Selenonium Polyelectrolyte Synthesis through Post-Polymerization Modifications of Poly (Glycidyl Methacrylate) Scaffolds
Abstract
1. Introduction
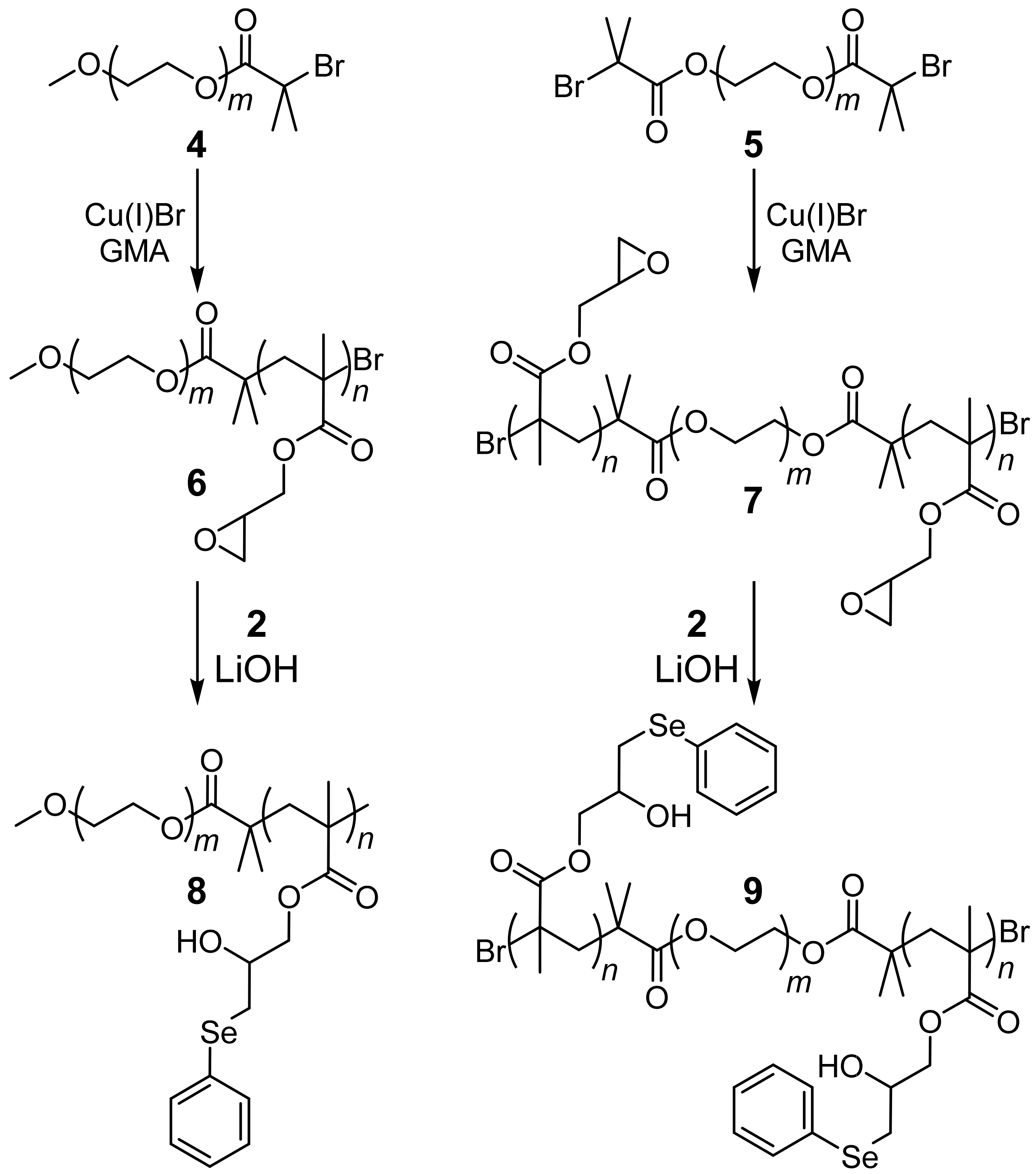
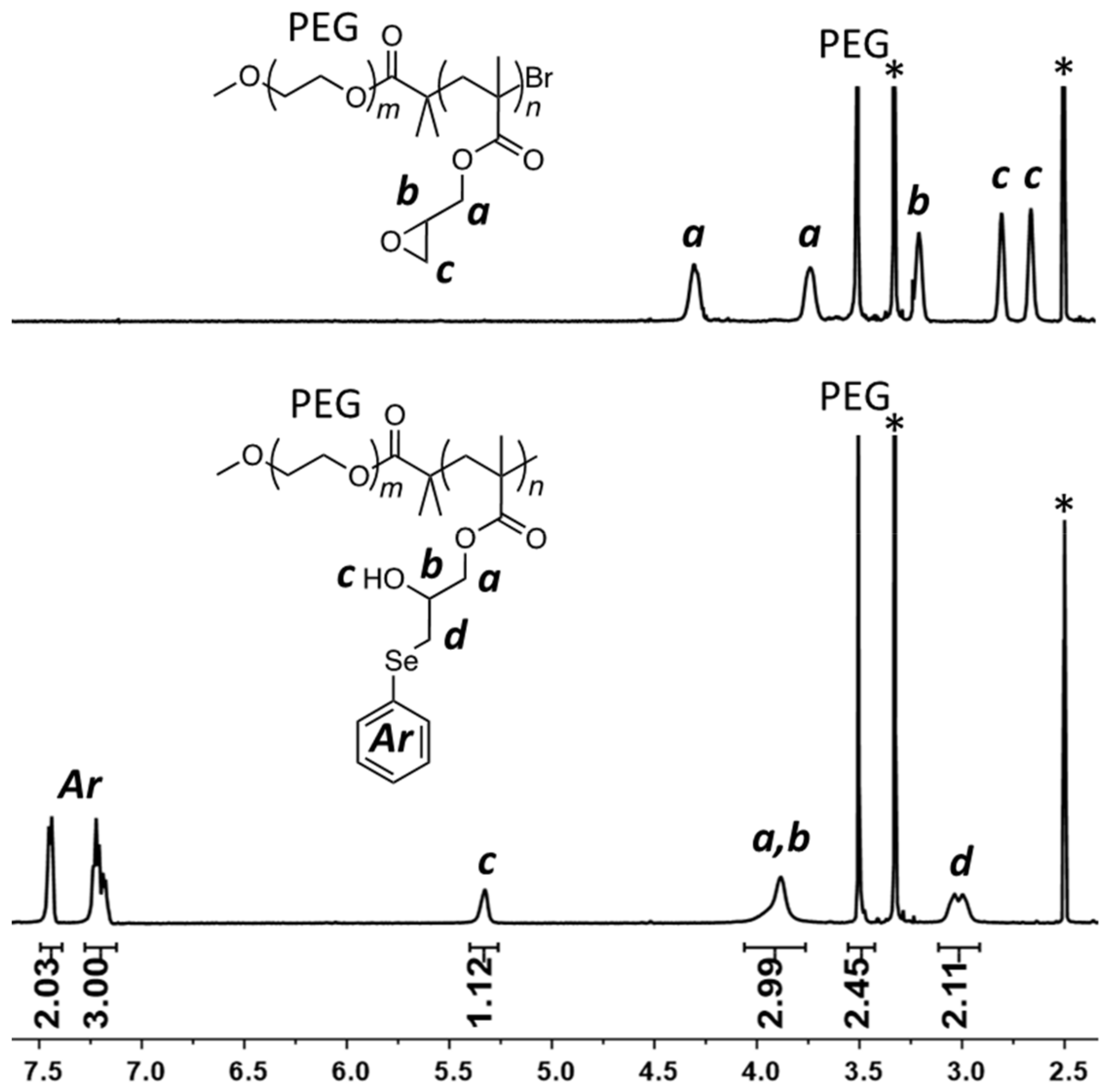
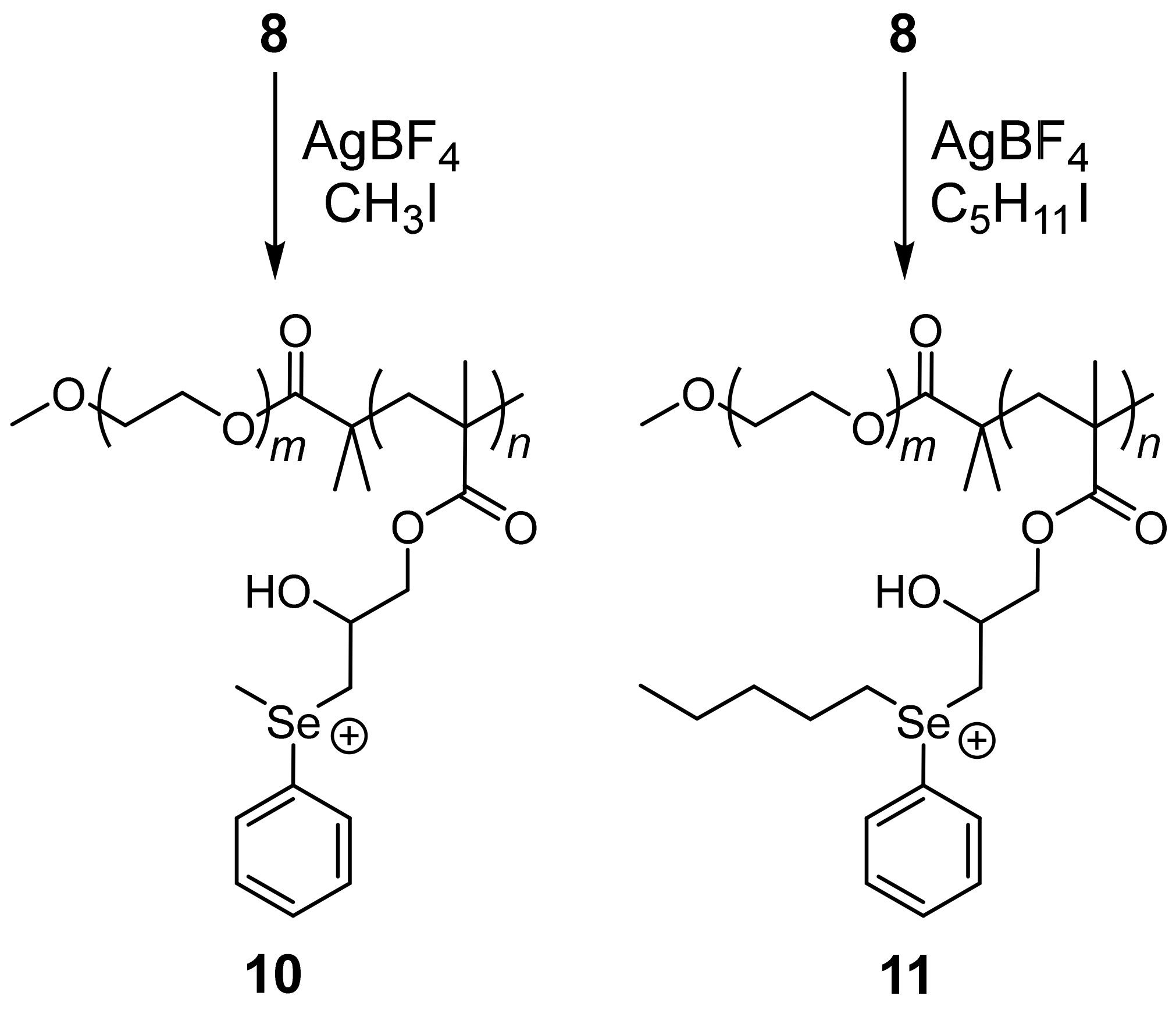
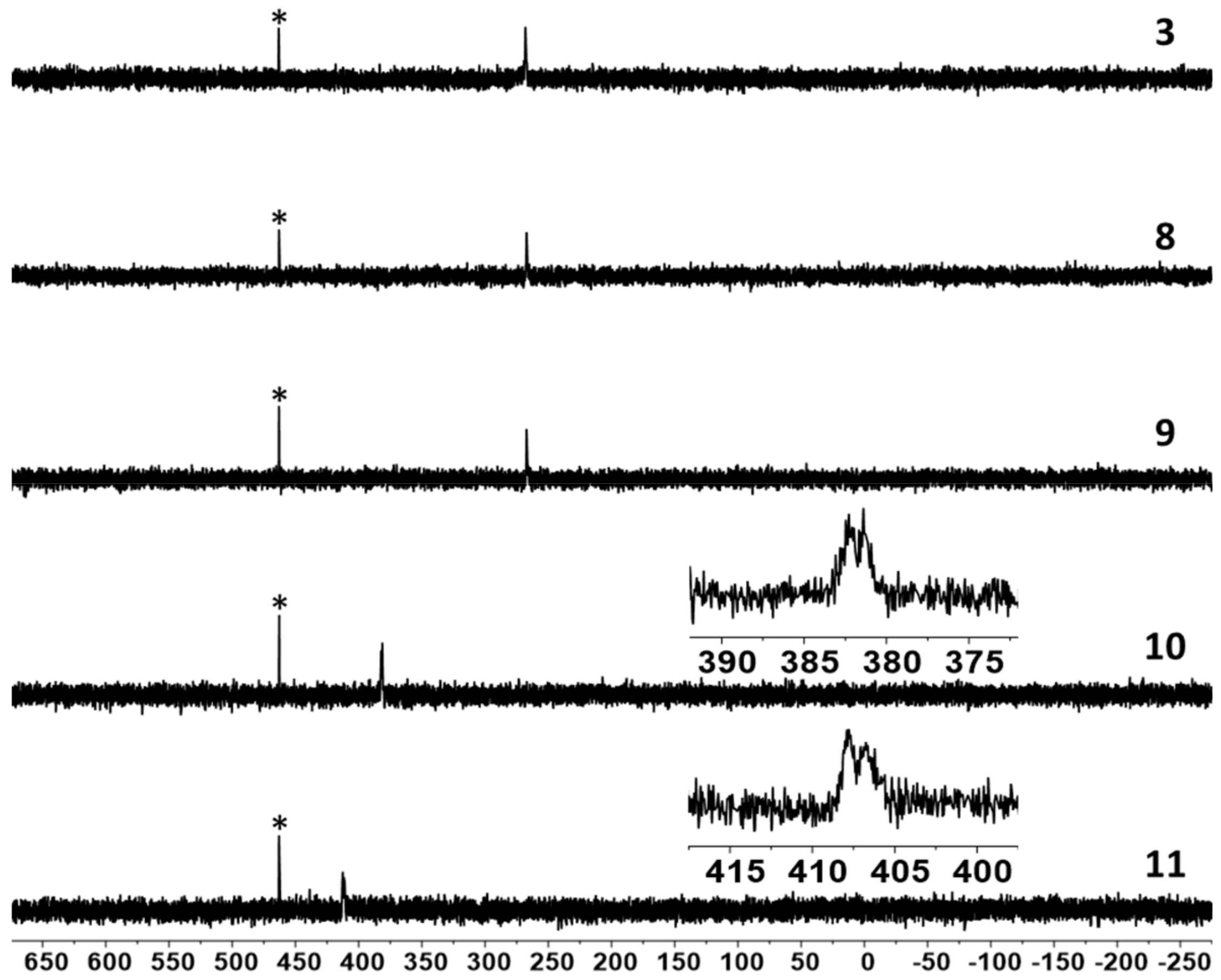
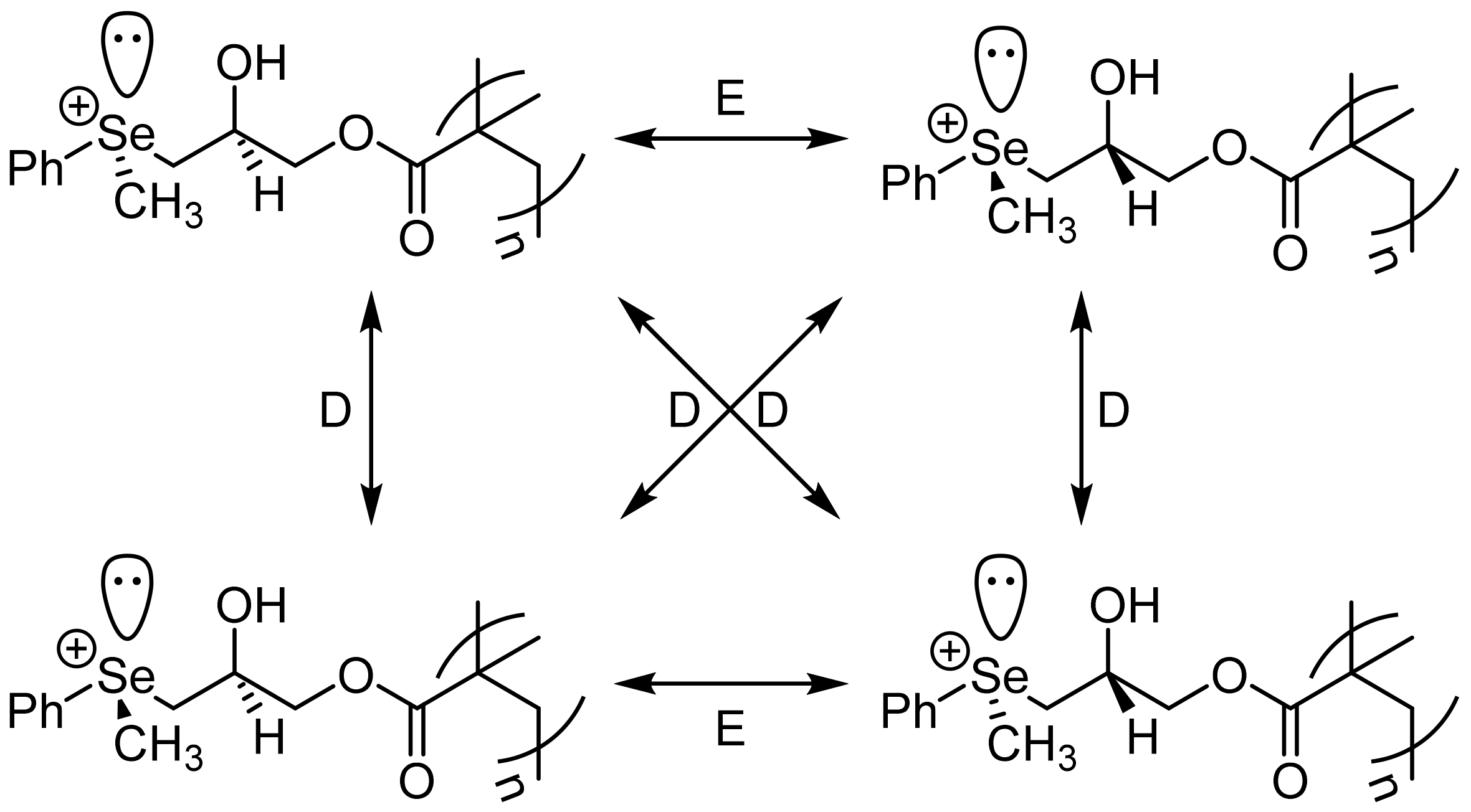
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eom, T.; Khan, A. Polyselenonium salts: Synthesis through sequential selenium-epoxy ‘click’ chemistry and Se-alkylation. Chem. Commun. 2020. [Google Scholar] [CrossRef]
- Eom, T.; Khan, A. Selenium-Epoxy ‘Click’ Reaction and Se-Alkylation—Efficient Access to Organo-Selenium and Selenonium Compounds. Chemistry 2020, 2, 827–836. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A. Ring opening reactions of heterocycles with selenium and tellurium nucleophiles. New J. Chem. 2019, 43, 11451–11468. [Google Scholar] [CrossRef]
- Pan, X.Q.; Driessen, F.; Zhu, X.L.; Du Prez, F.E. Selenolactone as a Building Block toward Dynamic Diselenide-Containing Polymer Architectures with Controllable Topology. ACS Macro Lett. 2017, 6, 89–92. [Google Scholar] [CrossRef]
