Novel Electrospun Pullulan Fibers Incorporating Hydroxypropyl-β-Cyclodextrin: Morphology and Relation with Rheological Properties
Abstract
1. Introduction
2. Materials and Methods
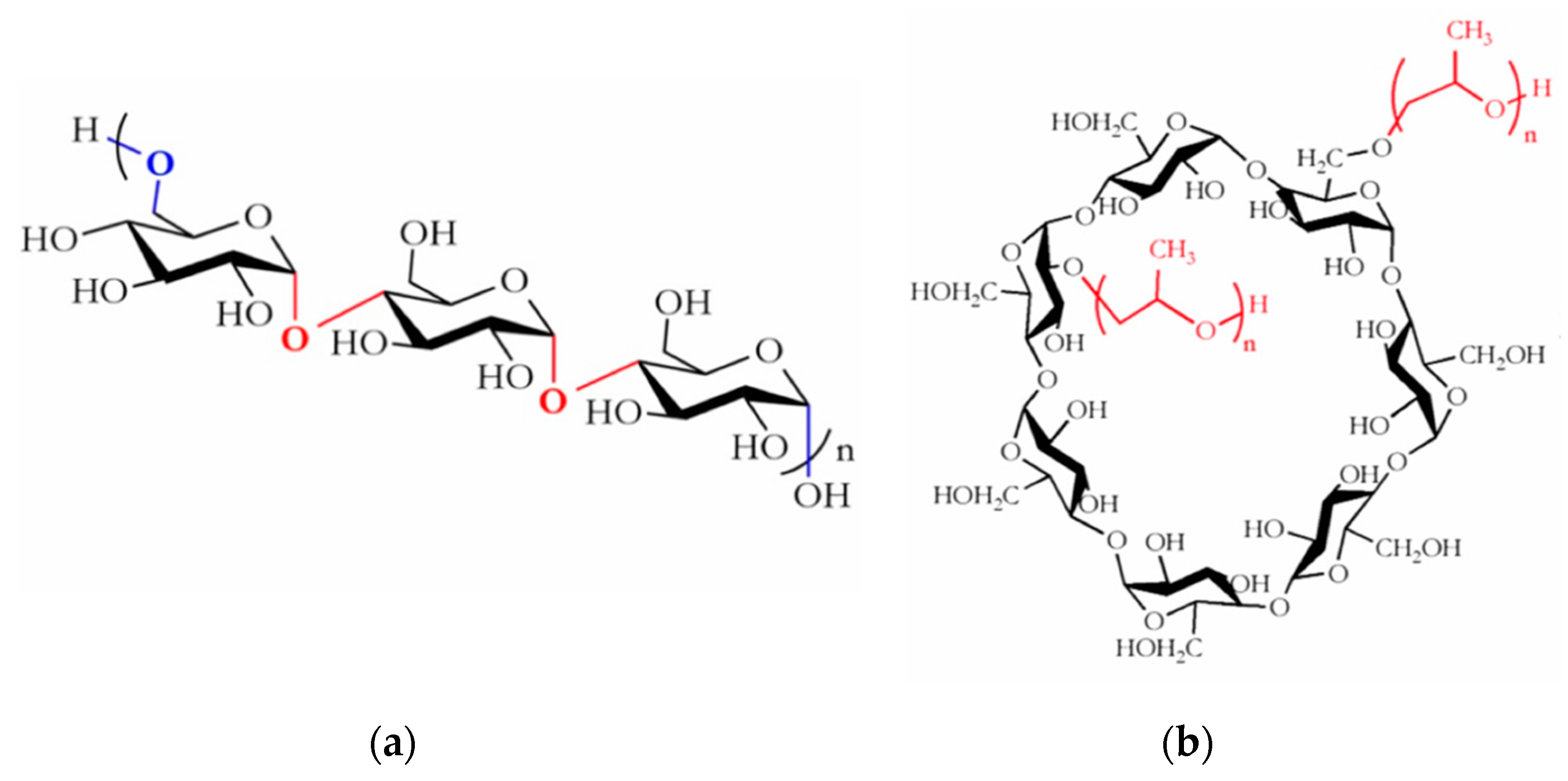
2.1. Materials
2.2. Preparation of Pullulan/HP-β-CD Binary Solutions for Electrospinning
2.3. Rheological Characterization of Solutions for Electrospinning
2.4. Dynamic Light Scattering Measurements
2.5. Electrical Conductivity Measurements
2.6. Fabrication of Electrospun Fibers
2.7. Characterization of Electrospun Nanofibers
2.7.1. Morphological Analysis
2.7.2. Thermal Analysis
2.7.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties of Electrospinning Solutions
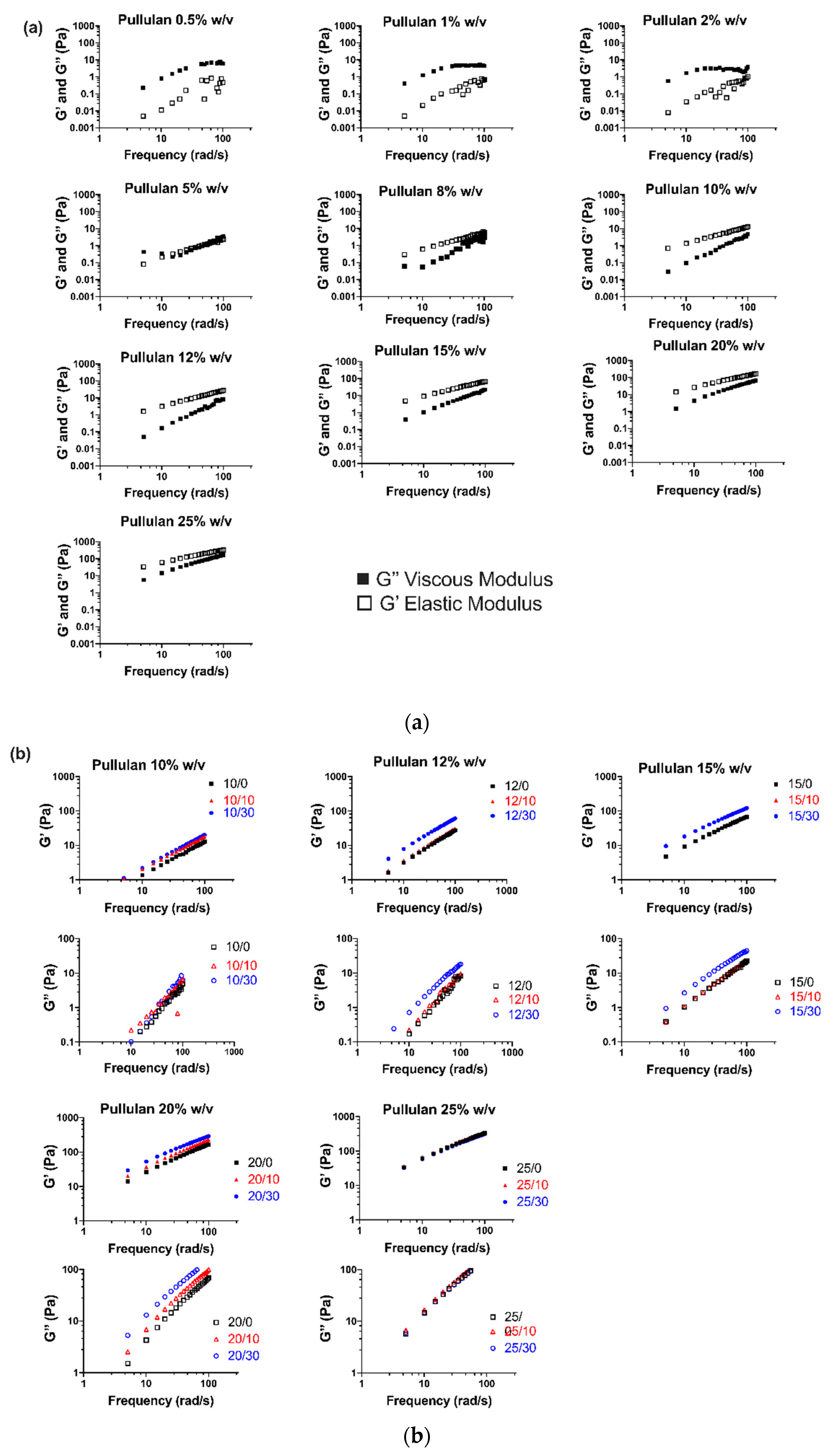
3.1.1. Viscoelastic Behavior
3.1.2. Flow Behavior
3.1.3. Entanglement Polymer Concentration (CE)
3.2. Electrospinning of Pullulan-HP-β-CD Fibers
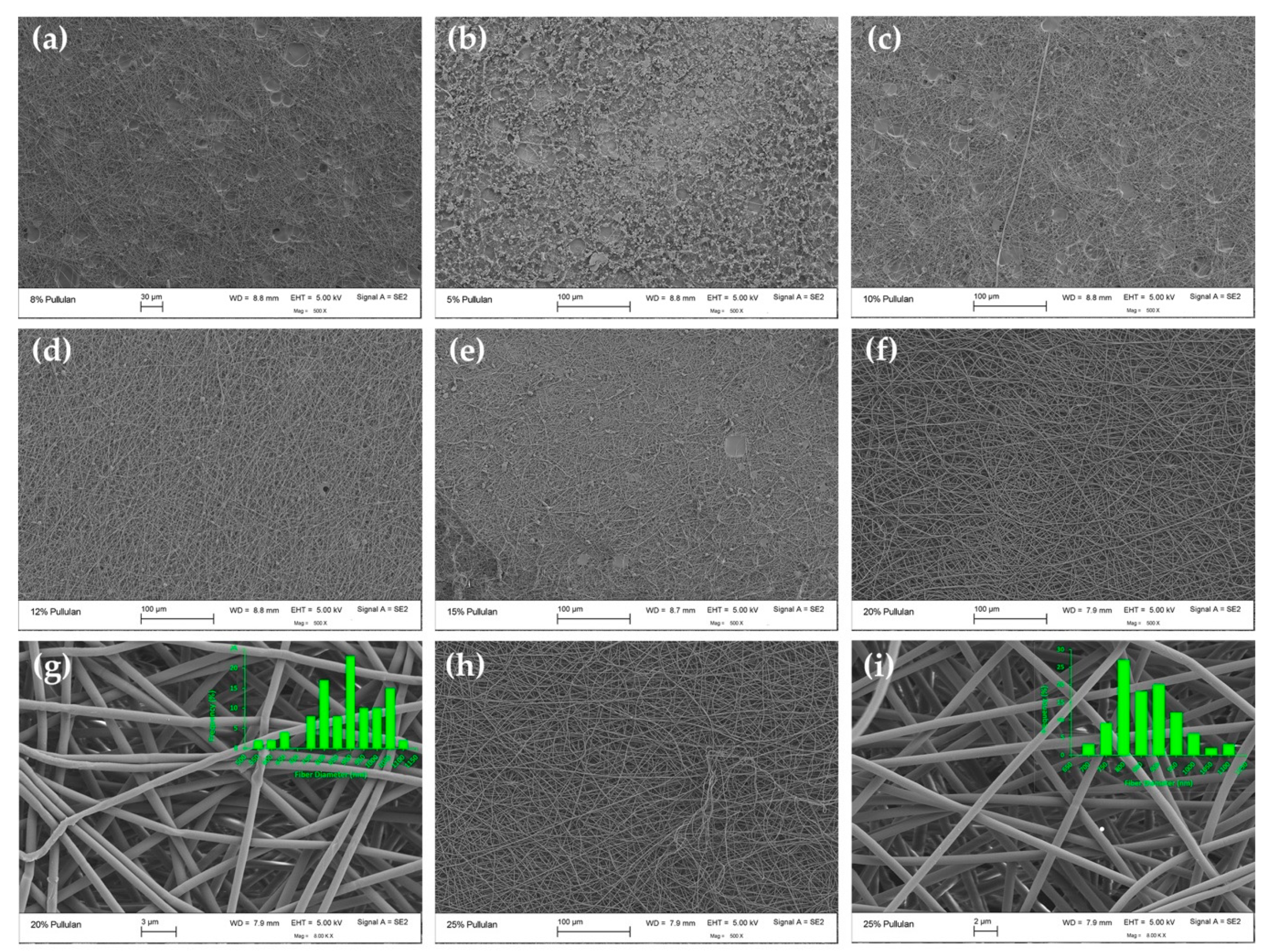
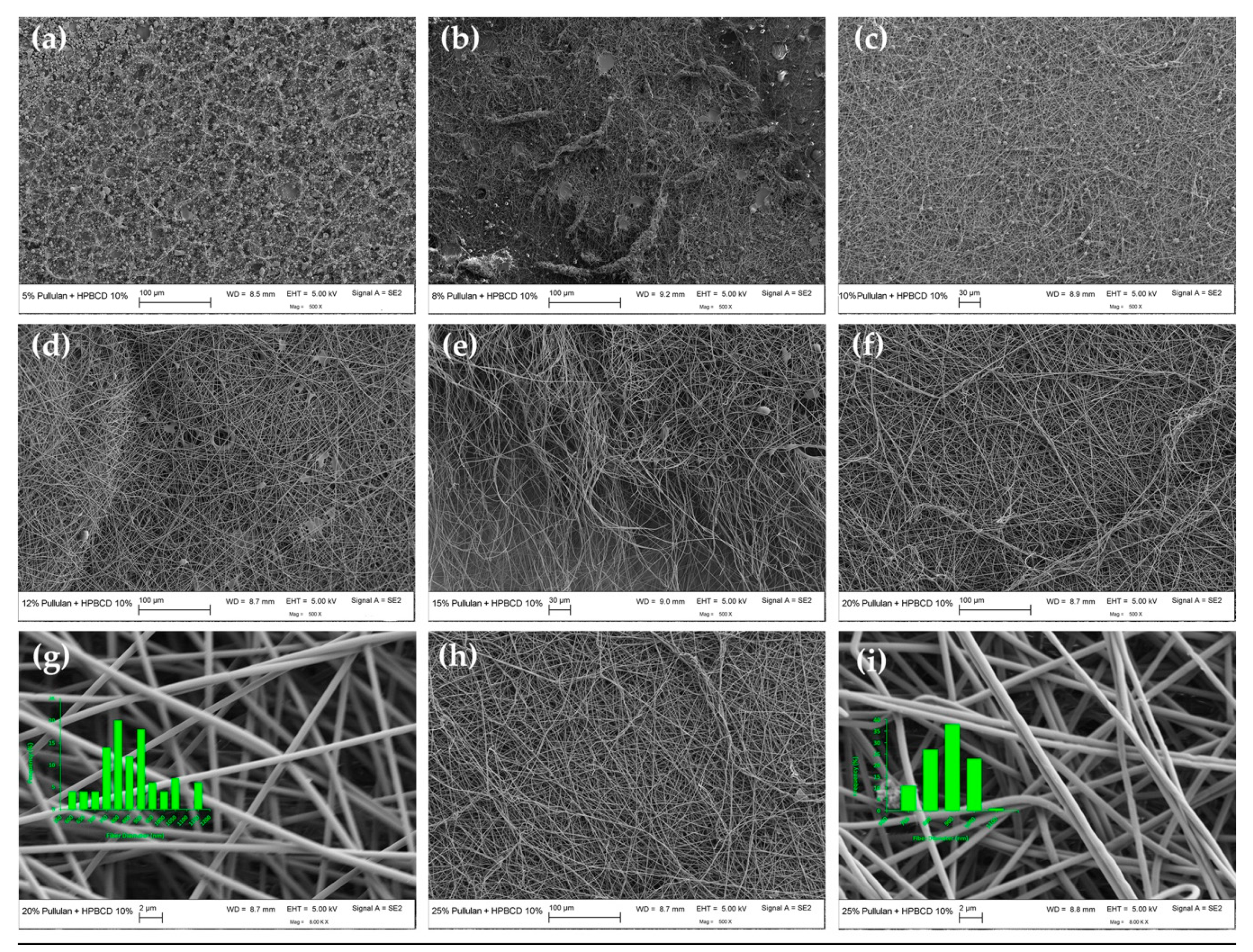
3.2.1. Electrospun Fiber Morphology and Correlation with CE
3.2.2. Thermal Characterization of Spunfibers
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Supaphol, P.; Suwantong, O.; Sangsanoh, P.; Srinivasan, S.; Jayakumar, R.; Nair, S.V. Electrospinning of Biocompatible Polymers and Their Potentials in Biomedical Applications, in Biomedical Applications of Polymeric Nanofibers; Jayakumar, R., Nair, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 213–239. [Google Scholar]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Wen, P.; Wen, Y.; Zong, M.