The Impact of the Addition of Compatibilizers on Poly (lactic acid) (PLA) Properties after Extrusion Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extrusion of PLA/Compatibilizer Mixtures
2.3. Physicochemical Characterization
2.4. Thermal Analysis
2.5. Size Exclusion Chromatography (SEC)
2.6. Mechanical Testing Measurements
3. Results and Discussion
3.1. Structural Analysis of the Extruded Materials
3.2. Thermal Properties
3.2.1. Termogravimetric Analysis
3.2.2. Influence of Temperature Processing on PLA Crystallization
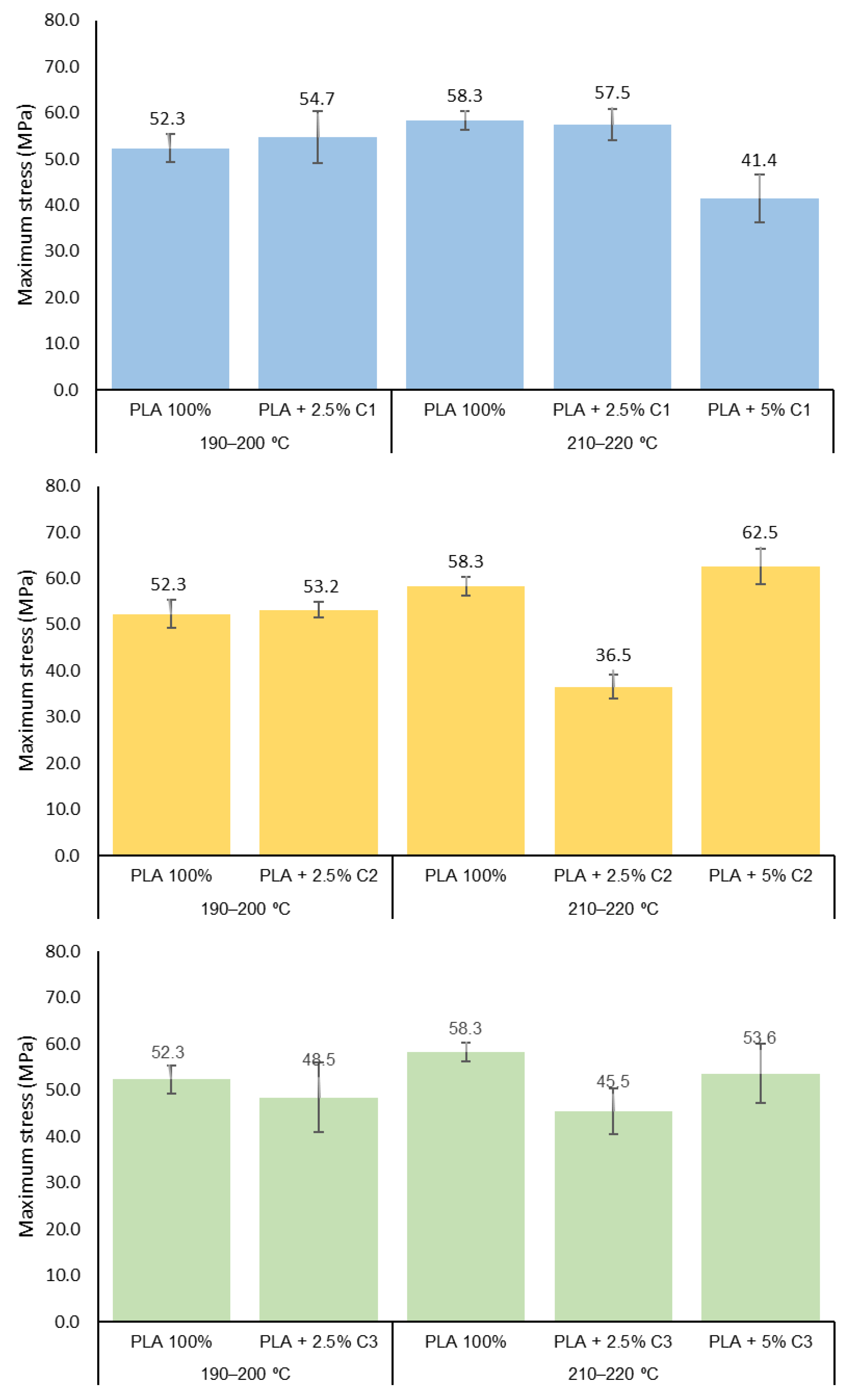
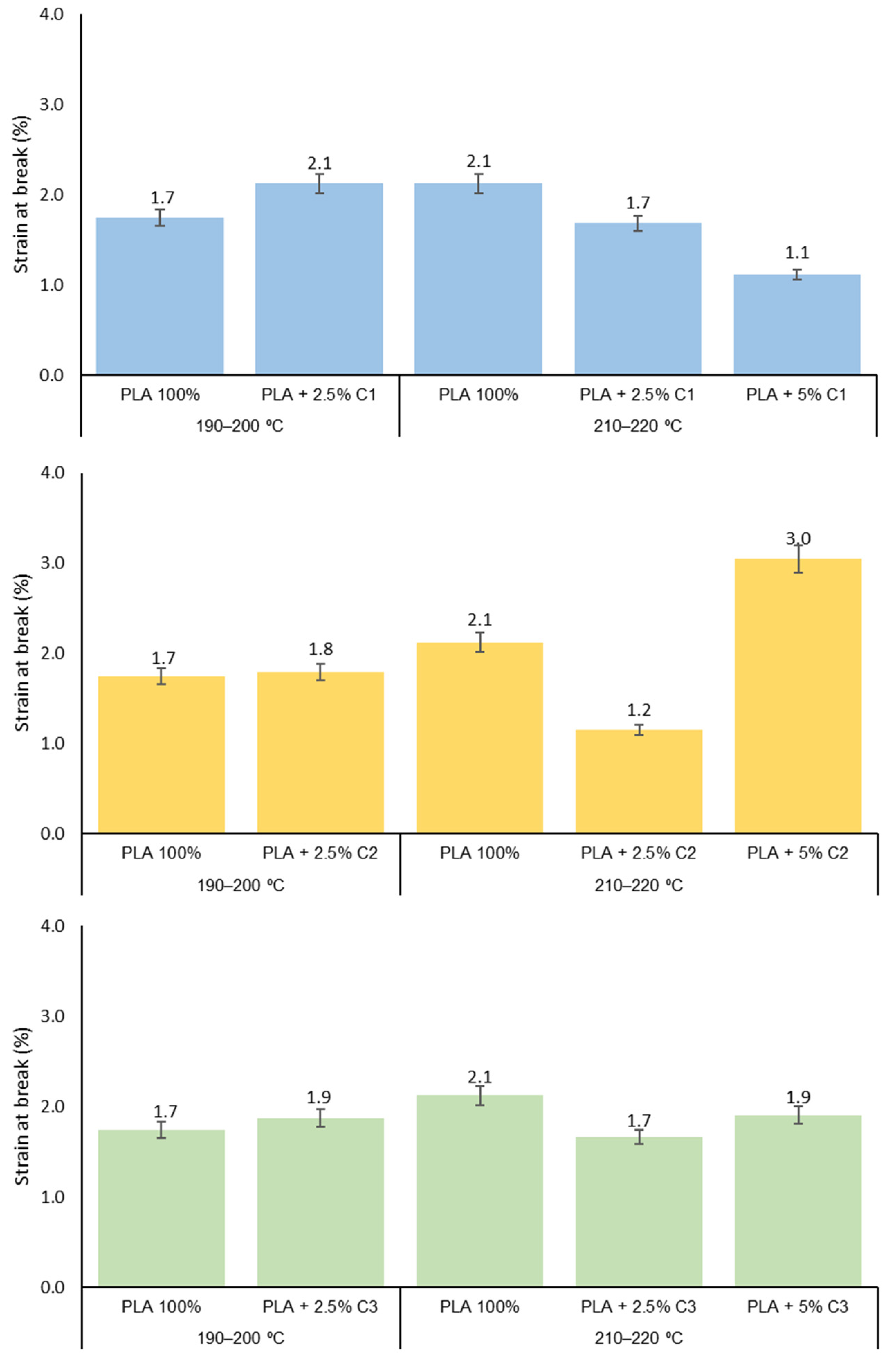
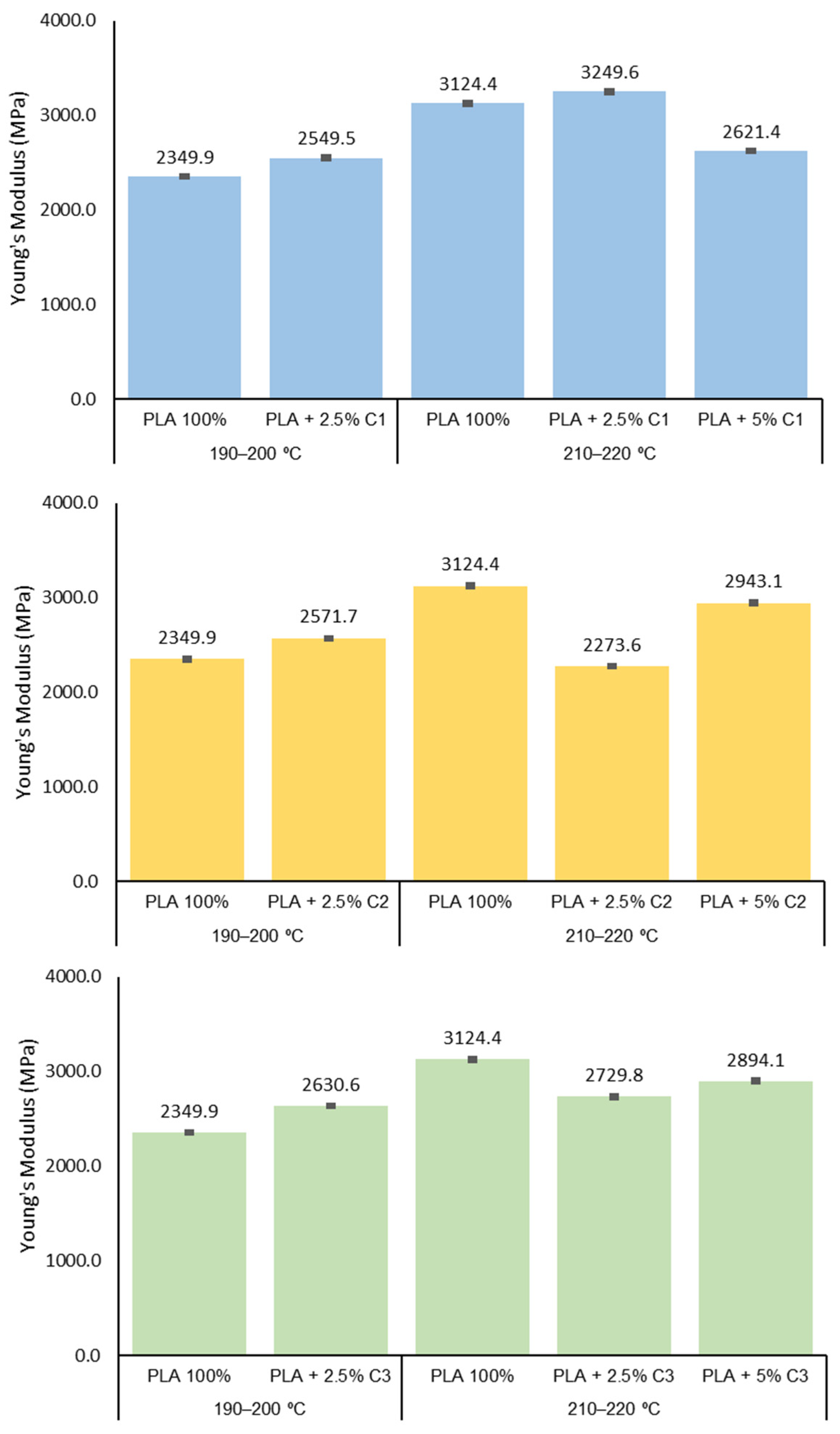
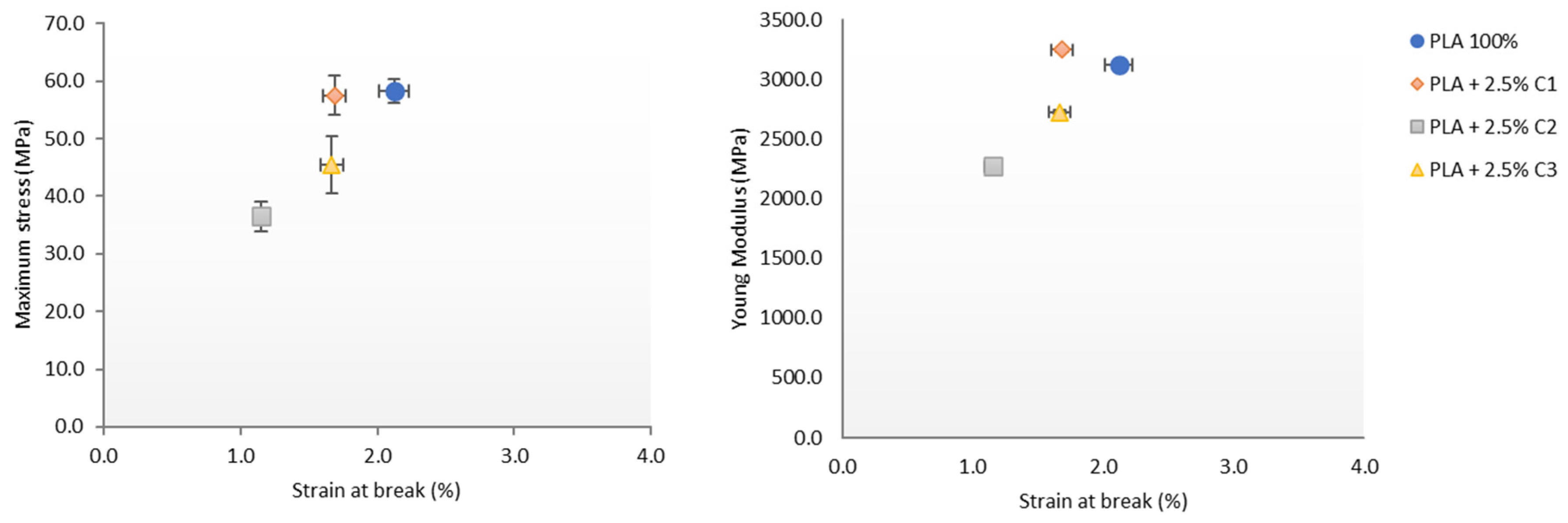
3.3. Mechanical Properties
