Chain Entanglement of 2-Ethylhexyl Hydrogen-2-Ethylhexylphosphonate into Methacrylate-Grafted Nonwoven Fabrics for Applications in Separation and Recovery of Dy (III) and Nd (III) from Aqueous Solution
Abstract
1. Introduction
2. Experimental
2.1. Materials
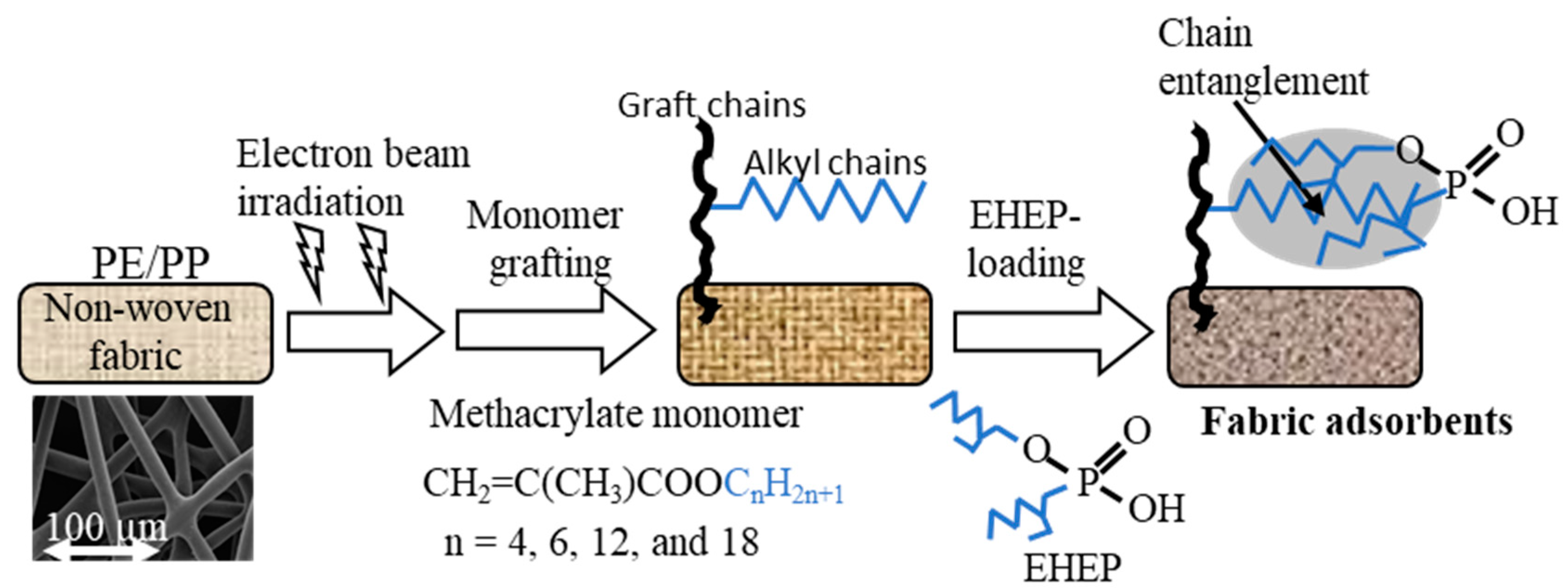
2.2. Graft Polymerization of Methacrylate Monomers
2.3. Loading of EHEP onto the Grafted Fabrics
2.4. Characterization
2.5. Batch Adsorption Tests
2.6. Column Adsorption Tests
3. Results and Discussion