- Chen, S.; Pan, X.; Zhu, J.; Zhu, X. Synthesis of selenide-containing polymers by multicomponent polymerization based on γ-butyroselenolactone. Polym. Chem. 2019, 10, 6395–6400. [Google Scholar] [CrossRef]
- Zhang, C.J.; Cao, X.H.; Zhang, X.H. Metal-Free Alternating Copolymerization of Nonstrained gamma-Selenobutyrolactone with Epoxides for Selenium-Rich Polyesters. Macromolecules 2020, 53, 203–211. [Google Scholar] [CrossRef]
- Wang, Y.N.; Lin, X.; Zhang, Z.; Zhu, J.; Pan, X.; Zhu, X. A Novel Synthesis of Poly (Ester-Alt-Selenide) s by Ring-Opening Copolymerization of γ-Selenobutyrolactone and Epoxy Monomer. Polymers 2020, 12, 1203. [Google Scholar] [CrossRef]
- Lu, W.; An, X.; Gao, F.; Zhu, J.; Zhou, N.; Zhang, Z.; Pan, X.; Zhu, X. Highly Efficient Chain End Derivatization of Selenol-Ended Polystyrenes by Nucleophilic Substitution Reactions. Macromol. Chem. Phys. 2017, 218, 1600485. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Organoselenium chemistry-based polymer synthesis. Org. Chem. Front. 2020, 7, 2815–2841. [Google Scholar] [CrossRef]
