Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Gigantochloa Levis Bamboo-MCC (GL-MCC)
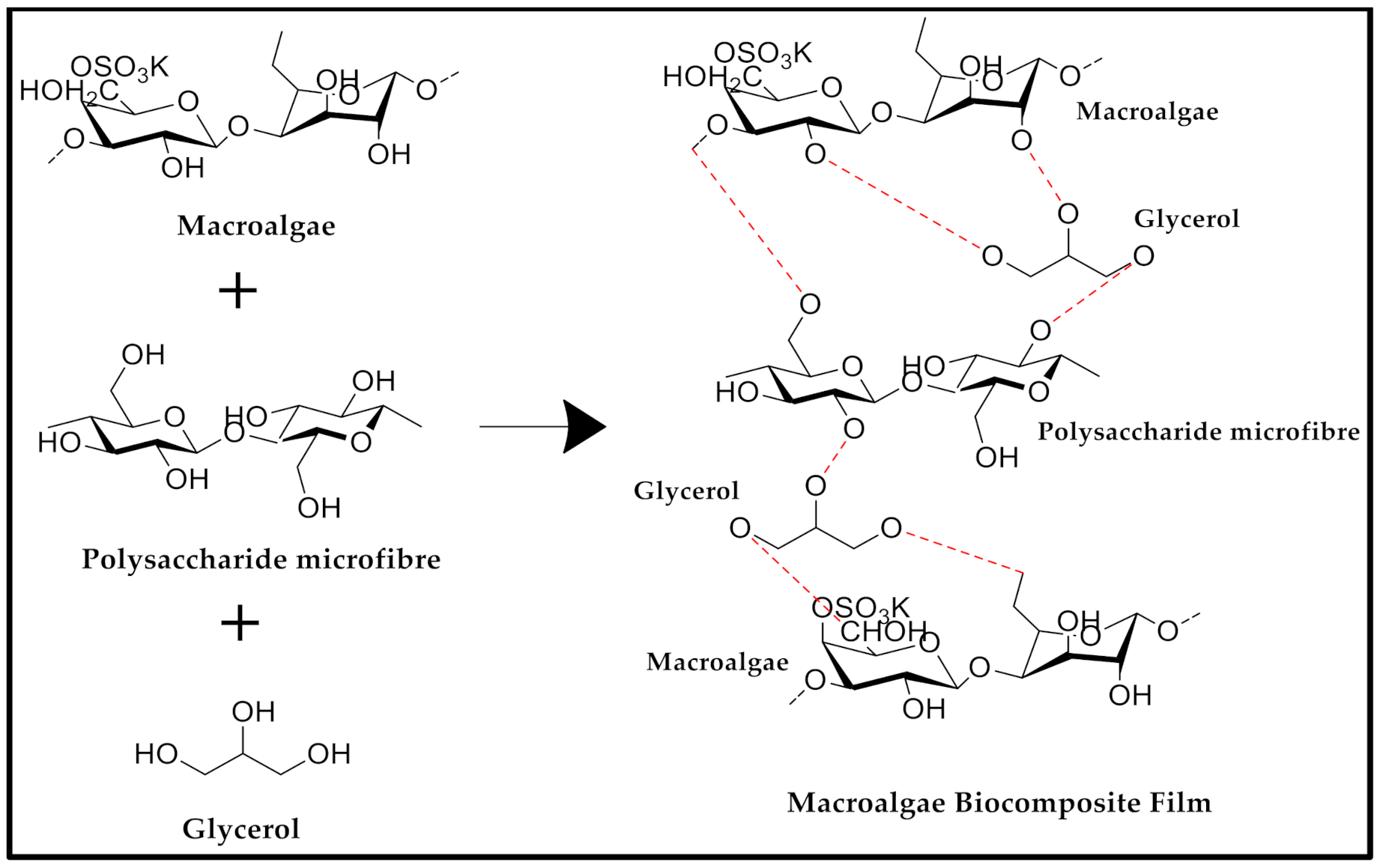
2.2. Preparation of Macroalgae (SW)/Polysaccharide Microfibre (GL-MCC) Biopolymer Film
2.3. Moisture Content (MC)
2.4. Water Absorption Capacity (WSC)
2.5. Film Thickness
2.6. Morphological Property
2.7. X-Ray Diffraction Micrographs (X-RD)
2.8. Thermogravimetric Analysis
2.9. Tensile Properties
2.10. Water Contact Angle (WCA)
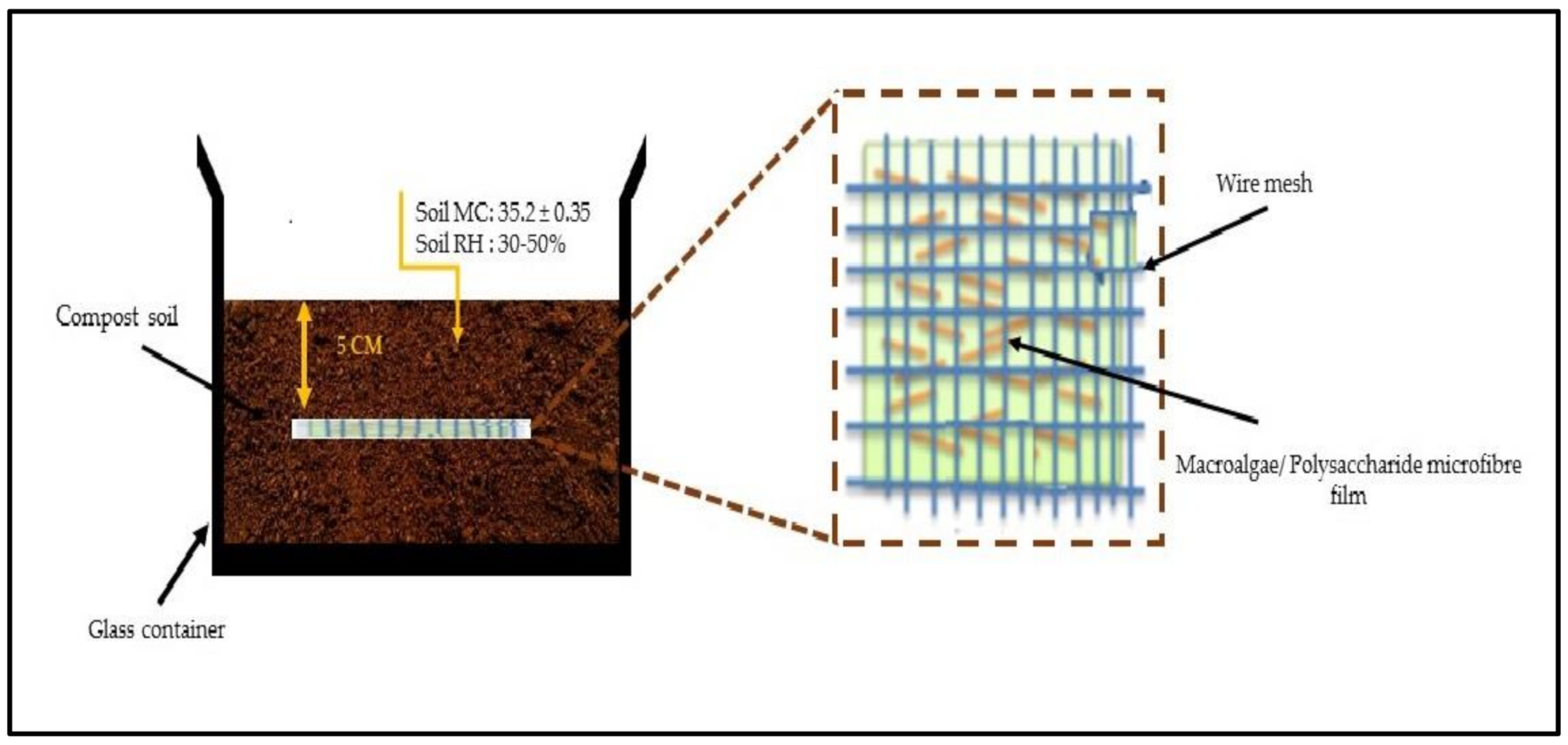
2.11. Soil Burial Test of Macroalgae (SW)/Polysaccharide Microfibre (GL-MCC) Biopolymer Films