3.1. Synthesis of EHEP-Loaded Adsorbent
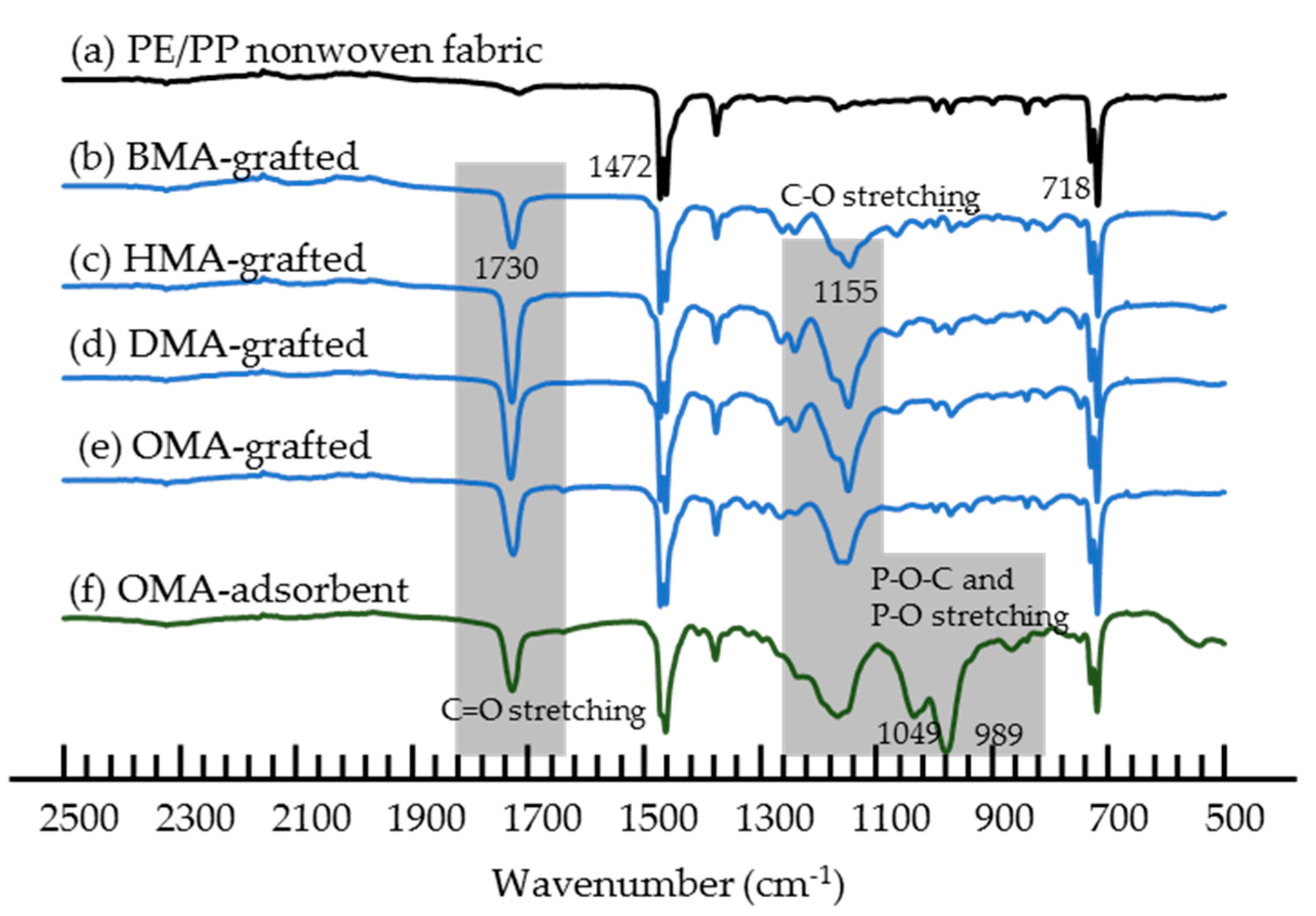
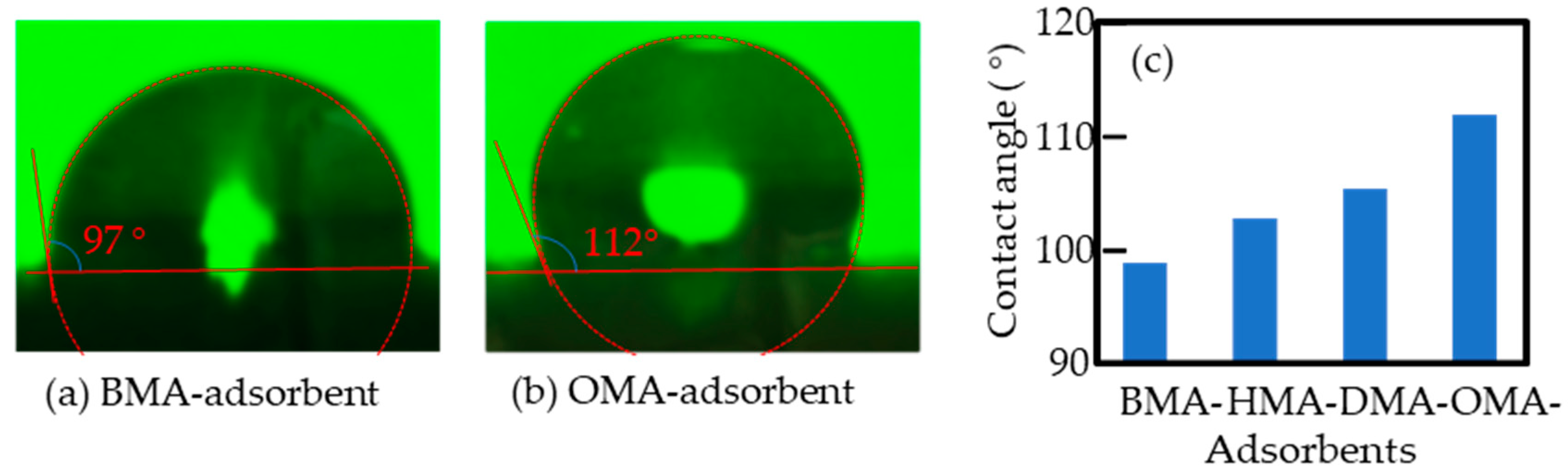
3.2. Materials Characterization
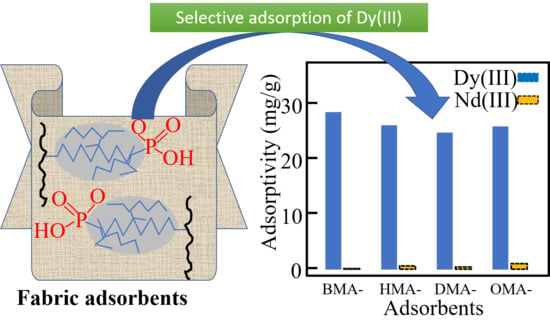
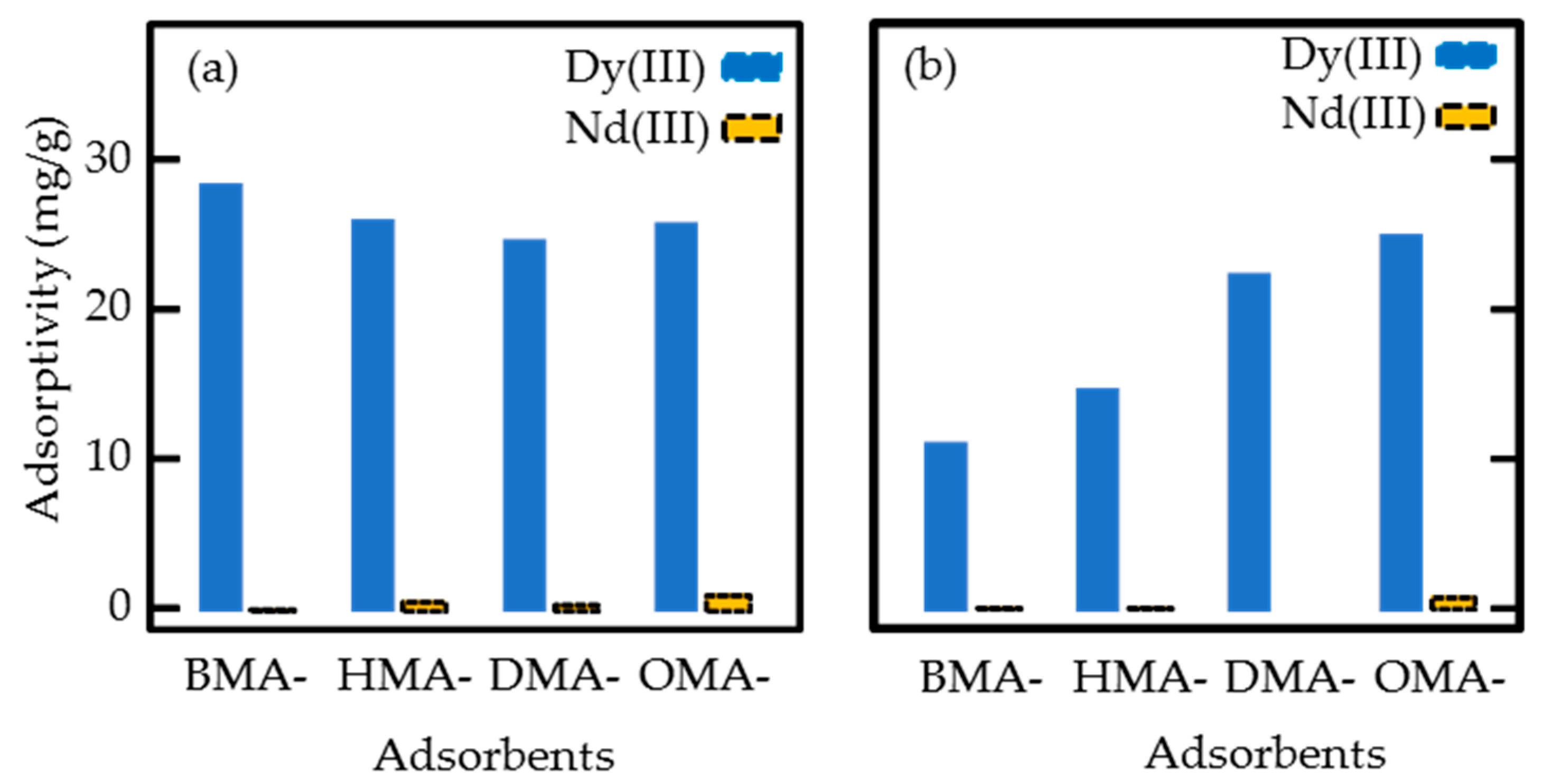
3.3. Batch Adsorption Tests
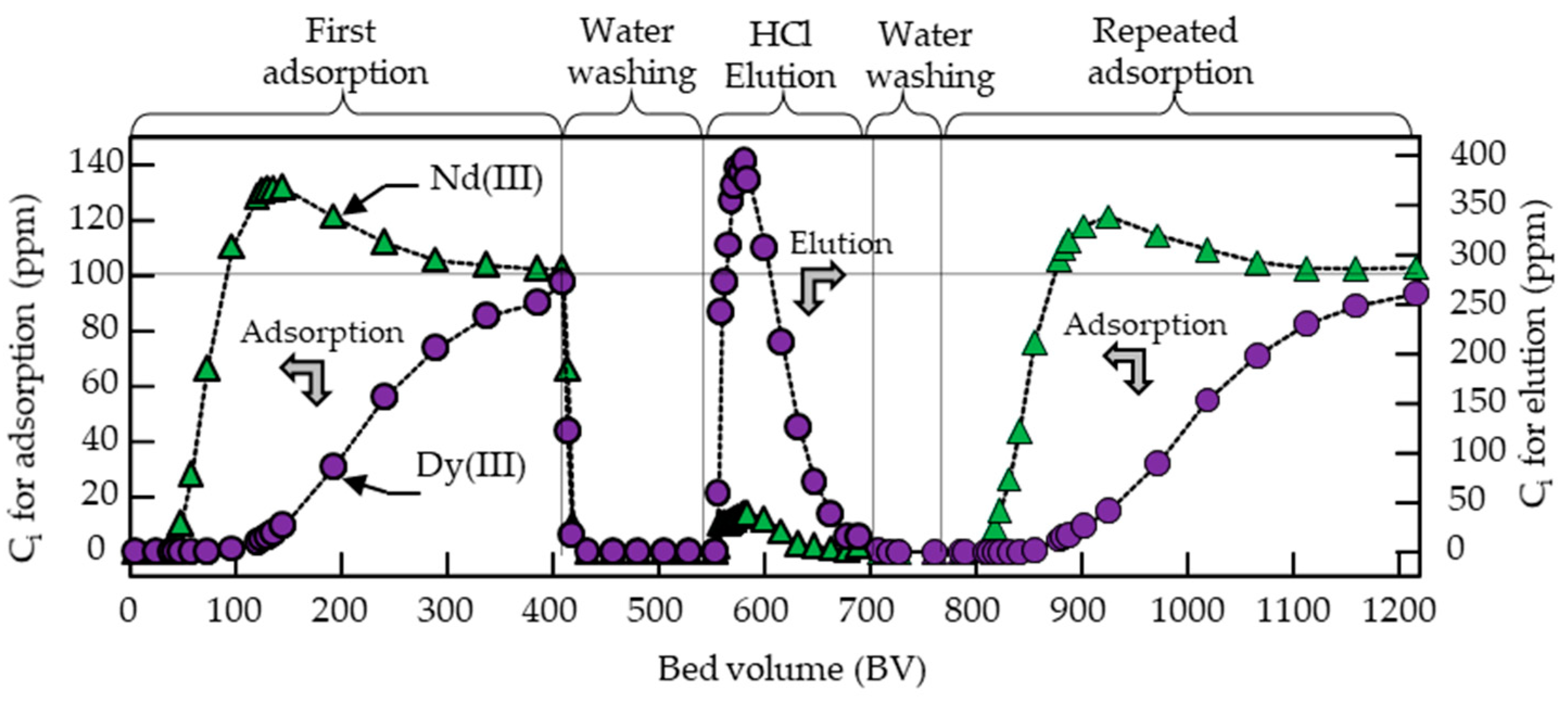
3.4. Column Adsorption Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schreiber, A.; Marx, J.; Zapp, P.; Hake, J.F.; Voßenkaul, D.; Friedrich, B. Environmental impacts of rare earth mining and separation based on eudialyte: A new European way. Resources 2016, 5, 32. [Google Scholar] [CrossRef]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Abhilash; Meshram, P. Overview on extraction and separation of rare earth elements from red mud: Focus on scandium. Min. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- Golev, A.; Scott, M.; Erskine, P.D.; Ali, S.H.; Ballantyne, G.R. Rare earths supply chains: Current status, constraints and opportunities. Resour. Policy 2014, 41, 52–59. [Google Scholar] [CrossRef]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Gerven, T.V.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Machacek, E.; Richter, J.L.; Habib, K.; Klossek, P. Recycling of rare earths from fluorescent lamps: Value analysis of closing-the-loop under demand and supply uncertainties. Resour. Conserv. Recycl. 2015, 104, 76–93. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Critical Materials Strategy; U.S. Department of Energy: Washington, DC, USA, 2011; pp. 1–191.