-H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.Z.; Ziegler, R.G.; Bhosale, R. Fibers spun from polysaccharides. In Handbook of Carbohydrate Polymers; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; pp. 1–43. [Google Scholar]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An exopolysaccharide and its various applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial sources, production and applications. Carbohydr. Polym. 2008, 73, 515–531. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G. Water sorption and thermo-mechanical properties of water/sorbitol-plasticized composite biopolymer films: Caseinate–pullulan bilayers and blends. Food Hydrocoll. 2006, 20, 1057–1071. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G.; Kontogiorgos, V. Molecular weight effects on solution rheology of pullulan and mechanical properties of its films. Carbohydr. Polym. 2003, 52, 151–166. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Rheological aspects in fabricating pullulan fibers by electro-wet-spinning. Food Hydrocoll. 2014, 38, 220–226. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of cyclodextrin functionalized polyethylene oxide (PEO) nanofibers. Eur. Polym. J. 2009, 45, 1032–1037. [Google Scholar] [CrossRef]
- Qian, Y.; Qi, M.; Zheng, L.; King, M.W.; Lv, L.; Ye, F. Incorporation of Rutin in Electrospun Pullulan/PVA Nanofibers for Novel UV-Resistant Properties. Materials 2016, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Vázquez, G.; Loarca-Piña, G.; Figueroa-Cárdenas, J.D.; Mendoza, S. Electrospun fibers from blends of pea (Pisum sativum) protein and pullulan. Food Hydrocoll. 2018, 83, 173–181. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Lagaron, J.M.; López-Rubio, A. Development and characterization of food-grade electrospun fibers from amaranth protein and pullulan blends. Food Res. Int. 2013, 54, 667–674. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocoll. 2018, 77, 726–735. [Google Scholar] [CrossRef]
