A Critical Review on Metal-Organic Frameworks and Their Composites as Advanced Materials for Adsorption and Photocatalytic Degradation of Emerging Organic Pollutants from Wastewater
Abstract
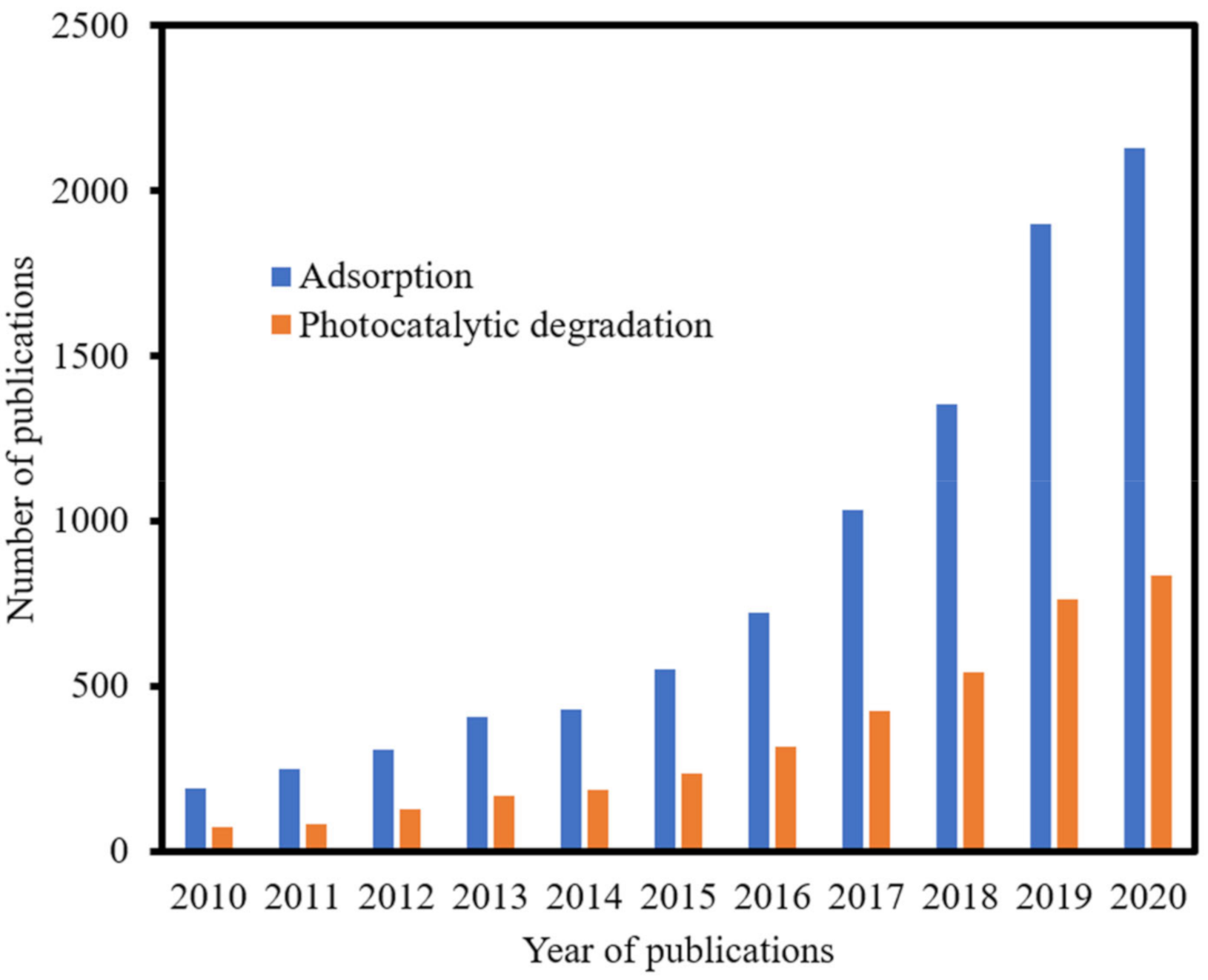
1. Introduction
1.1. Adsorption
1.2. Photocatalysis
- (i)
- Ability to degrade pollutants within a short time with the help of light or solar energy.
- (ii)
- Operates under ambient conditions.
- (iii)
- Mineralization of organic pollutants into carbon dioxide and water; thus, no secondary pollutants are produced.
1.3. Metal-Organic Frameworks
2. MOFs for Remediation of Emerging Pollutants in Water
2.1. MOFs for Adsorption
2.2. MOFs for Photocatalysis
2.3. MOF Composites for Adsorption and Photocatalytic Degradation
3. MOFs and Composites for Adsorption and Photocatalytic Degradation of Emerging Pollutants in Water
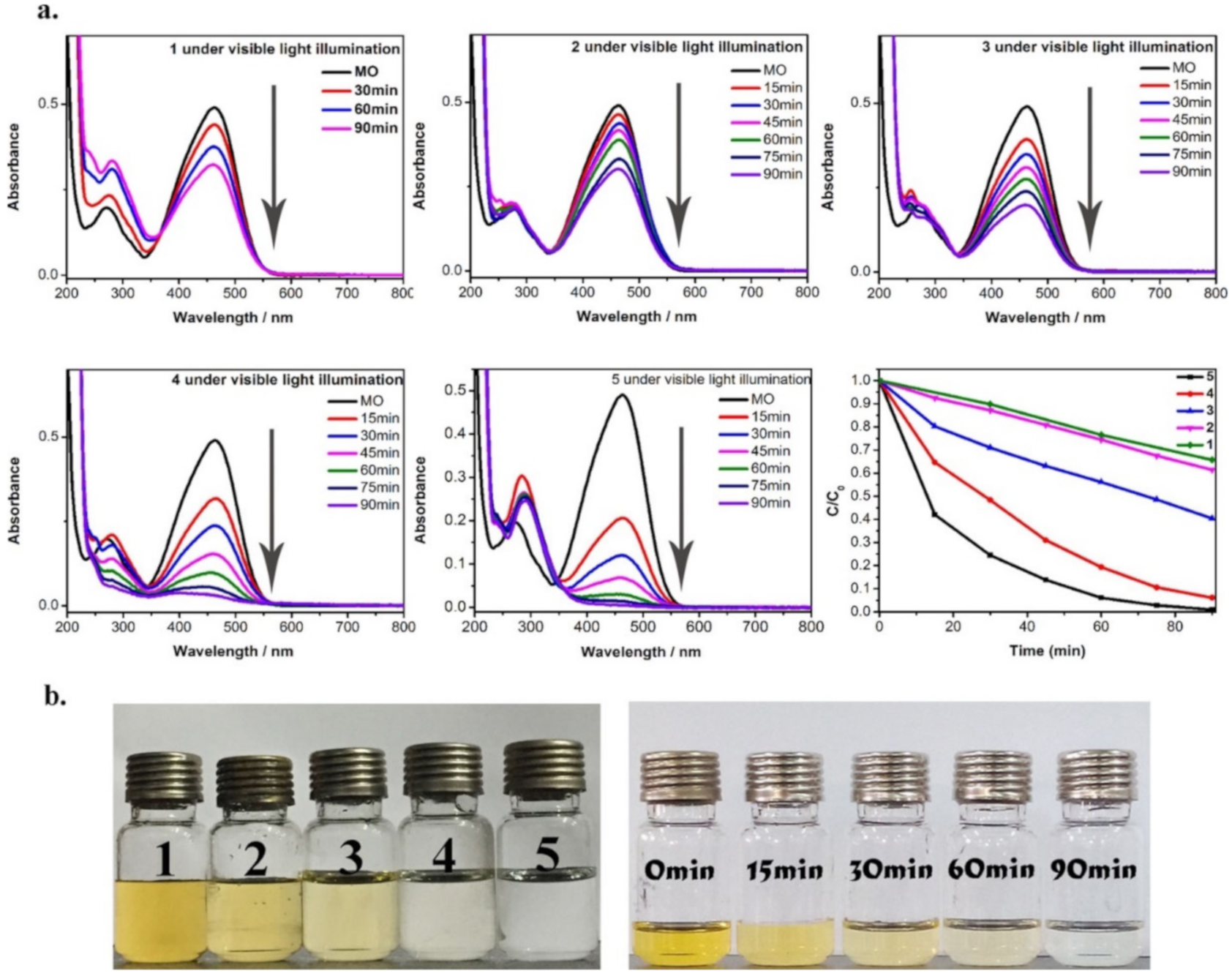
3.1. MOFs and Composites for Adsorption and Photocatalytic Degradation of Dyes
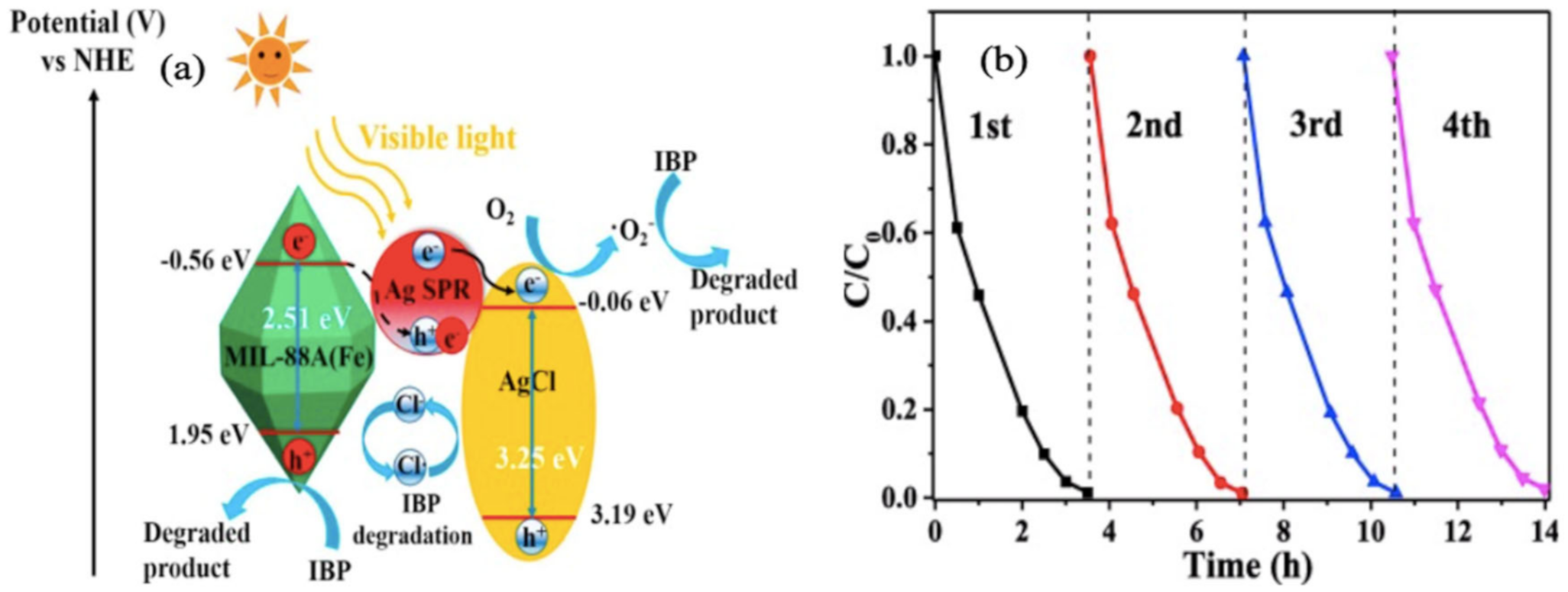
3.2. MOFs and Composites for Adsorptive Removal and Photocatalytic Degradation of Phenols and Other Miscellaneous Emerging Pollutants
| Type of MOF | Synthesis Method | Surface Area (m2 g−1) | Pollutants | Concentration (mg L−1) | % Removal | Qe (mg g−1) | Equilibrium Time | Reused | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Phenolics | |||||||||
| MIL-53(Al) MIL-53(Al)-F127 | Hydrothermal | 931 1008 | Bisphenol A | 250 | - | 329 473 | 90 min 30 min | 3 3 | [185] |
| MIL-68(Al)/PVDF | Casting | - | P-nitrophenol | 10 | 94 | 126 | 720 min | 6 | [125] |
| HKUST-1(Cu) | Microwave | - | P-nitrophenol | 200 | 400 | 30 min | - | [193] | |
| SiO2@MIL-68(Al) | Solvothermal | 1156 | Aniline | 3000 | - | 532 | 40 s | 5 | [194] |
| [Zn(ATA)(BPD)] MOF-VII | Ultrasound | 170 675 | 2,4-dichlorophenol | 60 | 68 91 | - - | 90 min 90 min | 5 | [195] |
| [Zn(TDC) MOF | Vapor-diffusion | 235 | 2, 4-dichloropheno | 60 | 95 | - | 180 min | - | [196] |
| MIL-68(Al) CNT@MIL-68(Al) | Solvothermal | 1283 1407 | Phenol Phenol | 1000 | - - | 118 257 | 120 min | 5 | [86] |
| NH2-UiO-66(Zr) | Solvothermal | 2,4,6-trinitrophenol Styphnic acid 2,4-dinitrotoluene | 100 | - | 23 24 0.5 2 | 36 h | - | [197] | |
| MIL–68(Al) MIL–68(Al)/GO | Solvothermal | 550 762 | p–nitrophenol | 300 | - - | 271 332 | 17 h 17 h | 5 | [198] |
| NH2-MIL-88(Fe) | Hydrothermal | 414 | 2,4,6-trinitrophenol | 35 | - | 164 | 40 min | 5 | [199] |
| MOF-199(Cu) | Solvothermal | 2271 | Phenol p-nitro phenol | 500 | 80 89 | 58 68 | 300 min 30 min | - - | [200] |
| Al-MOF/SA-CS | Hydrothermal | 688 | Bisphenol A | 50 | - | 137 | 18 h | 6 | [201] |
| Cu-BDC MOF Cu-BDC@GrO Cu-BDC@CNT | Solvothermal | - - - | Bisphenol A Bisphenol A Bisphenol A | 200 | 97 | 60 182 164 | 40 min | 5 | [202] |
| laccase@HKUST-1 | Immobilization | - | Bisphenol A | 200 | 74 | - | 4 h | NA | [203] |
| Pesticides | |||||||||
| M-MOF | Room temperature | 250 | Thiamethoxam Acetamiprid Nitenpyram Dinotefuran Clothianidin Thiacloprid | 100 | - | 3 3 3 3 2 3 | 60 min | - | [204] |
| MIL-101(Cr) | Hydrothermal | 2612 | Diazinon | 50 | 54 | 158 | 45 min | 4 | [205] |
| Cr-MIL-101-BTP | Hydrothermal | 1113 | Acetochlor | 120 | 100 | 322 | 200 min | 6 | [206] |
| MIL-101(Cr) TS-MIL-101(Cr) | Hydrothermal | - | Atrazine | 30 | 37 69 | - | 60 min | - | [207] |
| Herbicides | |||||||||
| HKUST-1(Cu) ZrO2@HKUST-1 | Room temperature | 1484 1152 | Cyhalothrin | 60 | - | 140 138 | 2 h | - | [208] |
| UiO-67(Zr) | Hydrothermal | 2172 | Glyphosate Glufosinate | 200 | 96 92 | 537 360 | 150 min 200 min | - | [209] |
| NU-100(Zr) UiO-67(Zr) | Solvothermal | N/A N/A | Glyphosate | 1117.5 | 100 100 | 1340 1500 | 20 min 60 min | - - | [184] |
| UiO-66(Zr) UiO-67(Zr) | Solvothermal | 1640 2345 | Atrazine | 25 | 20 98 | 3 12 | 50 min 2 min | 1 4 | [210] |
| DUT-52(Zr) NU-1008(Zr) NU-901(Zr) NU-1000(Zr) | Solvothermal | 1960 1400 2110 2110 | Atrazine | 10 | 82 69 85 93 | - | 1 min | 3 | [186] |
| PAHs | |||||||||
| Zn-BDC MOF Cu-BDC MOF | Mechanical Mechanical | - | Naphthalene Anthracene Naphthalene Anthracene | 100 | 88 50 84 52 | 87 52 84 52 | 210 min 120 min 210 min 120 min | 3 | [211] |
| UiO-66(Zr) NH2-UiO-66(Zr) | Solvothermal | 1420 985 | Anthracene Chrysene Anthracene Chrysene | 4 | 99 96 98 96 | 24 22 24 19 | 25 min 25 min 30 min 30 min | 5 5 | [187] |
| MIL-88(Fe) NH2-MIL-88(Fe) | Microwave | 1240 941 | Pyrene Pyrene | 4 | 99 96 | 24 23 | 40 min | 5 | [212] |
| MIL-88(Fe) NH2-MIL-88(Fe) | Microwave | 1240 941 | Chrysene Chrysene | 4 | 99 95 | 24 22 | 25 min | 5 | [188] |
| MIL-88(Fe) NH2-MIL-88(Fe) Mixed-MIL-88(Fe) | Microwave | 1240 941 1025 | Anthracene Anthracene Anthracene | 4 | 98 92 96 | 24 21 23 | 25 min | - | [189] |
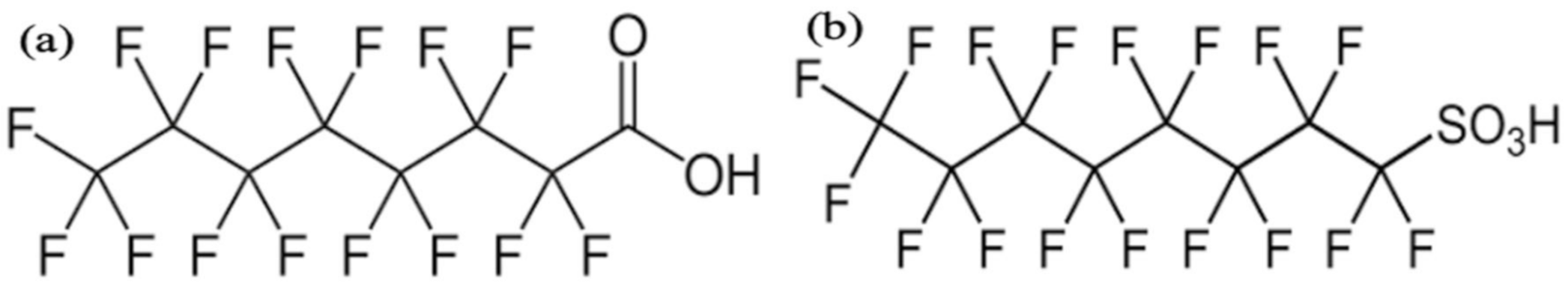
| PFCAs | |||||||||
| ZIF-7 ZIF-8 ZIF-L | Room temperature | 14 1291 12 | Perfluorooctanoic acid | 250 | 40 45 97 | 26 214 295 | 60 min | - | [213] |
| Basolite A-100 | Commercial | 630 | Perfluorooctanoic acid | 1 | 100 | 169 | 4 |
| MOF | Synthesis Method | Surface Area (m2 g−1) | Bandgap (eV) | Pollutants | Concentration (mg L−1) | Light Source | (%) Removal | Irradiation Time | Reused | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolics | ||||||||||
| NH2-MIL-125 (Ti)@Bi2M | Solvothermal | 88 | 1.89 | Dichlorophen | 10 | Visible | 93 | 180 min | - | [214] |
| [CoNi(m3-tp)2(m2-pyz)2] MOF/CuWO4 | Hydrothermal | 1054 801 | 2.5 2.4 | 4-nitrophenol | 10 | Visible | 24 81 | 105 min | 6 | [152] |
| MIL-88B(Fe) CNT@MIL-88B(Fe) | Hydrothermal Hydrothermal | 118 | - | Phenol | 25 | 55 100 | 30 min 10 min | 3 | [215] | |
| CdS@NH2-MIL-125(Ti) | Solvothermal | 1375 | 2.36 | Phenol | 180 | Visible | - | 120 min | 5 | [147] |
| HOQ@MOF-5(Zn) | Room temperature | - | 3.12 | Phenol | 1 | Visible | 100 | 70 min | 5 | [216] |
| MIL-100(Fe)@ZnO | Solvothermal | 654 | 2.63 | Phenol, Bisphenol A | 5 | Visible | 95 84 | 120 min | 5 | [191] |
| MIL-101-NH2@TpMA UiO-66-NH2@TpMA | Hydrothermal Hydrothermal | 129 531 | 2.12 2.01 | Bisphenol A | 50 | Visible | 99 82 | 240 min 240 min | 5 5 | [192] |
| MIL-88(Fe)/PS/UV | Microwave | - | 1.78 | Bisphenol A | 10 | Visible | 100 | 30 min | 3 | [217] |
| MIL-101(Fe) Pd@MIL-100(Fe) | Hydrothermal | 2006 2102 | - | Bisphenol A | 20 | Visible | 47 68 | 240 min | 4 | [218] |
| Cu-hemin-MOFs/BN | Room temperature | - | - | Bisphenol A | 40 | Visible | 99 | 30 min | 4 | [219] |
| laccase@HKUST-1(Cu) | Immobilization | - | - | Bisphenol A | 200 | Visible | 100 | 4 h | 10 | [203] |
| AQS-NH-MIL-101(Fe) | Solvothermal | - | - | Bisphenol A | 60 | Visible | 98 | 180 min | 3 | [220] |
| Pesticides | ||||||||||
| UiO- 66@WG | Solvothermal | 380 | 2.3 | Malathion | 20 | Visible | 83 | 70 min | 4 | [221] |
| AgIO3/MIL-53(Fe) | Room temperature | 208 | 2.43 | Malathion Chlorpyrifos | 20 | Solar | 93 98 | 120 min | - | [222] |
| Fe3O4@MOF-2 | Room temperature | - | - | Diazinon | 30 | Visible | 99 | 60 min | 15 | [223] |
| MIL-53(Fe) | Solvothermal | 668 | 2.89 | Thiamethoxam | 5 | Visible | 96 | 60 min | - | [190] |
| HKUST-1(Cu) ZrO2@HKUST-1(Cu) | Room temperature Solvothermal | 1484 1152 | 3.87 2.27 | Cyhalothrin | 60 | Visible | 34 100 | 6 h | 4 | [208] |
| Herbicides | ||||||||||
| MIL-100(Fe)@ZnO | Solvothermal | 654 | 2.63 | Atrazine | 5 | Visible | 79 | 120 min | 5 | [191] |
| TiO2@NH2-MIL-101(Cr) | Solvothermal | - | - | Atrazine | 30 | Visible | 45 | 60 min | - | [84] |
3.3. MOFs and Composites for Adsorption and Photocatalytic Degradation of Pharmaceutical and Personal Care Products (PPCPs)
4. Patent Search
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BaWO4 | Barium tungstate |
| BET | Brunner Emmett Teller |
| COF | Covalent organic framework |
| CNTs | Carbon nanotubes |
| CPs | Coordination polymers |
| CQDs | Carbon quantum dots |
| EDCs | Endocrine disrupting compounds |
| GO | Graphene oxide |
| HKUST | Hongkong University of Science and Technology |
| HOMO | Highest occupied molecular orbital |
| HPLC | High performance liquid chromatography |
| LMCT | ligand to metal cluster charge transition |
| LUMO | Lowest occupied molecular orbital |
| MIL | Material institute Lavoisier |
| MIPs | Molecularly impregnated polymers |
| MNPs | Metal-oxide nanoparticles |
| MOFs | Metal-organic frameworks |
| PAHs | Polycyclic aromatic hydrocarbons |
| PDI | Pyromellitic diimide |
| PFAS | Perfluoroalkyl substances |
| PFCs | Perfluorinated compounds |
| PFCAs | Perfluoro carboxylic acids |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctane sulfonates |
| PPCPs | Pharmaceutical and Personal Care Products |
| RGO | Reduced graphene oxide |
| UiO | Universiti i Oslo |
| USEPA | United-states environmental protection agency |
| ZIFs | Zeolite imidazole framework |
References
- Chen, Y.; Zhai, B.; Liang, Y. Enhanced degradation performance of organic dyes removal by semiconductor/MOF/graphene oxide composites under visible light irradiation. Diam. Relat. Mater. 2019, 98. [Google Scholar] [CrossRef]
- Zango, Z.U.; Shehu Imam, S. Evaluation of Microcrystalline Cellulose from Groundnut Shell for the Removal of Crystal Violet and Methylene Blue. Nanosci. Nanotechnol. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Huang, H.; Zhong, C. Flexibility induced high-performance MOF-based adsorbent for nitroimidazole antibiotics capture. Chem. Eng. J. 2018, 333, 678–685. [Google Scholar] [CrossRef]
- Seo, P.W.; Bhadra, B.N.; Ahmed, I.; Khan, N.A.; Jhung, S.H. Adsorptive Removal of Pharmaceuticals and Personal Care Products from Water with Functionalized Metal-organic Frameworks: Remarkable Adsorbents with Hydrogen-bonding Abilities. Sci. Rep. 2016, 6, 34462. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, Q.; Ma, A. Biodegradation of Phenolic Contaminants: Current Status and Perspectives. IOP Conf. Ser. Earth Environ. Sci. 2018, 111. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Seczkowska, M.; Tarasiuk, B. Phenoxyacid pesticide adsorption on activated carbon – Equilibrium and kinetics. Chemosphere 2019, 214, 349–360. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Ramli, A.; Hana, N.; Abu, H.; Saad, B.; Nur, M.; Rozaini, H.; Isiyaka, H.A.; et al. An Overview and Evaluation of Highly Porous Adsorbent Materials for Polycyclic Aromatic Hydrocarbons and Phenols Removal from Wastewater. Water 2020, 12, 2921. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Hwang, Y.; Andersen, H.R.; Stasinakis, A.S.; Thomaidis, N.S.; Aloupi, M. Reductive degradation of perfluorinated compounds in water using Mg-aminoclay coated nanoscale zero valent iron. Chem. Eng. J. 2015, 262, 133–139. [Google Scholar] [CrossRef]
