastes. Carbohydr. Polym. 2018, 189, 250–256. [Google Scholar] [CrossRef]
- Flórez-Pardo, L.M.; González-Córdoba, A.; Mendoza, J.G.S. Evaluation of different methods for efficient extraction of hemicelluloses leaves and tops of sugarcane. DYNA 2018, 85, 18–27. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, J.; Treasure, T.; Skotty, J.; Floyd, T.; Kelley, S.S.; Park, S. Alkaline extraction and characterization of residual hemicellulose in dissolving pulp. Cellulose 2018, 26, 1323–1333. [Google Scholar] [CrossRef]
- Mohtar, S.S.; Busu, T.N.Z.T.M.; Noor, A.M.M.; Shaari, N.; Mat, H. An ionic liquid treatment and fractionation of cellulose, hemicellulose and lignin from oil palm empty fruit bunch. Carbohydr. Polym. 2017, 166, 291–299. [Google Scholar] [CrossRef]
- Krogell, J.; Korotkova, E.; Eränen, K.; Pranovich, A.; Salmi, T.; Murzin, D.; Willför, S. Intensification of hemicellulose hot-water extraction from spruce wood in a batch extractor—Effects of wood particle size. Bioresour. Technol. 2013, 143, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Doner, L.W.; Hicks, K.B. Isolation of Hemicellulose from Corn Fiber by Alkaline Hydrogen Peroxide Extraction. Cereal Chem. J. 1997, 74, 176–181. [Google Scholar] [CrossRef]
- Yuan, Y.; Zou, P.; Zhou, J.; Geng, Y.; Fan, J.; Clark, J.; Li, Y.-Q.; Zhang, C.S. Microwave-assisted hydrothermal extraction of non-structural carbohydrates and hemicelluloses from tobacco biomass. Carbohydr. Polym. 2019, 223, 115043. [Google Scholar] [CrossRef] [PubMed]
- Väisänen, T.; Kilpeläinen, P.; Kitunen, V.; Lappalainen, R.; Tomppo, L. Effect of steam treatment on the chemical composition of hemp (Cannabis sativa L.) and identification of the extracted carbohydrates and other compounds. Ind. Crop. Prod. 2019, 131, 224–233. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Ebringerová, A. Structural Diversity and Application Potential of Hemicelluloses. Macromol. Symp. 2005, 232, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Q.; Yan, Y.; Peng, F.; Sun, R.; Ren, J. Hemicellulose from Plant Biomass in Medical and Pharmaceutical Application: A Critical Review. Curr. Med. Chem. 2019, 26, 2430–2455. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.A.; Hubbe, M.; Taha, M.; Becquart, F.; Ayoub, A. A Review of Water-Resistant Hemicellulose-Based Materials: Processing and Applications. ChemSusChem 2017, 10, 305–323. [Google Scholar] [CrossRef]
- Laine, C.; Harlin, A.; Hartman, J.; Hyvärinen, S.; Kammiovirta, K.; Krogerus, B.; Pajari, H.; Rautkoski, H.; Setälä, H.; Sievänen, J.; et al. Hydroxyalkylated xylans—Their synthesis and application in coatings for packaging and paper. Ind. Crop. Prod. 2013, 44, 692–704. [Google Scholar] [CrossRef]
- Zoldners, J.; Kiseleva, T. Modification of hemicelluloses with polycarboxylic acids. Holzforschung 2013, 67, 567–571. [Google Scholar] [CrossRef]
- Peng, X.; Ren, J.; Sun, R. An efficient method for the synthesis of hemicellulosic derivatives with bifunctional groups in butanol/water medium and their rheological properties. Carbohydr. Polym. 2011, 83, 1922–1928. [Google Scholar] [CrossRef]
- Xu, W.; Pranovich, A.; Uppstu, P.; Wang, X.; Kronlund, D.; Hemming, J.; Öblom, H.; Moritz, N.; Preis, M.; Sandler, N.; et al. Novel biorenewable composite of wood polysaccharide and polylactic acid for three dimensional printing. Carbohydr. Polym. 2018, 187, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, Y.; Chang, Z.; Yan, S.; Liu, S.; Han, S. A new method of synthesizing hemicellulose-derived porous activated carbon for high-performance supercapacitors. Microporous Mesoporous Mater. 2020, 292, 109707. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D. Barley beta-glucan aerogels via supercritical CO2 drying. Food Res. Int. 2012, 48, 442–448. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D. Barley β-glucan aerogels as a carrier for flax oil via supercritical CO2. J. Food Eng. 2012, 111, 625–631. [Google Scholar] [CrossRef]
- Marquez-Escalante, J.A.; Carvajal-Millán, E.; Miki-Yoshida, M.; Álvarez-Contreras, L.; Toledo-Guillén, A.R.; Lizardi-Mendoza, J.; Rascón-Chu, A. Water Extractable Arabinoxylan Aerogels Prepared by Supercritical CO2 Drying. Molecules 2013, 18, 5531–5542. [Google Scholar] [CrossRef] [Green Version]
- Berglund, L.; Forsberg, F.; Jonoobi, M.; Oksman, K. Promoted hydrogel formation of lignin-containing arabinoxylan aerogel using cellulose nanofibers as a functional biomaterial. RSC Adv. 2018, 8, 38219–38228. [Google Scholar] [CrossRef] [Green Version]
- Jaafar, Z.; Quelennec, B.; Moreau, C.; Lourdin, D.; Maigret, J.; Pontoire, B.; D’Orlando, A.; Coradin, T.; Duchemin, B.; Fernandes, F.; et al. Plant cell wall inspired xyloglucan/cellulose nanocrystals aerogels produced by freeze-casting. Carbohydr. Polym. 2020, 247, 116642. [Google Scholar] [CrossRef]
- Köhnke, T.; Lin, A.; Elder, T.; Theliander, H.; Ragauskas, A.J. Nanoreinforced xylan–cellulose composite foams by freeze-casting. Green Chem. 2012, 14, 1864. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Zhang, A.; Liu, C.; Sun, R. Direct preparation of green and renewable aerogel materials from crude bagasse. Cellulose 2016, 23, 1325–1334. [Google Scholar] [CrossRef] [Green Version]
- Aaltonen, O.; Jauhiainen, O. The preparation of lignocellulosic aerogels from ionic liquid solutions. Carbohydr. Polym. 2009, 75, 125–129. [Google Scholar] [CrossRef]
- Sescousse, R.; Smacchia, A.; Budtova, T. Influence of lignin on cellulose-NaOH-water mixtures properties and on Aerocellulose morphology. Cellulose 2010, 17, 1137–1146. [Google Scholar] [CrossRef]
- Geng, S.; Wei, J.; Jonasson, S.; Hedlund, J.; Oksman, K. Multifunctional Carbon Aerogels with Hierarchical Anisotropic Structure Derived from Lignin and Cellulose Nanofibers for CO2 Capture and Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 7432–7441. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, O.; Budtova, T. All-cellulose composite aerogels and cryogels. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106027. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Jiang, B.; Zhang, H.; He, N.; Yang, S.; Tang, D.; Song, Y. Flexible and Mildew-Resistant Wood-Derived Aerogel for Stable and Efficient Solar Desalination. ACS Appl. Mater. Interfaces 2020, 12, 28179–28187. [Google Scholar] [CrossRef]