- Qin, Z.-Y.; Jia, X.-W.; Liu, Q.; Kong, B.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Qian, Y.; Zhang, Z.; Lyu, L.; Wang, Y.; Ye, F. Study on the Electrospinning of Gelatin/Pullulan Composite Nanofibers. Polymers 2019, 11, 1424. [Google Scholar] [CrossRef]
- Del Valle, E. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Burns, N.A.; Burroughs, M.C.; Gracz, H.; Pritchard, C.Q.; Brozena, A.H.; Willoughby, J.; Khan, S.A. Cyclodextrin facilitated electrospun chitosan nanofibers. RSC Adv. 2015, 5, 7131–7137. [Google Scholar] [CrossRef]
- Kayaci, F.; Sen, H.S.; Durgun, E.; Uyar, T. Functional electrospun polymeric nanofibers incorporating geraniol–cyclodextrin inclusion complexes: High thermal stability and enhanced durability of geraniol. Food Res. Int. 2014, 62, 424–431. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Uyar, T.; Havelund, R.; Hacaloglu, J.; Besenbacher, F.; Kingshott, P. Functional Electrospun Polystyrene Nanofibers Incorporating α-, β-, and γ-Cyclodextrins: Comparison of Molecular Filter Performance. ACS Nano 2010, 4, 5121–5130. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Uyar, T. Electrospun zein nanofibers incorporating cyclodextrins. Carbohydr. Polym. 2012, 90, 558–568. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.G.; Wilkes, G.L.; Colby, R.H.; Long, T.E. Correlations of Solution Rheology with Electrospun Fiber Formation of Linear and Branched Polyesters. Macromolecules 2004, 37, 1760–1767. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Role of Molecular Entanglements in Starch Fiber Formation by Electrospinning. Biomacromolecules 2012, 13, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Improved viscoelasticity of xanthan gum through self-association with surfactant: β-cyclodextrin inclusion complexes for applications in enhanced oil recovery. Polym. Eng. Sci. 2014, 55, 523–532. [Google Scholar] [CrossRef]