- Lath, S.; Navarro, D.A.; Losic, D.; Kumar, A.; Mclaughlin, M.J. Sorptive remediation of perfluorooctanoic acid (PFOA) using mixed mineral and graphene/carbon-based materials. Environ. Chem. 2018, 15, 472–480. [Google Scholar] [CrossRef]
- Jun, B.M.; Hwang, H.S.; Heo, J.; Han, J.; Jang, M.; Sohn, J.; Park, C.M.; Yoon, Y. Removal of selected endocrine-disrupting compounds using Al-based metal organic framework: Performance and mechanism of competitive adsorption. J. Ind. Eng. Chem. 2019, 79, 345–352. [Google Scholar] [CrossRef]
- Canle, M.; Fernández Pérez, M.I.; Santaballa, J.A. Photocatalyzed degradation/abatement of endocrine disruptors. Curr. Opin. Green Sustain. Chem. 2017, 6, 101–138. [Google Scholar] [CrossRef]
- Imam, S.S.; Zango, Z.U. Magnetic Nanoparticle (Fe3O4) Impregnated onto Coconut Shell Activated Carbon for the Removal of Ni (II) from Aqueous Solution. Int. J. Res. Chem. Environ. 2018, 8, 9–15. [Google Scholar]
- Hsieh, H.Y.; Huang, K.C.; Cheng, J.O.; Lo, W.T.; Meng, P.J.; Ko, F.C. Environmental effects on the bioaccumulation of PAHs in marine zooplankton in Gaoping coastal waters, Taiwan: Concentration, distribution, profile, and sources. Mar. Pollut. Bull. 2019, 144, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Yali, Z.P.; Jadid, A.P.; Samin, L.A. Modeling of retention time for polychlorinated biphenyl congeners in human adipose tissue using quantitative structure–retention relationship methodology. Int. J. Environ. Sci. Technol. 2017, 14, 2357–2366. [Google Scholar] [CrossRef]
- Ho, Y.C.; Norli, I.; Alkarkhi, A.F.M.; Morad, N. Characterization of biopolymeric flocculant (pectin) and organic synthetic flocculant (PAM): A comparative study on treatment and optimization in kaolin suspension. Bioresour. Technol. 2010, 101, 1166–1174. [Google Scholar] [CrossRef]
- Ho, Y.C. New Vegetal Biopolymeric Flocculant: A Degradation and Flocculation Study. Iran. J. Energy Environ. 2014, 5, 2–3. [Google Scholar] [CrossRef][Green Version]
- Rosińska, A.; Dabrowska, L. Selection of coagulants for the removal of chosen PAH from drinking water. Water 2018, 10, 886. [Google Scholar] [CrossRef]
- Hussaini Jagaba, A. Wastewater Treatment Using Alum, the Combinations of Alum-Ferric Chloride, Alum-Chitosan, Alum-Zeolite and Alum-Moringa Oleifera as Adsorbent and Coagulant. Int. J. Eng. Manag. 2018, 2, 67. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Rajan, P.S. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Guo, D.; Wang, H.; Fu, P.; Huang, Y.; Liu, Y.; Lv, W.; Wang, F. Diatomite precoat filtration for wastewater treatment: Filtration performance and pollution mechanisms. Chem. Eng. Res. Des. 2018, 137, 403–411. [Google Scholar] [CrossRef]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Pervov, A.; Tikhonov, K.; Makisha, N. Application of reverse osmosis techniques to treat and reuse biologically treated wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2018, 365. [Google Scholar] [CrossRef]
- Jafarinejad, S. A Comprehensive Study on the Application of Reverse Osmosis (RO) Technology for the Petroleum Industry Wastewater Treatment. J. Water Environ. Nanotechnol. 2017, 2, 243–264. [Google Scholar] [CrossRef]
- Zeneli, A.; Kastanaki, E.; Simantiraki, F.; Gidarakos, E. Monitoring the biodegradation of TPH and PAHs in refinery solid waste by biostimulation and bioaugmentation. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Siipola, V.; Pflugmacher, S.; Romar, H.; Wendling, L.; Koukkari, P. Low-Cost Biochar Adsorbents for Water Purification Including Microplastics Removal. Appl. Sci. 2020, 10, 788. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Kumar, S.; Lee, S.-S.; Kim, K.-H.; Kumar, V. Metal organic frameworks as potent treatment media for odorants and volatiles in air. Environ. Res. 2018, 168, 336–356. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Ma, H.; Wang, Z.-H.; Li, C.-Y.; Zhao, N.; Wang, S. Simultaneous adsorption of methyl orange and methylene blue from aqueous solution using amino functionalized Zr-based MOFs. Microporous Mesoporous Mater. 2019, 282, 179–187. [Google Scholar] [CrossRef]
- Fu, L.; Wang, S.; Lin, G.; Zhang, L.; Liu, Q.; Fang, J.; Wei, C.; Liu, G. Post-functionalization of UiO-66-NH 2 by 2,5-Dimercapto-1,3,4-thiadiazole for the high efficient removal of Hg(II) in water. J. Hazard. Mater. 2019, 368, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhang, Y.; Xie, L.; Liu, H.; Zhang, X.; Ruan, B.; Ding, H.; Wu, J.; Shi, D.; Jiang, T.; et al. Magnetically treated Zr-based UiO-type porous coordination polymers study on adsorption of azo dye. Microporous Mesoporous Mater. 2020, 110291. [Google Scholar] [CrossRef]
- Xin, S.; Yang, N.; Gao, F.; Zhao, J.; Li, L.; Teng, C. Three-dimensional polypyrrole-derived carbon nanotube framework for dye adsorption and electrochemical supercapacitor. Appl. Surf. Sci. 2017, 414, 218–223. [Google Scholar] [CrossRef]
- Jung, C.; Son, A.; Her, N.; Zoh, K.D.; Cho, J.; Yoon, Y. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: A review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.; Belver, C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. C J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate–Adsorbent Interactions. J. Carbon Res. 2018, 4, 62. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zango, Z.U.; Babando, A.A.; Galadima, A. Competitive adsorption of dyes onto granular activated carbon. J. Chem. Pharm. Res. 2015, 7, 710–717. [Google Scholar]
- Sophia, A.C.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Garba, Z.N.; Tanimu, A.; Zango, Z.U. Borassus aethiopum shell-based activated carbon as efficient adsorbent for carbofuran. Bull. Chem. Soc. Ethiop. 2019, 33, 425–436. [Google Scholar] [CrossRef]
- Kaur, S.; Rani, S.; Mahajan, R.K.; Asif, M.; Gupta, V.K. Synthesis and adsorption properties of mesoporous material for the removal of dye safranin: Kinetics, equilibrium, and thermodynamics. J. Ind. Eng. Chem. 2015, 22, 19–27. [Google Scholar] [CrossRef]
- Peres, E.C.; Slaviero, J.C.; Cunha, A.M.; Hosseini-Bandegharaei, A.; Dotto, G.L. Microwave synthesis of silica nanoparticles and its application for methylene blue adsorption. J. Environ. Chem. Eng. 2018, 6, 649–659. [Google Scholar] [CrossRef]
- Zango, Z.U.; Abu Bakar, N.H.H.; Tan, W.L.; Bakar, M.A. Enhanced removal efficiency of methyl red via the modification of halloysite nanotubes by copper oxide. J. Dispers. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Zango, Z.U.; Garba, Z.N.; Abu Bakar, N.H.H.; Tan, W.L.; Abu Bakar, M. Adsorption studies of Cu2+–Hal nanocomposites for the removal of 2,4,6-trichlorophenol. Appl. Clay Sci. 2016, 132–133, 68–78. [Google Scholar] [CrossRef]
- Park, C.M.; Wang, D.; Han, J.; Heo, J.; Su, C. Evaluation of the colloidal stability and adsorption performance of reduced graphene oxide–elemental silver/magnetite nanohybrids for selected toxic heavy metals in aqueous solutions. Appl. Surf. Sci. 2019, 471, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Krupadam, R.J. Nanoporous Polymeric Material for Remediation of PAHs Polluted Water. Polycycl. Aromat. Compd. 2012, 32, 313–333. [Google Scholar] [CrossRef]
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Wang, X.; Chen, L. Magnetic copper-based metal organic framework as an effective and recyclable adsorbent for removal of two fluoroquinolone antibiotics from aqueous solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef]
- Zango, Z.U.; Ramli, A.; Jumbri, K.; Soraya, N.; Ahmad, H.I.; Hana, N.; Abu, H.; Saad, B. Optimization studies and artificial neural network modeling for pyrene adsorption onto UiO-66(Zr) and NH2-UiO-66(Zr) metal organic frameworks. Polyhedron 2020, 192, 114857. [Google Scholar] [CrossRef]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef]
- Sharma, K.; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, P.; Singh, P. Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. J. Ind. Eng. Chem. 2019, 78, 1–20. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Zhang, X.; Huang, Y. Highly efficient Fenton and enzyme-mimetic activities of NH2-MIL-88B(Fe) metal organic framework for methylene blue degradation. Sci. Rep. 2018, 8, 5159. [Google Scholar] [CrossRef]
- Debnath, D.; Gupta, A.K.; Ghosal, P.S. Recent advances in the development of tailored functional materials for the treatment of pesticides in aqueous media: A review. J. Ind. Eng. Chem. 2019, 70, 51–69. [Google Scholar] [CrossRef]
- García, E.; Medina, R.; Lozano, M.; Hernández Pérez, I.; Valero, M.; Franco, A. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron-Benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, Y.; Liu, J.; Gu, C.; Wu, D. High CO2 adsorption capacities in UiO type MOFs comprising heterocyclic ligand. Microporous Mesoporous Mater. 2018, 256, 25–31. [Google Scholar] [CrossRef]
- Fan, Y.H.; Zhang, S.W.; Qin, S.-B.; Li, X.S.; Qi, S.H. An enhanced adsorption of organic dyes onto NH2 functionalization titanium-based metal-organic frameworks and the mechanism investigation. Microporous Mesoporous Mater. 2018, 263, 120–127. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and Construction of a New Class of Scaffolding-like Materials Comprising Infinite Polymeric Frameworks of 3D-Linked Molecular Rods. A Reappraisal of the Zn(CN)2 and Cd(CN)2 Structures and the Synthesis and Structure of the Diamond-Related Framework. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Neshastehgar, M.; Rahmani, P.; Shojaei, A.; Molavi, H. Enhanced adsorption removal performance of UiO-66 by rational hybridization with nanodiamond. Microporous Mesoporous Mater. 2020, 296. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.; Zhao, S.; Rong, H.; Tian, Y.; Zhu, G. Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water. Chem. Eng. J. 2020, 382, 122893. [Google Scholar] [CrossRef]
- Hu, M.L.; Masoomi, M.Y.; Morsali, A. Template strategies with MOFs. Coord. Chem. Rev. 2019, 387, 415–435. [Google Scholar] [CrossRef]