3.4. Influence of Compatibilizers Type on PLA Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Y.; Romain, C.; Williams, C. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Mohanty, S.; Nayak, S.K.; Unnikrishnan, L. Poly(L-lactide)/polypropylene blends: Evaluation of mechanical, thermal, and morphological characteristics. J. Appl. Polym. Sci. 2011, 121, 3223–3237. [Google Scholar] [CrossRef]
- Statista. Global Plastic Production Statistics. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 1 December 2019).
- Schmack, G.; Tändler, B.; Vogel, R.; Beyreuther, R.; Jacobsen, S.; Fritz, H.G. Biodegradable fibers of poly(L-lactide) produced by high-speed melt spinning and spin drawing. J. Appl. Polym. Sci. 1999, 73, 2785–2797. [Google Scholar] [CrossRef]
- Reichert, C.; Bugnicourt, E.; Coltelli, M.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martinez, B.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Yu, L.; Chen, L. Polymeric Materials from Renewable Resources. In Biodegradable Polymer Blends and Composites from Renewable Resources; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–15. [Google Scholar]
- Soulestin, J.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Bioplastics Based Nanocomposites for Packaging Applications. In Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 76–119. [Google Scholar]
- Nagarajan, V.; Mohanty, A.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Bioplastics, E. Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market/ (accessed on 1 December 2019).