- Rademaker, J.H.; Kleijn, R.; Yang, Y. Recycling as a strategy against rare earth element criticality: A systemic evaluation of the potential yield of NdFeB magnet recycling. Environ. Sci. Technol. 2013, 47, 10129–10136. [Google Scholar] [CrossRef]
- Thakur, N.V.; Jayawant, D.V.; Iyer, N.S.; Koppiker, K.S. Separation of neodymium from lighter rare earths using alkyl phosphonic acid, PC 88A. Hydrometallurgy 1993, 34, 99–108. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, C.J.; Chung, K.W.; Kim, S.D.; Lee, J.Y.; Kumar, J.R. Solvent extraction, separation and recovery of dysprosium (Dy) and neodymium (Nd) from aqueous solutions: Waste recycling strategies for permanent magnet processing. Hydrometallurgy 2016, 165, 27–43. [Google Scholar] [CrossRef]
- Riaño, S.; Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Riaño, S.; Foltova, S.S.; Binnemans, K. Separation of neodymium and dysprosium by solvent extraction using ionic liquids combined with neutral extractants: Batch and mixer-settler experiments. RSC Adv. 2020, 10, 307–316. [Google Scholar] [CrossRef]
- Ding, Y.; Harvey, D.; Wang, N.L. Two-zone ligand-assisted displacement chromatography for producing high-purity praseodymium, neodymium, and dysprosium with high yield and high productivity from crude mixtures derived from waste magnets. Green Chem. 2020, 22, 3769–3783. [Google Scholar] [CrossRef]
- Alcaraz, L.; Escudero, M.E.; Alguacil, F.J.; Llorente, I.; Urbieta, A.; Fernández, P.; López, F.A. Dysprosium removal from water using active carbons obtained from spent coffee ground. Nanomaterials 2019, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Gergoric, M.; Ravaux, C.; Steenari, B.M.; Espegren, F.; Retegan, T. Leaching and recovery of rare-earth elements from neodymium magnet waste using organic acids. Metals 2018, 8, 721. [Google Scholar] [CrossRef]
- Kim, J.; Azimi, G. Recovery of scandium and neodymium from blast furnace slag using acid baking–water leaching. RSC Adv. 2020, 10, 31936–31946. [Google Scholar] [CrossRef]
- Demey, H.; Lapo, B.; Ruiz, M.; Fortuny, A.; Marchand, M.; Sastre, A.M. Neodymium recovery by chitosan/iron (III) hydroxide [ChiFer (III)] sorbent material: Batch and column systems. Polymers 2018, 10, 204. [Google Scholar] [CrossRef]
- Matsumiya, M.; Yamada, T.; Kikuchi, Y.; Kawakami, S. Removal of iron and boron by solvent extraction with ionic liquids and recovery of neodymium metal by direct electrodeposition. Solvent Extr. Ion Exch. 2016, 34, 522–534. [Google Scholar] [CrossRef]
- Riaño, S.; Petranikova, M.; Onghena, B.; Vander Hoogerstraete, T.; Banerjee, D.; Foreman, M.R.S.; Binnemans, K. Separation of rare earths and other valuable metals from deep-eutectic solvents: A new alternative for the recycling of used NdFeB magnets. RSC Adv. 2017, 7, 32100–32113. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Su, Z.; Liu, S.; Anderson, C.; Jiang, T. Hydrometallurgical recovery of rare earth elements from NdFeB permanent magnet scrap: A review. Metals 2020, 10, 841. [Google Scholar] [CrossRef]
- Ismail, N.A.; Aziz, M.A.A.; Yunus, M.Y.M.; Hisyam, A. Selection of extractant in rare earth solvent extraction system: A review. Int. J. Recent Technol. Eng. 2019, 8, 728–742. [Google Scholar]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, L.; Xiao, C.; Zeng, L. Direct solvent extraction of molybdenum(VI) from sulfuric acid leach solutions using PC-88A. Hydrometallurgy 2015, 158, 114–118. [Google Scholar] [CrossRef]