- Biserčić, M.S.; Marjanović, B.; Vasiljević, B.N.; Mentus, S.; Zasońska, B.A.; Ćirić-Marjanović, G. The quest for optimal water quantity in the synthesis of metal-organic framework MOF-5. Microporous Mesoporous Mater. 2019, 278, 23–29. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.H.; Lee, J.; Shang, J.; Khazi, M.I.; Kumar, N.; Lisak, G. Metal-organic framework for sorptive/catalytic removal and sensing applications against nitroaromatic compounds. J. Ind. Eng. Chem. 2020, 84, 87–95. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water Contaminant Elimination Based on Metal-Organic Frameworks and Perspective on Their Industrial Applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y.; Lester, E.; Wu, T. Optimized synthesis of nano-scale high quality HKUST-1 under mild conditions and its application in CO2 capture. Microporous Mesoporous Mater. 2018, 270, 249–257. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bansal, V.; Kim, K.H.; Kwon, E.E. Metal-organic frameworks (MOFs) as futuristic options for wastewater treatment. J. Ind. Eng. Chem. 2018, 62, 130–145. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.W.; Jeon, B.H. Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Jang, M.; Park, C.M.; Muñoz-Senmache, J.C.; Hernández-Maldonado, A.J.; Heyden, A.; Yu, M.; Yoon, Y. Removal of contaminants of emerging concern by metal-organic framework nanoadsorbents: A review. Chem. Eng. J. 2019, 369, 928–946. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, G. Green synthesis of Sn(II)-BDC MOF: Preferential and efficient adsorption of anionic dyes. Microporous Mesoporous Mater. 2020, 297. [Google Scholar] [CrossRef]
- Xu, F.; Yu, Y.; Yan, J.; Xia, Q.; Wang, H.; Li, J.; Li, Z. Ultrafast room temperature synthesis of GrO@HKUST-1 composites with high CO2 adsorption capacity and CO2/N2 adsorption selectivity. Chem. Eng. J. 2016, 303, 231–237. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kim, S.J.; Han, S. Novel metal–organic framework of UTSA-16(Zn) synthesized by a microwave method: Outstanding performance for CO2 capture with improved stability to acid gases. J. Ind. Eng. Chem. 2020, 87, 250–263. [Google Scholar] [CrossRef]
- Jang, S.; Song, S.; Lim, J.H.; Kim, H.S.; Phan, B.T.; Ha, K.T.; Park, S.; Park, K.H. Application of various metal-organic frameworks (MOFs) as catalysts for air and water pollution environmental remediation. Catalysts 2020, 10, 195. [Google Scholar] [CrossRef]
- Petit, C. Present and future of MOF research in the field of adsorption and molecular separation. Curr. Opin. Chem. Eng. 2018, 20, 132–142. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Wang, Z.; Wu, T.; Zhang, M. Synthesis of MIL-101(Cr) and its water adsorption performance. Microporous Mesoporous Mater. 2020, 297. [Google Scholar] [CrossRef]
- Yoon, S.; Calvo, J.J.; So, M.C. Removal of acid orange 7 from aqueous solution by metal-organic frameworks. Crystals 2019, 9, 17. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.Ø.; Llabr, F.X. Semiconductor Behavior of a Metal-Organic Framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef]
- Quan, X.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. Polyethyleneimine (PEI) incorporated Cu-BTC composites: Extended applications in ultra-high efficient removal of congo red. J. Solid State Chem. 2019, 270, 231–241. [Google Scholar] [CrossRef]
- Qin, J.-S.; Yuan, S.; Lollar, C.; Pang, J.; Alsalme, A.; Zhou, H.C. Stable metal–organic frameworks as a host platform for catalysis and biomimetics. Chem. Commun. 2018, 54, 4231–4249. [Google Scholar] [CrossRef]
- Zahn, G.; Schulze, H.A.; Lippke, J.; König, S.; Sazama, U.; Fröba, M.; Behrens, P. A water-born Zr-based porous coordination polymer: Modulated synthesis of Zr-fumarate MOF. Microporous Mesoporous Mater. 2015, 203, 186–194. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xu, Y.; Chen, Y.; Liu, Y.; Lu, Y.; Shao, L. Fabrication of MIL-101(Cr/Al) with flower-like morphology and its catalytic performance. Appl. Catal. A Gen. 2018, 559, 138–145. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Huang, D.; Cheng, M.; Zeng, G.; Lai, C.; Zhang, C.; Zhou, C.; Wang, W.; Jiang, D.; et al. Metal or metal-containing nanoparticle@MOF nanocomposites as a promising type of photocatalyst. Coord. Chem. Rev. 2019, 388, 63–78. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Shahat, M. Fabrication of ZIF-67@MIL-125-NH2 nanocomposite with enhanced visible light photoreduction activity. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Han, T.; Xiao, Y.; Tong, M.; Huang, H.; Liu, D.; Wang, L.; Zhong, C. Synthesis of CNT@MIL-68(Al) composites with improved adsorption capacity for phenol in aqueous solution. Chem. Eng. J. 2015, 275, 134–141. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, B.; Li, M.; Liu, X.; Li, S.; Su, B. Molecular imprinted materials PDA/Fe-MOFs/RGO for the selective and high removal of phenol. Desalin. Water Treat. 2019, 169, 279–286. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Liang, X.; Lei, C.; Gao, L.; Hao, R.; Lu, D.; Lei, Z. Fabrication of Ce doped UiO-66/graphene nanocomposites with enhanced visible light driven photoactivity for reduction of nitroaromatic compounds. Appl. Surf. Sci. 2017, 420, 276–285. [Google Scholar] [CrossRef]
- Alfonso-Herrera, L.A.; Huerta-Flores, A.M.; Torres-Martínez, L.M.; Rivera-Villanueva, J.M.; Ramírez-Herrera, D.J. Hybrid SrZrO3-MOF heterostructure: Surface assembly and photocatalytic performance for hydrogen evolution and degradation of indigo carmine dye. J. Mater. Sci. Mater. Electron. 2018, 29, 10395–10410. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.; Liang, X.; Dong, X.; Zhang, X. Supporting carbon quantum dots on NH2-MIL-125 for enhanced photocatalytic degradation of organic pollutants under a broad spectrum irradiation. Appl. Surf. Sci. 2019, 467–468, 320–327. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Cao, Z.; Li, N.; Chen, D.; Xu, Q.; Lu, J. Construction of g-C3N4/PDI@MOF heterojunctions for the highly efficient visible light-driven degradation of pharmaceutical and phenolic micropollutants. Appl. Catal. B Environ. 2019, 250, 150–162. [Google Scholar] [CrossRef]
- He, S.; Rong, Q.; Niu, H.; Cai, Y. Platform for molecular-material dual regulation: A direct Z-scheme MOF/COF heterojunction with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2019, 247, 49–56. [Google Scholar] [CrossRef]
- Ramezanalizadeh, H.; Zakeri, F.; Manteghi, F. Immobilization of BaWO4 nanostructures on a MOF-199-NH2: An efficient separable photocatalyst for the degradation of organic dyes. Optik 2018, 174, 776–786. [Google Scholar] [CrossRef]
- Ayati, A.; Shahrak, M.N.; Tanhaei, B.; Sillanpää, M. Emerging adsorptive removal of azo dye by metal–organic frameworks. Chemosphere 2016, 160, 30–44. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, M.; Wang, H.; Zeng, G.; Huang, D.; Cheng, M.; Liu, Y.; Xue, W.; Wang, Z.W. The application of different typological and structural MOFs-based materials for the dyes adsorption. Coord. Chem. Rev. 2019, 380, 471–483. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, J.; Wang, D.; Sun, W.; Gao, X.; Yu, H.; Liu, H.; Liu, Z. Construction and Photocatalytic Activities of a Series of Isostructural Co2+/Zn2+ Metal-Doped Metal-Organic Frameworks. Cryst. Growth Des. 2017, 17, 1096–1102. [Google Scholar] [CrossRef]
- Yang, J.M.; Yang, B.C.; Zhang, Y.; Yang, R.N.; Ji, S.S.; Wang, Q.; Quan, S.; Zhang, R.Z. Rapid adsorptive removal of cationic and anionic dyes from aqueous solution by a Ce(III)-doped Zr-based metal–organic framework. Microporous Mesoporous Mater. 2020, 292. [Google Scholar] [CrossRef]
- Tong, M.; Liu, D.; Yang, Q.; Devautour-Vinot, S.; Maurin, G.; Zhong, C. Influence of framework metal ions on the dye capture behavior of MIL-100 (Fe,Cr) MOF type solids. J. Mater. Chem. A 2013, 1, 8534–8537. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sert, E.; Atalay, F.S. Synthesis, characterization of a metal organic framework: MIL-53(Fe) and adsorption mechanisms of methyl red onto MIL-53(Fe). J. Taiwan Inst. Chem. Eng. 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Guo, Z.G. Facile modification of NH2-MIL-125(Ti) to enhance water stability for efficient adsorptive removal of crystal violet from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 541, 58–67. [Google Scholar] [CrossRef]
- Shen, T.; Luo, J.; Zhang, S.; Luo, X. Hierarchically mesostructured MIL-101 metal-organic frameworks with different mineralizing agents for adsorptive removal of methyl orange and methylene blue from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 1372–1383. [Google Scholar] [CrossRef]
- Karmakar, S.; Roy, D.; Janiak, C.; De, S. Insights into multi-component adsorption of reactive dyes on MIL-101-Cr metal organic framework: Experimental and modeling approach. Sep. Purif. Technol. 2019, 215, 259–275. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Gao, Z.; Gao, H.; Xie, Z.; Du, X.; Huang, H. Reversing the Dye Adsorption and Separation Performance of Metal-Organic Frameworks via Introduction of -SO3H Groups. Ind. Eng. Chem. Res. 2017, 56, 4496–4501. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Metal organic framework (MOF) porous octahedral nanocrystals of Cu-BTC: Synthesis, properties and enhanced absorption properties. Mater. Res. Bull. 2019, 109, 124–133. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef]
- Azhdari, R.; Mojtaba, S.; Alireza, S.; Bahrani, S. Decorated graphene with aluminum fumarate metal organic framework as a superior non-toxic agent for e ffi cient removal of Congo Red dye from wastewater. J. Environ. Chem. Eng. 2019, 7, 103437. [Google Scholar] [CrossRef]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef]
- Niu, P.; Lu, N.; Liu, J.; Jia, H.; Zhou, F.; Fan, B.; Li, R. Water-induced synthesis of hierarchical Zr-based MOFs with enhanced adsorption capacity and catalytic activity. Microporous Mesoporous Mater. 2019, 281, 92–100. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Sun, Q. Rapid and selective adsorption of cationic dyes by a unique metal-organic framework with decorated pore surface. Appl. Surf. Sci. 2018, 440, 1219–1226. [Google Scholar] [CrossRef]
- Tian, S.; Xu, S.; Liu, J.; He, C.; Xiong, Y.; Feng, P. Highly efficient removal of both cationic and anionic dyes from wastewater with a water-stable and eco-friendly Fe-MOF via host-guest encapsulation. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, M.; Guan, Q.; Li, W. Kinetic and thermodynamic studies on the adsorption of xylenol orange onto MIL-101(Cr). Chem. Eng. J. 2012, 183, 60–67. [Google Scholar] [CrossRef]