- Karamanlioglu, M.; Preziosi, R.; Robson, G. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Beltran, F.; Lorenzo, V.; de la Orden, M.; Martinez-Urreaga, J. Effect of different mechanical recycling processes on the hydrolytic degradation of poly(L-lactic acid). Polym. Degrad. Stab. 2016, 133, 339–348. [Google Scholar] [CrossRef]
- Cuadri, A.; Martin-Alfonso, J. Thermal, thermo-oxidative and thermomechanical degradation of PLA: A comparative study based on rheological, chemical and thermal properties. Polym. Degrad. Stab. 2018, 150, 37–45. [Google Scholar] [CrossRef]
- Ramlee, N.; Tominaga, Y. Mechanical and degradation properties in alkaline solution of poly(ethylene carbonate)/poly(lactic acid) blends. Polymer 2019, 166, 44–49. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/ polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Ratner, B.D. Surface modification of polymers: Chemical, biological and surface analytical challenges. Biosens. Bioelectron. 1995, 10, 797–804. [Google Scholar] [CrossRef]
- Burg, K.J.L.; Holder, W.D.; Culberson, C.R.; Beiler, R.J.; Greene, K.G.; Loebsack, A.B.; Roland, W.D.; Mooney, D.J.; Halberstadt, C.R. Parameters affecting cellular adhesion to polylactide films. J. Biomater. Sci. Polym. Ed. 1999, 10, 147–161. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Rasal, R.M.; Hirt, D.E. Toughness decrease of PLA-PHBHHx blend films upon surface-confined photopolymerization. J. Biomed. Mater. Res. Part A 2009, 88, 1079–1086. [Google Scholar] [CrossRef]
- Hiljanen-Vainio, M.; Varpomaa, P.; Seppälä, J.; Törmälä, P. Modification of poly(L-lactides) by blending: Mechanical and hydrolytic behavior. Macromol. Chem. Phys. 1996, 197, 1503–1523. [Google Scholar] [CrossRef]
- Grijpma, D.W.; Nijenhuis, A.J.; van Wijk, P.G.T.; Pennings, A.J. High impact strength as-polymerized PLLA. Polym. Bull. 1992, 29, 571–578. [Google Scholar] [CrossRef]
- Herrera, N.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid/cellulose nanocomposites prepared using melt-extrusion and liquid feeding: Mechanical, thermal and optical properties. Compos. Sci. Technol. 2015, 106, 149–155. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. Preparation and Properties of Plasticized Poly(lactic acid) Films. Biomacromolecules 2005, 6, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.-B.; Maggiore, I.D.; Bertoldo, M.; Signori, F.; Bronco, S.; Ciardelli, F. Poly(lactic acid) properties as a consequence of poly(butylene adipate-co-terephthalate) blending and acetyl tributyl citrate plasticization. J. Appl. Polym. Sci. 2008, 110, 1250–1262. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J.; Wolcott, M.P. Comparison of polylactide/nano-sized calcium carbonate and polylactide/montmorillonite composites: Reinforcing effects and toughening mechanisms. Polymer 2007, 48, 7632–7644. [Google Scholar] [CrossRef]
- Li, B.; Dong, F.-X.; Wang, X.-L.; Yang, J.; Wang, D.-Y.; Wang, Y.-Z. Organically modified rectorite toughened poly(lactic acid): Nanostructures, crystallization and mechanical properties. Eur. Polym. J. 2009, 45, 2996–3003. [Google Scholar] [CrossRef]
- Greco, A.; Ferrari, F.; Maffezzoli, A. Mechanical properties of poly(lactid acid) plasticized by cardanol derivatives. Polym. Degrad. Stab. 2019, 159, 199–204. [Google Scholar] [CrossRef]
- Spoerk, M.; Holzer, C.; Gonzalez-Gutierrez, J. Material extrusion-based additive manufacturing of polypropylene: A review on how to improve dimensional inaccuracy and warpage. J. Appl. Polym. Sci. 2020, 137, 48545. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Rheological and mechanical characterization of poly(lactic acid)/polypropylene polymer blends. J. Polym. Res. 2011, 18, 1799–1806. [Google Scholar] [CrossRef]
- Ploypetchara, N.; Suppakul, P.; Atong, D.; Pechyen, C. Blend of Polypropylene/Poly(lactic acid) for Medical Packaging Application: Physicochemical, Thermal, Mechanical, and Barrier Properties. Energy Procedia 2014, 56, 201–210. [Google Scholar] [CrossRef]
- Koning, C.; van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Paul, D.R. Interfacial Agents (“Compatibilizers”) for Polymer Blends. In Polymer Blends; Academic Press: Cambridge, MA, USA, 1978; pp. 35–62. [Google Scholar]
- Patel, J.; Xiang, Z.; Hsu, S.; Schoch, A.; Carleen, S.; Matsumoto, D. Path to Achieving Molecular Dispersion in a Dense Reactive Mixture. J. Polym. Sci. Part B-Polym. Phys. 2015, 53, 1519–1526. [Google Scholar] [CrossRef]
- Patel, J.; Zhao, C.; Deshmukh, S.; Zou, G.; Wamuo, O.; Hsu, S.; Schoch, A.; Carleen, S.; Matsumoto, D. An analysis of the role of reactive plasticizers in the crosslinking reactions of a rigid resin. Polymer 2016, 107, 12–18. [Google Scholar] [CrossRef]
- Vlachopoulos, J.; Strutt, D. Polymer processing. Mater. Sci. Technol. 2003, 19, 1161–1169. [Google Scholar] [CrossRef]
- Vera-Sorroche, J.; Kelly, A.; Brown, E.; Coates, P.; Karnachi, N.; Harkin-Jones, E.; Li, K.; Deng, J. Thermal optimisation of polymer extrusion using in-process monitoring techniques. Appl. Therm. Eng. 2013, 53, 405–413. [Google Scholar] [CrossRef]
- Obuchi, S.; Ogawa, S. Packaging and Other Commercial Applications. In Poly(Lactic Acid); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 457–467. [Google Scholar]
- Nishida, H. Thermal Degradation. In Poly(Lactic Acid); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 401–412. [Google Scholar]
- Pruthtikul, R.; Liewchirakorn, P. Preparation of Polypropylene Graft Maleic Anhydride (PP-g-MA) via Twin Screw Extrusion. Adv. Mater. Res. 2010, 93–94, 451–454. [Google Scholar] [CrossRef]
- NatureWorks. Ingeo™ Biopolymer 2500HP Technical Data Sheet. Available online: https://www.natureworksllc.com/~/media/Technical_Resources/Technical_Data_Sheets/TechnicalDataSheet_2500HP_extrusion_pdf.pdf?la=en&la=en (accessed on 1 December 2019).