- Tran, D.T.; Nguyen, S.T.; Do, N.D.; Thai, N.N.T.; Thai, Q.B.; Huynh, H.K.P.; Nguyen, V.T.T.; Phan, A.N. Green aerogels from rice straw for thermal, acoustic insulation and oil spill cleaning applications. Mater. Chem. Phys. 2020, 253, 123363. [Google Scholar] [CrossRef]
- Mussana, H.; Yang, X.; Tessima, M.; Han, F.; Iqbal, N.; Liu, L. Preparation of lignocellulose aerogels from cotton stalks in the ionic liquid-based co-solvent system. Ind. Crop. Prod. 2018, 113, 225–233. [Google Scholar] [CrossRef]
- Ainsworth, C.H.; Paris, C.B.; Perlin, N.; Dornberger, L.N.; Iii, W.F.P.; Chancellor, E.; Murawski, S.; Hollander, D.; Daly, K.; Romero, I.C.; et al. Impacts of the Deepwater Horizon oil spill evaluated using an end-to-end ecosystem model. PLoS ONE 2018, 13, e0190840. [Google Scholar] [CrossRef]
- Hadji, E.M.; Fu, B.; Abebe, A.; Bilal, H.M.; Wang, J. Sponge-based materials for oil spill cleanups: A review. Front. Chem. Sci. Eng. 2020, 14, 749–762. [Google Scholar] [CrossRef]
- Chhajed, M.; Yadav, C.; Agrawal, A.K.; Maji, P.K. Esterified superhydrophobic nanofibrillated cellulose based aerogel for oil spill treatment. Carbohydr. Polym. 2019, 226, 115286. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Bidgoli, H.; Mortazavi, Y.; Khodadadi, A.A. A functionalized nano-structured cellulosic sorbent aerogel for oil spill cleanup: Synthesis and characterization. J. Hazard. Mater. 2019, 366, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dong, F.; Yang, X.; Liu, H.; Guo, L.; Qian, Y.; Wang, A.; Wang, S.; Luo, J. Preparation and Characterization of Cellulose Grafted with Epoxidized Soybean Oil Aerogels for Oil-Absorbing Materials. J. Agric. Food Chem. 2019, 67, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Aalbers, G.; Boott, C.E.; D’Acierno, F.; Lewis, L.; Ho, J.; Michal, C.A.; Hamad, W.Y.; MacLachlan, M.J. Post-modification of Cellulose Nanocrystal Aerogels with Thiol–Ene Click Chemistry. Biomacromolecules 2019, 20, 2779–2785. [Google Scholar] [CrossRef]
- Fauziyah, M.; Widiyastuti, W.; Setyawan, H. A hydrophobic cellulose aerogel from coir fibers waste for oil spill application. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012019. [Google Scholar] [CrossRef]
- Lazzari, L.K.; Zampieri, V.B.; Zanini, M.; Zattera, A.J.; Baldasso, C. Sorption capacity of hydrophobic cellulose cryogels silanized by two different methods. Cellulose 2017, 24, 3421–3431. [Google Scholar] [CrossRef]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Rafieian, F.; Hosseini, M.; Jonoobi, M.; Yu, Q. Development of hydrophobic nanocellulose-based aerogel via chemical vapor deposition for oil separation for water treatment. Cellulose 2018, 25, 4695–4710. [Google Scholar] [CrossRef]
- Yagoub, H.; Zhu, L.; Shibraen, M.H.M.A.; Altam, A.A.; Babiker, D.M.D.; Liang, S.; Jin, Y.; Yang, S. Complex Aerogels Generated from Nano-Polysaccharides and Its Derivatives for Oil–Water Separation. Polymer 2019, 11, 1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2 Problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; Albahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Zhang, Y.; Shen, M.; Zhang, J. Gas phase synthesis of aminated nanocellulose aerogel for carbon dioxide adsorption. Cellulose 2020, 27, 2953–2958. [Google Scholar] [CrossRef]
- Jiang, X.; Kong, Y.; Zou, H.; Zhao, Z.; Zhong, Y.; Shen, X. Amine grafted cellulose aerogel for CO2 capture. J. Porous Mater. 2020, 1–5. [Google Scholar] [CrossRef]
- Sepahvand, S.; Jonoobi, M.; Ashori, A.; Gauvin, F.; Brouwers, H.J.H.; Oksman, K.; Yu, Q. A promising process to modify cellulose nanofibers for carbon dioxide (CO2) adsorption. Carbohydr. Polym. 2020, 230, 115571. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Jiang, H.; Wang, X.; Zhang, T.; Yao, Y. High CO2 adsorption by amino-modified bio-spherical cellulose nanofibres aerogels. Environ. Chem. Lett. 2018, 16, 605–614. [Google Scholar] [CrossRef]
- Li, Y.; Jia, P.; Xu, J.; Wu, Y.; Jiang, H.; Li, Z. The Aminosilane Functionalization of Cellulose Nanofibrils and the Mechanical and CO2 Adsorption Characteristics of Their Aerogel. Ind. Eng. Chem. Res. 2020, 59, 2874–2882. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Nie, J.; Yang, J.-H.; Qi, X.-D.; Zhou, Z.-W.; Wang, Y. Bio-inspired functionalization of microcrystalline cellulose aerogel with high adsorption performance toward dyes. Carbohydr. Polym. 2018, 198, 546–555. [Google Scholar] [CrossRef]
- Saeed, R.M.Y.; Bano, Z.; Sun, J.; Wang, F.; Ullah, N.; Wang, Q. CuS-functionalized cellulose based aerogel as biocatalyst for removal of organic dye. J. Appl. Polym. Sci. 2019, 136, 47404. [Google Scholar] [CrossRef]
- Hasan, M.; Gopakumar, D.A.; Arumughan, V.; Pottathara, Y.B.; Sisanth, S.K.; Pasquini, D.; Bračič, M.; Seantier, B.; Nzihou, A.; Thomas, S.; et al. Robust Superhydrophobic Cellulose Nanofiber Aerogel for Multifunctional Environmental Applications. Polymer 2019, 11, 495. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Zhu, M.; Zhu, Y.; Zhao, Y.; Yang, M.; Miao, Z.; Ren, H.; Ma, Q.; Qian, L. Zeolitic imidazolate framework-67 functionalized cellulose hybrid aerogel: An environmentally friendly candidate for dye removal. Cellulose 2019, 27, 2161–2172. [Google Scholar] [CrossRef]
- Guo, D.-M.; An, Q.-D.; Xiao, Z.; Zhai, S.-R.; Shi, Z. Polyethylenimine-functionalized cellulose aerogel beads for efficient dynamic removal of chromium(vi) from aqueous solution. RSC Adv. 2017, 7, 54039–54052. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zuo, K.; Wu, W.; Xu, Z.; Yi, Y.; Jing, Y.; Xiao, H.; Fang, G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydr. Polym. 2018, 196, 376–384. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Liu, H. A novel carbon aerogel prepared for adsorption of copper(II) ion in water. J. Porous Mater. 2017, 24, 1575–1580. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, S.; Cui, S.; Tang, Y.; Pei, Z.; Duan, H. Magnetic-controlled aerogels from carboxylated cellulose and MnFe2O4 as a novel adsorbent for removal of Cu(II). Cellulose 2019, 26, 5051–5063. [Google Scholar] [CrossRef]
- Giese, M.; Blusch, L.K.; Schlesinger, M.; Meseck, G.R.; Hamad, W.Y.; Arjmand, M.; Sundararaj, U.; MacLachlan, M.J. Magnetic Mesoporous Photonic Cellulose Films. Langmuir 2016, 32, 9329–9334. [Google Scholar] [CrossRef]