- He, J.; Li, J.; Du, W.; Han, Q.; Wang, Z.; Li, M. A mesoporous metal-organic framework: Potential advances in selective dye adsorption. J. Alloys Compd. 2018, 750, 360–367. [Google Scholar] [CrossRef]
- Qi, Z.P.; Kang, Y.S.; Guo, F.; Sun, W.Y. Controlled synthesis of NbO-type metal-organic framework nano/microcrystals with superior capacity and selectivity for dye adsorption from aqueous solution. Microporous Mesoporous Mater. 2019, 273, 60–66. [Google Scholar] [CrossRef]
- Yang, M.; Bai, Q. Flower-like hierarchical Ni-Zn MOF microspheres: Efficient adsorbents for dye removal. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 582. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, C.; Guan, H.; Li, L.; Fan, L.; Wang, Y.; Liu, L.; Meng, Q.; Zhang, R. Magnetic metal organic frameworks (MOFs) composite for removal of lead and malachite green in wastewater. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 539, 382–390. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Wei, F.; Chen, N.; Liang, Z.; Luo, Y. Removal of Congo red dye from aqueous solution with nickel-based metal-organic framework/graphene oxide composites prepared by ultrasonic wave-assisted ball milling. Ultrason. Sonochem. 2017, 39, 845–852. [Google Scholar] [CrossRef]
- Haque, E.; Lee, J.E.; Jang, I.T.; Hwang, Y.K.; Chang, J.S.; Jegal, J.; Jhung, S.H. Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates. J. Hazard. Mater. 2010, 181, 535–542. [Google Scholar] [CrossRef]
- Azad, F.N.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Pezeshkpour, V. Ultrasonically assisted hydrothermal synthesis of activated carbon-HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: Taguchi optimization. Ultrason. Sonochem. 2016, 31, 383–393. [Google Scholar] [CrossRef]
- Hamedi, A.; Zarandi, M.B.; Nateghi, M.R. Highly efficient removal of dye pollutants by MIL-101(Fe) metal-organic framework loaded magnetic particles mediated by Poly L-Dopa. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Liu, X.; Gong, W.; Luo, J.; Zou, C.; Yang, Y.; Yang, S. Selective adsorption of cationic dyes from aqueous solution by polyoxometalate-based metal-organic framework composite. Appl. Surf. Sci. 2016, 362, 517–524. [Google Scholar] [CrossRef]
- Aslam, S.; Zeng, J.; Subhan, F.; Li, M.; Lyu, F.; Li, Y.; Yan, Z. In situ one-step synthesis of Fe3O4@MIL-100(Fe) core-shells for adsorption of methylene blue from water. J. Colloid Interface Sci. 2017, 505, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Oveisi, M.; Asadi, E. Synthesis of NENU metal-organic framework-graphene oxide nanocomposites and their pollutant removal ability from water using ultrasound. J. Clean. Prod. 2019, 211, 198–212. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. A new MOFs/polymer hybrid membrane: MIL-68 (Al)/PVDF, fabrication and application in high-efficient removal of p-nitrophenol and methylene blue. Sep. Purif. Technol. 2019, 68, 217–226. [Google Scholar] [CrossRef]
- Tambat, S.N.; Sane, P.K.; Suresh, S.; Varadan, O.N.; Pandit, A.B.; Sontakke, S.M. Hydrothermal synthesis of NH2-UiO-66 and its application for adsorptive removal of dye. Adv. Powder Technol. 2018, 29, 2626–2632. [Google Scholar] [CrossRef]
- Chang, N.; Zhang, H.; Shi, M.S.; Li, J.; Yin, C.J.; Wang, H.T.; Wang, L. Regulation of the adsorption affinity of metal-organic framework MIL-101 via a TiO2 coating strategy for high capacity adsorption and efficient photocatalysis. Microporous Mesoporous Mater. 2018, 266, 47–55. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, Y.M.; Wang, T.; Wu, Y.L.; He, Y.L.; Yang, R.; Zheng, S.R. Regulation of the surface area and surface charge property of MOFs by multivariate strategy: Synthesis, characterization, selective dye adsorption and separation. Microporous Mesoporous Mater. 2018, 272, 101–108. [Google Scholar] [CrossRef]
- Jalali, S.; Rahimi, M.R.; Dashtian, K.; Ghaedi, M.; Mosleh, S. One step integration of plasmonic Ag2CrO4/Ag/AgCl into HKUST-1-MOF as novel visible-light driven photocatalyst for highly efficient degradation of mixture dyes pollutants: Its photocatalytic mechanism and modeling. Polyhedron 2019, 166, 217–225. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.J. Nanoparticle/metal-organic framework composites for catalytic applications: Current status and perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Song, H.; Chen, C.; Han, F.; Wen, C. Protonated graphitic carbon nitride coated metal-organic frameworks with enhanced visible-light photocatalytic activity for contaminants degradation. Appl. Surf. Sci. 2018, 441, 85–98. [Google Scholar] [CrossRef]
- Xu, W.T.; Ma, L.; Ke, F.; Peng, F.M.; Xu, G.S.; Shen, Y.H.; Zhu, J.F.; Qiu, L.G.; Yuan, Y.P. Metal-organic frameworks MIL-88A hexagonal microrods as a new photocatalyst for efficient decolorization of methylene blue dye. Dalt. Trans. 2014, 43, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Alinia Asli, M.; Vossoughi, M. Metal-organic framework (MIL-100(Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Prince, G.; Nikhil, R.; Dhabarde, P.C. Rapid synthesis of Titanium based Metal Organic framework (MIL-125) via crossmark microwave route and its performance evaluation in photocatalysis. Mater. Lett. 2017, 186, 151–154. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Liu, Z.; Wang, R.; Liu, H. Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation. Appl. Surf. Sci. 2016, 369, 130–136. [Google Scholar] [CrossRef]
- Wan, Y.; Wan, J.; Ma, Y.; Wang, Y.; Luo, T. Sustainable synthesis of modulated Fe-MOFs with enhanced catalyst performance for persulfate to degrade organic pollutants. Sci. Total Environ. 2020, 701. [Google Scholar] [CrossRef]
- Huang, J.; Song, H.; Chen, C.; Yang, Y.; Xu, N.; Ji, X.; Li, C.; You, J.A. Facile synthesis of N-doped TiO2 nanoparticles caged in MIL-100(Fe) for photocatalytic degradation of organic dyes under visible light irradiation. J. Environ. Chem. Eng. 2017, 5, 2579–2585. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Li, H.; Jin, X.; Wang, H.; Zhang, L.; Zhang, Y. NH2-MIL-53(Al) nanocrystals anchored on the surface of RGO hollow spheres and its visible light degradation of methylene blue. Mater. Lett. 2017, 197, 17–20. [Google Scholar] [CrossRef]
- Abdpour, S.; Kowsari, E.; Reza, M.; Moghaddam, A.; Schmolke, L.; Janiak, C. Mil-100(Fe) nanoparticles supported on urchin like Bi2S3 structure for improving photocatalytic degradation of rhodamine-B dye under visible light irradiation. J. Solid State Chem. 2018, 266, 54–62. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J. Nanoporous metal-organic framework (MOF-199): Synthesis, characterization and photocatalytic degradation of Basic Blue 41. Microchem. J. 2019, 144, 436–442. [Google Scholar] [CrossRef]
- Chang, N.; Zhang, H.; Shi, M.S.; Li, J.; Shao, W.; Wang, H.T. Metal-organic framework templated synthesis of TiO2@MIL-101 core-shell architectures for high-efficiency adsorption and photocatalysis. Mater. Lett. 2017, 200, 55–58. [Google Scholar] [CrossRef]
- Wu, W.; Li, B.; Gu, C.; Wang, J.; Singh, A.; Kumar, A. Luminescent sensing of Cu2+, CrO24 and photocatalytic degradation of methyl violet by Zn (II) metal-organic framework (MOF) having 5,5-(1H-2,3,5-triazole-1,4-diyl) diisophthalic acid ligand. J. Mol. Struct. 2017, 1148, 531–536. [Google Scholar] [CrossRef]
- Du, X.; He, H.; Du, L.; Li, W.; Wang, Y.; Jiang, Q.; Yang, L.; Zhang, J.; Guo, S. Porous Pr(III)-based organic framework for dye-adsorption and photo degradation with (4,5)-c net. Polyhedron 2019, 171, 221–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Feng, Q.; Chen, X.; Hu, Z. Visible light photocatalytic degradation of MB using UiO-66/g-C3N4 heterojunction nanocatalyst. Chemosphere 2018, 212, 523–532. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Z.; He, C.; Tong, X.; Li, Y.; Niu, X.; Zhang, H. UiO-66(Zr) coupled with Bi2MoO6 as photocatalyst for visible-light promoted dye degradation. J. Colloid Interface Sci. 2017, 497, 126–133. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Gan, C.; Liu, Y.; Chen, L.; Zhang, C.; Fang, Y. Synthesis of In2S3/UiO-66 hybrid with enhanced photocatalytic activity towards methyl orange and tetracycline hydrochloride degradation under visible-light irradiation. Mater. Sci. Semicond. Process. 2019, 91, 212–221. [Google Scholar] [CrossRef]
- Wang, H.; Cui, P.H.; Shi, J.X.; Tan, J.Y.; Zhang, J.Y.; Zhang, N.; Zhang, C. Controllable self-assembly of CdS@NH2-MIL-125(Ti) heterostructure with enhanced photodegradation efficiency for organic pollutants through synergistic effect. Mater. Sci. Semicond. Process. 2019, 97, 91–100. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Soheili, H.Z.; Hosseinifard, M.; Gholami, M.R. Preparation and characterization of novel Ag3VO4/Cu-MOF/rGO heterojunction for photocatalytic degradation of organic pollutants. Mater. Res. Bull. 2020, 121. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; He, X.; Xu, Z.; Wang, Y.; Jia, L. Synthesis of AgBr@MOFs nanocomposite and its photocatalytic activity for dye degradation. Polyhedron 2019, 165, 31–37. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.R.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Wang, S. Ag3PO4/AgBr/Ag-HKUST-1-MOF composites as novel blue LED light active photocatalyst for enhanced degradation of ternary mixture of dyes in a rotating packed bed reactor. Chem. Eng. Process. Process. Intensif. 2017, 114, 24–38. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.R.; Ghaedi, M.; Dashtian, K. Sonophotocatalytic degradation of trypan blue and vesuvine dyes in the presence of blue light active photocatalyst of Ag3PO4/Bi2S3-HKUST-1-MOF: Central composite optimization and synergistic effect study. Ultrason. Sonochem. 2016, 32, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Ramezanalizadeh, H.; Manteghi, F. Synthesis of a novel MOF/CuWO4 heterostructure for efficient photocatalytic degradation and removal of water pollutants. J. Clean. Prod. 2016, 172, 2655–2666. [Google Scholar] [CrossRef]
- Kaur, R.; Vellingiri, K.; Kim, K.H.; Paul, A.K.; Deep, A. Efficient photocatalytic degradation of rhodamine 6G with a quantum dot-metal organic framework nanocomposite. Chemosphere 2016, 154, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Araya, T.; Chen, C.C.; Jia, M.K.; Johnson, D.; Li, R.; Huang, Y. ping Selective degradation of organic dyes by a resin modified Fe-based metal-organic framework under visible light irradiation. Opt. Mater. 2017, 64, 512–523. [Google Scholar] [CrossRef]