- Xia, J.; Li, T.; Lu, C.; Xu, H. Selenium-Containing Polymers: Perspectives toward Diverse Applications in Both Adaptive and Biomedical Materials. Macromolecules 2018, 51, 7435–7455. [Google Scholar] [CrossRef]
- Ji, S.B.; Xia, J.H.; Xu, H.P. Dynamic Chemistry of Selenium: Se-N and Se-Se Dynamic Covalent Bonds in Polymeric Systems. ACS Macro Lett. 2016, 5, 78–82. [Google Scholar] [CrossRef]
- Xu, H.; Cao, W.; Zhang, X. Selenium-Containing Polymers: Promising Biomaterials for Controlled Release and Enzyme Mimics. Accounts Chem. Res. 2013, 46, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; Van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Criddle, R. Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch. Biochem. Biophys. 1967, 122, 164–173. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Bencherif, S.A.; Matyjaszewski, K. Graft Copolymers by a Combination of ATRP and Two Different Consecutive Click Reactions. Macromolecules 2007, 40, 4439–4445. [Google Scholar] [CrossRef]
- Jiang, H.; Pan, X.; Li, N.; Zhang, Z.; Zhu, J.; Zhu, X. Selenide-containing high refractive index polymer material with adjustable refractive index and Abbe’s number. React. Funct. Polym. 2017, 111, 1–6. [Google Scholar] [CrossRef]
- Theato, P.; Klok, H.-A. Functional Polymers By Post-Polymerization Modification: Concepts, Guidelines and Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Günay, K.A.; Theato, P.; Klok, H.A. Standing on the shoulders of hermann staudinger: Post-polymerization modification from past to present. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 1–28. [Google Scholar] [CrossRef]
- Iha, R.K.; Wooley, K.L.; Nyström, A.M.; Burke, D.J.; Kade, M.J.; Hawker, C.J. Applications of Orthogonal "Click" Chemistries in the Synthesis of Functional Soft Materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef]
- Goldmann, A.S.; Glassner, M.; Inglis, A.J.; Barner-Kowollik, C. Post-Functionalization of Polymers via Orthogonal Ligation Chemistry. Macromol. Rapid Commun. 2013, 34, 810–849. [Google Scholar] [CrossRef]