2.12. Fourier Transforms Infrared Spectroscopy (FT-IR) Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Moisture Content (MC)
3.2. Water Sorption Capacity (WSC)
3.3. Morphological Properties of Polysaccharide Microfibre (GL-MCC) and Macroalgae (SW)/Polysaccharide Microfibre (GL-MCC) Biopolymer Films
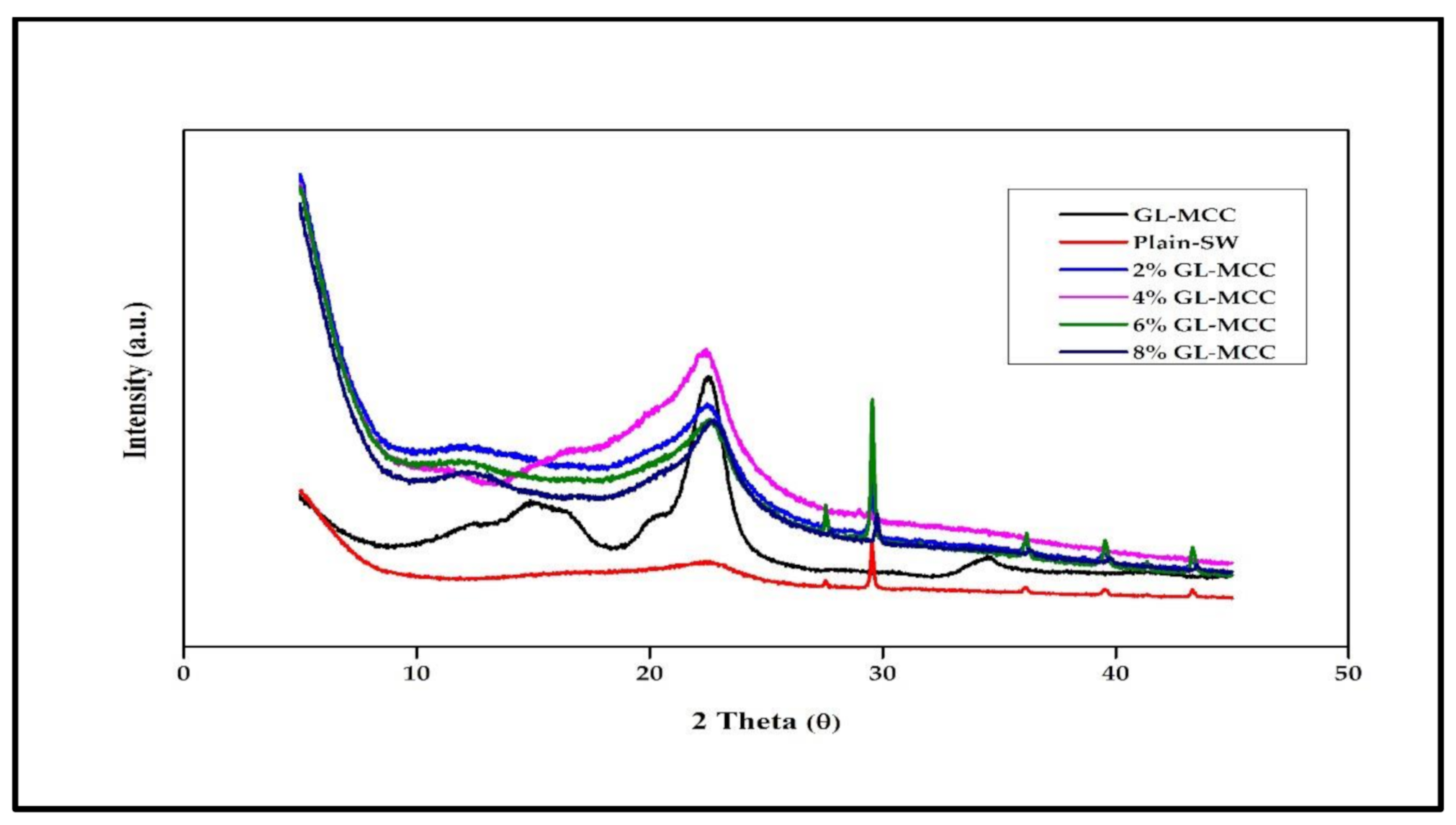
3.4. The Crystallinity Index of GL-MCC and SW/GL-MCC Biopolymer Films
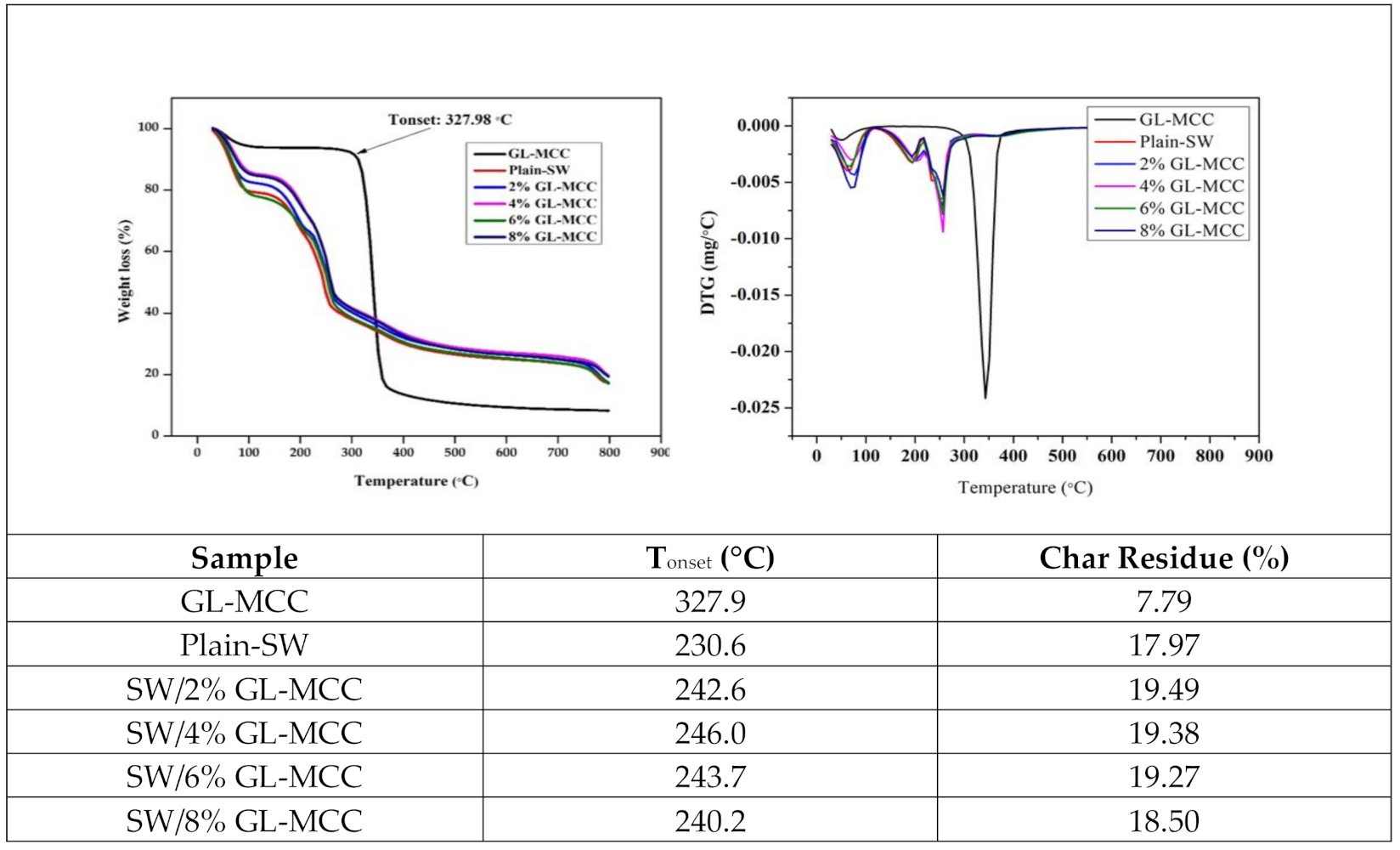
3.5. Thermal Properties of GL-MCC and Macroalgae (SW)/GL-MCC Biopolymer Films
3.6. Tensile Properties of Macroalgae (SW)/GL-MCC Biopolymer Films
3.7. Wettability of Macroalgae (SW)/Polysaccharide Microfibre (GL-MCC) Biopolymer Films