- Zanata, D.D.M.; Battirola, L.C.; Gonçalves, M.D.C. Chemically cross-linked aerogels based on cellulose nanocrystals and polysilsesquioxane. Cellulose 2018, 25, 7225–7238. [Google Scholar] [CrossRef]
- Qian, L.; Yang, M.; Chen, H.; Xu, Y.; Zhang, S.; Zhou, Q.; He, B.; Bai, Y.; Song, W. Preparation of a poly(ionic liquid)-functionalized cellulose aerogel and its application in protein enrichment and separation. Carbohydr. Polym. 2019, 218, 154–162. [Google Scholar] [CrossRef]
- Keshipour, S.; Khezerloo, M. Au-dimercaprol functionalized cellulose aerogel: Synthesis, characterization and catalytic application. Appl. Organomet. Chem. 2018, 32, e4255. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, S.; Goenaga, G.A.; Meng, X.; Zawodzinski, T.A.; Ragauskas, A.J. Chemically Cross-Linked Cellulose Nanocrystal Aerogels for Effective Removal of Cation Dye. Front. Chem. 2020, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Zheng, L.; Liu, H. A novel graphene aerogel synthesized from cellulose with high performance for removing MB in water. J. Mater. Sci. Technol. 2020, 41, 68–75. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Wang, Z.; Pu, J. Facile fabrication of an effective nanocellulose-based aerogel and removal of methylene blue from aqueous system. J. Water Process. Eng. 2020, 37, 101511. [Google Scholar] [CrossRef]
- Balboa, E.; Moure, A.; Domínguez, H. Valorization of Sargassum muticum Biomass According to the Biorefinery Concept. Mar. Drugs 2015, 13, 3745–3760. [Google Scholar] [CrossRef]
- Seghetta, M.; Hou, X.; Simone, B.; Bjerre, A.-B.; Thomsen, M. Life cycle assessment of macroalgal biorefinery for the production of ethanol, proteins and fertilizers—A step towards a regenerative bioeconomy. J. Clean. Prod. 2016, 137, 1158–1169. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Rehm, B.H.; Moradali, M.F. Alginates and their Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Kim, S. Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Baudron, V.; Gurikov, P.; Smirnova, I. A continuous approach to the emulsion gelation method for the production of aerogel micro-particle. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 58–69. [Google Scholar] [CrossRef]
- Şahin, I.; Uzunlar, E.; Erkey, C. Investigation of the effect of gel properties on supercritical drying kinetics of ionotropic alginate gel particles. J. Supercrit. Fluids 2019, 152, 104571. [Google Scholar] [CrossRef]
- Hatami, T.; Viganó, J.; Mei, L.H.I.; Martínez, J. Production of alginate-based aerogel particles using supercritical drying: Experiment, comprehensive mathematical model, and optimization. J. Supercrit. Fluids 2020, 160, 104791. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics. Molecules 2019, 24, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, P.; Siqueira, É.; De Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterial 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.; Aquino, R.; Del Gaudio, P. Prilling and supercritical drying: A successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef]
- Li, X.-L.; He, Y.-R.; Qin, Z.-M.; Chen, M.-J.; Chen, H.-B. Facile fabrication, mechanical property and flame retardancy of aerogel composites based on alginate and melamine-formaldehyde. Polymer 2019, 181, 121783. [Google Scholar] [CrossRef]
- Shan, C.; Wang, L.; Li, Z.; Zhong, X.; Hou, Y.; Zhang, L.; Shi, F. Graphene oxide enhanced polyacrylamide-alginate aerogels catalysts. Carbohydr. Polym. 2019, 203, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, N.; Brovko, O.; Palamarchuk, I.; Bogolitsyn, K.; Bogdanovich, N.; Ivakhnov, A.; Chukhchin, D.; Arkhilin, M. Formation of supramolecular structure in alginate/chitosan aerogel materials during sol-gel synthesis. J. Sol-Gel Sci. Technol. 2020, 95, 101–108. [Google Scholar] [CrossRef]
- Zhai, Z.; Ren, B.; Xu, Y.; Wang, S.; Zhang, L.; Liu, Z. The preparation of Fe-doped carbon aerogels from sodium alginate. IOP Conf. Ser. Earth Environ. Sci. 2020, 508, 012137. [Google Scholar] [CrossRef]
- Zhai, Z.; Ren, B.; Xu, Y.; Wang, S.; Zhang, L.; Liu, Z. Green and facile fabrication of Cu-doped carbon aerogels from sodium alginate for supercapacitors. Org. Electron. 2019, 70, 246–251. [Google Scholar] [CrossRef]
- Batista, M.; Gonçalves, V.; Gaspar, F.; Nogueira, I.; Matias, A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and hybrid alginate-hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- Dos Santos, P.; Viganó, J.; Furtado, G.D.F.; Cunha, R.L.; Hubinger, M.D.; Rezende, C.A.; Martínez, J. Production of resveratrol loaded alginate aerogel: Characterization, mathematical modeling, and study of impregnation. J. Supercrit. Fluids 2020, 163, 104882. [Google Scholar] [CrossRef]
- Lovskaya, D.; Menshutina, N. Alginate-Based Aerogel Particles as Drug Delivery Systems: Investigation of the Supercritical Adsorption and In Vitro Evaluations. Material 2020, 13, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viganó, J.; Meirelles, A.A.; Náthia-Neves, G.; Baseggio, A.M.; Cunha, R.L.; Junior, M.R.M.; Meireles, M.A.A.; Gurikov, P.; Smirnova, I.; Martínez, J. Impregnation of passion fruit bagasse extract in alginate aerogel microparticles. Int. J. Biol. Macromol. 2020, 155, 1060–1068. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Zhou, X.; Li, S. Efficient Removal of Heavy Metal Ions in Wastewater by Using a Novel Alginate-EDTA Hybrid Aerogel. Appl. Sci. 2019, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Zhuang, Y.; Han, K.; Shi, B. Enhanced tetracycline adsorption using alginate-graphene-ZIF67 aerogel. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124360. [Google Scholar] [CrossRef]
- Tao, E.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloy. Compd. 2020, 832, 154833. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, X.; Zheng, H. Removal of Methylene Blue from Water by Copper Alginate/Activated Carbon Aerogel: Equilibrium, Kinetic, and Thermodynamic Studies. J. Polym. Environ. 2020, 28, 200–210. [Google Scholar] [CrossRef]
- Jiao, C.; Li, T.; Wang, J.; Wang, H.; Zhang, X.; Han, X.; Du, Z.; Shang, Y.; Chen, Y. Efficient Removal of Dyes from Aqueous Solution by a Porous Sodium Alginate/gelatin/graphene Oxide Triple-network Composite Aerogel. J. Polym. Environ. 2020, 28, 1492–1502. [Google Scholar] [CrossRef]