- Stuparu, M.C.; Khan, A. Thiol-epoxy “click” chemistry: Application in preparation and postpolymerization modification of polymers. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3057–3070. [Google Scholar] [CrossRef]
- Muzammil, E.M.; Khan, A.; Stuparu, M.C. Post-polymerization modification reactions of poly(glycidyl methacrylate)s. RSC Adv. 2017, 7, 55874–55884. [Google Scholar] [CrossRef]
- Gadwal, I.; Stuparu, M.C.; Khan, A. Homopolymer bifunctionalization through sequential thiol–epoxy and esterification reactions: An optimization, quantification, and structural elucidation study. Polym. Chem. 2015, 6, 1393–1404. [Google Scholar] [CrossRef]
- De, S.; Khan, A. Efficient synthesis of multifunctional polymers via thiol-epoxy “click” chemistry. Chem. Commun. 2012, 48, 3130–3132. [Google Scholar] [CrossRef]
- Buerkli, C.; Lee, S.H.; Moroz, E.; Stuparu, M.C.; Leroux, J.-C.; Khan, A. Amphipathic Homopolymers for siRNA Delivery: Probing Impact of Bifunctional Polymer Composition on Transfection. Biomacromolecules 2014, 15, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Ma, N.; Ren, H.; Xu, H.; Li, Z.; Wang, Z.; Zhang, X. Oxidation-Responsive Micelles Based on a Selenium-Containing Polymeric Superamphiphile. Langmuir 2010, 26, 14414–14418. [Google Scholar] [CrossRef]
- Ren, H.; Wu, Y.; Ma, N.; Xu, H.; Zhang, X. Side-chain selenium-containing amphiphilic block copolymers: Redox-controlled self-assembly and disassembly. Soft Matter 2012, 8, 1460–1466. [Google Scholar] [CrossRef]
- De, S.; Stelzer, C.; Khan, A. A general synthetic strategy to prepare poly(ethylene glycol)-based multifunctional copolymers. Polym. Chem. 2012, 3, 2342–2345. [Google Scholar] [CrossRef]
- Duddeck, H. Selenium-77 nuclear magnetic resonance spectroscopy. Prog. Nucl. Mag. Res. Spectrosc. 1995, 27, 1–323. [Google Scholar] [CrossRef]
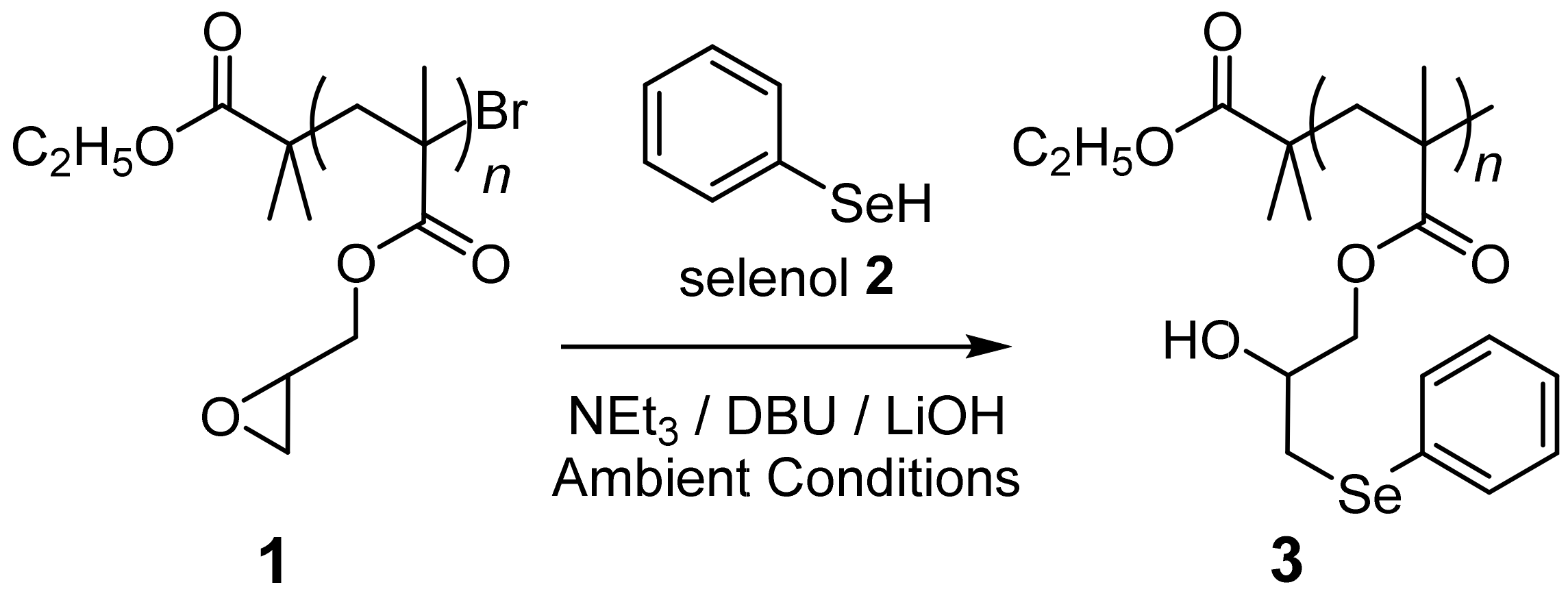
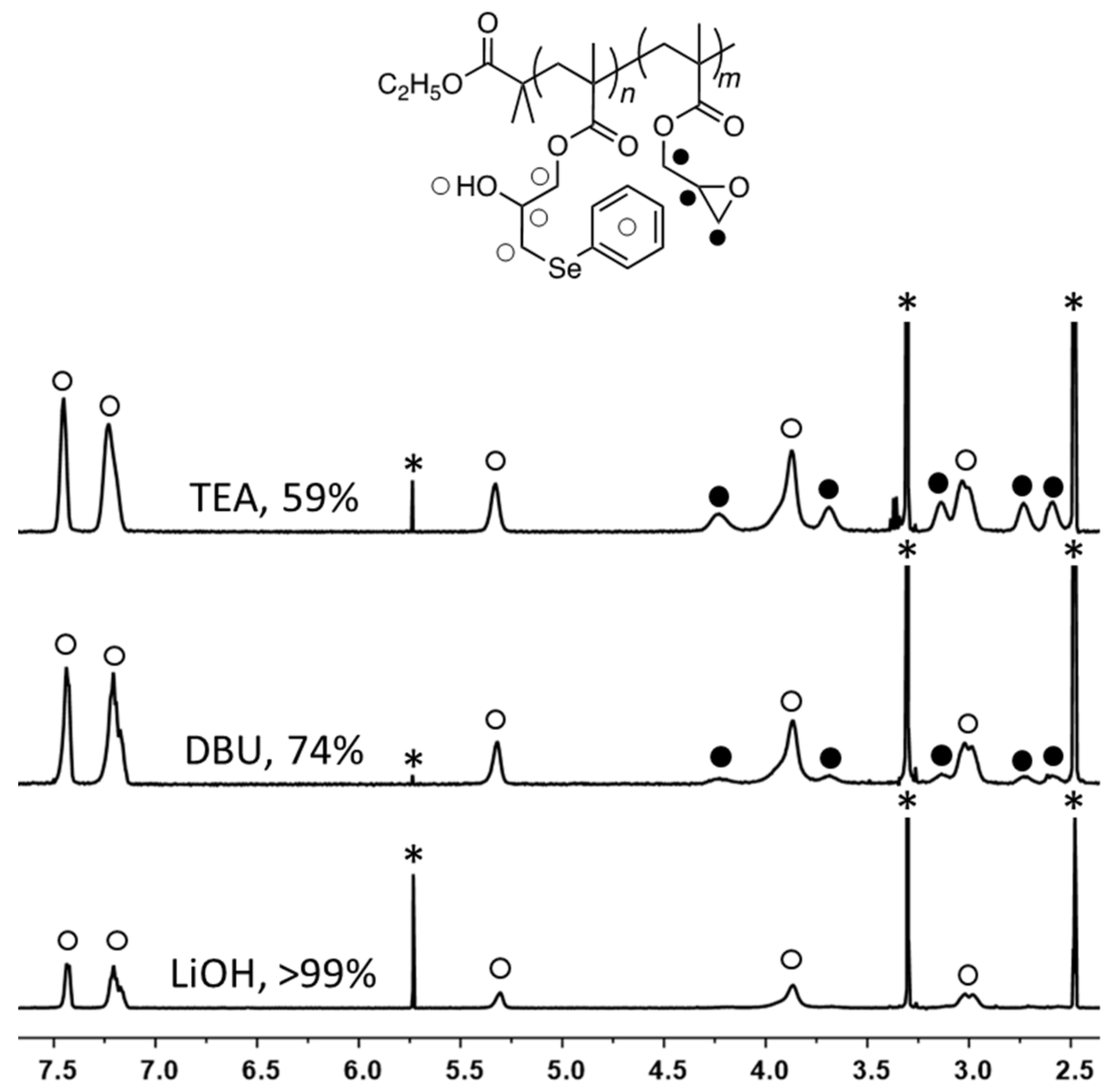



| Entry | Solvent | Selenol (2) | Catalyst | Catalyst (mol%) a | Catalyst (eq./SeH) | Time (hour) | Ring-Opening (%) |
|---|---|---|---|---|---|---|---|