- Beheshti, N.; Zhu, K.; Kjøniksen, A.-L.; Nyström, B. Effects of β-cyclodextrin and β-cyclodextrin polymer addition and temperature on the modulation of hydrophobic interactions in aqueous solutions of two hydrophobically modified biopolymers. J. Non Cryst. Solids 2007, 353, 3906–3914. [Google Scholar] [CrossRef]
- McKee, M.G.; Hunley, M.T.; Layman, J.M.; Long, T.E. Solution Rheological Behavior and Electrospinning of Cationic Polyelectrolytes. Macromolecules 2006, 39, 575–583. [Google Scholar] [CrossRef]
- Gadkari, S. Influence of Polymer Relaxation Time on the Electrospinning Process: Numerical Investigation. Polymers 2017, 9, 501. [Google Scholar] [CrossRef]
- Straube, E. Scaling concepts in polymer physics. Von, P. G. DE GENNES. Ithaca/London: Cornell University Press 1980. Geb., £ 23,–, $ 48.–. Acta Polym. 1981, 32, 290. [Google Scholar] [CrossRef]
- Morris, E.R.; Cutler, A.N.; Ross-Murphy, S.B.; Rees, D.A.; Price, J. Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydr. Polym. 1981, 1, 5–21. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Electrospun nanofibers from native cyclodextrins. J. Colloid Interface Sci. 2013, 404, 1–7. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, A.; Sumnu, G.; Sahin, S. A novel electrospun hydroxypropyl methylcellulose/polyethylene oxide blend nanofibers: Morphology and physicochemical properties. Carbohydr. Polym. 2018, 181, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Lee, H.W.; Kim, R.; Ji, B.C.; Cho, J.W.; Son, T.W.; Oh, W.; Yeum, J.H. Preparation and characterization of electrospun pullulan/montmorillonite nanofiber mats in aqueous solution. Carbohydr. Polym. 2009, 78, 336–342. [Google Scholar]