- Wang, S.-J.; Bu, H.; Chen, H.-J.; Hu, T.; Chen, W.-Z.; Wu, J.-H.; Hu, H.-J.; Lin, M.-Z.; Li, Y.; Jiang, G.-B. Floatable magnetic aerogel based on alkaline residue used for the convenient removal of heavy metals from wastewater. Chem. Eng. J. 2020, 399, 125760. [Google Scholar] [CrossRef]
- Shang, K.; Liao, W.; Wang, J.; Wang, Y.-Z.; Schiraldi, D.A. Nonflammable Alginate Nanocomposite Aerogels Prepared by a Simple Freeze-Drying and Post-Cross-Linking Method. ACS Appl. Mater. Interfaces 2015, 8, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhou, X.; Xu, T.; Dai, C.; Gu, Y.; Yun, S.; Hu, T.; Guan, G.; Chen, J. Ultralight and Hydrophobic Palygorskite-based Aerogels with Prominent Thermal Insulation and Flame Retardancy. ACS Appl. Mater. Interfaces 2020, 12, 11815–11824. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Chen, M.-J.; Chen, H.-B. Facile fabrication of mechanically-strong and flame retardant alginate/clay aerogels. Compos. Part B Eng. 2019, 164, 18–25. [Google Scholar] [CrossRef]
- Gurikov, P.; Raman, S.P.; Weinrich, D.; Fricke, M.; Smirnova, I. A novel approach to alginate aerogels: Carbon dioxide induced gelation. RSC Adv. 2015, 5, 7812–7818. [Google Scholar] [CrossRef]
- Agostinho, D.A.; Paninho, A.I.; Cordeiro, T.; Nunes, A.V.; Fonseca, I.M.; Pereira, C.; Matias, A.; Ventura, M.G. Properties of κ-carrageenan aerogels prepared by using different dissolution media and its application as drug delivery systems. Mater. Chem. Phys. 2020, 253, 123290. [Google Scholar] [CrossRef]
- Xiao, Y.; Fu, M.; Wu, D.; Xue, Z.; Xia, Y. Preparation of Carrageenan Aerogel from Extraction of Chondrus and Application in Oil/Organic Solvents Absorption. J. Appl. Sci. Eng. Innov. 2020, 7, 44–48. [Google Scholar]
- Ganesan, K.; Ratke, L. Facile preparation of monolithic κ-carrageenan aerogels. Soft Matter 2014, 10, 3218–3224. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr. Polym. 2018, 180, 264–275. [Google Scholar] [CrossRef]
- Abdellatif, F.H.H.; Abdellatif, M.M. Bio-based i-carrageenan aerogels as efficient adsorbents for heavy metal ions and acid dye from aqueous solution. Cellulose 2020, 27, 441–453. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Guo, R.; Li, D.; Lv, C.; Wang, Y.; Zhang, H.; Xia, Y.; Yang, D.; Zhao, X. Porous Ni3S4/C Aerogels Derived from Carrageenan-Ni Hydrogels for High-Performance Sodium-Ion Batteries Anode. Electrochim. Acta 2019, 299, 72–79. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structure of oleogels from κ-carrageenan templates as affected by supercritical-CO2-drying, freeze-drying and lettuce-filler addition. Food Hydrocoll. 2019, 96, 1–10. [Google Scholar] [CrossRef]
- Lv, D.; Li, Y.; Wang, L. Carbon aerogels derived from sodium lignin sulfonate embedded in carrageenan skeleton for methylene-blue removal. Int. J. Biol. Macromol. 2020, 148, 979–987. [Google Scholar] [CrossRef]
- Manzocco, L.; Valoppi, F.; Calligaris, S.; Andreatta, F.; Spilimbergo, S.; Nicoli, M.C. Exploitation of κ-carrageenan aerogels as template for edible oleogel preparation. Food Hydrocoll. 2017, 71, 68–75. [Google Scholar] [CrossRef]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Broussignac, P. Chitosan: A Natural Polymer Not Well Known by the Industry. Chim. Ind. Genie Chim. 1968, 99, 1241–1247. [Google Scholar]
- Kurita, K.; Tomita, K.; Tada, T.; Ishii, S.; Nishimura, S.-I.; Shimoda, K. Squid chitin as a potential alternative chitin source: Deacetylation behavior and characteristic properties. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 485–491. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Daya, M.R.; Worley, J.A. Experience with Chitosan Dressings in a Civilian EMS System. J. Emerg. Med. 2009, 37, 1–7. [Google Scholar] [CrossRef]
- Prashanth, K.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.; Abd-Elaal, A.A.; Badr, E.A.; Kana, M.T.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, N.G.; Zhao, S.; Adilien, N.; Koebel, M.M.; Lattuada, M.; Malfait, W.J. Strong, Machinable, and Insulating Chitosan–Urea Aerogels: Toward Ambient Pressure Drying of Biopolymer Aerogel Monoliths. ACS Appl. Mater. Interfaces 2020, 12, 22037–22049. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Gurikov, P.; Monteiro, F.J.; Smirnova, I.; Alvarez-Lorenzo, C.; García-González, C. Jet Cutting Technique for the Production of Chitosan Aerogel Microparticles Loaded with Vancomycin. Polymer 2020, 12, 273. [Google Scholar] [CrossRef] [Green Version]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Obaidat, R.M.; Tashtoush, B.M.; Bayan, M.F.; Al Bustami, R.T.; Alnaief, M. Drying Using Supercritical Fluid Technology as a Potential Method for Preparation of Chitosan Aerogel Microparticles. Aaps Pharmscitech 2015, 16, 1235–1244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y.; Li, L. Ultra-low shrinkage chitosan aerogels trussed with polyvinyl alcohol. Mater. Des. 2018, 156, 398–406. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Jeong, E.; Fischer, B.; Zhang, Y.; Xu, H.; Angelica, E.; Risen, W.M.; Suggs, J.W.; Koebel, M.M. Facile One-Pot Synthesis of Mechanically Robust Biopolymer–Silica Nanocomposite Aerogel by Cogelation of Silicic Acid with Chitosan in Aqueous Media. ACS Sustain. Chem. Eng. 2016, 4, 5674–5683. [Google Scholar] [CrossRef]
- Takeshita, S.; Akasaka, S.; Yoda, S. Structural and acoustic properties of transparent chitosan aerogel. Mater. Lett. 2019, 254, 258–261. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Chang, X.; Chen, D.; Jiao, X. Chitosan-Based Aerogels with High Adsorption Performance. J. Phys. Chem. B 2008, 112, 7721–7725. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, Y.; Dong, Z.; Wang, J.; Hou, X. Hydrophobic and nanoporous chitosan-silica composite aerogels for oil absorption. J. Appl. Polym. Sci. 2015, 132, 132. [Google Scholar] [CrossRef]
- Diosa, J.; Guzman, F.; Bernal, C.; Mesa, M. Formation mechanisms of chitosan-silica hybrid materials and its performance as solid support for KR-12 peptide adsorption: Impact on KR-12 antimicrobial activity and proteolytic stability. J. Mater. Res. Technol. 2020, 9, 890–901. [Google Scholar] [CrossRef]