- Araya, T.; Jia, M.; Yang, J.; Zhao, P.; Cai, K.; Ma, W.; Huang, Y. Resin modified MIL-53(Fe) MOF for improvement of photocatalytic performance. Appl. Catal. B Environ. 2017, 203, 768–777. [Google Scholar] [CrossRef]
- Du, J.; Yuan, Y.; Sun, J.; Peng, F.; Jiang, X.; Qiu, L. New photocatalysts based on MIL-53 metal—Organic frameworks for the decolorization of methylene blue dye. J. Hazard. Mater. 2011, 190, 945–951. [Google Scholar] [CrossRef]
- Liu, N.; Jing, C.; Li, Z.; Huang, W.; Gao, B.; You, F.; Zhang, X. Effect of synthesis conditions on the photocatalytic degradation of Rhodamine B of MIL-53(Fe). Mater. Lett. 2019, 237, 92–95. [Google Scholar] [CrossRef]
- Pu, M.; Guan, Z.; Ma, Y.; Wan, J.; Wang, Y.; Brusseau, M.L. General Synthesis of iron-based metal-organic framework MIL-53 as an efficient catalyst to activate persulfate for the degradation of Orange G in aqueous solution. Appl. Catal. A 2017, 549, 82–92. [Google Scholar] [CrossRef]
- Abdpour, S.; Kowsari, E.; Reza, M.; Moghaddam, A. Synthesis of MIL-100(Fe)@ MIL-53(Fe) as a novel hybrid photocatalyst and evaluation photocatalytic and photoelectrochemical performance under visible light irradiation. J. Solid State Chem. 2018, 262, 172–180. [Google Scholar] [CrossRef]
- Zhang, R.; Du, B.; Li, Q.; Cao, Z.; Feng, G.; Wang, X. α-Fe2O3 nanoclusters confined into UiO-66 for efficient visible-light photodegradation performance. Appl. Surf. Sci. 2019, 466, 956–963. [Google Scholar] [CrossRef]
- Chen, J.; Chao, F.; Ma, X.; Zhu, Q.; Jiang, J.; Ren, J.; Guo, Y.; Lou, Y. Synthesis of flower-like CuS/UiO-66 composites with enhanced visible-light photocatalytic performance. Inorg. Chem. Commun. 2019, 104, 223–228. [Google Scholar] [CrossRef]
- Huu, V.; Giang, L.; Thi, Q.; Bui, P.; Duy, T. Composite photocatalysts containing MIL-53(Fe) as a heterogeneous photo-Fenton catalyst for the decolorization of rhodamine B under visible light irradiation. J. Environ. Chem. Eng. 2018, 53, 2–9. [Google Scholar]
- Liu, X.; Dang, R.; Dong, W.; Huang, X.; Tang, J.; Gao, H.; Wang, G. A sandwich-like heterostructure of TiO2 nanosheets with MIL-100(Fe): A platform for efficient visible-light-driven photocatalysis. Appl. Catal. B Environ. 2017, 209, 506–513. [Google Scholar] [CrossRef]
- Michałowicz, J.; Włuka, A.; Cyrkler, M.; Maćczak, A.; Sicińska, P.; Mokra, K. Phenol and chlorinated phenols exhibit different apoptotic potential in human red blood cells (in vitro study). Environ. Toxicol. Pharmacol. 2018, 61, 95–101. [Google Scholar] [CrossRef]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michałowicz, J. Eryptosis-inducing activity of bisphenol A and its analogs in human red blood cells (in vitro study). J. Hazard. Mater. 2016, 307, 328–335. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef]
- De Roos, A.J.; Blair, A.; Rusiecki, J.A.; Hoppin, J.A.; Svec, M.; Dosemeci, M.; Sandler, D.P.; Alavanja, M.C. Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study. Environ. Health Perspect. 2005, 113, 49–54. [Google Scholar] [CrossRef]
- Drout, R.J.; Robison, L.; Chen, Z.; Islamoglu, T.; Farha, O.K. Zirconium Metal–Organic Frameworks for Organic Pollutant Adsorption. Trends Chem. 2019, 1, 304–317. [Google Scholar] [CrossRef]
- Xiao, Y.; Tong, F.; Kuang, Y.; Chen, B. Distribution and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in forest soils from urban to rural areas in the Pearl River Delta of southern China. Int. J. Environ. Res. Public Health 2014, 11, 2642–2656. [Google Scholar] [CrossRef]
- Yali, Z.P.; Fatemi, M.H. Prediction of the sorption coefficient for the adsorption of PAHs on MWCNT based on hybrid QSPR-molecular docking approach. Adsorption 2019, 25, 737–743. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, S.; Shen, H.; Ma, J. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc. Natl. Acad. Sci. USA 2009, 106, 21063–21067. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1–89. [Google Scholar] [CrossRef]
- Mezzanotte, V.; Anzano, M.; Collina, E.; Marazzi, A.; Lasagni, M. Distribution and Removal of Polycyclic Aromatic Hydrocarbons in Two Italian Municipal Wastewater Treatment Plants in 2011–2013. Polycycl. Aromat. Compd. 2015. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Li, C.; Ji, R.; Tian, X. Metal nanoparticles by doping carbon nanotubes improved the sorption of perfluorooctanoic acid. J. Hazard. Mater. 2018, 351, 206–214. [Google Scholar] [CrossRef]
- Moody, C.A.; Kwan, W.C.; Martin, J.W.; Muir, D.C.G.; Mabury, S.A. Determination of perfluorinated surfactants in surface water samples by two independent analytical techniques: Liquid chromatography/tandem mass spectrometry and 19F NMR. Anal. Chem. 2002, 73, 2200–2206. [Google Scholar] [CrossRef]
- Enevoldsen, R.; Juhler, R.K. Perfluorinated compounds (PFCs) in groundwater and aqueous soil extracts: Using inline SPE-LC-MS/MS for screening and sorption characterisation of perfluorooctanesulphonate and related compounds. Anal. Bioanal. Chem. 2010, 398, 1161–1172. [Google Scholar] [CrossRef]
- Loewen, M.; Halldorson, T.; Wang, F.; Tomy, G. Fluorotelomer carboxylic acids and PFOS in rainwater from an urban center in Canada. Environ. Sci. Technol. 2005, 39, 2944–2951. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Taniyasu, S.; Yeung, L.W.Y.; Lam, P.K.S.; Wang, J.; Li, X.; Yamashita, N.; Dai, J. Distribution and fate of perfluoroalkyl substances in municipal wastewater treatment plants in economically developed areas of China. Environ. Pollut. 2013, 176, 10–17. [Google Scholar] [CrossRef]
- Mak, Y.L.; Taniyasu, S.; Yeung, L.W.; Lu, G.; Jin, L.; Yang, Y.; Lam, P.K.; Kannan, K.; Yamashita, N. Perfluorinated compounds in tap water from China and several other countries. Environ. Sci. Technol. 2009, 43, 4824–4829. [Google Scholar] [CrossRef]
- Zabaleta, I.; Bizkarguenaga, E.; Iparragirre, A.; Navarro, P.; Prieto, A.; Fernandez, L.A.; Zuloaga, O. Focused ultrasound solid-liquid extraction for the determination of perfluorinated compounds in fish, vegetables and amended soil. J. Chromatogr. A 2014, 1331, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Brox, J. An automated high-throughput SPE micro-elution method for perfluoroalkyl substances in human serum. Anal. Bioanal. Chem. 2015, 407, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.E.; Andaya, C.; Burant, A.; Condee, C.W.; Urtiaga, A.; Strathmann, T.J.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid and perfluorooctane sulfonate: Insights into mechanisms and application to groundwater treatment. Chem. Eng. J. 2017, 317, 424–432. [Google Scholar] [CrossRef]
- Pankajakshan, A.; Sinha, M.; Ojha, A.A.; Mandal, S. Water-Stable Nanoscale Zirconium-Based Metal-Organic Frameworks for the Effective Removal of Glyphosate from Aqueous Media. ACS Omega 2018, 3, 7832–7839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wu, Y.-N.; Qiao, J.; Zhang, J.; McDonald, A.; Li, G.; Li, F. The removal of bisphenol A from aqueous solutions by MIL-53(Al) and mesostructured MIL-53(Al). J. Colloid Interface Sci. 2013, 405, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, I.; Drout, R.J.; Islamoglu, T.; Kato, S.; Lyu, J.; Farha, O.K. Exploiting π-π Interactions to Design an Efficient Sorbent for Atrazine Removal from Water. ACS Appl. Mater. Interfaces 2019, 11, 6097–6103. [Google Scholar] [CrossRef] [PubMed]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Abu Bakar, N.H.H.; Abdullah, N.A.F.; Negim, E.S.M.; Saad, B. Experimental and molecular docking model studies for the adsorption of polycyclic aromatic hydrocarbons onto UiO-66(Zr) and NH2-UiO-66(Zr) metal-organic frameworks. Chem. Eng. Sci. 2020, 220, 115608. [Google Scholar] [CrossRef]
- Zango, Z.U.; Abu Bakar, N.H.H.; Sambudi, N.S.; Jumbri, K.; Abdullah, N.A.F.; Abdul Kadir, E.; Saad, B. Adsorption of chrysene in aqueous solution onto MIL-88(Fe) and NH2-MIL-88(Fe) metal-organic frameworks: Kinetics, isotherms, thermodynamics and docking simulation studies. J. Environ. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Abu Bakar, N.H.H.; Abdullah, N.A.F.; Basheer, C.; Saad, B. Removal of anthracene in water by MIL-88(Fe), NH2-MIL-88(Fe), and mixed-MIL-88(Fe) metal–organic frameworks. RCS Adv. 2019, 9, 41490–41501. [Google Scholar] [CrossRef]
- Mei, W.; Song, H.; Tian, Z.; Zuo, S.; Li, D.; Xu, H.; Xia, D. Efficient photo-Fenton like activity in modified MIL-53(Fe) for removal of pesticides: Regulation of photogenerated electron migration. Mater. Res. Bull. 2019, 119. [Google Scholar] [CrossRef]
- Ahmad, M.; Chen, S.; Ye, F.; Quan, X.; Afzal, S.; Yu, H.; Zhao, X. Efficient photo-Fenton activity in mesoporous MIL-100(Fe) decorated with ZnO nanosphere for pollutants degradation. Appl. Catal. B Environ. 2018, 245, 428–438. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, J.M.; Li, C.Y.; Zhao, N.; Wang, Z.H.; Wang, S. Two novel MOFs@COFs hybrid-based photocatalytic platforms coupling with sulfate radical-involved advanced oxidation processes for enhanced degradation of bisphenol A. Chemosphere 2020, 243. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.A.; Hsieh, Y. Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water. J. Taiwan Inst. Chem. Eng. 2015, 50, 223–228. [Google Scholar]
- Han, T.; Li, C.; Guo, X.; Huang, H.; Liu, D.; Zhong, C. In-situ synthesis of SiO2@MOF composites for high-efficiency removal of aniline from aqueous solution. Appl. Surf. Sci. 2016, 390, 506–512. [Google Scholar] [CrossRef]
- Abazari, R.; Salehi, G.; Mahjoub, A.R. Ultrasound-assisted preparation of a nanostructured zinc(II) amine pillar metal-organic framework as a potential sorbent for 2,4-dichlorophenol adsorption from aqueous solution. Ultrason. Sonochem. 2018, 46, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Abazari, R.; Mahjoub, A.R. Ultrasound-assisted synthesis of Zinc(II)-based metal organic framework nanoparticles in the presence of modulator for adsorption enhancement of 2,4-dichlorophenol and amoxicillin. Ultrason. Sonochem. 2018, 42, 577–584. [Google Scholar] [CrossRef]
- Xu, Z.; Wen, Y.; Tian, L.; Li, G. Efficient and selective adsorption of nitroaromatic explosives by Zr-MOF. Inorg. Chem. Commun. 2017, 77, 11–13. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhong, H.; Wang, H.; Zeng, G.; Chen, X.; Wang, H.; Zhang, L.; Shao, J. Enhanced adsorptive removal of p-nitrophenol from water by aluminum metal-organic framework/reduced graphene oxide composite. Sci. Rep. 2016, 6, 25638. [Google Scholar] [CrossRef]