- Fambri, L.; Migliaresi, C. Crystallization and Thermal Properties. In Poly(Lactic Acid); Grossman, R.F., Nwabunma, D., Auras, R., Lim, L.T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 113–124. [Google Scholar]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Rojas-Gonzalez, A.F.; Carrero-Mantilla, J.I. Thermal degradation kinetic of polylactic acid in multiple extrusions. Ing. Univ. 2015, 19, 189–206. [Google Scholar] [CrossRef]
- Zhai, W.; Ko, Y.; Zhu, W.; Wong, A.; Park, C. A Study of the Crystallization, Melting, and Foaming Behaviors of Polylactic Acid in Compressed CO2. Int. J. Mol. Sci. 2009, 10, 5381. [Google Scholar] [CrossRef]
- Lim, L.-T.; Cink, K.; Vanyo, T. Processing of Poly(Lactic Acid). In Poly(Lactic Acid); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 189–215. [Google Scholar]
- Persson, M.; Lorite, G.S.; Cho, S.-W.; Tuukkanen, J.; Skrifvars, M. Melt Spinning of Poly(lactic acid) and Hydroxyapatite Composite Fibers: Influence of the Filler Content on the Fiber Properties. ACS Appl. Mater. Interfaces 2013, 5, 6864–6872. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, F.; Raquez, J.-M.; Addiego, F.; Dubois, P.; Toniazzo, V.; Ruch, D. New approach on the development of plasticized polylactide (PLA): Grafting of poly(ethylene glycol) (PEG) via reactive extrusion. Eur. Polym. J. 2011, 47, 2134–2144. [Google Scholar] [CrossRef]