- Zhang, F.; Dai, J.; Wang, A.; Wu, W. Investigation of the synergistic extraction behavior between cerium (III) and two acidic organophosphorus extractants using FT-IR, NMR and mass spectrometry. Inorg. Chim. Acta 2017, 466, 333–342. [Google Scholar] [CrossRef]
- Tasaki-Handa, Y.; Abe, Y.; Ooi, K.; Narita, H.; Tanaka, M.; Wakisaka, A. Selective crystallization of phosphoester coordination polymer for the separation of neodymium and dysprosium: A thermodynamic approach. J. Phys. Chem. B 2016, 120, 12730–12735. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.A.; Lippincott, C.A.; Carroll, P.J.; Schelter, E.J. An operationally simple method for separating the rare-earth elements neodymium and dysprosium. Angew. Chem. Int. Ed. 2015, 54, 8222–8225. [Google Scholar] [CrossRef] [PubMed]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, J.; Yuan, W.; Yi, Y.; Zhao, L. Recovery of Au (III) by radiation synthesized aminomethyl pyridine functionalized adsorbents based on cellulose. Chem. Eng. J. 2016, 283, 504–513. [Google Scholar] [CrossRef]
- Hayashi, N.; Chen, J.; Seko, N. Nitrogen-containing fabric adsorbents prepared by radiation grafting for removal of chromium from wastewater. Polymers 2018, 10, 744. [Google Scholar] [CrossRef]
- Abney, C.W.; Mayes, R.T.; Saito, T.; Dai, S. Materials for the recovery of uranium from seawater. Chem. Rev. 2017, 117, 13935–14013. [Google Scholar] [CrossRef]
- Liu, S.; Xu, M.; Yu, T.; Han, D.; Peng, J.; Li, J.; Zhai, M. Radiation synthesis and performance of novel cellulose-based microsphere adsorbents for efficient removal of boron (III). Carbohyd. Polym. 2017, 174, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Zhang, S.; Wen, J.; Xiong, J.; Hu, S. Functional polymer brushes for highly efficient extraction of uranium from seawater. J. Mater. Sci. 2019, 54, 3572–3585. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Zhang, M.; Xing, Z.; Ma, H.; Wu, G. Phosphate-based ultrahigh molecular weight polyethylene fibers for efficient removal of uranium from carbonate solution containing fluoride ions. Molecules 2018, 23, 1245. [Google Scholar] [CrossRef]
- Tran, T.H.; Okabe, H.; Hidaka, Y.; Hara, K. Removal of metal ions from aqueous solutions using carboxymethyl cellulose/sodium styrene sulfonate gels prepared by radiation grafting. Carbohyd. Polym. 2017, 157, 335–343. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Peng, J.; Wu, H.; Li, J.; Zhai, M. Adsorption of Cr (VI) using cellulose microsphere-based adsorbent prepared by radiation-induced grafting. Radiat. Phys. Chem. 2012, 81, 967–970. [Google Scholar] [CrossRef]
- Ray, P.Z.; Shipley, H.J. Inorganic nano-adsorbents for the removal of heavy metals and arsenic: A review. RSC Adv. 2015, 5, 29885–29907. [Google Scholar] [CrossRef]
- Topel, S.D.; Legaria, E.P.; Tiseanu, C.; Rocha, J.; Nedelec, J.M.; Kessler, V.G.; Seisenbaeva, G.A. Hybrid silica nanoparticles for sequestration and luminescence detection of trivalent rare-earth ions (Dy3+ and Nd3+) in solution. J. Nanopart. Res. 2014, 16, 2783. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Nasef, M.M.; Gürsel, S.A.; Karabelli, D.; Güven, O. Radiation-grafted materials for energy conversion and energy storage applications. Progr. Polym. Sci. 2016, 63, 1–41. [Google Scholar] [CrossRef]