- Guo, H.; Niu, B.; Wu, X.; Zhang, Y.; Ying, S. Effective removal of 2, 4, 6-Trinitrophenol over hexagonal metal—Organic framework NH2--MIL--88B(Fe). Appl. Organomet. Chem. 2018, 33, e4580. [Google Scholar] [CrossRef]
- Giraldo, L.; Bastidas-Barranco, M.; Húmpola, P.; Moreno-Piraján, J.C. Design, synthesis and characterization of MOF-199 and ZIF-8: Applications in the adsorption of phenols derivatives in aqueous solution. Eur. J. Chem. 2017, 8, 293–304. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, H.; Wu, S.; Yang, C.; Cheng, J. Enhanced removal of bisphenol A from aqueous solution by aluminum-based MOF/sodium alginate-chitosan composite beads. Chemosphere 2019, 237. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.A.; Jabbari, V.; Islam, M.T.; Turley, R.S.; Dominguez, N.; Kim, H.; Castro, E.; Hernandez-Viezcas, J.A.; Curry, M.L.; Lopez, J.; et al. Sustainable synthesis and remarkable adsorption capacity of MOF/graphene oxide and MOF/CNT based hybrid nanocomposites for the removal of Bisphenol A from water. Sci. Total Environ. 2019, 673, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, L.; Han, J.; Wu, J.; Li, C.; Ni, L.; Wang, Y. Improving laccase activity and stability by HKUST-1 with cofactor via one-pot encapsulation and its application for degradation of bisphenol A. J. Hazard. Mater. 2020, 383. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, L.; Xu, D.; Huang, X.; Xu, X.; Zheng, S.; Zhang, Y.; Lin, H. Metal–organic framework preparation using magnetic graphene oxide–β-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr. Polym. 2017, 175, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Mirsoleimani-azizi, S.M.; Setoodeh, P.; Samimi, F.; Shadmehr, J. Diazinon removal from aqueous media by mesoporous MIL-101(Cr) in a continuous fi xed-bed system. J. Environ. Chem. Eng. 2018, 6, 4653–4664. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.; Lu, Y.; Wang, W.; Wang, Q.; Dong, X.; Zhao, J. Rapid and ef ficient removal of acetochlor from environmental water using Cr-MIL-101 sorbent modi fi ed with 3, 5-Bis (trifluoromethyl) phenyl isocyanate. Sci. Total Environ. 2019, 710, 135512. [Google Scholar]
- Moeini, Z.; Azhdarpoor, A.; Yousefinejad, S.; Hashemi, H. Removal of atrazine from water using titanium dioxide encapsulated in salicylaldehyde–NH 2 –MIL-101(Cr): Adsorption or oxidation mechanism. J. Clean. Prod. 2019, 224, 238–245. [Google Scholar] [CrossRef]
- Fan, C.; Dong, H.; Liang, Y.; Yang, J.; Tang, G.; Zhang, W.; Cao, Y. Sustainable synthesis of HKUST-1 and its composite by biocompatible ionic liquid for enhancing visible-light photocatalytic performance. J. Clean. Prod. 2019, 208, 353–362. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Yang, J.; Li, Y.; Zhao, W.; Shi, J.; Gu, J. Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-Based MOFs of UiO-67. ACS Appl. Mater. Interfaces 2015, 7, 223–231. [Google Scholar] [CrossRef]
- Akpinar, I.; Yazaydin, A.O. Adsorption of Atrazine from Water in Metal-Organic Framework Materials. J. Chem. Eng. Data 2018, 63, 2368–2375. [Google Scholar] [CrossRef]
- Okoro, H.K.; Tella, A.C.; Ajibola, O.A.; Zvinowanda, C.; Ngila, J.C. Adsorptive removal of naphthalene and anthracene from aqueous solution with zinc and copper-terephthalate metal-organic frameworks. Bull. Chem. Soc. Ethiop. 2019, 33, 229–241. [Google Scholar] [CrossRef]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Abu Bakar, N.H.H.; Saad, B. Removal of Pyrene from Aqueous Solution Using Fe-based Metal-organic Frameworks. IOP Conf. Ser. Earth Environ. Sci. 2020, 549, 012061. [Google Scholar] [CrossRef]
- Chen, M.J.; Yang, A.C.; Wang, N.H.; Chiu, H.C.; Li, Y.L.; Kang, D.Y.; Lo, S.L. Influence of crystal topology and interior surface functionality of metal-organic frameworks on PFOA sorption performance. Microporous Mesoporous Mater. 2016, 236, 202–210. [Google Scholar] [CrossRef]
- Zhang, S.; Du, M.; Kuang, J.; Xing, Z.; Li, Z.; Pan, K.; Zhu, Q.; Zhou, W. Surface-defect-rich mesoporous NH2-MIL-125(Ti)@Bi2MoO6 core-shell heterojunction with improved charge separation and enhanced visible-light-driven photocatalytic performance. J. Colloid Interface Sci. 2019, 554, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, S.; Zhang, H.; Fan, X.; Gao, C.; Yu, H.; Quan, X. Carbon nanotubes-incorporated MIL-88B-Fe as highly ef fi cient Fenton-like catalyst for degradation of organic pollutants. Front. Environ. Sci. Eng. 2019, 13, 18. [Google Scholar] [CrossRef]
- Thakare, S.R.; Ramteke, S.M. Postmodification of MOF-5 using secondary complex formation using 8-hydroxyquinoline (HOQ) for the development of visible light active photocatalysts. J. Phys. Chem. Solids 2018, 116, 264–272. [Google Scholar] [CrossRef]
- Lin, J.; Hu, Y.; Wang, L.; Liang, D.; Ruan, X.; Shao, S. M88/PS/Vis system for degradation of bisphenol A: Environmental factors, degradation pathways, and toxicity evaluation. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Liang, R.; Luo, S.; Jing, F.; Shen, L.; Qin, N.; Wu, L. A simple strategy for fabrication of Pd@MIL-100(Fe) nanocomposite as a visible-light-driven photocatalyst for the treatment of pharmaceuticals and personal care products (PPCPs). Appl. Catal. B Environ. 2015, 176–177, 240–248. [Google Scholar] [CrossRef]
- Ke, Q.; Shi, Y.; Liu, Y.; Chen, F.; Wang, H.; Wu, X.L.; Lin, H.; Chen, J. Enhanced catalytic degradation of bisphenol A by hemin-MOFs supported on boron nitride via the photo-assisted heterogeneous activation of persulfate. Sep. Purif. Technol. 2019, 229. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Liu, Z.; Wang, R.; Liu, H. Quinone-modified NH2-MIL-101(Fe) composite as a redox mediator for improved degradation of bisphenol A. J. Hazard. Mater. 2017, 324, 665–672. [Google Scholar] [CrossRef]
- Fakhri, H.; Bagheri, H. Highly efficient Zr-MOF@WO3/graphene oxide photocatalyst: Synthesis, characterization and photodegradation of tetracycline and malathion. Mater. Sci. Semicond. Process. 2020, 107. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Vaziri, R.; Abureesh, M.A. Highly robust AgIO3/MIL-53(Fe) nanohybrid composites for degradation of organophosphorus pesticides in single and binary systems: Application of artificial neural networks modelling. J. Taiwan Inst. Chem. Eng. 2018, 83, 133–142. [Google Scholar] [CrossRef]
- Sajjadi, S.; Khataee, A.; Bagheri, N.; Kobya, M.; Şenocak, A.; Demirbas, E.; Karaoğlu, A.G. Degradation of diazinon pesticide using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite under ultrasound irradiation. J. Ind. Eng. Chem. 2019, 77, 280–290. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, Y.; Yun, Y.S. Highly Effective Removal of Nonsteroidal Anti-inflammatory Pharmaceuticals from Water by Zr(IV)-Based Metal-Organic Framework: Adsorption Performance and Mechanisms. ACS Appl. Mater. Interfaces 2018, 10, 28076–28085. [Google Scholar] [CrossRef]
- Jun, B.M.; Heo, J.; Park, C.M.; Yoon, Y. Comprehensive evaluation of the removal mechanism of carbamazepine and ibuprofen by metal organic framework. Chemosphere 2019, 235, 527–537. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, R.; Zeng, S.; Liu, K.; Huang, S.; Liu, Y.; Xu, P.; Lin, C.; Cheng, Y.; Liu, M. Removal of p-arsanilic acid by an amino-functionalized indium-based metal–organic framework: Adsorption behavior and synergetic mechanism. Chem. Eng. J. 2018, 339, 359–368. [Google Scholar] [CrossRef]
- Li, S.; Cui, J.; Wu, X.; Zhang, X.; Hu, Q.; Hou, X. Rapid in situ microwave synthesis of Fe3O4 @MIL-100(Fe) for aqueous diclofenac sodium removal through integrated adsorption and photodegradation. J. Hazard. Mater. 2019, 373, 408–416. [Google Scholar] [CrossRef]
- Hasan, Z.; Choi, E.J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, J.; Liu, D.; Kang, R.; Yu, G.; Deng, S. Synthesis of mixed-linker Zr-MOFs for emerging contaminant adsorption and photodegradation under visible light. Chem. Eng. J. 2019, 378, 122118. [Google Scholar] [CrossRef]
- Liu, W.; Shen, X.; Han, Y.; Liu, Z.; Dai, W.; Dutta, A.; Kumar, A.; Liu, J. Selective adsorption and removal of drug contaminants by using an extremely stable Cu(II)-based 3D metal-organic framework. Chemosphere 2019, 215, 524–531. [Google Scholar] [CrossRef]
- Tella, A.C.; Owalude, S.O.; Olatunji, S.J.; Adimula, V.O.; Elaigwu, S.E.; Alimi, L.O.; Ajibade, P.A.; Oluwafemi, O.S. Synthesis of zinc-carboxylate metal-organic frameworks for the removal of emerging drug contaminant (amodiaquine) from aqueous solution. J. Environ. Sci. 2018, 64, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Abazari, R.; Mahjoub, A.R.; Shariati, J. Synthesis of a nanostructured pillar MOF with high adsorption capacity towards antibiotics pollutants from aqueous solution. J. Hazard. Mater. 2019, 366, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, Y.; Zhao, H.; Gao, Z.; Zhang, Y. Functionalized metal-organic frameworks for effective removal of rocephin in aqueous solutions. J. Colloid Interface Sci. 2018, 514, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Mirsoleimani-Azizi, S.M.; Setoodeh, P.; Zeinali, S.; Rahimpour, M.R. Tetracycline antibiotic removal from aqueous solutions by MOF-5: Adsorption isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2018, 6, 6118–6130. [Google Scholar] [CrossRef]
- Gao, Y.; Kang, R.; Xia, J.; Yu, G.; Deng, S. Understanding the adsorption of sulfonamide antibiotics on MIL-53s: Metal dependence of breathing effect and adsorptive performance in aqueous solution. J. Colloid Interface Sci. 2019, 535, 159–168. [Google Scholar] [CrossRef]
- Naeimi, S.; Faghihian, H. Application of novel metal organic framework, MIL-53(Fe) and its magnetic hybrid: For removal of pharmaceutical pollutant, doxycycline from aqueous solutions. Environ. Toxicol. Pharmacol. 2017, 53, 121–132. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, K.; Kang, R.; Xia, J.; Yu, G.; Deng, S. A comparative study of rigid and fl exible MOFs for the adsorption of pharmaceuticals: Kinetics, isotherms and mechanisms. J. Hazard. Mater. 2018, 359, 248–257. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, Z.; Li, X.; Zeng, G.; Xiao, R.; Yang, Z.; Xu, H.; Chen, H.; Cao, J.; Zhou, C.; et al. Ni-doped MIL-53(Fe) nanoparticles for optimized doxycycline removal by using response surface methodology from aqueous solution. Chemosphere 2019, 232, 186–194. [Google Scholar] [CrossRef]