- Gao, X.-D.; Huang, Y.-D.; Zhang, T.-T.; Wu, Y.-Q.; Li, X.-M. Amphiphilic SiO 2 hybrid aerogel: An effective absorbent for emulsified wastewater. J. Mater. Chem. A 2017, 5, 12856–12862. [Google Scholar] [CrossRef]
- Keshipour, S.; Mirmasoudi, S.S. Cross-linked chitosan aerogel modified with Au: Synthesis, characterization and catalytic application. Carbohydr. Polym. 2018, 196, 494–500. [Google Scholar] [CrossRef]
- Rinki, K.; Dutta, P.K.; Hunt, A.J.; MacQuarrie, D.J.; Clark, J.H. Chitosan Aerogels Exhibiting High Surface Area for Biomedical Application: Preparation, Characterization, and Antibacterial Study. Int. J. Polym. Mater. 2011, 60, 988–999. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Nanostructured chitosan-gelatin hybrid aerogels produced by supercritical gel drying. Polym. Eng. Sci. 2017, 58, 1494–1499. [Google Scholar] [CrossRef]
- Valchuk, N.A.; Brovko, O.S.; Palamarchuk, I.A.; Boitsova, T.A.; Bogolitsyn, K.G.; Ivakhnov, A.D.; Chukhchin, D.G.; Bogdanovich, N.I. Preparation of Aerogel Materials Based on Alginate–Chitosan Interpolymer Complex Using Supercritical Fluids. Russ. J. Phys. Chem. B 2019, 13, 1121–1124. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Scognamiglio, M.; Reverchon, E. A new tool to produce alginate-based aerogels for medical applications, by supercritical gel drying. J. Supercrit. Fluids 2019, 146, 152–158. [Google Scholar] [CrossRef]
- Frindy, S.; El Kadib, A.; Lahcini, M.; Primo, A.; García, H. Copper Nanoparticles Stabilized in a Porous Chitosan Aerogel as a Heterogeneous Catalyst for C?S Cross-coupling. ChemCatChem 2015, 7, 3307–3315. [Google Scholar] [CrossRef]
- Anouar, A.; Katir, N.; Lahcini, M.; Primo, A.; Garcia, H. Palladium Supported on Porous Chitosan-Graphene Oxide Aerogels as Highly Efficient Catalysts for Hydrogen Generation from Formate. Molecules 2019, 24, 3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokin, A.B.; Quignard, F.; Valentin, R.; Mangematin, S. Chitosan supported phthalocyanine complexes: Bifunctional catalysts with basic and oxidation active sites. Appl. Catal. A Gen. 2006, 309, 162–168. [Google Scholar] [CrossRef]
- Kayser, H.; Müller, C.R.; García-González, C.; Smirnova, I.; Leitner, W.; De María, P.D. Dried chitosan-gels as organocatalysts for the production of biomass-derived platform chemicals. Appl. Catal. A Gen. 2012, 445, 180–186. [Google Scholar] [CrossRef]
- Raman, S.; Gurikov, P.; Smirnova, I. Hybrid alginate based aerogels by carbon dioxide induced gelation: Novel technique for multiple applications. J. Supercrit. Fluids 2015, 106, 23–33. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y. Formation of enhanced gelatum using ethanol/water binary medium for fabricating chitosan aerogels with high specific surface area. Chem. Eng. J. 2017, 309, 700–707. [Google Scholar] [CrossRef]
- Di Renzo, F.; Valentin, R.; Boissiere, M.; Tourrette, A.; Sparapano, G.; Molvinger, K.; Devoisselle, J.M.; Gérardin, C.; Quignard, F. Hierarchical Macroporosity Induced by Constrained Syneresis in Core–Shell Polysaccharide Composites. Chem. Mater. 2005, 17, 4693–4699. [Google Scholar] [CrossRef]
- Ricci, A.; Bernardi, L.; Gioia, C.; Vierucci, S.; Robitzer, M.; Quignard, F. Chitosan Aerogel: A Recyclable, Heterogeneous Organocatalyst for the Asymmetric Direct Aldol Reaction in Water. Chem. Commun. 2010, 46, 6288–6290. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Sanz, Y.; Alonso, J.L.; Parajó, J.C.; Gullón, B. Prebiotic potential of a refined product containing pectic oligosaccharides. LWT Food Sci. Technol. 2011, 44, 1687–1696. [Google Scholar] [CrossRef]
- Koubala, B.; Mbome, L.; Kansci, G.; Mbiapo, F.T.; Crepeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chem. 2008, 106, 1202–1207. [Google Scholar] [CrossRef]
- De Vries, R.P.; Visser, J. Aspergillus Enzymes Involved in Degradation of Plant Cell Wall Polysaccharides. Microbiol. Mol. Biol. Rev. 2001, 65, 497–522. [Google Scholar] [CrossRef] [Green Version]
- Müller-Maatsch, J.; Caligiani, A.; Tedeschi, T.; Elst, K.; Sforza, S. Simple and Validated Quantitative1H NMR Method for the Determination of Methylation, Acetylation, and Feruloylation Degree of Pectin. J. Agric. Food Chem. 2014, 62, 9081–9087. [Google Scholar] [CrossRef]
- Srivastava, P.; Malviya, R. Sources of Pectin, Extraction and its Applications in Pharmaceutical Industry—An Overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Canteri-Schemin, M.H.; Fertonani, H.C.R.; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Braz. Arch. Biol. Technol. 2005, 48, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Massih, R.M.; Baydoun, E.; Waldron, K.W.; Brett, C.T. Effects of partial enzymic degradation of sugar beet pectin on oxidative coupling of pectin-linked ferulates in vitro. Phytochemistry 2007, 68, 1785–1790. [Google Scholar] [CrossRef]
- Willats, W.G.T.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Industrial Gums: Polysaccharides and Their Derivatives; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Yapo, B.M.; Lerouge, P.; Thibault, J.-F.; Ralet, M.-C.J. Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydr. Polym. 2007, 69, 426–435. [Google Scholar] [CrossRef]
- Taylor, S. The Chemistry and Technology of Pectin; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Harholt, J.; Suttangkakul, A.; Scheller, H.V. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-S.; Mu, T.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, F.M.; Thibault, J.-F. Feruloylated pectic substances from sugar-beet pulp. Carbohydr. Res. 1986, 154, 177–187. [Google Scholar] [CrossRef]
- Khodaei, N.; Karboune, S. Enzymatic generation of galactose-rich oligosaccharides/oligomers from potato rhamnogalacturonan I pectic polysaccharides. Food Chem. 2016, 197, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr. Polym. 2016, 142, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, G.; Negi, P. Isolation of pectin from kinnow peels and its characterization. Food Bioprod. Process. 2020, 124, 342–353. [Google Scholar] [CrossRef]
- Buathongjan, C.; Israkarn, K.; Sangwan, W.; Outrequin, T.; Gamonpilas, C.; Methacanon, P. Studies on chemical composition, rheological and antioxidant properties of pectin isolated from Riang (Parkia timoriana (DC.) Merr.) pod. Int. J. Biol. Macromol. 2020, 164, 4575–4582. [Google Scholar] [CrossRef]