- Zhilyaeva, N.A.; Lytkina, A.A.; Mironova, E.Y.; Ermilova, M.M.; Orekhova, N.V.; Shevlyakova, N.V.; Tverskoy, V.A.; Yaroslavtsev, A.B. Polyethylene with radiation-grafted sulfonated polystyrene membranes for butane and butenes separation. Chem. Eng. Res. Des. 2020, 161, 253–259. [Google Scholar] [CrossRef]
- Sherazi, T.A.; Guiver, M.D.; Kingston, D.; Ahmad, S.; Kashmiri, M.A.; Xue, X. Radiation-grafted membranes based on polyethylene for direct methanol fuel cells. J. Power Sour. 2010, 195, 21–29. [Google Scholar] [CrossRef]
- Mandal, D.V.; Bhunia, H.; Bajpai, P.K.; Bhalla, V.K. Thermal degradation kinetics and estimation of lifetime of radiation grafted polypropylene films. Radiat. Phys. Chem. 2017, 136, 1–8. [Google Scholar] [CrossRef]
- Bondar, Y.; Kim, H.J.; Yoon, S.H.; Lim, Y.J. Synthesis of cation-exchange adsorbent for anchoring metal ions by modification of poly (glycidyl methacrylate) chains grafted onto polypropylene fabric. React. Funct. Polym. 2004, 58, 43–51. [Google Scholar] [CrossRef]
- Chen, J.; Seko, N. Cleavage of the graft bonds in PVDF-g-St films by boiling xylene extraction and the determination of the molecular weight of the graft chains. Polymers 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Seko, N.; Basuki, F.; Tamada, M.; Yosaii, F. Rapid removal of arsenic(V) by zirconium(IV) loaded phosphoric chelate adsorbent synthesized by radiation induced graft polymerization. React. Funtc. Polym. 2004, 59, 235–241. [Google Scholar] [CrossRef]
- Ha, H.; Wu, L.; Tai, H.; Zhang, Z.; Wei, J.; Wu, J. Study of radiation grafting of styrene on cotton cellulose. Radiat. Phys. Chem. 1995, 46, 823–827. [Google Scholar]
- Gad, H.M.H.; Hamed, M.M.; Eldahab, H.A.; Moustafa, M.E.; El-Reefy, S.A. Radiation-induced grafting copolymerization of resin onto the surface of silica extracted from rice husk ash for adsorption of gadolinium. J. Mol. Liq. 2017, 231, 45–55. [Google Scholar] [CrossRef]
- Ueki, Y.; Seko, N. Synthesis of Fibrous metal adsorbent with a piperazinyl—Dithiocarbamate group by radiation-induced grafting and its performance. ACS OMEGA 2020, 5, 2947–2956. [Google Scholar] [CrossRef]
- Shibata, T.; Seko, N.; Amada, H.; Kasai, N.; Saiki, S.; Hoshina, H.; Ueki, Y. Evaluation of a cesium adsorbent grafted with ammonium 12-molybdophosphate. Radiat. Phys. Chem. 2016, 119, 247–252. [Google Scholar] [CrossRef]
- Othman, N.A.F.; Selambakkannu, S.; Yamanobe, T.; Hoshina, H.; Seko, N.; Abdullah, T.A.T. Radiation grafting of DMAEMA and DEAEMA-based adsorbents for thorium adsorption. J. Radioanal. Nucl. Chem. 2020, 324, 429–440. [Google Scholar] [CrossRef]
- Zhang, W.; Avdibegović, D.; Koivula, R.; Hatanpää, T.; Hietala, S.; Regadío, M.; Harjula, R. Titanium alkylphosphate functionalised mesoporous silica for enhanced uptake of rare-earth ions. J. Mater. Chem. A 2017, 5, 23805–23814. [Google Scholar] [CrossRef]
- Ueki, Y.; Saiki, S.; Hoshina, H.; Seko, N. Biodiesel fuel production from waste cooking oil using radiation-grafted fibrous catalysts. Radiat. Phys. Chem. 2018, 143, 41–46. [Google Scholar] [CrossRef]
- Fonseca, J.L.C.; Apperley, D.C.; Plasma, J.P.S. Plasma polymerization of tetramethylsilane. Chem. Mater. 1993, 5, 1676–1682. [Google Scholar] [CrossRef]
- Nava-Ortiz, C.A.B.; Burillo, G.; Bucio, E.; Alvarez-Lorenzo, C. Modification of polyethylene films by radiation grafting of glycidyl methacrylate and immobilization of β-cyclodextrin. Radiat. Phys. Chem. 2009, 78, 19–24. [Google Scholar] [CrossRef]