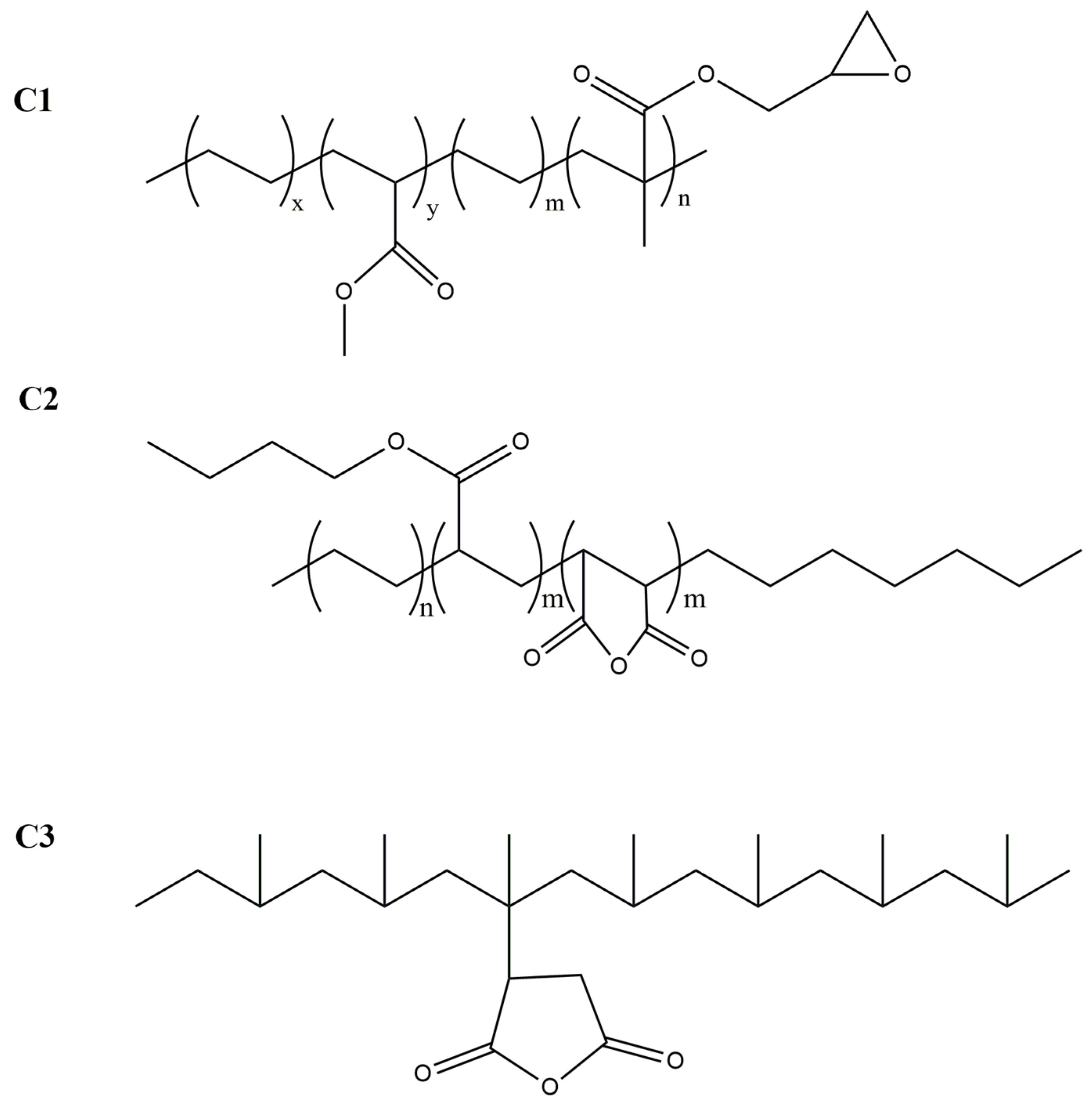
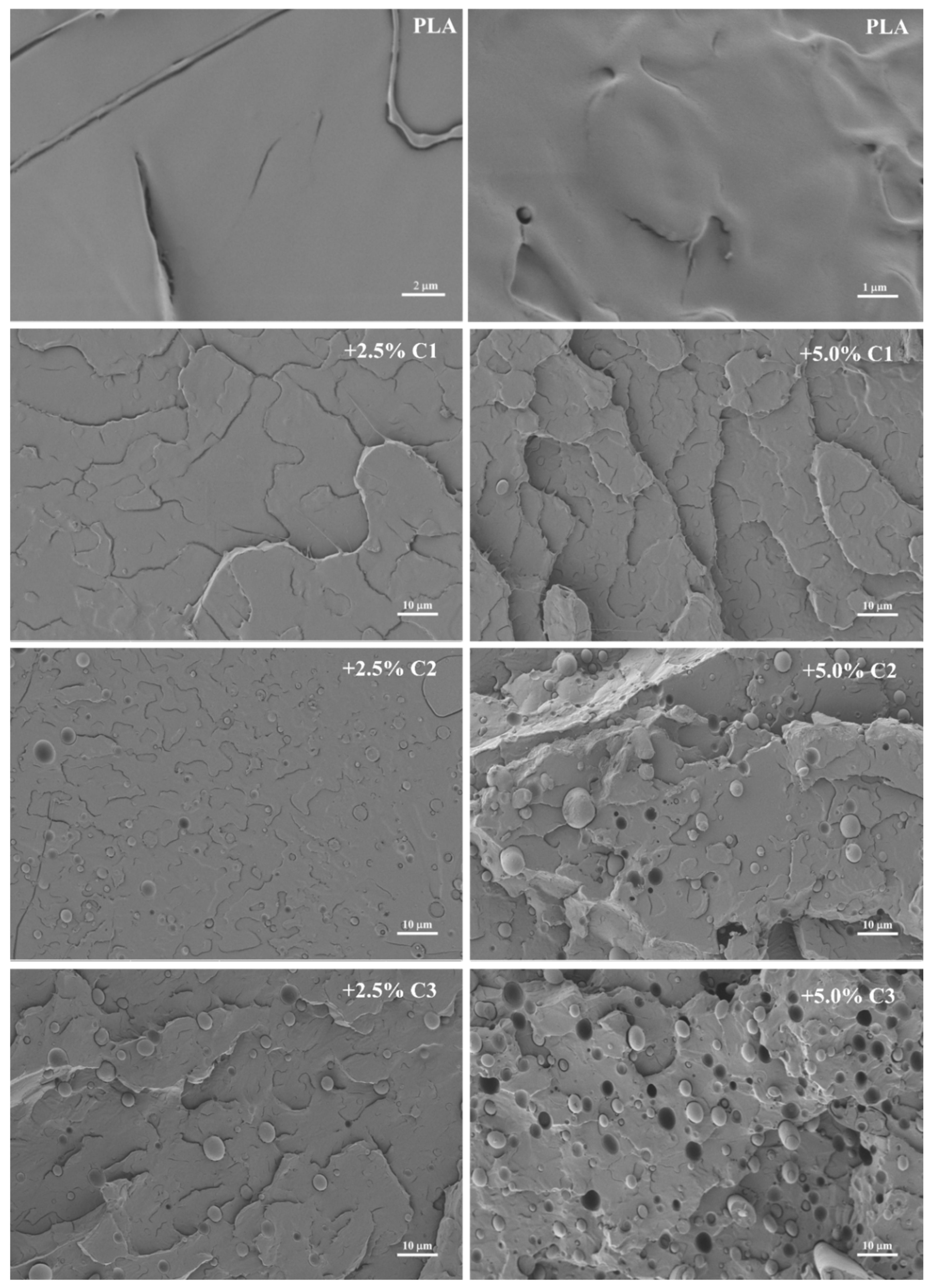
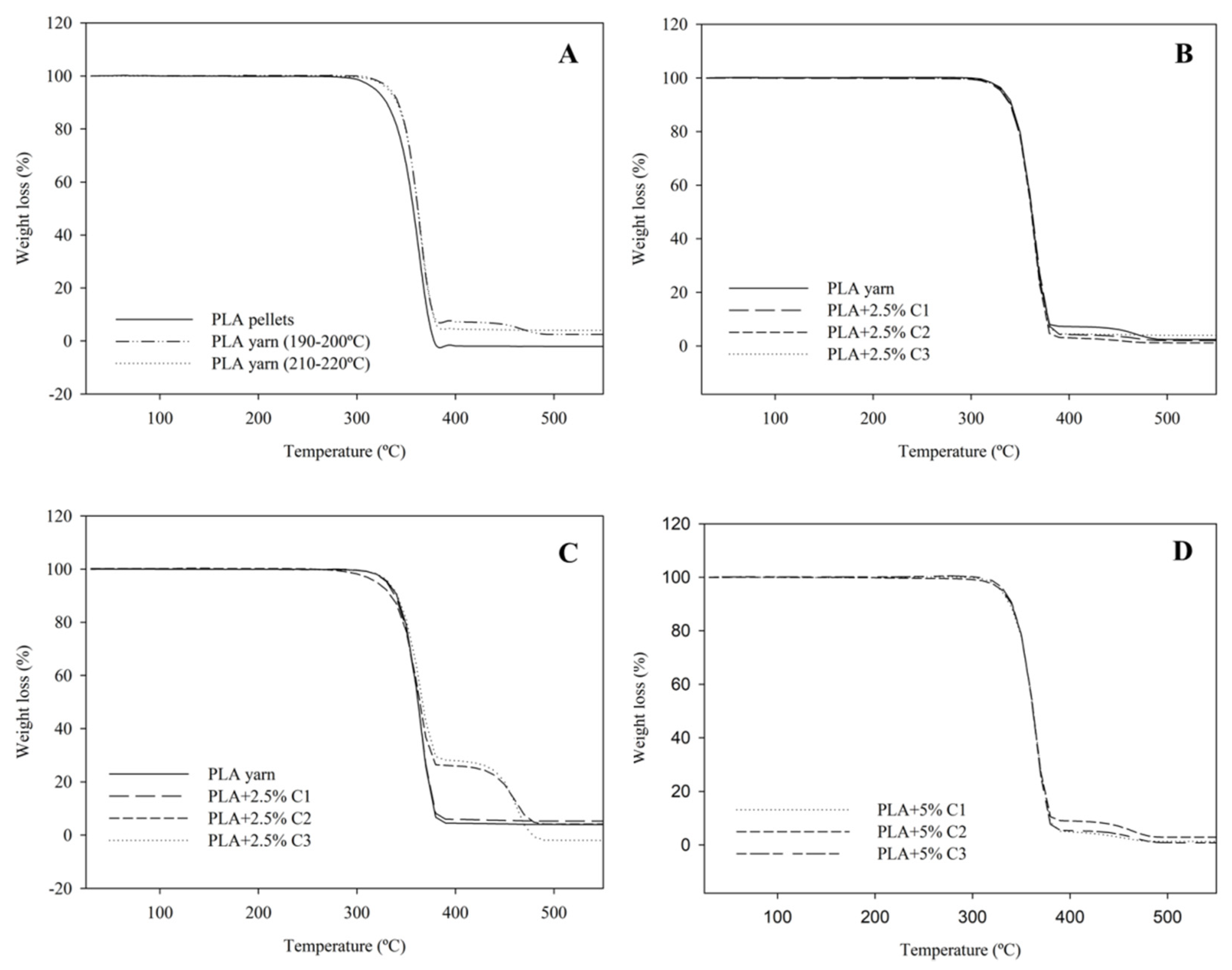
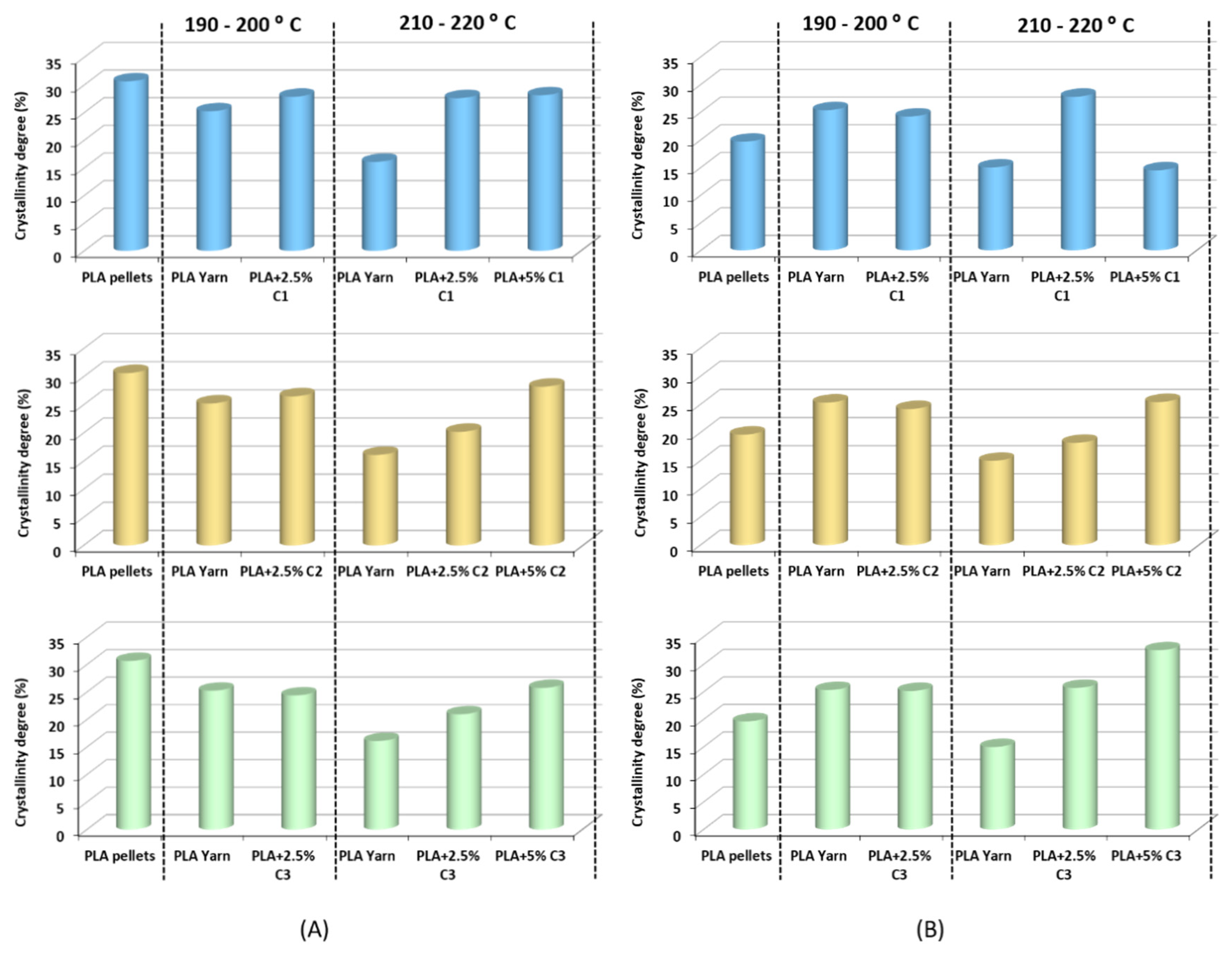
| Samples | Processing T. Range (°C) | Mn | PD |
|---|---|---|---|
| Pellets PLA Ingeo 2500 HP | Not processed | 138,000 | 1.5 |
| 100% PLA | 190–200 | 132,000 | 1.5 |
| 100% PLA | 210–220 | 125,000 | 1.5 |
| PLA + 2.5%C1 | 86,800 | 1.6 | |
| PLA + 2.5%C2 | 65,200 | 1.6 | |
| PLA + 2.5% C3 | 81,000 | 1.5 | |
| PLA + 5%C1 | 107,235 | 1.5 | |
| PLA + 5%C2 | 101,808 | 1.5 | |
| PLA + 5%C3 | 95,653 | 1.5 |
| Samples | Processing T. Range (°C) | T5% | T10% | Ton |
|---|---|---|---|---|
| PLA | 190–200 | 333.9 | 342.2 | 346.7 |
| PLA + 2.5%C1 | 331.1 | 340.2 | 346.8 | |
| PLA + 2.5%C2 | 332.4 | 349.7 | 346.6 | |
| PLA + 2.5%C3 | 333.5 | 341.9 | 347.0; 436.8 | |
| 100% PLA | 210–220 | 331.8 | 341.0 | 346.4 |
| PLA + 2.5%C1 | 330.8 | 339.1 | 346.0 | |
| PLA + 2.5%C2 | 320.7 | 335.4 | 342.3; 440.8 | |
| PLA + 2.5%C3 | 333.3 | 341.4 | 345.2; 444.6 | |
| PLA + 5%C1 | 329.7 | 338.6 | 346.2 | |
| PLA + 5%C2 | 330.9 | 340.3 | 346.2 | |
| PLA + 5%C3 | 333.1 | 341.0 | 346.2 |
| Sample | Processing Temperature (°C) | Tg (°C) | Tc (°C) | Tm (°C) | ΔHc (kJ/kg) | ΔHm (kJ/kg) | XC (%) |
|---|---|---|---|---|---|---|---|