- Chan, S.-Y.; Choo, W.-S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013, 141, 3752–3758. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef]
- Yeoh, S.; Shi, J.; Langrish, T. Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination 2008, 218, 229–237. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. In Chemistry and Chemical Technologies in Waste Valorization; Springer: Berlin/Heidelberg, Germany, 2018; pp. 229–282. [Google Scholar]
- Khodaei, N.; Karboune, S.; Orsat, V. Microwave-assisted alkaline extraction of galactan-rich rhamnogalacturonan I from potato cell wall by-product. Food Chem. 2016, 190, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Pińkowska, H.; Złocińska, A. Pektyny–występowanie, budowa chemiczna i właściwości. Wiad. Chem. 2014, 68, 685–700. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A. Quality of french fried potatoes as influenced by coating with hydrocolloids. Food Chem. 1999, 66, 201–208. [Google Scholar] [CrossRef]
- Zaitseva, O.; Khudyakov, A.; Sergushkina, M.; Solomina, O.; Polezhaeva, T. Pectins as a universal medicine. Fitoterapia 2020, 146, 104676. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymer 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Olano-Martin, E.; Gibson, G.; Rastall, R. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Wikiera, A.; Irla, M.; Mika, M. Health-promoting properties of pectin. Postępy Hig. Med. Dosw. 2014, 68, 590–596. [Google Scholar] [CrossRef]
- Sánchez-Infantes, D.; Muguerza, B.; Moulay, L.; Hernandez, R.; Miguel, M.; Aleixandre, A. Highly Methoxylated Pectin Improves Insulin Resistance and Other Cardiometabolic Risk Factors in Zucker Fatty Rats. J. Agric. Food Chem. 2008, 56, 3574–3581. [Google Scholar] [CrossRef]
- Schwab, U.S.; Louheranta, A.; Törrönen, A.; Uusitupa, M. Impact of sugar beet pectin and polydextrose on fasting and postprandial glycemia and fasting concentrations of serum total and lipoprotein lipids in middle-aged subjects with abnormal glucose metabolism. Eur. J. Clin. Nutr. 2006, 60, 1073–1080. [Google Scholar] [CrossRef]
- Sudheesh, S.; Vijayalakshmi, N. Lipid-lowering action of pectin from Cucumis sativus. Food Chem. 1999, 67, 281–286. [Google Scholar] [CrossRef]
- Jackson, C.L.; Dreaden, T.M.; Theobald, L.K.; Tran, N.M.; Beal, T.L.; Eid, M.; Gao, M.Y.; Shirley, R.B.; Stoffel, M.T.; Kumar, M.V.; et al. Pectin induces apoptosis in human prostate cancer cells: Correlation of apoptotic function with pectin structure. Glycobioloy 2007, 17, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, B.S.; Barsett, H. Bioactive Pectic Polysaccharides. In Polysaccharides I; Springer: Berlin/Heidelberg, Germany, 2005; pp. 69–101. [Google Scholar]
- Salman, H.; Bergman, M.; Djaldetti, M.; Orlin, J.; Bessler, H. Citrus pectin affects cytokine production by human peripheral blood mononuclear cells. Biomed. Pharm. 2008, 62, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Sheu, M.-T.; Chen, T.-F.; Wang, Y.-C.; Hou, W.-C.; Liu, D.-Z.; Chung, T.-C.; Liang, Y.-C. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem. Pharm. 2006, 72, 1001–1009. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Tuning structure and properties of pectin aerogels. Eur. Polym. J. 2018, 108, 250–261. [Google Scholar] [CrossRef]
- García-González, C.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.L.; Clark, J.H. Pectin-Derived Porous Materials. Chem. A Eur. J. 2010, 16, 1326–1335. [Google Scholar] [CrossRef]
- Veronovski, A.; Tkalec, G.; Knez, Ž.; Novak, Z. Characterisation of biodegradable pectin aerogels and their potential use as drug carriers. Carbohydr. Polym. 2014, 113, 272–278. [Google Scholar] [CrossRef]
- García-González, C.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of biocompatible magnetic pectin aerogel monoliths and microspheres. RSC Adv. 2012, 2, 9816. [Google Scholar] [CrossRef]
- García-González, C.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Tkalec, G.; Knez, Z.; Novak, Z.; Gabrijela, T.; Željko, K.; Novak, Z. Encapsulation of pharmaceuticals into pectin aerogels for controlled drug release. Adv. Technol. 2015, 4, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Malfait, W.J.; Demilecamps, A.; Zhang, Y.; Brunner, S.; Huber, L.; Tingaut, P.; Rigacci, A.; Budtova, T.; Koebel, M. Strong, Thermally Superinsulating Biopolymer–Silica Aerogel Hybrids by Cogelation of Silicic Acid with Pectin. Angew. Chem. Int. Ed. 2015, 54, 14282–14286. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, G.; Knez, Ž.; Novak, Z. PH sensitive mesoporous materials for immediate or controlled release of NSAID. Microporous Mesoporous Mater. 2016, 224, 190–200. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Fast production of high-methoxyl pectin aerogels for enhancing the bioavailability of low-soluble drugs. J. Supercrit. Fluids 2015, 106, 16–22. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Formation of polysaccharide aerogels in ethanol. RSC Adv. 2015, 5, 77362–77371. [Google Scholar] [CrossRef]
- Horvat, G.; Xhanari, K.; Finšgar, M.; Gradišnik, L.; Maver, U.; Knez, Ž.; Novak, Z. Novel ethanol-induced pectin–xanthan aerogel coatings for orthopedic applications. Carbohydr. Polym. 2017, 166, 365–376. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Chen, M.; Chen, H.-B. Thermally Insulating and Flame-Retardant Polyaniline/Pectin Aerogels. ACS Sustain. Chem. Eng. 2017, 5, 7012–7019. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Encapsulation and drug release of poorly water soluble nifedipine from bio-carriers. J. Non-Cryst. Solids 2018, 481, 486–493. [Google Scholar] [CrossRef]
- Chen, H.-B.; Li, X.-L.; Chen, M.-J.; He, Y.-R.; Zhao, H.-B. Self-cross-linked melamine-formaldehyde-pectin aerogel with excellent water resistance and flame retardancy. Carbohydr. Polym. 2019, 206, 609–615. [Google Scholar] [CrossRef]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Preparation and characterization of polysaccharide—Silica hybrid aerogels. Sci. Rep. 2019, 9, 16492. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-B.; Chiou, B.-S.; Wang, Y.-Z.; Schiraldi, D.A. Biodegradable Pectin/Clay Aerogels. ACS Appl. Mater. Interfaces 2013, 5, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yuen, A.C.Y.; Ping, P.; Wei, R.-C.; Hua, L.; Zhu, Z.; Li, A.; Zhu, S.-E.; Wang, L.-L.; Liang, J.; et al. Pectin-assisted dispersion of exfoliated boron nitride nanosheets for assembled bio-composite aerogels. Compos. Part A Appl. Sci. Manuf. 2019, 119, 196–205. [Google Scholar] [CrossRef]