- Seo, P.W.; Khan, N.A.; Jhung, S.H. Removal of nitroimidazole antibiotics from water by adsorption over metal—Organic frameworks modified with urea or melamine. Chem. Eng. J. 2017, 315, 92–100. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, Z.; Li, X.; Zeng, G.; Xiao, R.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Hu, L.; et al. Multi-walled carbon nanotube/amino-functionalized MIL-53(Fe) composites: Remarkable adsorptive removal of antibiotics from aqueous solutions. Chemosphere 2018, 210, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C.; et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH 2: Batch experiment and DFT calculation. Chem. Eng. 2018, 360, 645–653. [Google Scholar] [CrossRef]
- Dong, W.; Wang, D.; Wang, H.; Li, M.; Chen, F.; Jia, F.; Yang, Q.; Li, X.; Yuan, X.; Gong, J.; et al. Facile synthesis of In2S3/UiO-66 composite with enhanced adsorption performance and photocatalytic activity for the removal of tetracycline under visible light irradiation. J. Colloid Interface Sci. 2019, 535, 444–457. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Periasamy, V.; Sun, H.; Tade, M.O.; Wang, S. Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. J. Colloid Interface Sci. 2017, 500, 88–95. [Google Scholar] [CrossRef]
- Yang, W.; Han, Y.; Li, C.; Zhu, L.; Shi, L.; Tang, W.; Wang, J.; Yue, T.; Li, Z. Shapeable three-dimensional CMC aerogels decorated with Ni/Co-MOF for rapid and highly efficient tetracycline hydrochloride removal. Chem. Eng. J. 2019, 375. [Google Scholar] [CrossRef]
- Yu, L.L.; Cao, W.; Wu, S.C.; Yang, C.; Cheng, J.H. Removal of tetracycline from aqueous solution by MOF/graphite oxide pellets: Preparation, characteristic, adsorption performance and mechanism. Ecotoxicol. Environ. Saf. 2018, 164, 289–296. [Google Scholar] [CrossRef]
- Sarker, M.; Song, J.Y.; Jhung, S.H. Adsorptive removal of anti-inflammatory drugs from water using graphene oxide/metal-organic framework composites. Chem. Eng. J. 2018, 335, 74–81. [Google Scholar] [CrossRef]
- Gautam, R.K.; Banerjee, S.; Sanroman, M.A.; Chattopadhyaya, M.C. Synthesis of copper coordinated dithiooxamide metal organic framework and its performance assessment in the adsorptive removal of tartrazine from water. J. Environ. Chem. Eng. 2017, 5, 328–340. [Google Scholar] [CrossRef]
- Huang, W.; Jing, C.; Zhang, X.; Tang, M.; Tang, L.; Wu, M.; Liu, N. Integration of plasmonic effect into spindle-shaped MIL-88A(Fe): Steering charge flow for enhanced visible-light photocatalytic degradation of ibuprofen. Chem. Eng. J. 2018, 349, 603–612. [Google Scholar] [CrossRef]
- Jiang, D.; Zhu, Y.; Chen, M.; Huang, B.; Zeng, G.; Huang, D.; Song, B.; Qin, L.; Wang, H.; Wei, W. Modified crystal structure and improved photocatalytic activity of MIL-53 via inorganic acid modulator. Appl. Catal. B Environ. 2019, 255. [Google Scholar] [CrossRef]
- Rasheed, H.U.; Lv, X.; Zhang, S.; Wei, W.; Ullah, N.; Xie, J. Ternary MIL-100(Fe)@Fe3O4/CA magnetic nanophotocatalysts (MNPCs): Magnetically separable and Fenton-like degradation of tetracycline hydrochloride. Adv. Powder Technol. 2018, 29, 3305–3314. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Cai, M.; Huang, J.; Chen, P.; Liu, G. Improvement of Sulfamethazine photodegradation by Fe (III) assisted MIL-53 (Fe)/percarbonate system. Appl. Surf. Sci. 2018, 457, 726–734. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.; Tang, M.; Yin, C.; Gao, B.; Li, Z.; Tang, L.; Lei, J.; Cui, L.; Zhang, X. In-situ fabrication of needle-shaped MIL-53(Fe) with 1T-MoS2 and study on its enhanced photocatalytic mechanism of ibuprofen. Chem. Eng. J. 2019, 359, 254–264. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Jonidi Jafari, A.; Gholami, M.; Sobhi, H.R.; Nourbakhsh, M.; Akbari-Adergani, B. Photocatalytic degradation of cefixime with MIL-125(Ti)-mixed linker decorated by g-C3N4 under solar driven light irradiation. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 582. [Google Scholar] [CrossRef]
- Sun, J.; Feng, S.; Feng, S. composite with enhanced photocatalytic performance for ketoprofen. Inorg. Chem. Commun. 2020, 111, 107669. [Google Scholar] [CrossRef]
- Huo, Q.; Qi, X.; Li, J.; Liu, G.; Ning, Y.; Zhang, X.; Zhang, B.; Fu, Y.; Liu, S. Preparation of a direct Z-scheme A-Fe2O3/MIL-101(Cr) hybrid for degradation of carbamazepine under visible light irradiation. Appl. Catal. B Environ. 2019, 255. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; Liu, K.; Deng, S.; Wang, B.; Huang, J.; Wang, Y. Integrated adsorption and visible-light photodegradation of aqueous clofibric acid and carbamazepine by a Fe-based metal-organic framework. Chem. Eng. J. 2017, 330, 157–165. [Google Scholar] [CrossRef]







| Type of MOF | Synthesis Method | Surface Area (m2 g−1) | Pollutants | Concentration (mg L−1) | % Removal | Qe (mg g−1) | Equilibrium Time | Reused | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Fe-BTC | Solvothermal | 877 | Orange II | 50 | 92 | 207 | 80 min | 4 | [52] |
| MIL-53(Fe) | Solvothermal | 53 | Methyl orange | 100 | 77 | 77 | 60 min | 3 | [100] |
| MOF-235(Fe) | Solvothermal | - | Methyl orange Methylene blue | 30 | - | 477 187 | 250 min | - | [101] |
| MIL-125(Ti) | Solvothermal | 1108 | Crystal violet | 40 | - | 130 | 180 min | - | [102] |
| MIL-101(Cr) | Hydrothermal | 3514 | Methylene blue Methyl red | 30 300 | - | 11 247 | 30 min 30 min | - - | [103] |
| MIL-101(Cr) | Microwave | 2410 | Reactive yellow Reactive black Reactive red Reactive blue | 300 | 100 | 386 377 390 397 | 24 h | - | [104] |
| MIL-100(Fe) MIL-100(Cr) | Hydrothermal Hydrothermal | 17701760 | Methyl orange Methylene blue Methyl orange Methylene blue | 30 30 | 85 100 8 100 | 1045 736 212 645 | 3 days 22 days | - - | [99] |
| MIL-101(Cr) MIL-101(Cr)-SO3H | Hydrothermal Hydrothermal | 3016 1546 | Fluorescein sodium Safranine Fluorescein sodium Safranine | 100 100 | - - - - | 280 701 114 425 | 700 min 700 min 700 min 700 min | 4 4 | [105] [105] |
| Cu-BTC | Hydrothermal | 521 | Methylene blue | 200 | - | 96 | 40 min | 4 | [106] |
| Cu-BTC MOF Cu-BTC@GO Cu-BTC@CNT Fe3O4/Cu-BTC@GO | Solvothermal | 856508123176 | Methylene blue | 100 | - - - - | 67 152 172 136 | 12 h | - | [107] |
| Ce(III)-doped UiO-67 | Solvothermal | 1911 | Methylene blue Congo red Methyl orange | 100 | 95 96 | 399 800 401 | 80 min | 4 4 | [98] |
| AlF-MOF AlF-GO AlF-rGO | Hydrothermal | 973 918 952 | Congo red | 50 | 99 | 93 102 179 | 30 min | - | [108] |
| NH2 -MIL-125(Ti) | Solvothermal | 1350 | Basic blue Methylene blue Basic red | 20 | 93 97 99 | 1257 862 1296 | 30 min | 3 | [109] |
| NH2-UiO-66(Zr) | Solvothermal | 954 | Methylene blue | 200 | 88 | 321 | 15 min | 6 | [30] |
| UiO-66(Zr) | Solvothermal | 1244 | Rhodamine Blue | 20 | 91 | 90 | 200 min | 5 | [110] |
| Zn-MOF | Room temp | 1046 | Methylene blue | 10 | 98 | 326 | 60 min | 4 | [111] |
| CPM-97(Fe) | Solvothermal | 1397 | Congo red | 40 | 100 | 831 | 30 min | 3 | [112] |
| MIL-53(Fe) | Solvothermal | 23 | Methyl red | 100 | 78 | 76 | 60 min | 3 | [100] |
| MIL-101(Cr) | Hydrothermal | 2664 | Xylenol orange | 400 | 90 | 159 | 30 min | 3 | [113] |
| BTB-Mn | Solvothermal | 3143 | Methylene blue | 15 | 89 | 308 | 120 min | 6 | [114] |
| NOTT-102(Cu) | Solvothermal | 3006 | Methylene blue | 20 | 97 | 850 | 24 h | 3 | [115] |
| Ni-Zn-MOF | Solvothermal | 57 | Congo red | 30 | - | 461 | 300 min | 5 | [116] |
| Cu-MOF/Fe3O4 | Solvothermal | 34 | Malachite green | 50 | 90 | 114 | 60 min | 5 | [117] |
| Ni-MOF/GO | Ball milling | 70 | Congo red | 200 | - | 2489 | 300 min | - | [118] |
| PEI-modified Cu-BTC | Hydrothermal | 785 | Congo red Acid blue | 1200 100 | 100 100 | 2578 132 | 200 min | 6 6 | [78] |
| PED-MIL-101(Cr) PED-MIL-101(Cr) | Hydrothermal | 3491 3296 | Methyl orange Methyl orange | 50 50 | NA NA | 160 194 | 250 min 250 min | 3 3 | [119] |
| Ac-HKUST-1 | Solvothermal | - | Crystal violet Disulfine blue Quinoline yellow | 10 10 10 | 100 91 | 133 130 65 | 4 min | - | [120] |
| MIL-101(Fe)@PDopa@Fe3O4 | Solvothermal | - | Methyl red Malachite green | 100 100 | 92 100 | 833 1250 | 30 min 60 min | 4 4 | [121] |
| H6P2W18O62 /MOF-5 | Hydrothermal | 395 | Methylene blue | 20 | 97 | 52 | 10 min | - | [122] |
| Fe3O4@MIL-100(Fe) | Solvothermal | 730 | Methylene blue | 20 | 83 | 221 | 24 h | 4 | [123] |
| NENU/GO | Solvothermal | 380 | Basic red 46 | 5 | 88 | 130 | 6 min | - | [124] |
| MIL-68(Al)/PVDF | Casting | - | Methylene blue | 10 | 96 | 61 | 360 min | 6 | [125] |
| NH2-UiO-66(Zr) | Solvothermal | 247 | Safranin | 135 | 100 | 39 | 480 min | 4 | [126] |
| MIL-101(Cr) TiO2-MIL-101(Cr) | Hydrothermal | 2361 531 | Methylene blue | 20 | - | 9 21 | 50 min | - | [127] |
| MOF | Synthesis Method | Surface Area (m2 g−1) | Bandgap (eV) | Pollutants | Concentration (mg L−1) | Light Source | (%) Removal | Irradiation Time | Reused | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| MIL-88(Fe) | Hydrothermal | - | 2.05 | Methylene blue | 32 | Visible | - | 50 min | 4 | [132] |
| NH2-MIL-88(Fe) | Microwave | 164 | - | Methylene blue | 20 | Visible | 98 | 60 min | 5 | [50] |
| MIL-100(Fe) | Hydrothermal | 5 | - | Basic blue | 15 | Ultraviolet | 99 | 180 min | 3 | [133] |
| MIL-125(Ti) | Microwave | - | 3.14 | Methylene blue | - | Visible | 97 | 360 min | - | [134] |
| MIL-101(Fe) MIL-100(Fe) MIL-53(Fe) MIL-88B(Fe) | Solvothermal Solvothermal Solvothermal Solvothermal | 2986 1798 965 19 | - - - - | Acid orange | 80 | Visible | 95 88 62 23 | 120 min | 3 | [135] |
| MIL-53(Fe) Ni-MIL-53(Fe) | Solvothermal | 300 480 | 2.59 2.24 | Rhodamine blue | 14.4 | Visible | 81 91 | 180 min | - | [136] |
| MIL-101(Cr) TiO2-MIL-101(Cr) | Hydrothermal | 2361 531 | 2.3 2.59 | Methylene blue | 20 | Ultraviolet | 43 100 | 30 min | - | [127] |
| NH2-MIL-88B(Fe) | Microwave | 164 | - | Methylene blue | 20 | Visible | 98 | 45 min | 5 | [50] |
| NT/MIL-100(Fe) | Hydrothermal | 1414 | - - | Methylene blue Rhodamine blue | - | Visible | 99 94 | 180 min | 4 | [137] |
| PCN/MIL-100(Fe) | Hydrothermal | 1252 | - | Methylene blue Rhodamine blue | 10 | Visible | 75 80 | 200 min | - | [131] |
| TiO2@MIL-101(Fe) | Hydrothermal | 1919 | - | Methyl orange | 150 | Ultraviolet | 99 | 50 min | - | |
| NH2-MIL-125(Ti) CQDs/NH2-MIL-125(Ti) | Hydrothermal | 487 198 | 2.43 2.33 | Rhodamine blue | 10 10 | Visible | 64 100 | 120 min 120 min | 7 7 | [90] |
| NH2-MIL-53(Al) NH2-MIL-53(Al)/ RGO/PS | Hydrothermal | 1051 95 | 2.7 2.4 | Methylene blue | 30 | Visible | 41 59 | 210 min | 3 | [138] |