- Sun, X.B.; Jia, D.; Kang, W.M.; Cheng, B.W.; Li, Y.B. Research on Electrospinning Process of Pullulan Nanofibers. Appl. Mech. Mater. 2012, 268–270, 198–201. [Google Scholar] [CrossRef]
- Wang, H.; Ziegler, G.R. Electrospun nanofiber mats from aqueous starch-pullulan dispersions: Optimizing dispersion properties for electrospinning. Int. J. Biol. Macromol. 2019, 133, 1168–1174. [Google Scholar] [CrossRef]
- Aduba, D.C., Jr.; Zhang, K.; Kanitkar, A.; Sirrine, J.M.; Verbridge, S.S.; Long, T.E. Electrospinning of plant oil-based, non-isocyanate polyurethanes for biomedical applications. J. Appl. Polym. Sci. 2018, 135, 46464. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Mattoso, L.H.C.; Offeman, R.D.; Wood, D.F.; Orts, W.J. Effect of relative humidity on the morphology of electrospun polymer fibers. Can. J. Chem. 2008, 86, 590–599. [Google Scholar] [CrossRef]
- Uyar, T.; Havelund, R.; Hacaloglu, J.; Zhou, X.; Besenbacher, F.; Kingshott, P. The formation and characterization of cyclodextrin functionalized polystyrene nanofibers produced by electrospinning. Nanotechnology 2009, 20, 125605. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 2012, 4, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. JBT 2010, 21, 167–193. [Google Scholar] [PubMed]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar]
- Shao, P.; Niu, B.; Chen, H.; Sun, P. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Singh, D.; Kennedy, J.F. Investigating aqueous phase separation of pullulan from Aureobasidium pullulans and its characterization. Carbohydr. Polym. 2019, 223, 115103. [Google Scholar] [CrossRef]