- Horvat, G.; Fajfar, T.; Uzunalić, A.P.; Knez, Ž.; Novak, Z. Thermal properties of polysaccharide aerogels. J. Anal. Calorim. 2016, 127, 363–370. [Google Scholar] [CrossRef]
- Budtova, T. Bio-Based Aerogels: A New Generation of Thermal Superinsulating Materials. In Cellulose Science and Technology: Chemistry, Analysis, and Applications; John Wiley & Sons: Chichester, UK, 2018; pp. 371–392. [Google Scholar]
- Maleki, H.; Durães, L.; García-González, C.A.; Del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Rindlav-Westling, A.; Stading, M.; Gatenholm, P. Crystallinity and Morphology in Films of Starch, Amylose and Amylopectin Blends. Biomacromolecules 2002, 3, 84–91. [Google Scholar] [CrossRef]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Franco, P.; Aliakbarian, B.; Perego, P.; Reverchon, E.; De Marco, I. Supercritical Adsorption of Quercetin on Aerogels for Active Packaging Applications. Ind. Eng. Chem. Res. 2018, 57, 15105–15113. [Google Scholar] [CrossRef]
- Glenn, G.M.; Irving, D.W. Starch-Based Microcellular Foams. Cereal Chem. 1995, 72, 155–161. [Google Scholar]
- García-González, C.; Camino-Rey, M.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Ardao, I.; Starbird-Perez, R.; García-González, C. Conductive nanostructured materials based on poly-(3,4-ethylenedioxythiophene) (PEDOT) and starch/κ-carrageenan for biomedical applications. Carbohydr. Polym. 2018, 189, 304–312. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Zou, F.; Budtova, T. Tailoring the morphology and properties of starch aerogels and cryogels via starch source and process parameter. Carbohydr. Polym. 2020, 117344. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Moreau, R.; Rose, D.J.; Zhang, J.; Ciftci, O.N. Enhancing the Bioaccessibility of Phytosterols Using Nanoporous Corn and Wheat Starch Bioaerogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1700229. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, J.M.; García-González, C. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules 2019, 24, 871. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, K.; Xiao, M.; Riffat, S.B.; Su, Y.; Jiang, F. Thermal conductivity, structure and mechanical properties of konjac glucomannan/starch based aerogel strengthened by wheat straw. Carbohydr. Polym. 2018, 197, 284–291. [Google Scholar] [CrossRef]
- Ye, D.-D.; Wang, T.; Liao, W.; Wang, H.; Zhao, H.-B.; Wang, Y.-Z.; Xu, S. Ultrahigh-Temperature Insulating and Fire-Resistant Aerogels from Cationic Amylopectin and Clay via a Facile Route. ACS Sustain. Chem. Eng. 2019, 7, 11582–11592. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Bi, Y.; Shi, X.; Ren, H.; Wang, B. A novel starch-enhanced melamine-formaldehyde aerogel with low volume shrinkage and high toughness. J. Porous Mater. 2017, 24, 1303–1307. [Google Scholar] [CrossRef]
- Lovskaya, D.; Lebedev, A.; Menshutina, N. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Starch aerogel loaded with poorly water-soluble vitamins through supercritical CO2 adsorption. Chem. Eng. Res. Des. 2017, 119, 221–230. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Generating phytosterol nanoparticles in nanoporous bioaerogels via supercritical carbon dioxide impregnation: Effect of impregnation conditions. J. Food Eng. 2017, 207, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Ubeyitogullari, A.; Ciftci, O.N. Phytosterol nanoparticles with reduced crystallinity generated using nanoporous starch aerogels. RSC Adv. 2016, 6, 108319–108327. [Google Scholar] [CrossRef]
- Goimil, L.; Braga, M.E.; Dias, A.M.; Gómez-Amoza, J.L.; Concheiro, A.; Diaz-Rodriguez, P.; De Sousa, H.C.; García-González, C.A. Supercritical processing of starch aerogels and aerogel-loaded poly(ε-caprolactone) scaffolds for sustained release of ketoprofen for bone regeneration. J. CO2 Util. 2017, 18, 237–249. [Google Scholar] [CrossRef]
- Miao, Z.; Ding, K.; Wu, T.; Liu, Z.; Han, B.; An, G.; Miao, S.; Yang, G. Fabrication of 3D-networks of native starch and their application to produce porous inorganic oxide networks through a supercritical route. Microporous Mesoporous Mater. 2008, 111, 104–109. [Google Scholar] [CrossRef]
- Starbird-Perez, R.; García-González, C.A.; Smirnova, I.; Krautschneider, W.H.; Bauhofer, W. Synthesis of an organic conductive porous material using starch aerogels as template for chronic invasive electrodes. Mater. Sci. Eng. C 2014, 37, 177–183. [Google Scholar] [CrossRef]
- Anas, M.; Gönel, A.G.; Bozbag, S.E.; Erkey, C. Thermodynamics of Adsorption of Carbon Dioxide on Various Aerogels. J. CO2 Util. 2017, 21, 82–88. [Google Scholar] [CrossRef]
- Loveday, S.M. Food Proteins: Technological, Nutritional, and Sustainability Attributes of Traditional and Emerging Proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structural characterization of oleogels from whey protein aerogel particles. Food Res. Int. 2020, 132, 109099. [Google Scholar] [CrossRef]
- Selmer, I.; Karnetzke, J.; Kleemann, C.; Lehtonen, M.; Mikkonen, K.S.; Kulozik, U.; Smirnova, I. Encapsulation of fish oil in protein aerogel micro-particles. J. Food Eng. 2019, 260, 1–11. [Google Scholar] [CrossRef]
- Andlinger, D.J.; Bornkeßel, A.C.; Jung, I.; Schröter, B.; Smirnova, I.; Kulozik, U. Microstructures of potato protein hydrogels and aerogels produced by thermal crosslinking and supercritical drying. Food Hydrocoll. 2020, 106305. [Google Scholar] [CrossRef]
- Betz, M.; García-González, C.; Subrahmanyam, R.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Kleemann, C.; Selmer, I.; Smirnova, I.; Kulozik, U. Tailor made protein based aerogel particles from egg white protein, whey protein isolate and sodium caseinate: Influence of the preceding hydrogel characteristics. Food Hydrocoll. 2018, 83, 365–374. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Qiu, K.; Preuss, K.; Marinovic, A.; Sevilla, M.; Sillanpää, M.; Guo, X.; Titirici, M.-M.; Sevilla, M. Soy protein directed hydrothermal synthesis of porous carbon aerogels for electrocatalytic oxygen reduction. Carbon 2016, 96, 622–630. [Google Scholar] [CrossRef]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of egg white protein aerogels as new matrix material for microencapsulation in food. J. Supercrit. Fluids 2015, 106, 42–49. [Google Scholar] [CrossRef]