| 1 | CHCl3 | 1.25 | TEA | 3.6 | 0.03 | 12 | 65 |
| 2 | CHCl3 | 1.25 | DBU | 3.6 | 0.03 | 12 | 80 |
| 3 | CHCl3 | 1.25 | LiOH | 3.6 | 0.03 | 12 | 84 |
| 4 | THF | 1.25 | TEA | 3.6 | 0.03 | 12 | 59 |
| 5 | THF | 1.25 | DBU | 3.6 | 0.03 | 12 | 74 |
| 6 | THF/H2O | 1.25 | LiOH | 3.6 | 0.03 | 12 | >99 |
| 7 | CHCl3 | 1.25 | TEA | 5.9 | 0.05 | 12 | 91 |
| 8 | CHCl3 | 1.25 | DBU | 5.9 | 0.05 | 12 | 91 |
| 9 | CHCl3 | 1.25 | LiOH | 5.9 | 0.05 | 12 | >99 |
| 10 | THF | 1.25 | TEA | 5.9 | 0.05 | 12 | 91 |
| 11 | THF | 1.25 | DBU | 5.9 | 0.05 | 12 | >99 |
| 12 | THF/H2O | 1.25 | LiOH | 5.9 | 0.05 | 12 | >99 |
| 13 | CHCl3 | 1.25 | TEA | 5.9 | 0.05 | 3 | 78 |
| 14 | CHCl3 | 1.25 | DBU | 5.9 | 0.05 | 3 | 88 |
| 15 | CHCl3 | 1.25 | LiOH | 5.9 | 0.05 | 3 | >99 |
| 16 | THF | 1.25 | TEA | 5.9 | 0.05 | 3 | 83 |
| 17 | THF | 1.25 | DBU | 5.9 | 0.05 | 3 | 83 |
| 18 | THF/H2O | 1.25 | LiOH | 5.9 | 0.05 | 3 | >99 |
| 19 | CHCl3 | 1.25 | TEA | 5.9 | 0.05 | 1 | 64 |
| 20 | CHCl3 | 1.25 | DBU | 5.9 | 0.05 | 1 | 68 |
| 21 | CHCl3 | 1.25 | LiOH | 5.9 | 0.05 | 1 | 77 |