- Karim, M.R.; Islam, M.S. Thermal Behavior with Mechanical Property of Fluorinated Silane Functionalized Superhydrophobic Pullulan/Poly(vinyl alcohol) Blends by Electrospinning Method. J. Nanomater. 2011, 2011, 979458. [Google Scholar] [CrossRef]
- Teramoto, N.; Shibata, M. Synthesis and properties of pullulan acetate. Thermal properties, biodegradability, and a semi-clear gel formation in organic solvents. Carbohydr. Polym. 2006, 63, 476–481. [Google Scholar] [CrossRef]
- Scandola, M.; Ceccorulli, G.; Pizzoli, M. Molecular motions of polysaccharides in the solid state: Dextran, pullulan and amylose. Int. J. Biol. Macromol. 1991, 13, 254–260. [Google Scholar] [CrossRef]
- D’Aria, F.; Serri, C.; Niccoli, M.; Mayol, L.; Quagliariello, V.; Iaffaioli, R.V.; Biondi, M.; Giancola, C. Host–guest inclusion complex of quercetin and hydroxypropyl-β-cyclodextrin. J. Therm. Anal. Calorim. 2017, 130, 451–456. [Google Scholar] [CrossRef]
- Venuti, V.; Stancanelli, R.; Acri, G.; Crupi, V.; Paladini, G.; Testagrossa, B.; Tommasini, S.; Ventura, C.A.; Majolino, D. “Host-guest” interactions in Captisol®/Coumestrol inclusion complex: UV–vis, FTIR-ATR and Raman studies. J. Mol. Struct. 2017, 1146, 512–521. [Google Scholar] [CrossRef]
- Savic, I.M.; Nikolic, V.D.; Savic-Gajic, I.; Nikolic, L.B.; Radovanovic, B.C.; Mladenovic, J.D. Investigation of properties and structural characterization of the quercetin inclusion complex with (2-hydroxypropyl)0≤-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 383–394. [Google Scholar] [CrossRef]

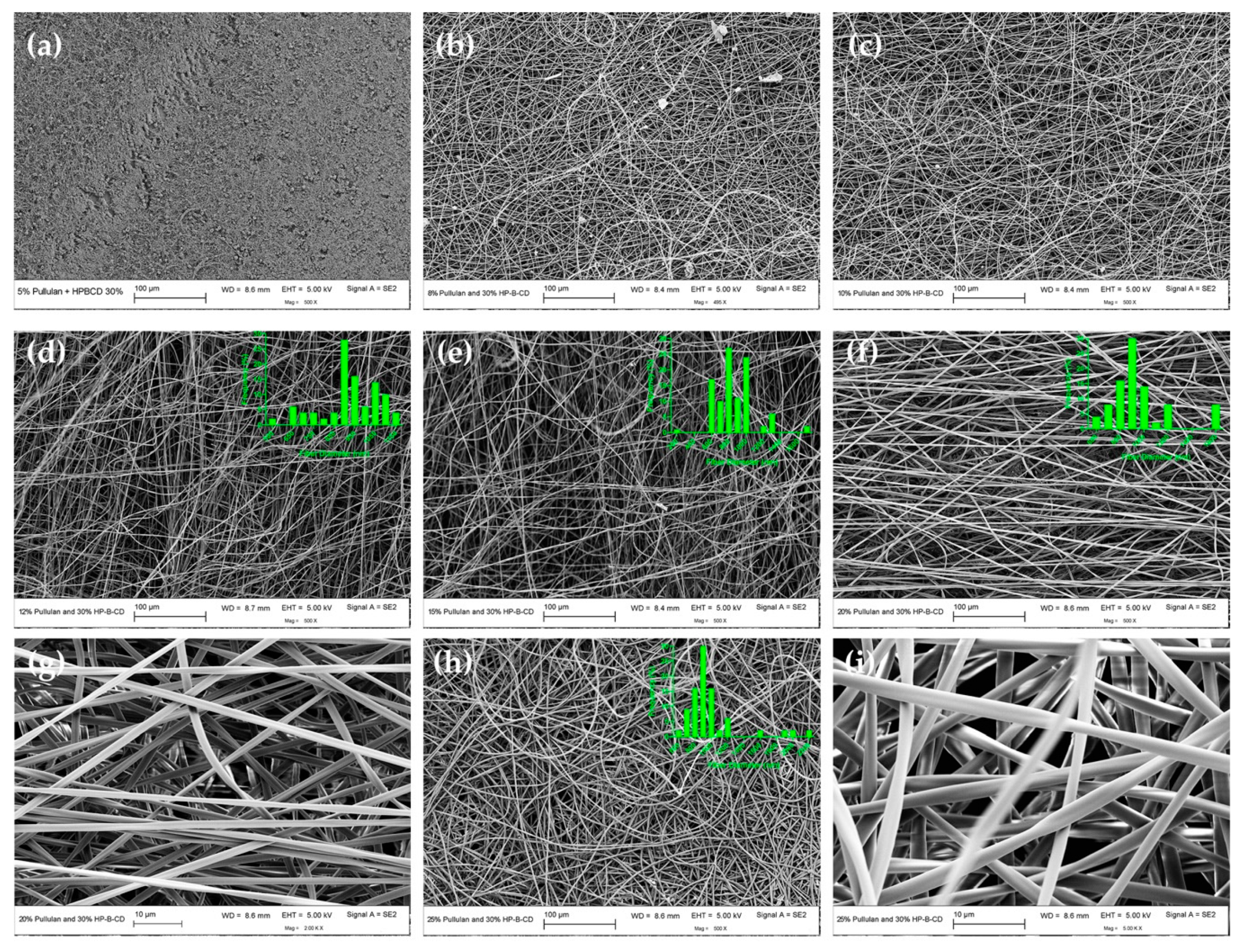
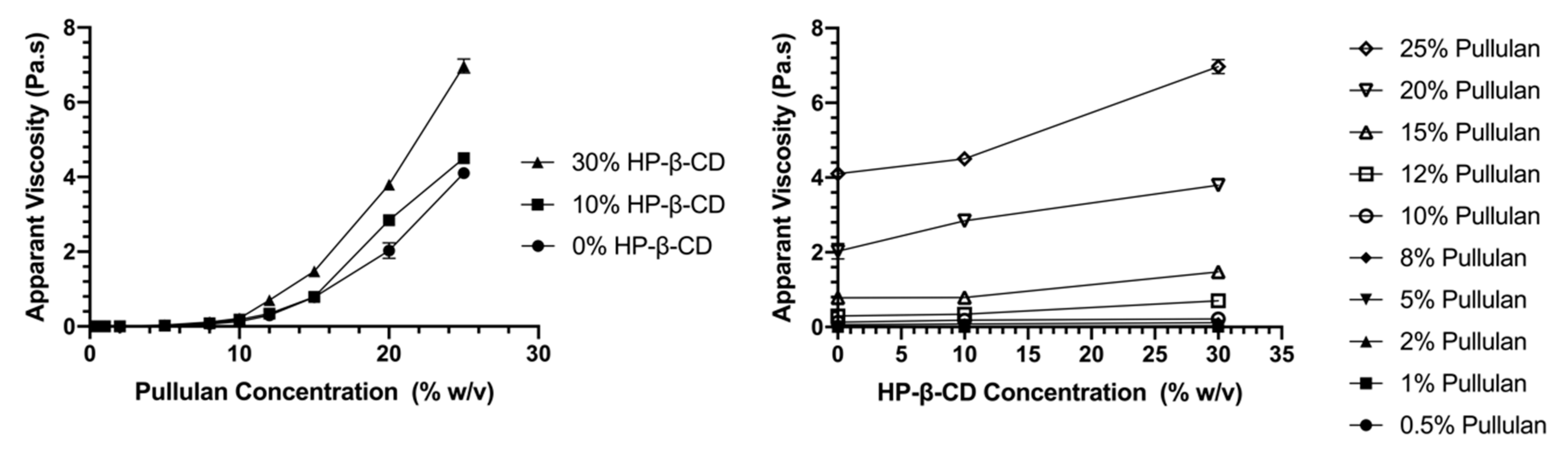
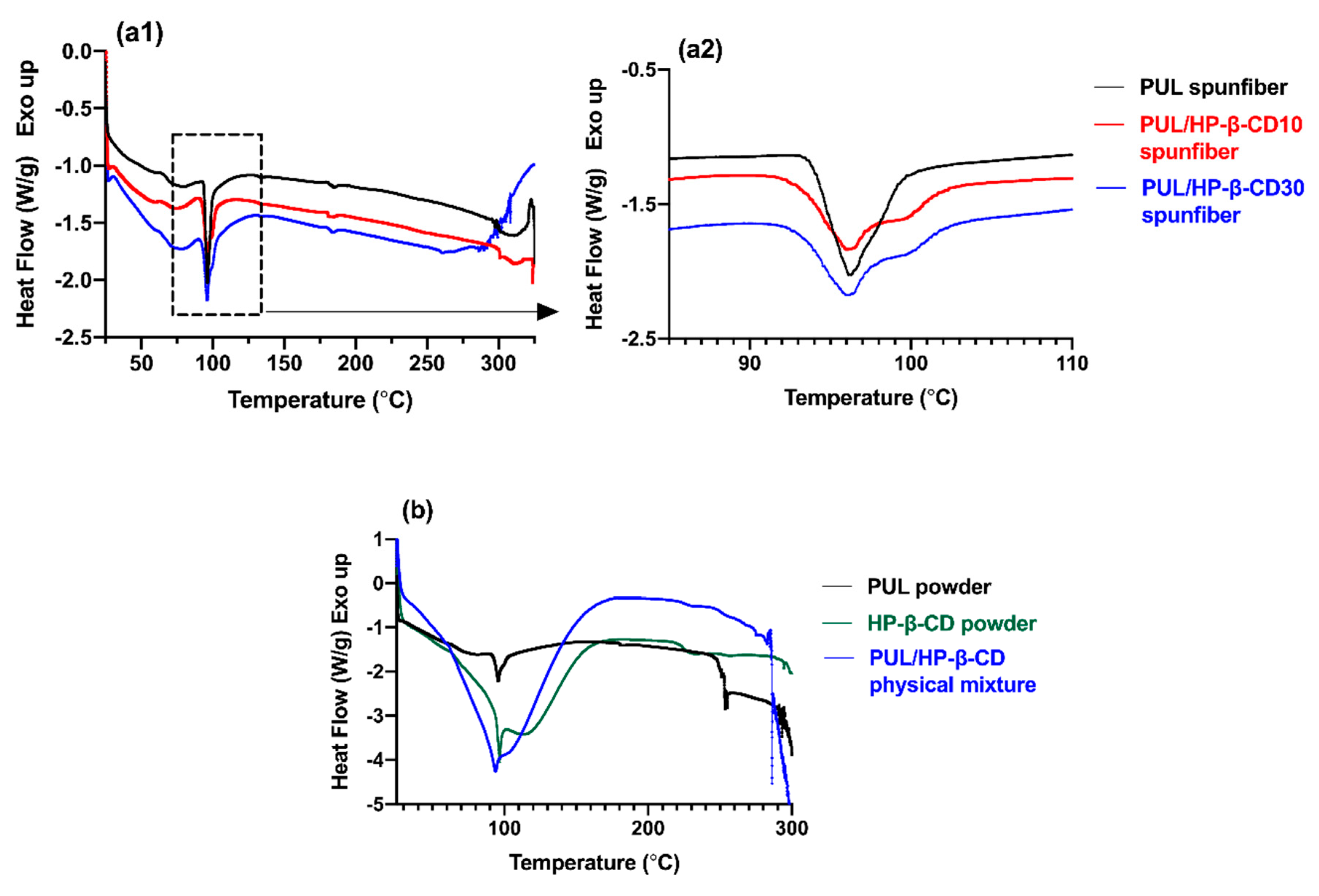
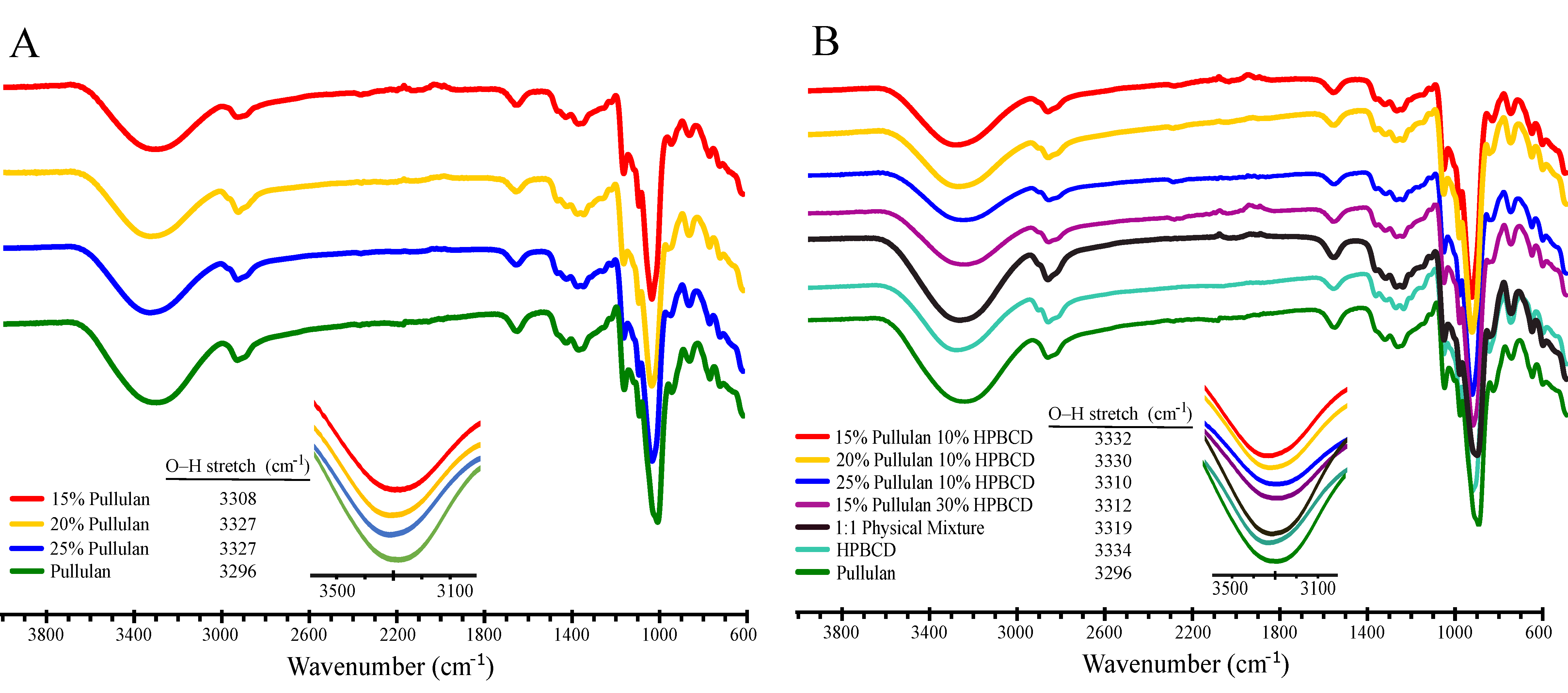
| Solutions (Pullulan/HP-β-CD) (% w/v) | k (mPa.s n) | n | Fiber Morphology | Average Fiber Diameter, ADF (nm) |