- Maleki, H.; Whitmore, L.; Hüsing, N. Novel multifunctional polymethylsilsesquioxane–silk fibroin aerogel hybrids for environmental and thermal insulation applications. J. Mater. Chem. A 2018, 6, 12598–12612. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, D.; Duraipandy, N.; Srivatsan, K.V.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Fabrication of Hybrid Collagen Aerogels Reinforced with Wheat Grass Bioactives as Instructive Scaffolds for Collagen Turnover and Angiogenesis for Wound Healing Applications. ACS Appl. Mater. Interfaces 2017, 9, 16939–16950. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Placin, F.; Desvergne, J.-P.; Cansell, F. Organic low molecular weight aerogel formed in supercritical fluids. J. Mater. Chem. 2000, 10, 2147–2149. [Google Scholar] [CrossRef]
- Jamart-Grégoire, B.; Son, S.; Allix, F.; Felix, V.; Barth, D.; Jannot, Y.; Pickaert, G.; DeGiovanni, A. Monolithic organic aerogels derived from single amino-acid based supramolecular gels: Physical and thermal properties. RSC Adv. 2016, 6, 102198–102205. [Google Scholar] [CrossRef]
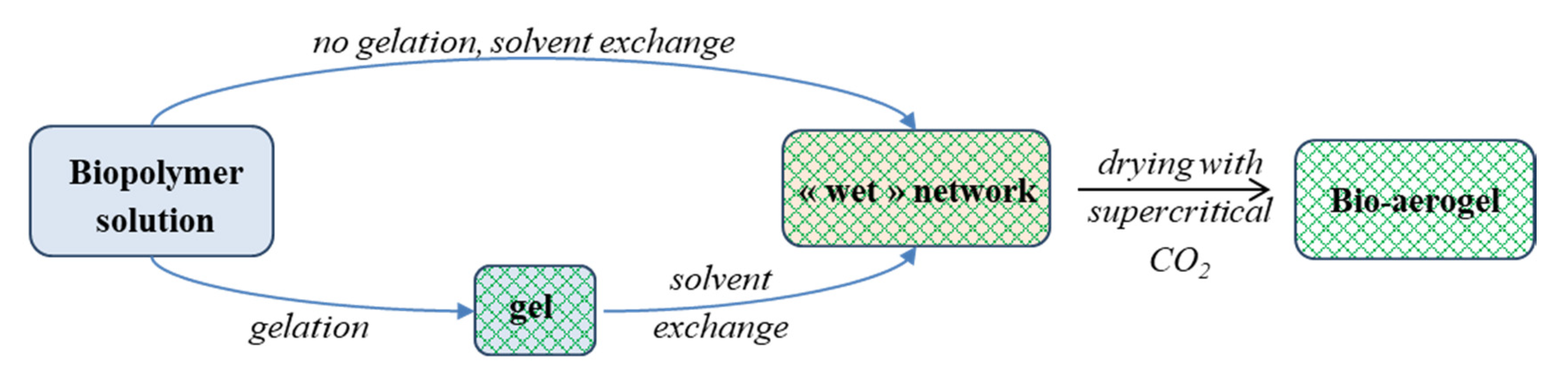
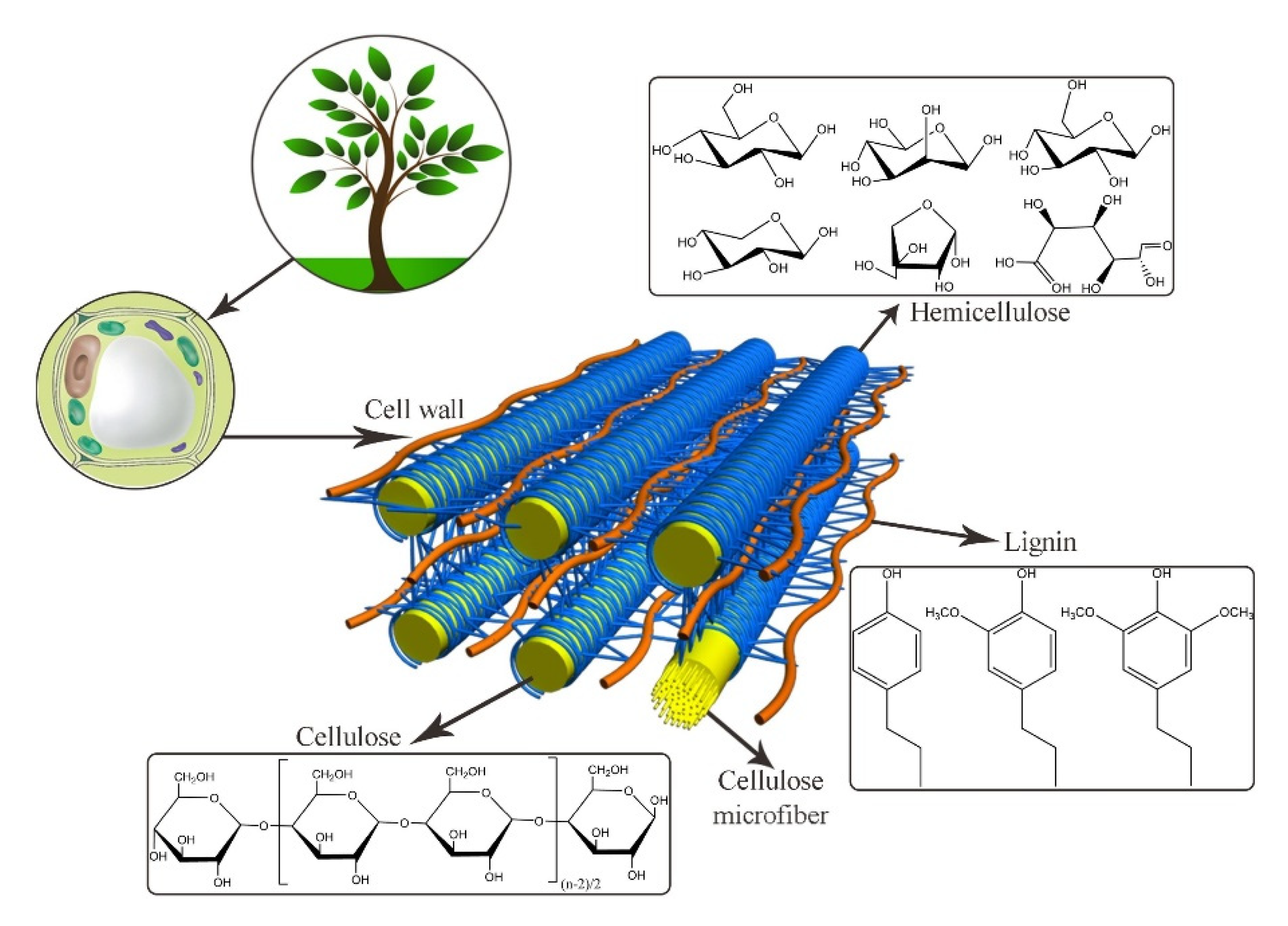
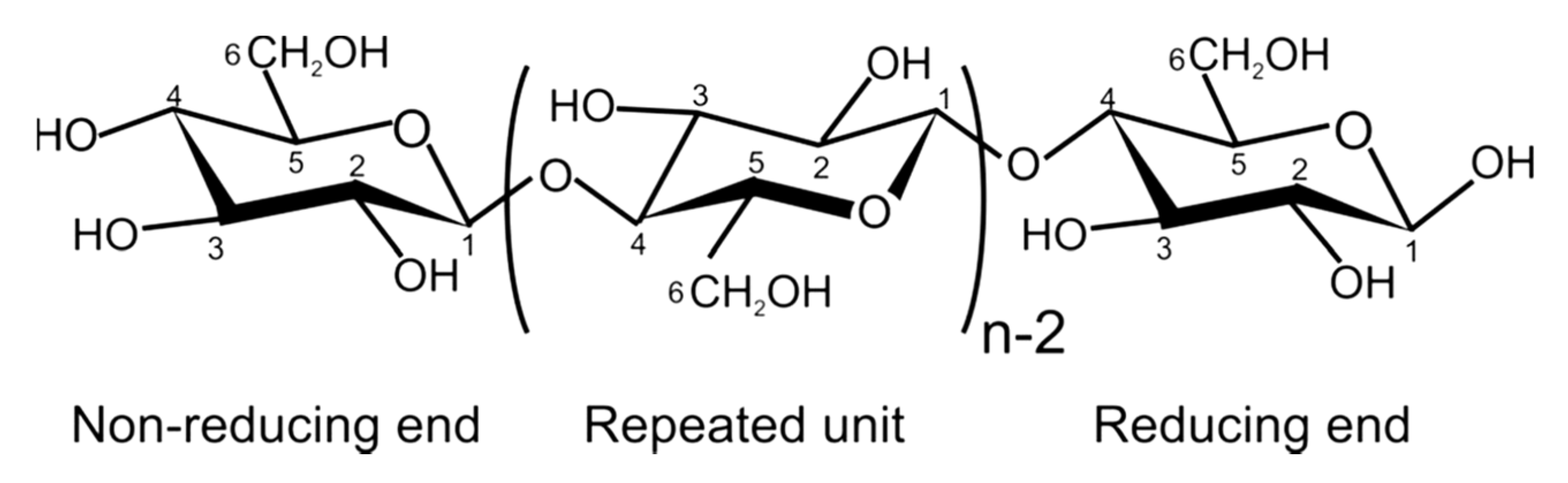


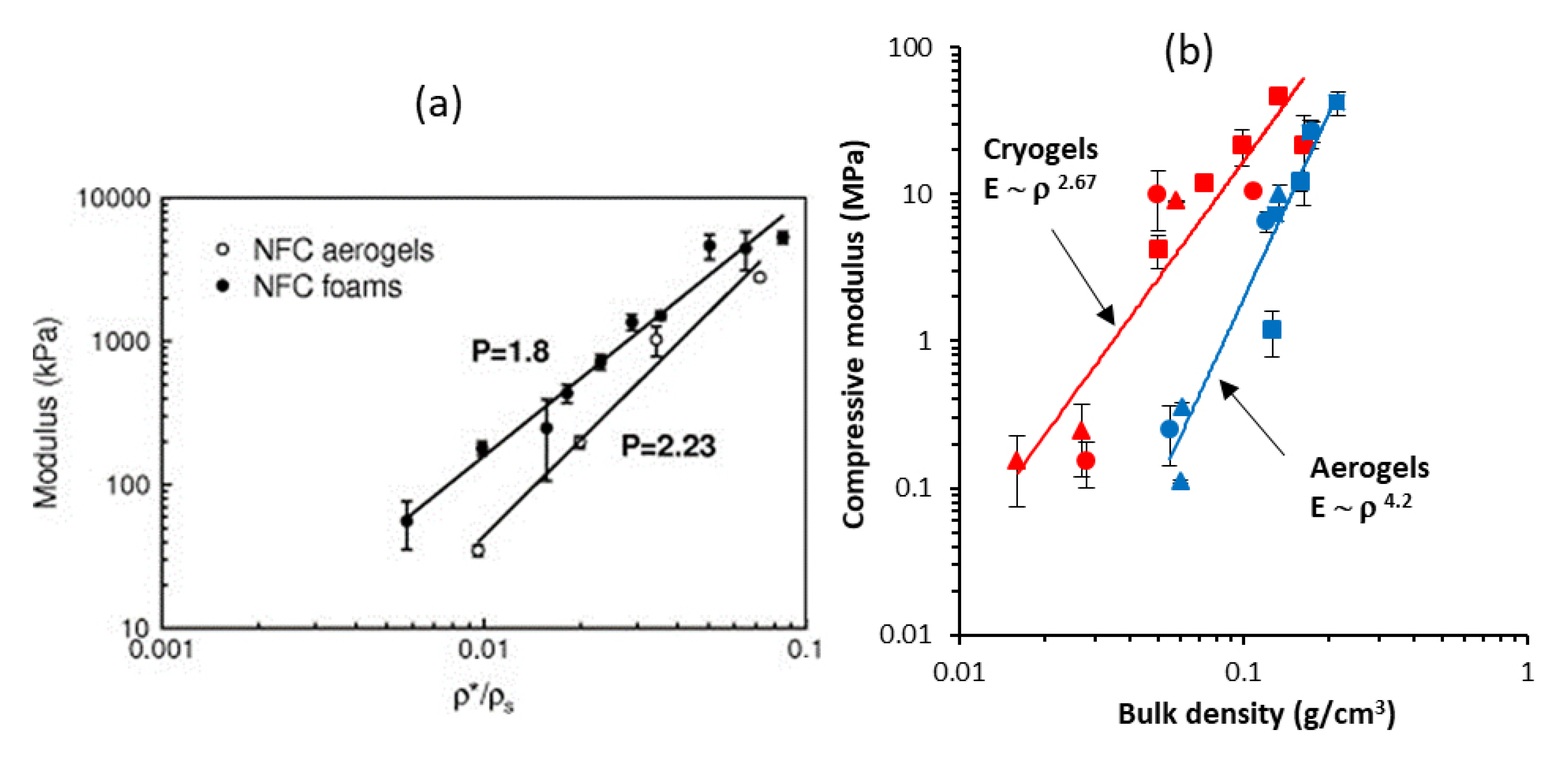
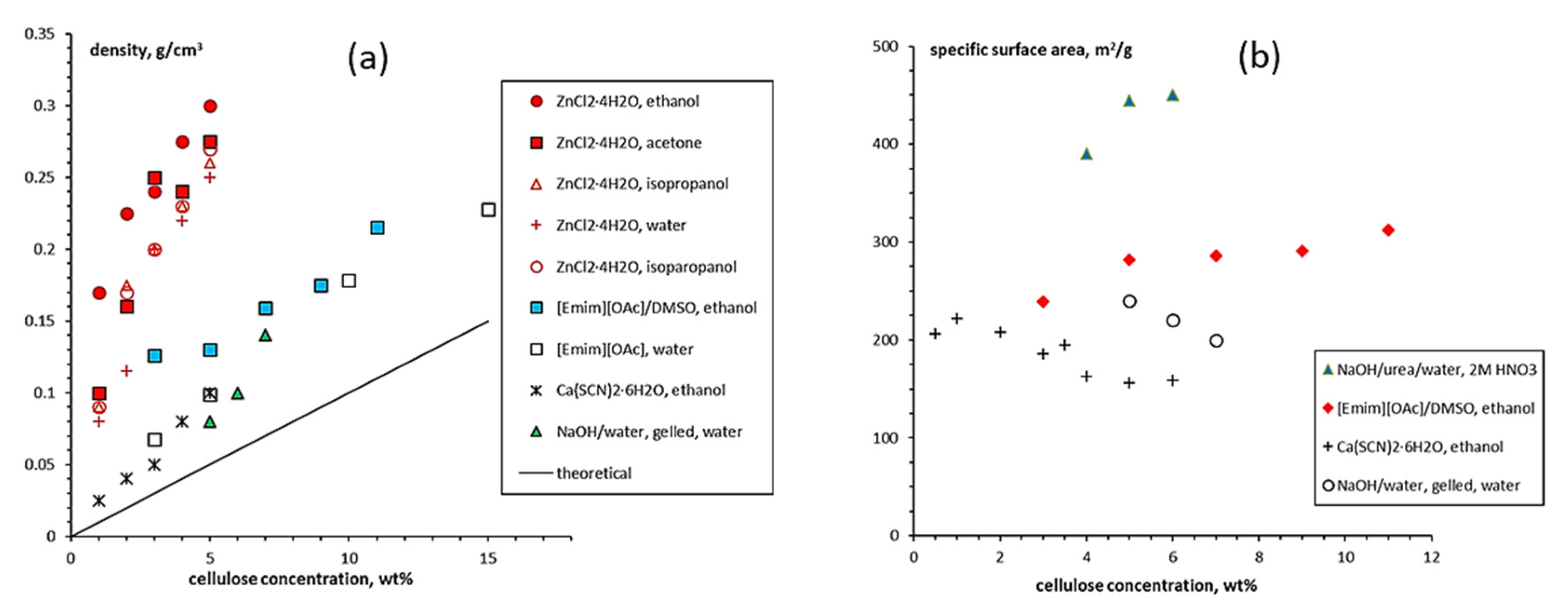
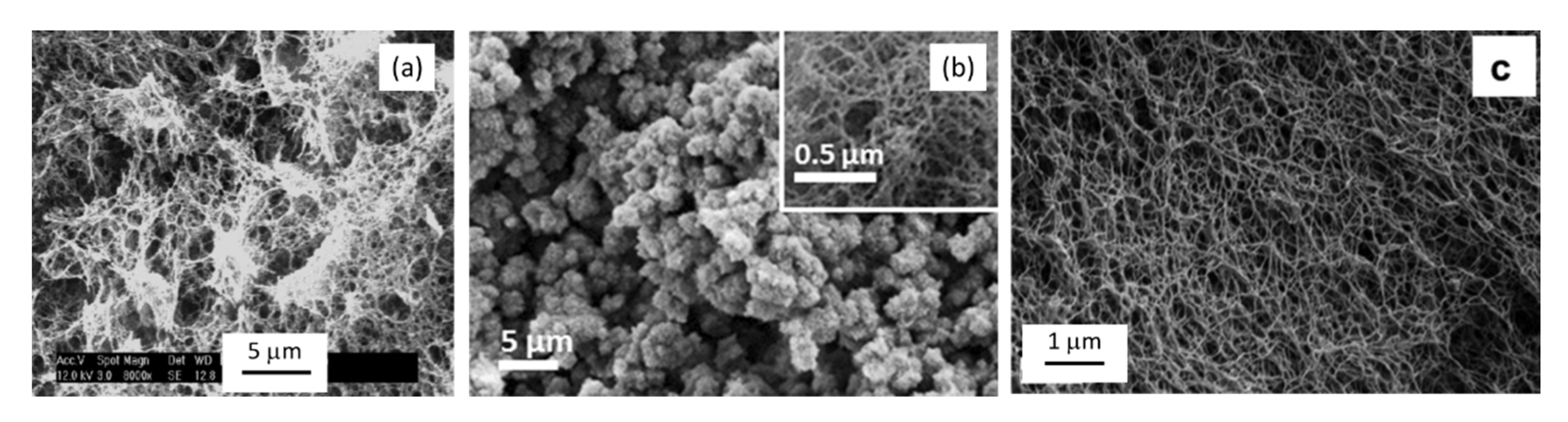

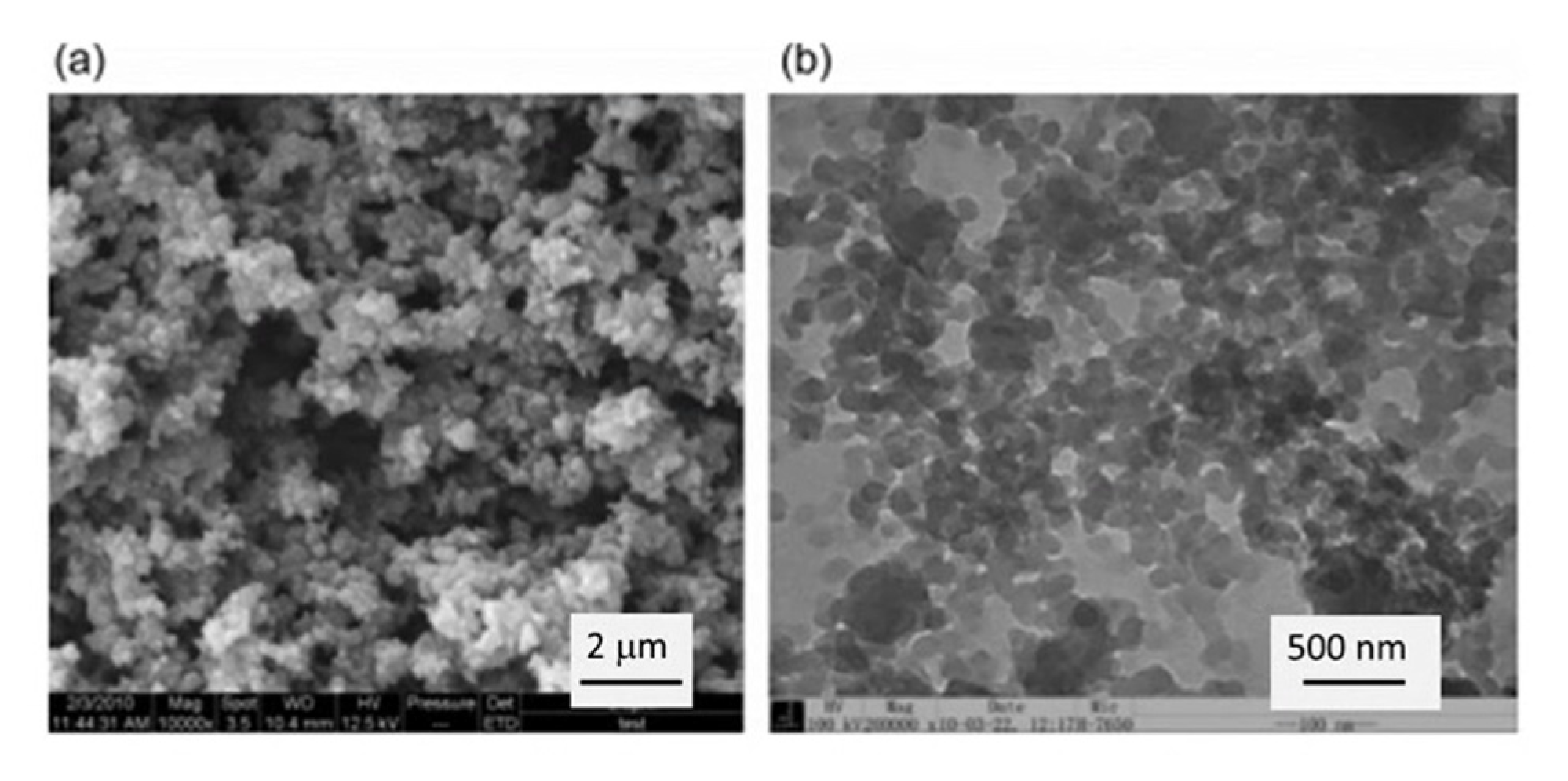
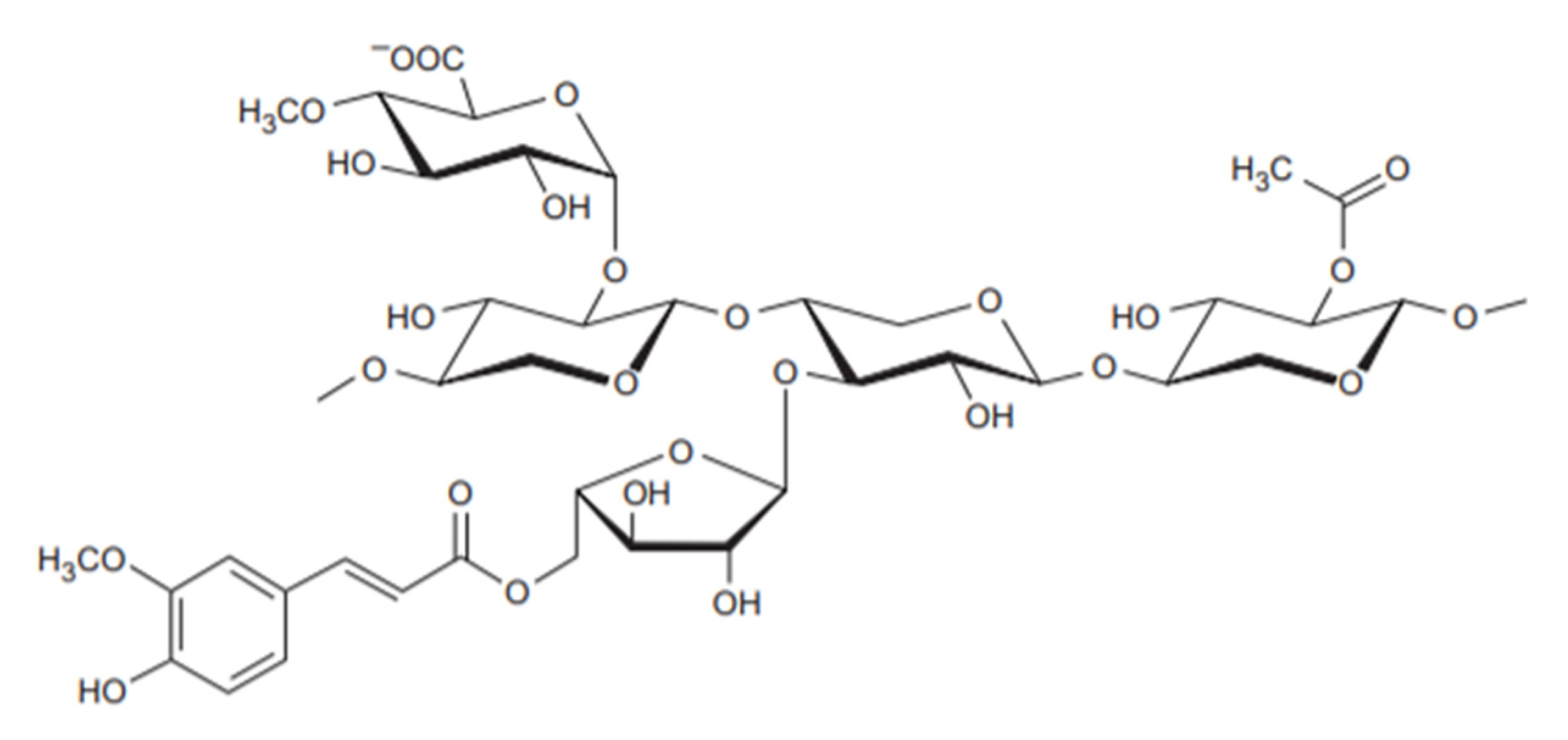
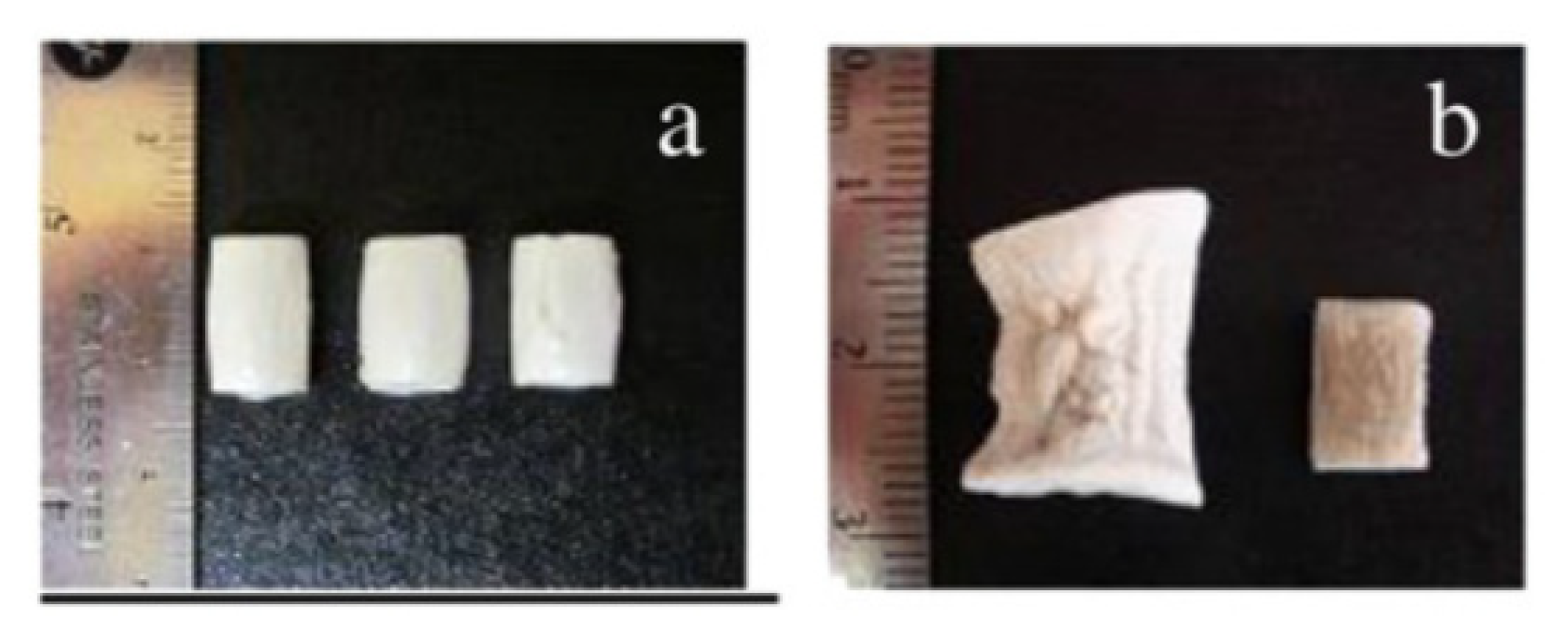
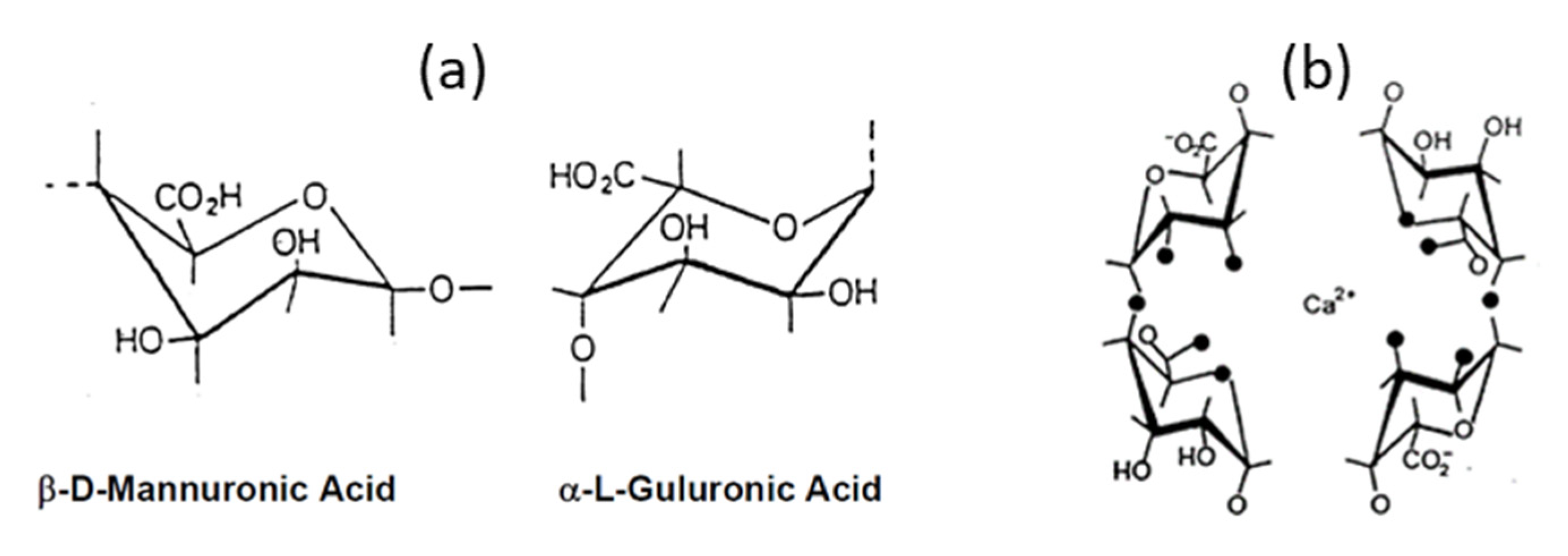
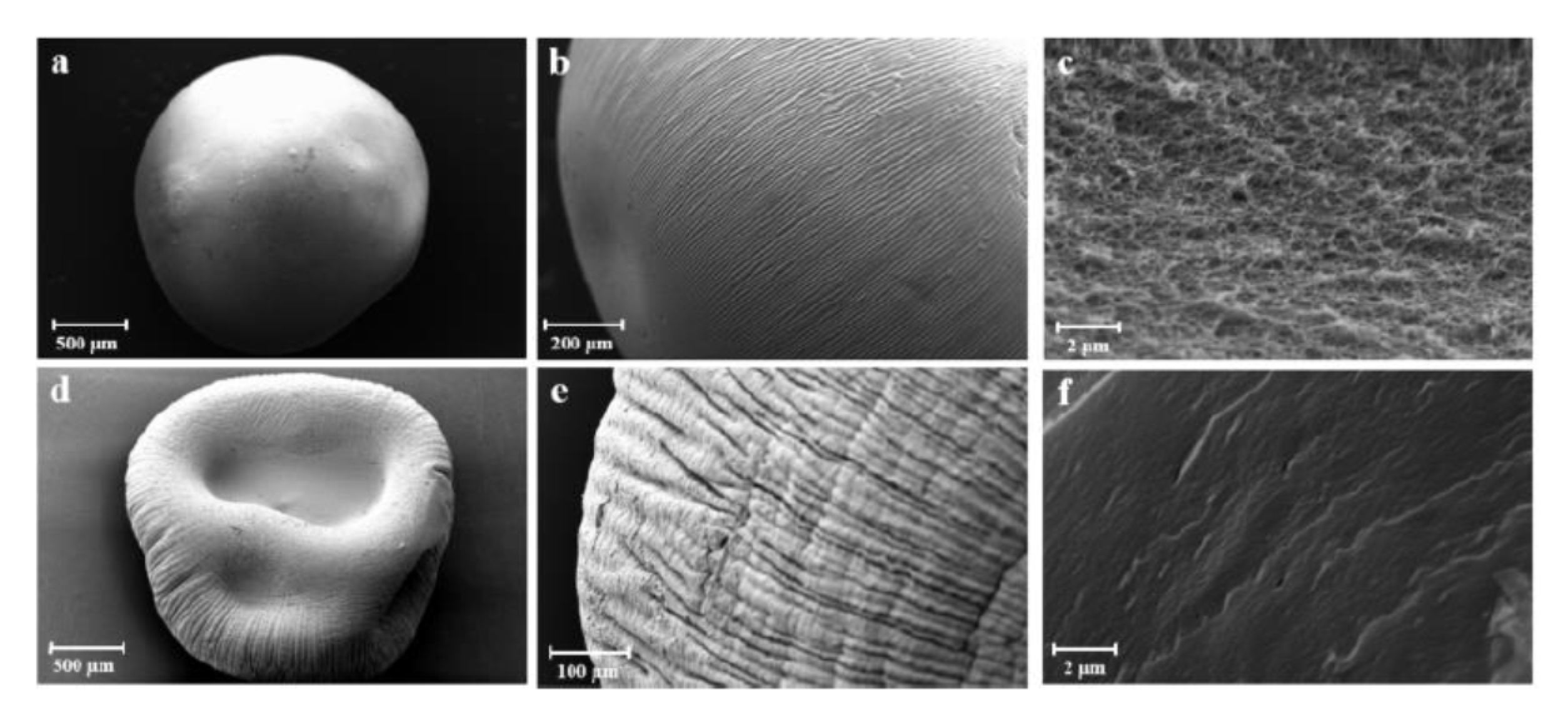
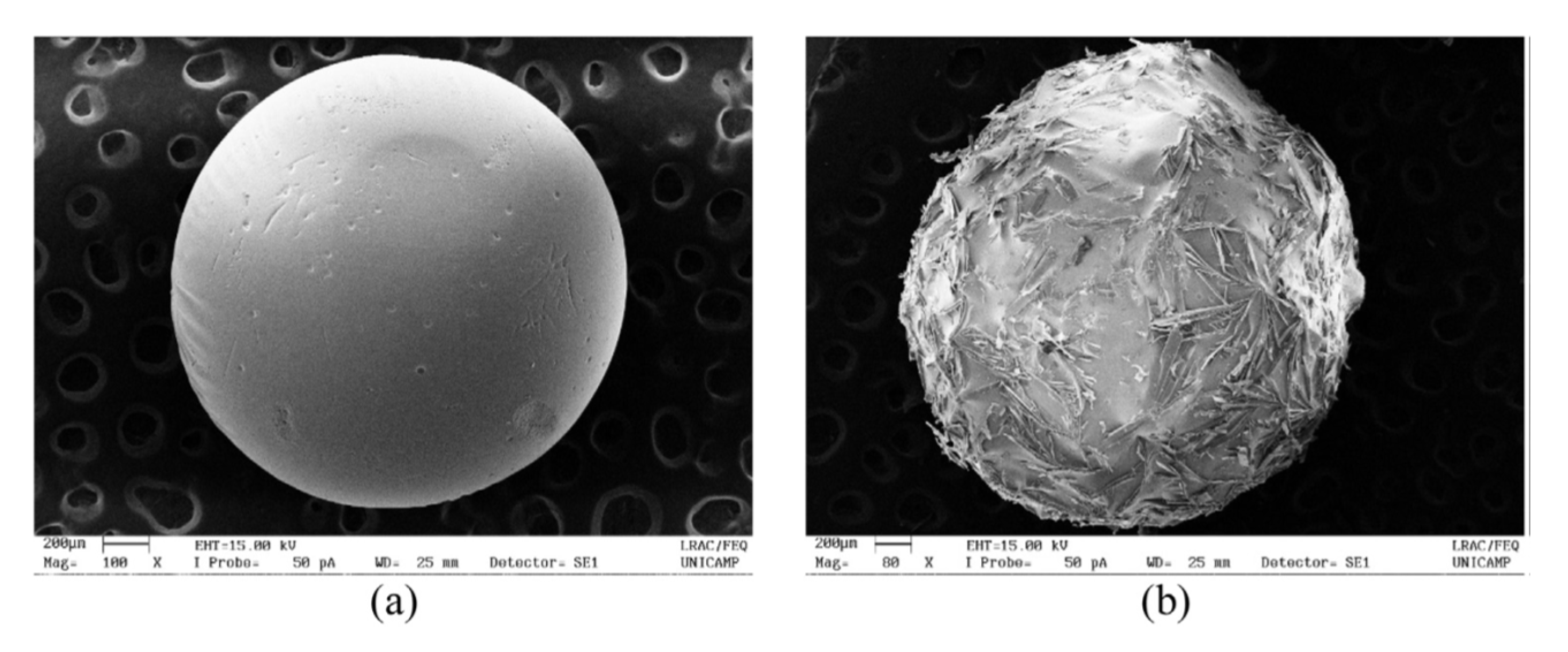
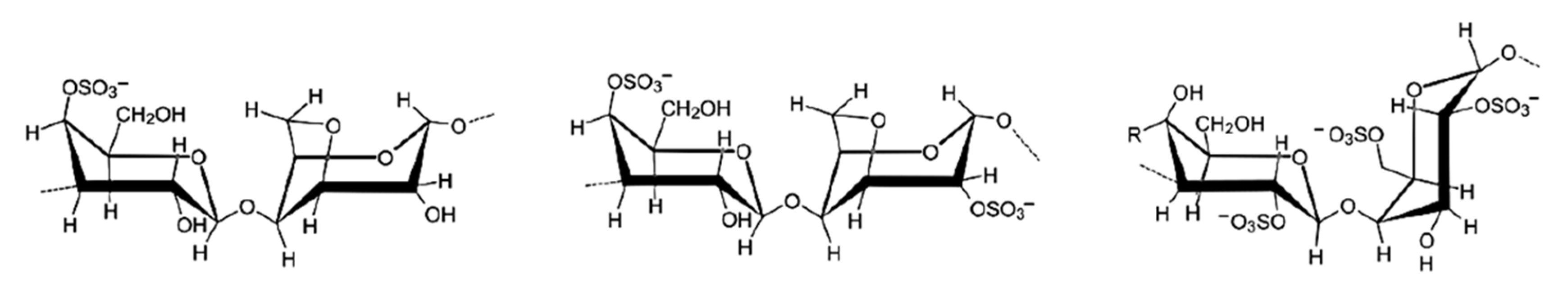

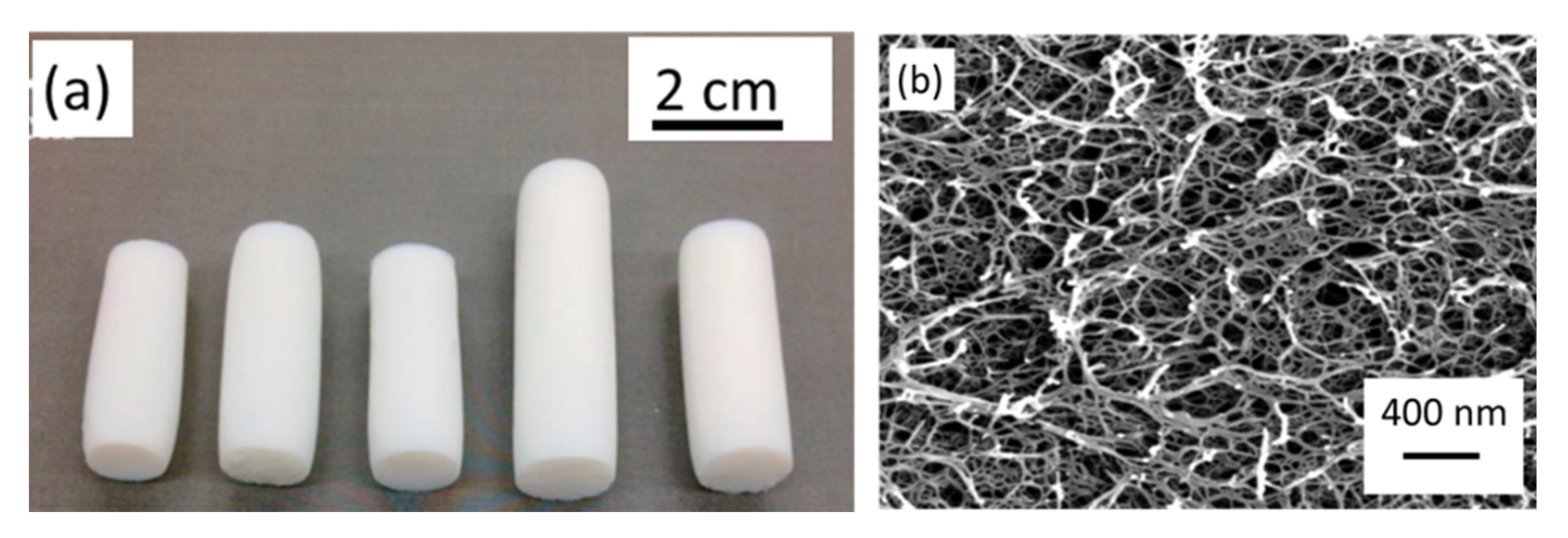
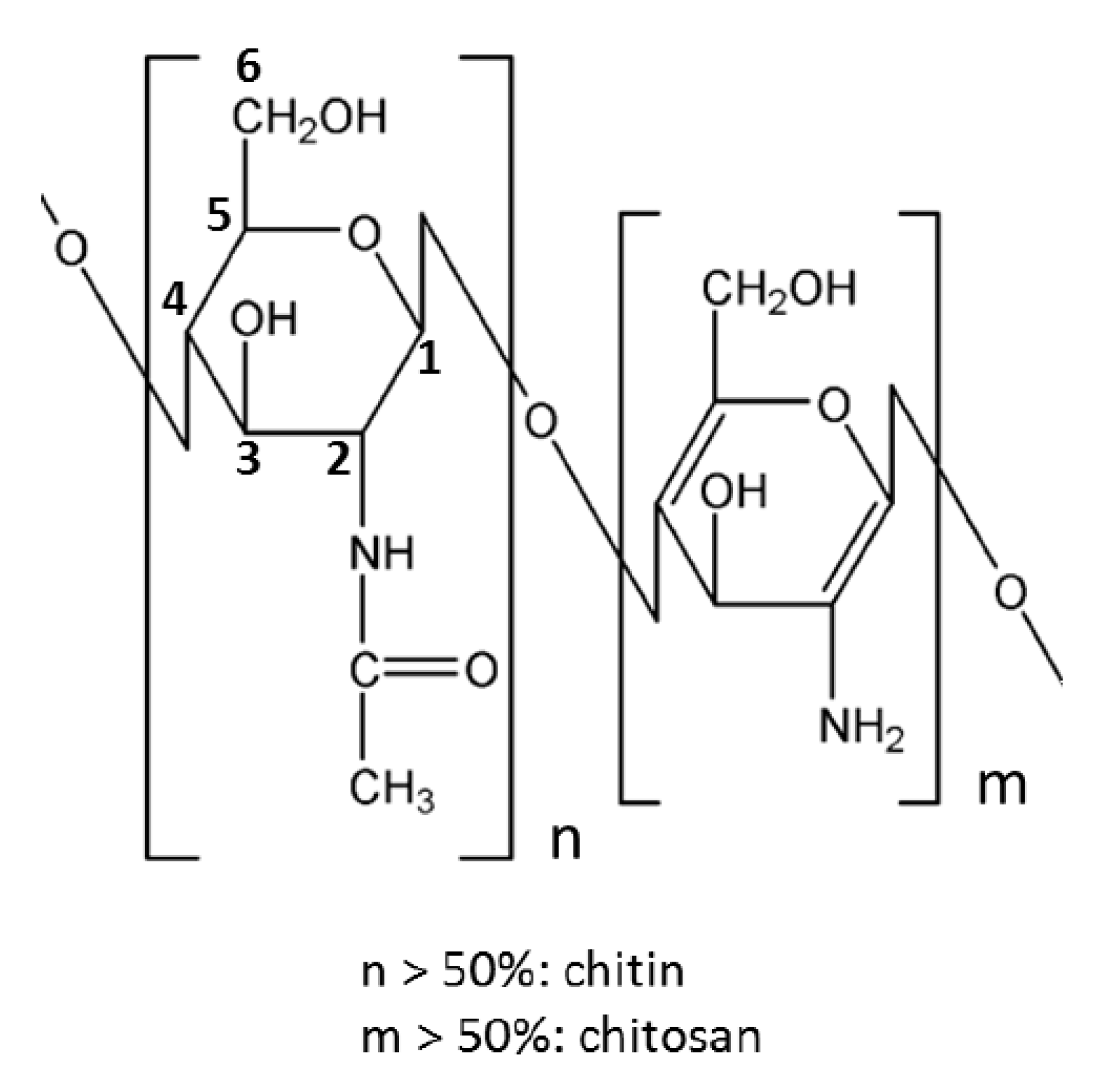

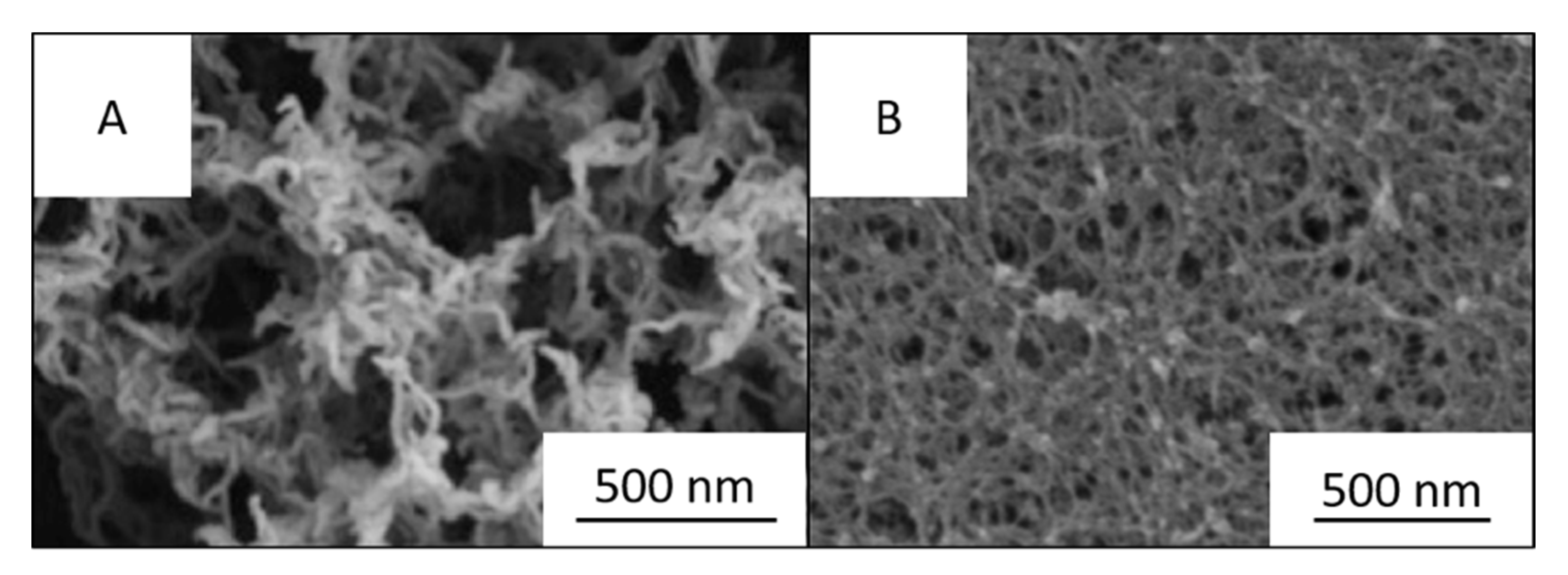
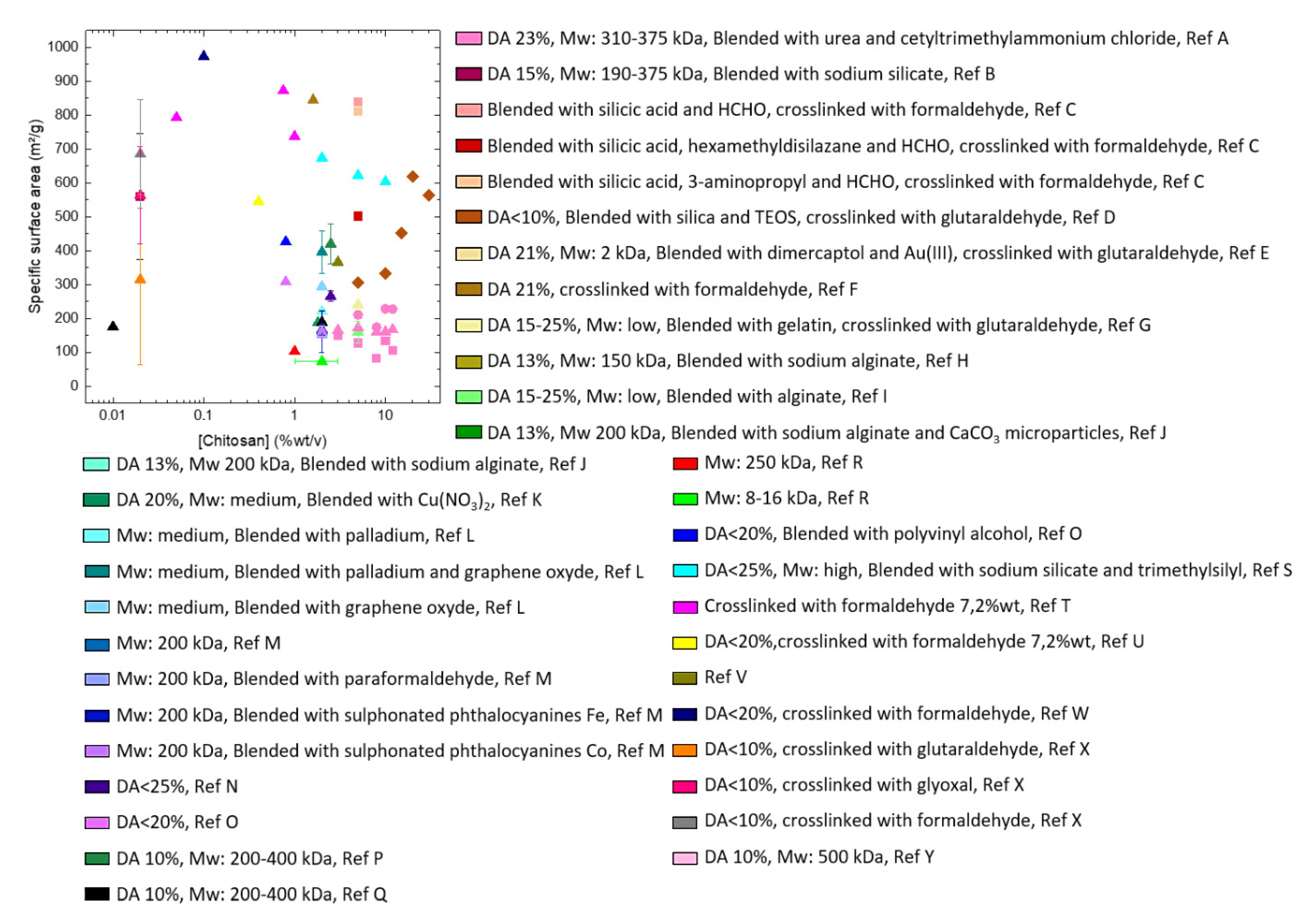
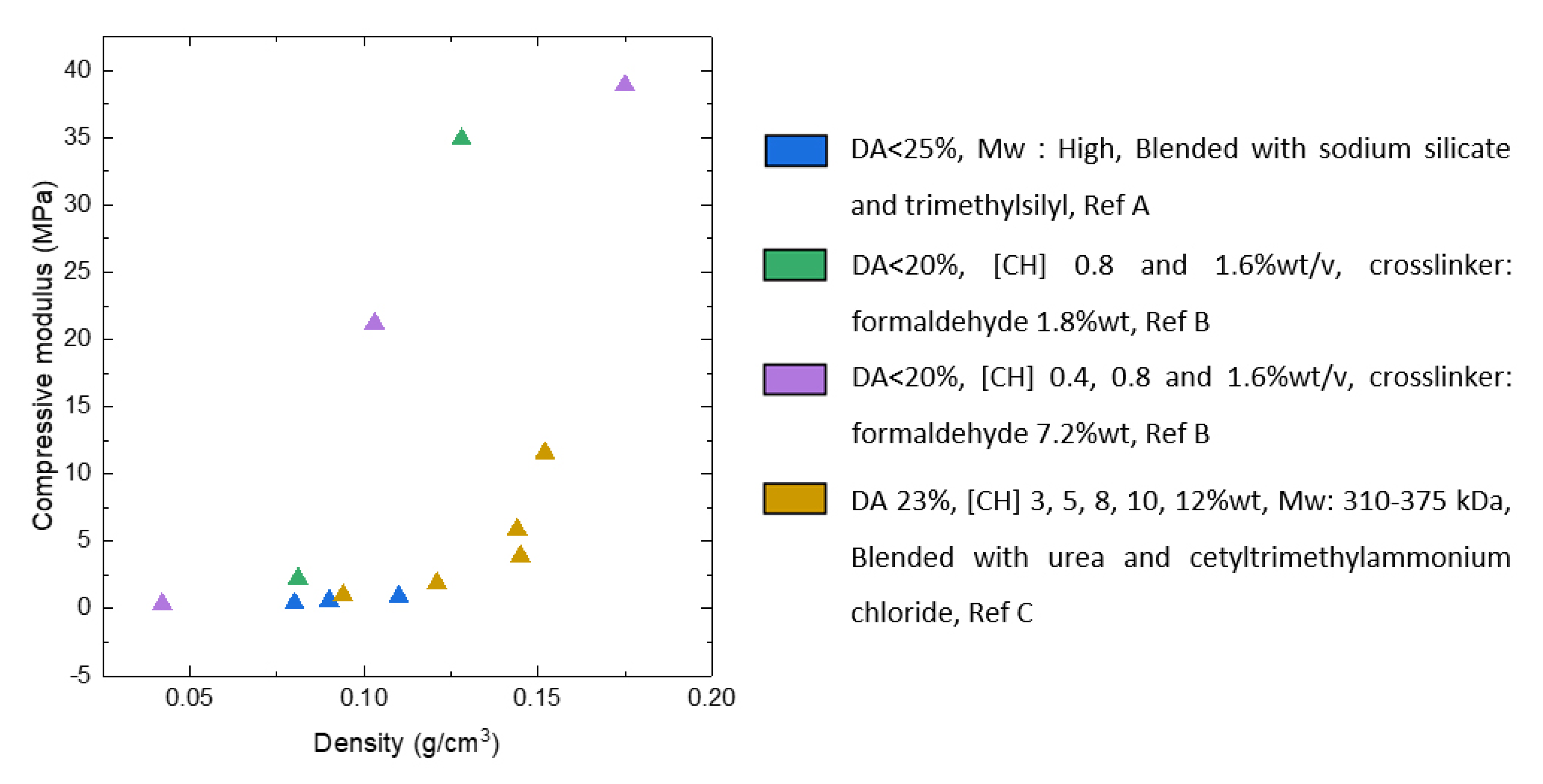
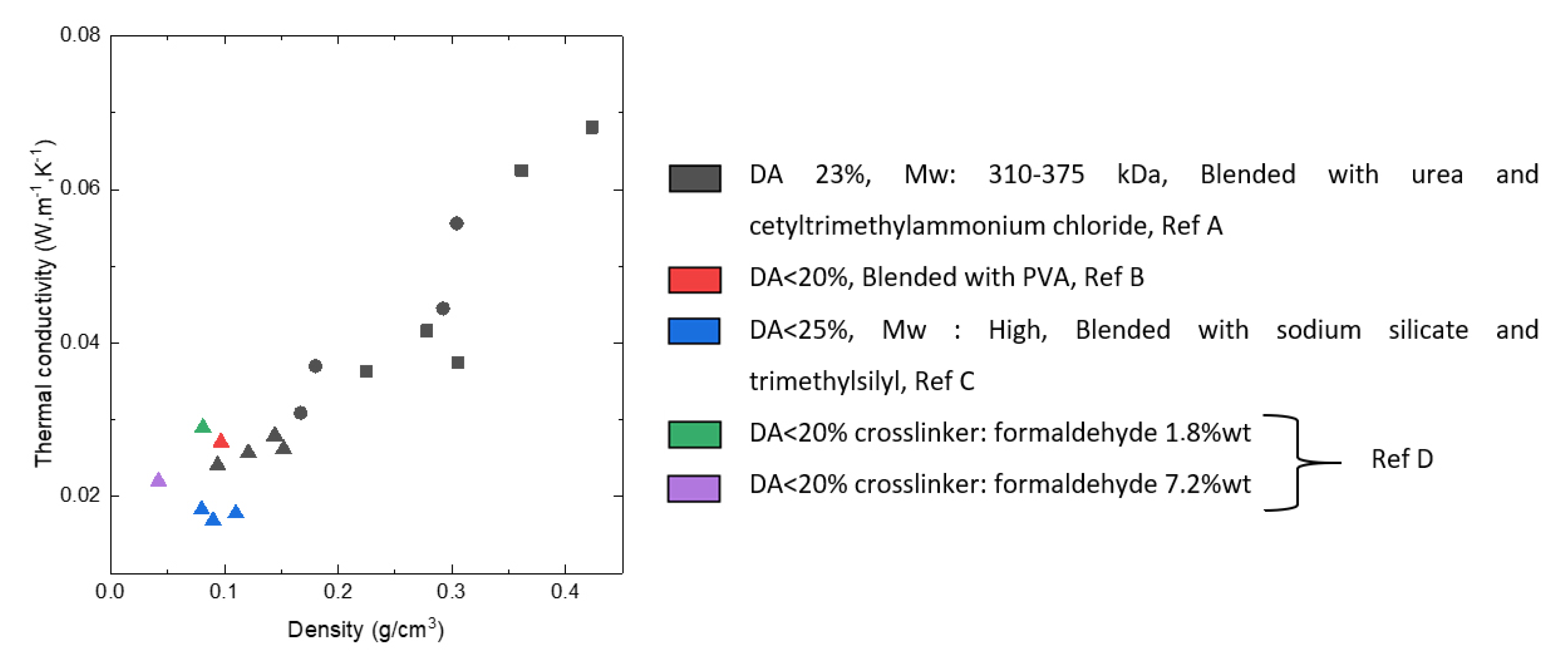

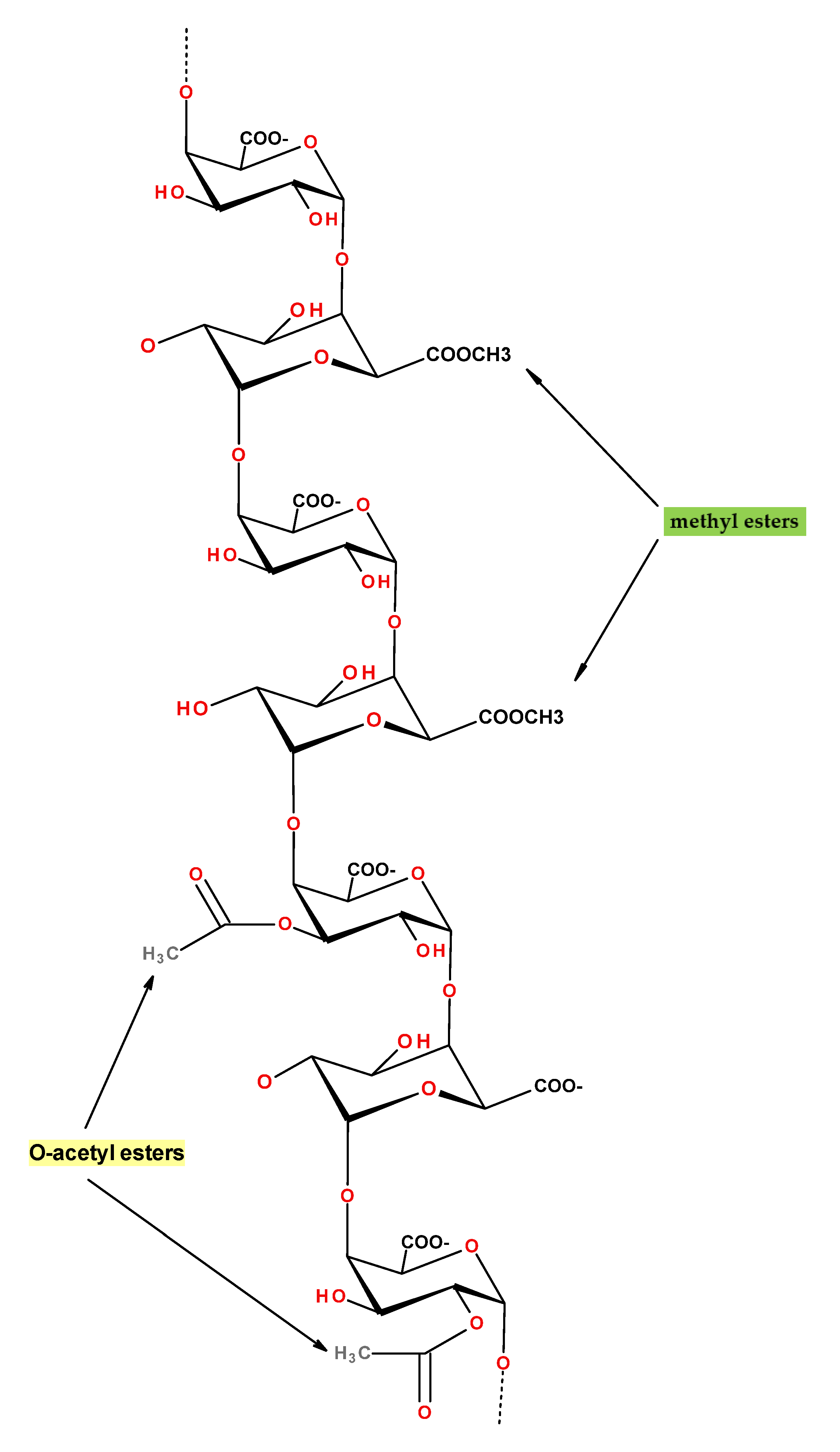
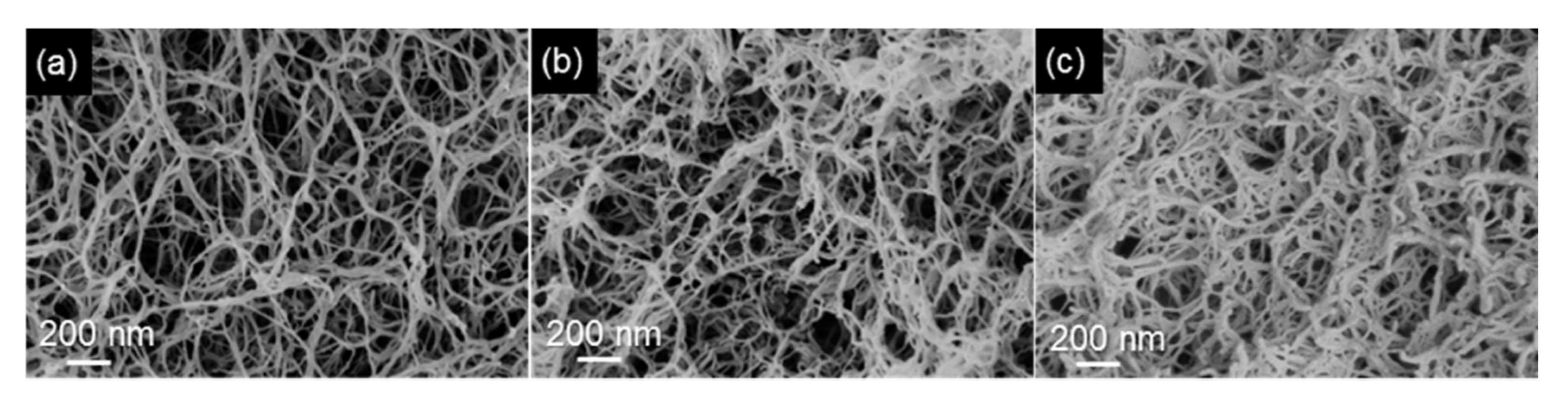
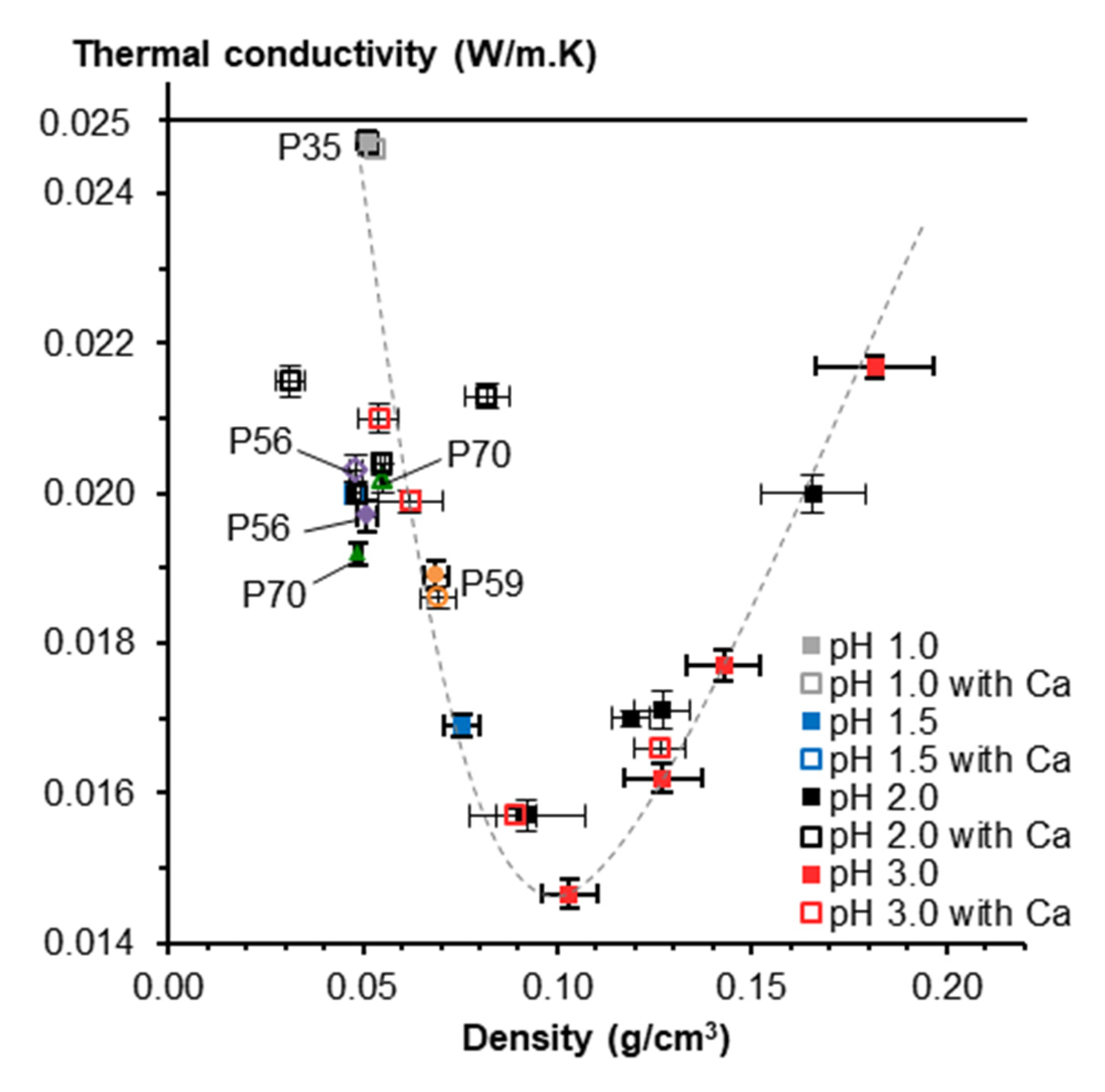
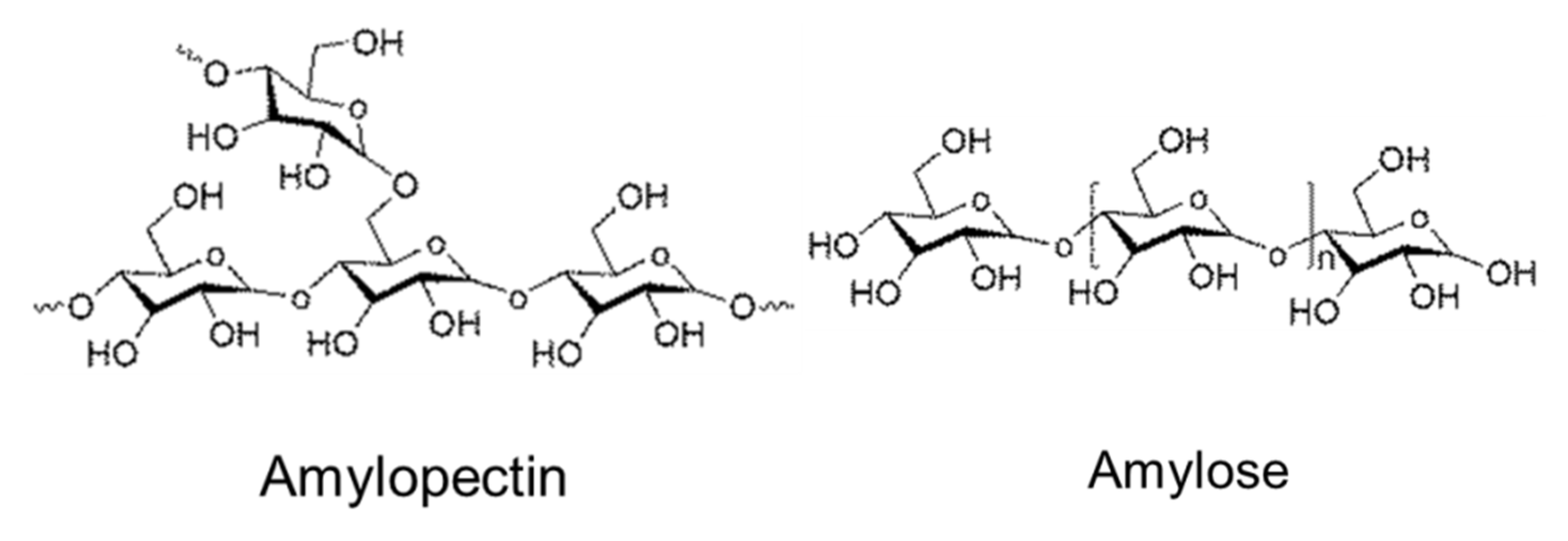


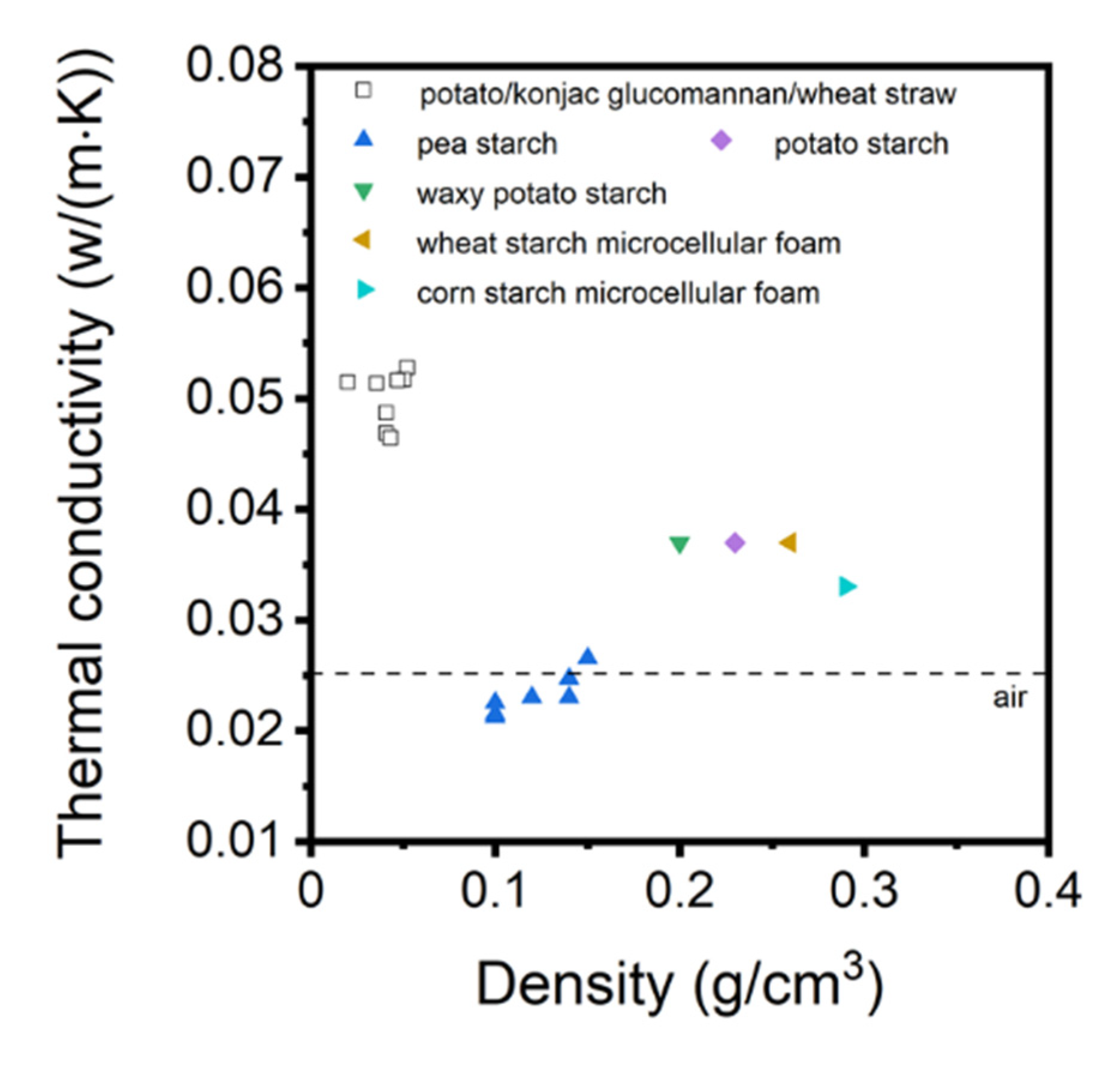
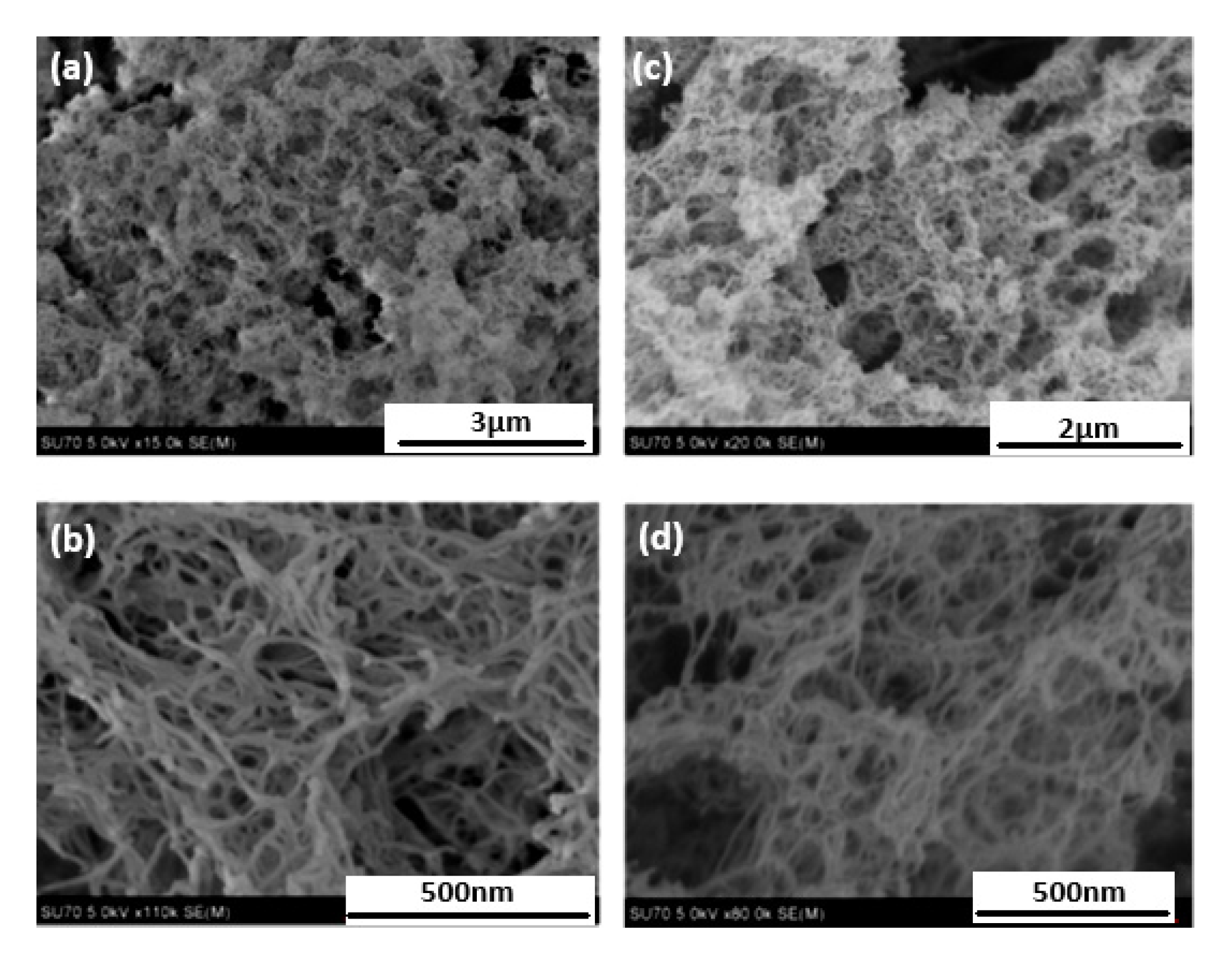
| Cellulose Source | Modification Type | Absorbed Substance | Absorption Capacity, g/g | Cycles | Contact Angle | Reference |
|---|---|---|---|---|---|---|
| Coir fibers | Trimethylchlorosilane, Hexamethyldisilazane | Lubricant oil | 10 | N/A | 148°, 140° | [195] |
| Mango wood scraps | CNF; CNF/PVA composite aerogel, esterification with stearic acid chloride | Various oils, organic solvents | 58–65 35–95 | 15 | 159° | [190] |
| Bark of Abutilon theophrasti | CNF, Chitin, Cationic guar gums; Methyltrichlorosilane | Corn oil, organic solvents | 6.8 9.4–21.9 | >10 | 155° | [199] |
| Sisal leaves | In-situ Cu nanoparticles | Various oils, organic solvents | 92–100 67.8–164.5 | >10 | 150.3° | [191] |
| Cotton cellulose | Esterification with octanoyl chloride; crosslinking with hexamethylene-diisocyanate | Various oils, organic solvents | 49.9–55.8, 40.8–48.7 | N/A | 138.7° | [192] |
| Hardwood cellulose pulp | 1,4-butanediol diglycidyl ether, epoxidized soybean oil | Crude oil, Engine oil, Pump oil | 37, 30, 28 | 30 | 132.6° | [193] |
| Canola straw | Hexadecyltrimethoxysilane | Motor oil, Sunflower oil | 79, 162 | 20 | 139° | [198] |
| Paper waste; cotton fibers | Methyltrimethoxysilane | Various oils, organic solvents | 68–78, 40–94 | 5 | 142.8° | [197] |
| Pinus elliottii | Methyltrimethoxysilane | Petroleum | 68.4 | N/A | 119.85° | [196] |
| Cellulose Source | Modification Type | CO2 Adsorption Amount, mmol/g | Cycles | Reference |
|---|---|---|---|---|
| Microcrystalline cellulose (MCC, officinal class) | N-(2-Aminoethyl)(3-aminopropyl)methyldimethoxysilane | 1.59 | 5 | [202] |
| Cellulose powder (C, α phase, ≤25 μm), | 3-aminopropyltriethoxysilane | 1.20 | 20 | [203] |
| Birch Kraft pulp | Phthalimide (1,3-dihydro-1,3-dioxisoindole) | 5.20 | N/A | [204] |
| CNF hydrogel | N-(2-Aminoethyl)(3-aminopropyl)methyldimethoxysilane | 1.02 chemical 0.35 physical | N/A | [206] |
| Eucalyptus pulp | N-(2-Aminoethyl)(3-aminopropyl)methyldimethoxysilane | 1.78 | 10 | [205] |
| Cellulose Source | Modification Type | Adsorbed Substance | Adsorption Capacity, mg/g | Cycles | Contact Angle | Reference |
|---|---|---|---|---|---|---|
| Kraft pulp | Methyltriethoxysilane (MTES) | Crystal violet dye | 150 | N/A | ~143° | [210] |
| Microcrystalline cellulose (MCC, with a trade name of C10583) | Dopamine (DA) | Methylene blue dye | 110 | N/A | N/A | [208] |
| Cotton linter pulp | Zeolitic imidazolate framework (ZIF-67); | Methyl orange dye | 617 | N/A | N/A | [211] |
| Wood pulp CNC | Poly (methyl vinyl ether-alt-maleic acid) (PMVEMA), Polyethylene glycol (PEG) | Methylene blue dye | 116.2 | 5 | N/A | [220] |
| Microcrystalline cellulose (MCC) | Phenolic resin (PF); carbonization | Methylene blue dye | 610.85 | >4 | N/A | [221] |
| Cellulose nanofibrils (CNF) | Graphene oxide (GO), Tetraethylorthosilicate | Methylene blue dye | 608.4 | N/A | N/A | [222] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budtova, T.; Aguilera, D.A.; Beluns, S.; Berglund, L.; Chartier, C.; Espinosa, E.; Gaidukovs, S.; Klimek-Kopyra, A.; Kmita, A.; Lachowicz, D.; et al. Biorefinery Approach for Aerogels. Polymers 2020, 12, 2779. https://doi.org/10.3390/polym12122779
Budtova T, Aguilera DA, Beluns S, Berglund L, Chartier C, Espinosa E, Gaidukovs S, Klimek-Kopyra A, Kmita A, Lachowicz D, et al. Biorefinery Approach for Aerogels. Polymers. 2020; 12(12):2779. https://doi.org/10.3390/polym12122779
Chicago/Turabian StyleBudtova, Tatiana, Daniel Antonio Aguilera, Sergejs Beluns, Linn Berglund, Coraline Chartier, Eduardo Espinosa, Sergejs Gaidukovs, Agnieszka Klimek-Kopyra, Angelika Kmita, Dorota Lachowicz, and et al. 2020. "Biorefinery Approach for Aerogels" Polymers 12, no. 12: 2779. https://doi.org/10.3390/polym12122779