| 22 | THF | 1.25 | TEA | 5.9 | 0.05 | 1 | 55 |
| 23 | THF | 1.25 | DBU | 5.9 | 0.05 | 1 | 62 |
| 24 | THF/H2O | 1.25 | LiOH | 5.9 | 0.05 | 1 | 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, T.; Khan, A. Selenonium Polyelectrolyte Synthesis through Post-Polymerization Modifications of Poly (Glycidyl Methacrylate) Scaffolds. Polymers 2020, 12, 2685. https://doi.org/10.3390/polym12112685
Eom T, Khan A. Selenonium Polyelectrolyte Synthesis through Post-Polymerization Modifications of Poly (Glycidyl Methacrylate) Scaffolds. Polymers. 2020; 12(11):2685. https://doi.org/10.3390/polym12112685
Chicago/Turabian StyleEom, Taejun, and Anzar Khan. 2020. "Selenonium Polyelectrolyte Synthesis through Post-Polymerization Modifications of Poly (Glycidyl Methacrylate) Scaffolds" Polymers 12, no. 11: 2685. https://doi.org/10.3390/polym12112685
APA StyleEom, T., & Khan, A. (2020). Selenonium Polyelectrolyte Synthesis through Post-Polymerization Modifications of Poly (Glycidyl Methacrylate) Scaffolds. Polymers, 12(11), 2685. https://doi.org/10.3390/polym12112685