|---|---|---|---|---|
| 0.5/0 | 0.81 ± 0.0006 a | 1.10 ± 0.00034 a | No fiber formation | - |
| 1/0 | 1.88 ± 0.07 b | 1.05 ± 0.0047 b | No fiber formation | - |
| 2/0 | 3.95 ± 0.18 c | 1.02 ± 0.0040 b | No fiber formation | - |
| 5/0 | 22.4 ± 2.7 e | 1.001 ± 0.0055 b | Beaded structures | - |
| 8/0 | 67.5 ± 11 f | 0.978 ± 0.0084 c | Fibers with many beads | - |
| 10/0 | 173 ± 19 g | 0.943 ± 0.0086 e | Fibers with many beads | - |
| 12/0 | 483 ± 83 h | 0.895 ± 0.0093 g | Fibers with bead structures | - |
| 15/0 | 1743 ± 402 i | 0.825 ± 0.012 i | Fibers with bead structures | - |
| 20/0 | 6170 ± 866 j | 0.754 ± 0.0089 k | Bead-free fibers | 886 ± 143 a |
| 25/0 | 16500 ± 50 l | 0.692 ± 0.0027 l | Bead-free fibers | 863 ± 87 a |
| 0.5/10 | 1.73 ± 0. 23 b | 1.04 ± 0.027 b | No fiber formation | - |
| 1/10 | 2.11 ± 0.02 b | 1.04 ± 0.00021 b | No fiber formation | - |
| 2/10 | 4.75 ± 0.11 c | 1.01 ± 0.0026 b | No fiber formation | - |
| 5/10 | 22.4 ± 1.55 e | 1.01 ± 0.00010 b | Bead structures with few fibers | - |
| 8/10 | 86.6 ± 6.39 f | 0.985 ± 0.00264 c | Fibers with many beads | - |
| 10/10 | 257 ± 44 g | 0.930 ± 0.0113 f | Fibers with many beads | - |
| 12/10 | 558 ± 76 h | 0.893 ± 0.00713 g | Fibers with few beads | - |
| 15/10 | 1710 ± 120 i | 0.831 ± 0.00298 i | Fibers with few beads | - |
| 20/10 | 8925 ± 61 j | 0.746 ± 0.0023 k | Bead-free fibers | 853 ± 130 a |
| 25/10 | 20179 ± 1171 m | 0.643 ± 0.016 m | Bead-free fibers | 823 ± 91 a |
| 0.5/30 | 3.66 ± 0.65 c | 1.01 ± 0.018 b | No fiber formation | - |
| 1/30 | 7.07 ± 0.31 d | 1.01 ± 0.0007 b | No fiber formation | - |
| 2/30 | 8.86 ± 0.08 d | 1.01 ± 0.00094 b | No fiber formation | - |