| PLA pellets | - | 61.2 | 101.3 | 177.6 | 18.9 | 47.6 | 30.7 |
| PLA Yarn | 190–200 | 62.1 | 97.9 | 180.0 | 27.4 | 51.0 | 25.2 |
| PLA + 2.5% C1 | 61.8 | 96.4 | 178.1 | 26.7 | 52.8 | 27.9 | |
| PLA + 2.5% C2 | 61.8 | 97.7 | 179.6 | 24.7 | 49.5 | 26.5 | |
| PLA + 2.5% C3 | 62.2 | 97.8 | 179.7 | 22.7 | 45.5 | 24.4 | |
| PLA Yarn | 210–220 | 61.6 | 98.6 | 178.4 | 13.9 | 28.9 | 16.1 |
| PLA + 2.5% C1 | 61.0 | 98.0 | 177.3 | 30.2 | 56.1 | 27.6 | |
| PLA + 2.5% C2 | 62.43 | 97.9 | 176.3 | 17.6 | 36.5 | 20.2 | |
| PLA + 2.5% C3 | 63.8 | 98.4 | 177.0 | 13.9 | 33.5 | 21.0 | |
| PLA + 5% C1 | 61.4 | 96.8 | 179.1 | 24.9 | 51.2 | 28.1 | |
| PLA + 5% C2 | 60.8 | 96.7 | 178.2 | 23.6 | 50.0 | 28.2 | |
| PLA + 5% C3 | 60.5 | 96.5 | 176.7 | 26.5 | 50.6 | 25.8 |
| Sample | Processing Temperature (°C) | Tg (°C) | Tc (°C) | Tm (°C) | ΔHc (kJ/kg) | ΔHm (kJ/kg) | XC (%) |
|---|---|---|---|---|---|---|---|
| PLA pellets | - | 61.4 | 98.1 | 175.3 | 28.7 | 47.0 | 19.6 |
| PLA Yarn | 190–200 | 63.8 | 99.8 | 176.5 | 26.3 | 50.0 | 25.3 |
| PLA + 2.5% C1 | 64.3 | 98.6 | 176.1 | 31.4 | 54.1 | 24.2 | |
| PLA + 2.5% C2 | 64.0 | 100.5 | 176.6 | 28.9 | 51.6 | 24.2 | |
| PLA + 2.5% C3 | 64.3 | 100.0 | 175.8 | 25.0 | 48.5 | 25.1 | |
| PLA Yarn | 210–220 | 64.0 | 100.3 | 176.1 | 15.4 | 29.4 | 15.0 |
| PLA + 2.5% C1 | 63.3 | 99.8 | 175.1 | 31.8 | 57.8 | 27.7 | |