- Ormond-Prout, J.; Dupin, D.; Armes, S.P.; Foster, N.J.; Burchell, M.J. Synthesis and characterization of polypyrrole-coated poly (methyl methacrylate) latex particles. J. Mater. Chem. 2009, 19, 1433–1442. [Google Scholar] [CrossRef]
- Yin, S.H.; Li, S.W.; Xie, F.; Zhang, L.B.; Peng, J.H. Study on the aqueous solution behavior and extraction mechanism of Nd (III) in the presence of the complexing agent lactic acid with di-(2-ethylhexyl) phosphoric acid. RSC Adv. 2015, 5, 64550–64556. [Google Scholar] [CrossRef]
- Pathak, S.K.; Tripathi, S.C.; Singh, K.K.; Mahtele, A.K.; Dwivedi, C.; Juby, K.A.; Bajaj, P.N. PC-88A–impregnated polymeric beads: Preparation, characterization and application for extraction of Pu (IV) from nitric acid medium. Radiochim. Acta 2013, 101, 761–771. [Google Scholar] [CrossRef]
| Grafting Conditions ** | |||||
|---|---|---|---|---|---|
| Name | Molecular Structures | M * | Dose *** (kGy) | Temp. (°C) | Time (min) |
| Butyl methacrylate (BMA) | CH2=CCH3COOC4H9 | 142 | 10 | 40 | 15 |
| Hexyl methacrylate (HMA) | CH2=CCH3COOC6H13 | 170 | 10 | 60 | 30 |
| Dodecyl methacrylate (DMA) | CH2=CCH3COOC12H25 | 254 | 300 | 60 | 180 |
| Octadecyl methacrylate (OMA) | CH2=CCH3COOC18H37 | 338 | 100 | 60 | 120 |
| Grafted Monomers | Degree of Grafting (%) | Alkyl Group Density * (mmol/g) | EHEP Loading ** (mmol/g) |
|---|---|---|---|
| Butyl methacrylate (BMA) | 51 | 2.39 | 1.24 |
| Hexyl methacrylate (HMA) | 62 | 2.24 | 1.26 |
| Dodecyl methacrylate (DMA) | 102 | 1.99 | 1.24 |
| Octadecyl methacrylate (OMA) | 219 | 2.03 | 1.22 |
| Adsorbents | 1st Adsorption * | Repeated Adsorption ** | Wa/Wb | ||||
|---|---|---|---|---|---|---|---|
| Wb (mg) | CDy-1 (mg/g) | CNd-1 (mg/g) | CDy-r (mg/g) | CNd-r (mg/g) | Wa (mg) | ||
| BMA | 54 | 28.6 | 0.1 | 11.4 | 0.1 | 40 | 0.74 |
| HMA | 66 | 26.2 | 0.6 | 15.0 | 0.1 | 51 | 0.77 |
| DMA | 80 | 24.9 | 0.4 | 22.7 | 0 | 74 | 0.93 |
| OMA | 86 | 26.0 | 1.0 | 25.3 | 0.8 | 82 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshina, H.; Chen, J.; Amada, H.; Seko, N. Chain Entanglement of 2-Ethylhexyl Hydrogen-2-Ethylhexylphosphonate into Methacrylate-Grafted Nonwoven Fabrics for Applications in Separation and Recovery of Dy (III) and Nd (III) from Aqueous Solution. Polymers 2020, 12, 2656. https://doi.org/10.3390/polym12112656
Hoshina H, Chen J, Amada H, Seko N. Chain Entanglement of 2-Ethylhexyl Hydrogen-2-Ethylhexylphosphonate into Methacrylate-Grafted Nonwoven Fabrics for Applications in Separation and Recovery of Dy (III) and Nd (III) from Aqueous Solution. Polymers. 2020; 12(11):2656. https://doi.org/10.3390/polym12112656
Chicago/Turabian StyleHoshina, Hiroyuki, Jinhua Chen, Haruyo Amada, and Noriaki Seko. 2020. "Chain Entanglement of 2-Ethylhexyl Hydrogen-2-Ethylhexylphosphonate into Methacrylate-Grafted Nonwoven Fabrics for Applications in Separation and Recovery of Dy (III) and Nd (III) from Aqueous Solution" Polymers 12, no. 11: 2656. https://doi.org/10.3390/polym12112656
APA StyleHoshina, H., Chen, J., Amada, H., & Seko, N. (2020). Chain Entanglement of 2-Ethylhexyl Hydrogen-2-Ethylhexylphosphonate into Methacrylate-Grafted Nonwoven Fabrics for Applications in Separation and Recovery of Dy (III) and Nd (III) from Aqueous Solution. Polymers, 12(11), 2656. https://doi.org/10.3390/polym12112656