| MIL-100(Fe)@Bi2S3 | Microwave | 702 | 1.75 | Rhodamine blue | 10 | Visible | 98 | 60 min | 4 | [139] |
| MOF-199 | Solvothermal | 343 | 5.43 | Basic blue | 20 | Ultraviolet | - | 180 min | - | [140] |
| MOF-199 MOF-199-NH2/BaWO4 | Hydrothermal | - - | 3.2 3 | Methyl orange | 10 10 | Ultraviolet | 38 98 | 50 min | - | [141] |
| MOF-1 | Solvothermal | - | 3.0 | Methyl violet | 10 | Ultraviolet | 74 | 100 min | - | [142] |
| HU11(Pr) | Solvothermal | - | 3.3 | Crystal blue | 220 | Visible | 100 | 24 h | - | [143] |
| UiO-66/g-C3N4 | Mechanical | 384 | 2.72 | Methylene blue | 10 | Visible | - | 180 min | 6 | [144] |
| Bi2MoO6/UiO-66(Zr) | Hydrothermal | 726 | 2.45 | Rhodamine blue | 10 | Visible | 96 | 120 min | 3 | [145] |
| In2S3/UiO-66(Zr) | Solvothermal | 802 | 1.4 | Methyl orange | 15 | Visible | 96 | 40 min | 5 | [146] |
| CdS@NH2-MIL-125(Ti) | Solvothermal | 1247 | 2.36 | Rhodamine blue | 180 | Visible | 97 | 120 min | - | [147] |
| Ag3VO4/Cu-MOF/GO | Room temperature | 6 | - | Acid blue | 10 | Visible | 100 | 120 min | 3 | [148] |
| BiVO4/Fe-MOF/GO | Microwave | 33 | 2.18 | Rhodamine blue | 15 | Visible | - | 60 min | 4 | [1] |
| AgBr@HPU-4 | Room temperature | - | - | Methylene blue Methyl orange | 12.75 12.75 | Visible | 95 92 | 60 min 120 min | 5 5 | [149] |
| BiVO4/MIL-53(Fe) | Solvothermal | 33 | 2.18 | Rhodamine blue | 15 | - | 60 min | 4 | [1] | |
| Ag3PO4/AgBr/Ag-HKUST-1 | Solvothermal | 1 | Methylene blue Acid orange Eosin red | 15 | Visible | 92 90 90 | 80 min | 3 | [150] | |
| Ag3PO4/Bi2S3-HKUST-1 | Solvothermal | - | 2.07 | Trypan blue vesuvine | 25 | Visible | 98 99 | 25 min | - | [151] |
| MOF/CuWO4 | Hydrothermal | 801 | 2.4 | Methylene blue | 10 | Visible | 98 | 135 min | 6 | [152] |
| QD/Eu-MOF | Room temperature | - | 2.29 | Rhodamine blue | 2 | Ultraviolet | 90 | 50 min | - | [153] |
| Resin/FeBTC | Hydrothermal | - | 2.31 | Rhodamine blue Methylene blue | 400 | Visible | 99 67 | 30 min | 5 | [154] |
| MIL-53(Fe) | Solvothermal | - | 2.43 | Rhodamine blue | 1580 | Visible | 85 | 120 min | 5 | [155] |
| MIL-53(Fe) | Solvothermal | - | 3.87 | Methylene blue | 128 | Visible | 99 | 20 min | 5 | [156] |
| MIL-53(Fe) | Solvothermal | 38 | 2.69 | Rhodamine blue | 10 | Visible | - | 180 min | - | [157] |
| MIL-53(Fe) | Solvothermal | 89 | - | Orange green | 0.2 | Visible | 98 | 90 min | 5 | [158] |
| MIL-100(Fe)@MIL-53(Fe) | Sonochemical | 315 | 1.84 | Methyl orange | 10 | Visible | 98 | 180 min | 5 | [159] |
| [CoNi(m3-tp)2 (m2-pyz)2] MOF/CuWO4 | Hydrothermal | 1054 801 | 2.5 2.4 | Methylene blue | 10 | Visible | 32 98 | 135 min | 6 | [152] |
| UiO-66(Zr) α-Fe2O3@UiO-66(Zr) | Solvothermal | 1487 1204 | - | Methylene blue | 128 | Visible | - | 50 min | 3 | [160] |
| UiO-66(Zr) CuS/UiO-66(Zr) | Solvothermal | - - | 3.5 2.01 | Rhodamine blue | 10 | Visible | 50 90 | 60 min | 3 | [161] |
| NiFe2O4/MIL-53(Fe) | Solvothermal | 43 | - | Rhodamine blue | 4.7 | Visible | 95 | 180 min | - | [162] |
| MIL-88(Fe) TiO2NS@MIL-100(Fe) | Hydrothermal | 1670 725 | 2.6 2.87 | Methylene blue | 50 | Visible | - | 60 min | 4 | [163] |
| Type of MOF | Synthesis Method | Surface Area (m2 g−1) | Pollutants | Concentration (mg L−1) | % Removal | Qe (mg g−1) | Equilibrium Time | Reused | Ref |
|---|---|---|---|---|---|---|---|---|---|
| A100(Al) MOF | Commercial | 630 | Carbamazepine Ibuprofen | 2 2 | 95 75 | 65 50 | 2 h 2 h | 4 | [225] |
| NH2-MIL-68(In) | Hydrothermal | 655 | p-arsanilic acid | 20 | 77 | 78 | 4 h | 4 | [226] |
| Fe3O4@MIL-100(Fe) | Microwave | 1245 | Diclofenac | 100 | 248 | 4 h | - | [227] | |
| MIL-101 (Cr) ED-MIL-101(Cr) AMSA-MIL-101(Cr) | Hydrothermal | 3014 2322 2255 | Naproxen Clofibric Naproxen Clofibric Naproxen Clofibric | 13 100 | - | 131 315 93 105 154 347 | 2 h | 4 | [228] |
| PCN-134(Zr) | Solvothermal | 756 | Diclofenac | 30 | - | - | 20 min | - | [229] |
| [Cu(BTTA)]n.2DMF | Solvothermal | Diclofenac Chlorpromazine Amodiaquine | 1200 1000 1000 | - - - | 650 67 72 | 7.5 h 5 h 5 h | 3 | [230] | |
| [Zn2(fum)2(bpy)] [Zn4O(bdc)3] | Mechanical Solvothermal | - | Amodiaquine | 25 | - | 0.5 48 | 3 h | - | [231] |
| [Zn6(IDC)4(OH)2(Hprz)2]n | Hydrothermal | 889 | Ampicillin Amoxicillin Cloxacillin | 60 | 93 88 89 | - | 4 h | 4 | [232] |
| PCN-222(Zr) | Solvothermal | 2917 | Chloramphenicol | 500 | 99 | 370 | 58 sec | - | [233] |
| PCN-128Y(Zr) | Solvothermal | Tetracycline | 44 | 56 | 400 | 30 min | - | [234] | |
| MIL-53(Al) | Hydrothermal | 1401 | Dimetridazole | 40 | 90 | 467 | 10 min | 5 | [3] |
| MOF-5 | Room temperature | 2510 | Tetracycline | 50 | 97 | 233 | 45 min | - | [235] |
| MIL-53(Cr) MIL-53(Al) | Solvothermal | 500 500 | Sulfonamide | 20 | 99 98 | 0.4 0.4 | 1 h | 3 3 | [236] |
| MIL-53(Fe)/Fe3O4. | Solvothermal | 76 | Doxycycline | 300 | 100 | 320 | 30 min | 5 | [237] |
| MIL-101(Cr) MIL-53(Cr) | Hydrothermal Hydrothermal | 2810 398 | Clofibric acid Carbamazepine Clofibric acid Carbamazepine | 20 | - | 144 35 137 31 | 1 h | - | [238] |
| MIL-101(Fe) MIL-100(Fe) MIL-53(Fe) | Hydrothermal Hydrothermal Solvothermal | 253 1203 21 | Tetracycline | 50 | 55.1 44 11 | 52 43 12 | 40 min | 4 | [239] |
| Ni-MIL-53(Fe) | Solvothermal | - | Doxycycline | 150 | 88 | 684 | 12 h | 5 | [240] |
| MIL-101(Cr) Urea-MIL-101(Cr) | Hydrothermal | 3030 1970 | Dimetridazole | 10 | - | 141 185 | 4 h | 4 | [241] |
| Pd@MIL-100(Fe) | Hydrothermal | 2102 | |||||||
| MWCNT/NH2-MIL-53(Fe) | Solvothermal | 126 | Tetracycline Chlortetracycline | 20 | - - | 368 254 | 12 h | 4 | [242] |
| MWCNT/MIL-53(Fe) | Solvothermal | 60 | Tetracycline Oxytetracycline Chlortetracycline | 20 | - | 364 326 181 | 10 h | 4 | [243] |
| UiO-66(Zr) NH2-UiO-66(Zr) | Solvothermal | 1171 646 | Ibuprofen Naproxen Ibuprofen naproxen | 9 | - - | 127 89 51 40 | 4 h 4 h | - | [244] |
| UiO-66(Zr) In2S3/UiO-66(Zr) | Solvothermal | 389 75 | Tetracycline | 40 | - | 51 61 | 1 h | 3 | [245] |
| UiO-66(Zr) | Solvothermal | 1155 | Sulfonamide | 100 | - | 417 | 10 min | 4 | [246] |
| Fe3O4/HKUST-1(Cu) | Solvothermal | 328 | CiprofloxacinNorfloxacin | 20 | 98 99 | 538 513 | 30 min | 10 | [46] |
| Zn(TDC)(4- BPMH)]n·n(H2O) | Sonochemical | 235 | Dichlorophenol Amoxicillin | 50 | 99 99 | - - | 3 h | - - | [196] |
| Ni/Co-MOF@CMC | Microwave | - | Tetracycline | 30 | 80 | 625 | 5 min | - | [247] |
| MIL-68(Al)/GO | Hydrothermal | 1267 | Tetracycline | 50 | - | 173 | 6 h | 3 | [248] |
| MIL-101(Cr) GnO/MIL-101(Cr) | Hydrothermal | - 3308 | Naproxen Ketoprofen Naproxen Ketoprofen | 50 | - | 112 80 171 140 | 12 h | 4 | [249] |
| Cu-DTO | Room temperature | 120 | Tartrazine | 200 | 98 | 255 | 40 min | 7 | [250] |
| MOF | Synthesis Method | Surface Area (m2 g−1) | Bandgap (eV) | Pollutants | Concentration (mg L−1) | Light Source | (%) Removal | Irradiation Time | Reused | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| MIL-53(Fe) | Solvothermal | 1890 | 2.75 | Tetracycline | 10 | Visible | 97 | 2 h | 4 | [252] |
| MIL-101(Fe) MIL-100(Fe) MIL-53(Fe) | Hydrothermal Hydrothermal Solvothermal | 253 1203 21 | 1.88 2.06 1.97 | Tetracycline | 50 | Visible | 97 57 41 | 3 h | [239] | |
| MIL-100(Fe)@Fe3O4 MIL-100(Fe)@Fe3O4/CA | Hydrothermal | 725 389 | 2.49 1.76 | Tetracycline | 10 | Visible | 42 85 | 3 h | 7 | [253] |
| MIL-88(Fe) Ag/AgCl@MIL-88(Fe) | Solvothermal | 26 139 | 2.51 2.23 | Ibuprofen | 10 | Visible | 45 93 | 3.5 h | 4 | [251] |
| CdS@NH2-MIL-125(Ti) | Solvothermal | 1375 | 2.36 | Oxytetracycline | 180 | Visible | - | 2 h | 5 | [147] |
| MIL-101(Fe) Pd@MIL-100(Fe) | Hydrothermal | 2006 2102 | - | Theophylline Ibuprofen Theophylline Ibuprofen | 20 | Visible | 88 92 100 100 | 2.5 h | 4 | [218] |
| UiO-66(Zr) In2S3/UiO-66(Zr) | Solvothermal | 389 75 | 3.70 1.92 | Tetracycline | 40 | Visible | 56 79 | 1 h | 3 | [245] |
| In2S3/UiO-66(Zr) | Solvothermal | 48 | 2.2 | Tetracycline | 30 | Visible | 85 | 1 h | 5 | [146] |
| MIL-100(Fe) Fe3O4@MIL-100(Fe) | Hydrothermal Microwave | 1766 1245 | - - | Diclofenac | 60 | visible | 100 99 | - - | [227] | |
| Vis/MIL-53(Fe)/Fe(III)/SPC | Solvothermal | - | 2.91 | Sulfamethazine | 0.2 | Visible | 90 | 1 h | - | [254] |
| 1T- MoS2@MIL-53(Fe) | Solvothermal | 337 | 0.7 | Ibuprofen | 10 | Visible | 100 | 2 h | 5 | [255] |
| MIL-68(In)-NH2 g-C3N4/MIL-68(In)-NH2 | Solvothermal | 659 537 | 2.81 2.65 | Ibuprofen | 20 | Visible | 93 68 | 2 h | - | [248] |
| MIL-125ML MIL-125ML/gCN | Solvothermal | 1001 725 | 2.86 2.68 | Cefixime | 20 | Visible | 48 74 | 2 h | 4 | [256] |
| UiO-66-NH2 CNT/N-TiO2/UiO-66-NH2 | Hydrothermal | 708 288 | 2.17 | Ketoprofen | 50 | Visible | 41 96 | 2 h | - | [257] |
| MIL-101(Cr) α-Fe2O3/MIL-101(Cr) | Hydrothermal | 2518 949 | 3.25 3.62 | Carbamazepine | 30 | Visible | - - | 3 h | 4 | [258] |
| MIL-53(Fe) | Solvothermal | 184 | - | Clofibric acid Carbamazepine | 40 | Visible | 98 90 | 4 h | 4 | [259] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Jagaba, A.H.; Aldaghri, O.; et al. A Critical Review on Metal-Organic Frameworks and Their Composites as Advanced Materials for Adsorption and Photocatalytic Degradation of Emerging Organic Pollutants from Wastewater. Polymers 2020, 12, 2648. https://doi.org/10.3390/polym12112648
Zango ZU, Jumbri K, Sambudi NS, Ramli A, Abu Bakar NHH, Saad B, Rozaini MNH, Isiyaka HA, Jagaba AH, Aldaghri O, et al. A Critical Review on Metal-Organic Frameworks and Their Composites as Advanced Materials for Adsorption and Photocatalytic Degradation of Emerging Organic Pollutants from Wastewater. Polymers. 2020; 12(11):2648. https://doi.org/10.3390/polym12112648
Chicago/Turabian StyleZango, Zakariyya Uba, Khairulazhar Jumbri, Nonni Soraya Sambudi, Anita Ramli, Noor Hana Hanif Abu Bakar, Bahruddin Saad, Muhammad Nur’ Hafiz Rozaini, Hamza Ahmad Isiyaka, Ahmad Hussaini Jagaba, Osamah Aldaghri, and et al. 2020. "A Critical Review on Metal-Organic Frameworks and Their Composites as Advanced Materials for Adsorption and Photocatalytic Degradation of Emerging Organic Pollutants from Wastewater" Polymers 12, no. 11: 2648. https://doi.org/10.3390/polym12112648
APA StyleZango, Z. U., Jumbri, K., Sambudi, N. S., Ramli, A., Abu Bakar, N. H. H., Saad, B., Rozaini, M. N. H., Isiyaka, H. A., Jagaba, A. H., Aldaghri, O., & Sulieman, A. (2020). A Critical Review on Metal-Organic Frameworks and Their Composites as Advanced Materials for Adsorption and Photocatalytic Degradation of Emerging Organic Pollutants from Wastewater. Polymers, 12(11), 2648. https://doi.org/10.3390/polym12112648