| PLA + 2.5% C2 | 62.8 | 98.6 | 173.7 | 19.4 | 36.4 | 18.2 | |
| PLA + 2.5% C3 | 63.8 | 98.4 | 174.7 | 15.1 | 39.1 | 25.7 | |
| PLA + 5% C1 | 63.8 | 106.4 | 175.8 | 33.3 | 46.9 | 14.5 | |
| PLA + 5% C2 | 63.6 | 100.3 | 174.9 | 26.5 | 50.3 | 25.4 | |
| PLA + 5% C3 | 63.3 | 98.9 | 173.9 | 23.1 | 53.5 | 32.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, F.A.M.M.; Cruz, S.M.A.; Coelho, J.F.J.; Serra, A.C. The Impact of the Addition of Compatibilizers on Poly (lactic acid) (PLA) Properties after Extrusion Process. Polymers 2020, 12, 2688. https://doi.org/10.3390/polym12112688
Gonçalves FAMM, Cruz SMA, Coelho JFJ, Serra AC. The Impact of the Addition of Compatibilizers on Poly (lactic acid) (PLA) Properties after Extrusion Process. Polymers. 2020; 12(11):2688. https://doi.org/10.3390/polym12112688
Chicago/Turabian StyleGonçalves, F.A.M.M., Sandra M. A. Cruz, Jorge F. J. Coelho, and Arménio C. Serra. 2020. "The Impact of the Addition of Compatibilizers on Poly (lactic acid) (PLA) Properties after Extrusion Process" Polymers 12, no. 11: 2688. https://doi.org/10.3390/polym12112688
APA StyleGonçalves, F. A. M. M., Cruz, S. M. A., Coelho, J. F. J., & Serra, A. C. (2020). The Impact of the Addition of Compatibilizers on Poly (lactic acid) (PLA) Properties after Extrusion Process. Polymers, 12(11), 2688. https://doi.org/10.3390/polym12112688