| 5/30 | 36.5 ± 0.96 e | 1.01 ± 0.00052 b | Fibers with many beads | - |
| 8/30 | 136 ± 7.61 g | 0.967 ± 0.00103 d | Fibers with few beads | - |
| 10/30 | 296 ± 17.5 g, h | 0.929 ± 0.00078 f | Fibers with few beads | - |
| 12/30 | 1380 ± 96 i | 0.852 ± 0.00502 h | Bead-free fibers | 866 ± 137 a |
| 15/30 | 3753 ± 147 k | 0.794 ± 0.0023 j | Bead-free fibers | 886 ± 225 a |
| 20/30 | 16392 ± 2441 l | 0.695 ± 0.015 l | Bead-free fibers | 882 ± 322 a |
| 25/30 | 44573 ± 9531 n | 0.617 ± 0.0108 n | Bead-free fibers | 1019 ± 200 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, D.; Swilley-Sanchez, S.; O’keefe, S.; Matson, J.; Long, T.; Fernández-Fraguas, C. Novel Electrospun Pullulan Fibers Incorporating Hydroxypropyl-β-Cyclodextrin: Morphology and Relation with Rheological Properties. Polymers 2020, 12, 2558. https://doi.org/10.3390/polym12112558
Poudel D, Swilley-Sanchez S, O’keefe S, Matson J, Long T, Fernández-Fraguas C. Novel Electrospun Pullulan Fibers Incorporating Hydroxypropyl-β-Cyclodextrin: Morphology and Relation with Rheological Properties. Polymers. 2020; 12(11):2558. https://doi.org/10.3390/polym12112558
Chicago/Turabian StylePoudel, Deepak, Sarah Swilley-Sanchez, Sean O’keefe, John Matson, Timothy Long, and Cristina Fernández-Fraguas. 2020. "Novel Electrospun Pullulan Fibers Incorporating Hydroxypropyl-β-Cyclodextrin: Morphology and Relation with Rheological Properties" Polymers 12, no. 11: 2558. https://doi.org/10.3390/polym12112558
APA StylePoudel, D., Swilley-Sanchez, S., O’keefe, S., Matson, J., Long, T., & Fernández-Fraguas, C. (2020). Novel Electrospun Pullulan Fibers Incorporating Hydroxypropyl-β-Cyclodextrin: Morphology and Relation with Rheological Properties. Polymers, 12(11), 2558. https://doi.org/10.3390/polym12112558