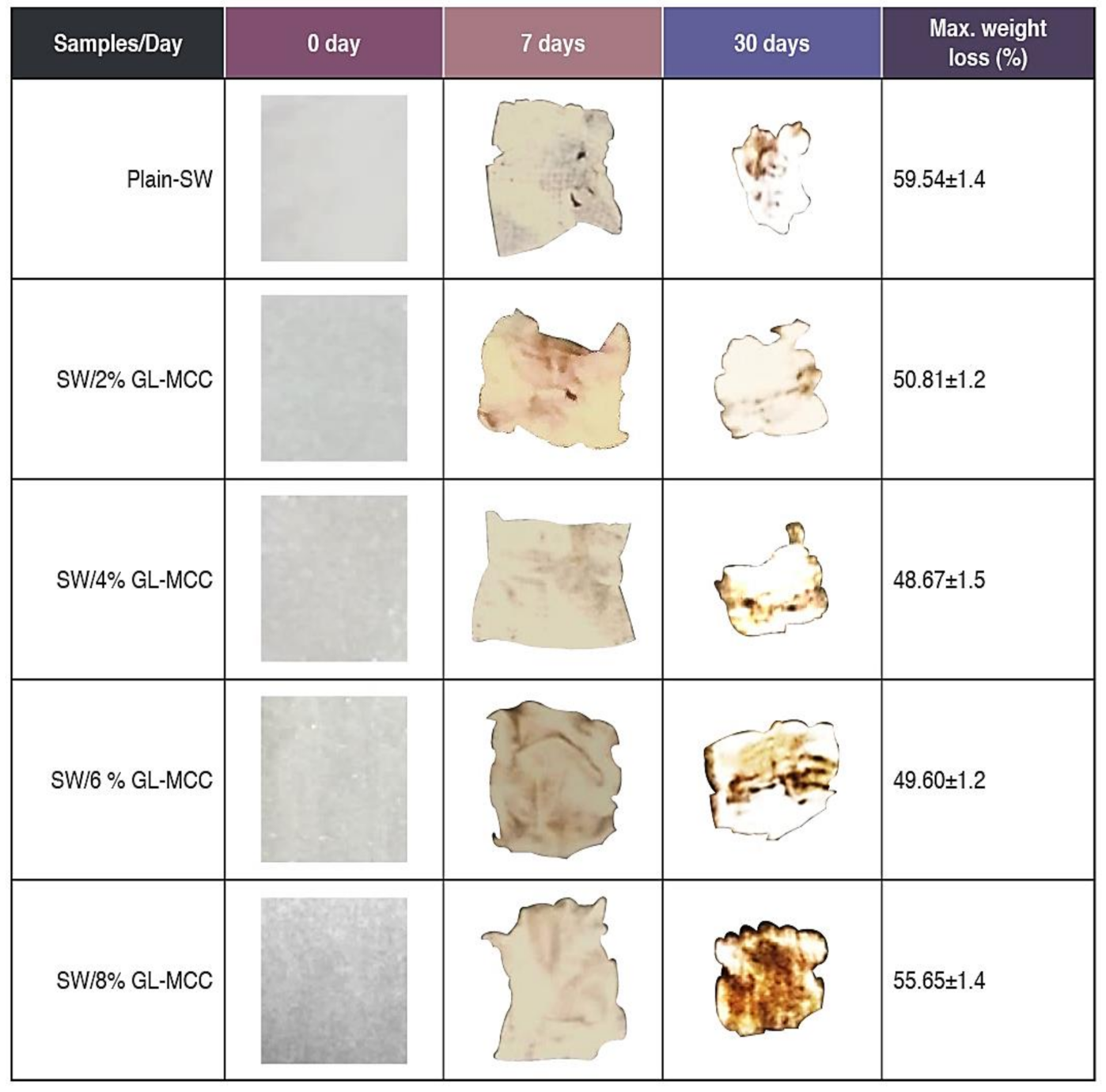
3.8. Biodegradability of Macroalgae (SW)/Polysaccharide Microfibre (GL-MCC) Biopolymer Films
3.9. FT-IR Characterisation of Macroalgae (SW)/Polysaccharide Microfibre (GL-MCC) Biopolymer Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Plackett, D. Biopolymers: New Materials for Sustainable Films and Coatings; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bahari, S.A.; Krause, A. Utilizing Malaysian bamboo for use in thermoplastic composites. J. Clean. Prod. 2016, 110, 16–24. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of nanocellulose from micro-crystalline cellulose: The effect on the performance and properties of agar-based composite films. Carbohydr. Polym. 2016, 135, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Yap, S.W.; Tye, Y.Y.; Tahir, P.M.; Rizal, S.; Fazita, M.N. Effects of corn starch and Kappaphycus alvarezii seaweed blend concentration on the optical, mechanical, and water vapor barrier properties of composite films. BioResources 2018, 13, 1157–1173. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Czech, Z. Citric acid modified potato starch films containing microcrystalline cellulose reinforcement—Properties and application. Starch 2014, 66, 660–667. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, H.; Cai, H.; Han, X.; Lin, X.; Qian, M.; Zhao, Y.; Huo, E.; Villota, E.M.; Mateo, W. Improvement on the properties of microcrystalline cellulose/polylactic acid composites by using activated biochar. J. Clean. Prod. 2020, 252, 119898. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Bhagabati, P.; Kumar, A.; Katiyar, V. Morphology and crystalline characteristics of polylactic acid [PLA]/linear low density polyethylene [LLDPE]/microcrystalline cellulose [MCC] fiber composite. Compos. Sci. Technol. 2019, 171, 54–61. [Google Scholar] [CrossRef]
- Wong, K.M. The Bamboos of Peninsular Malaysia; Malaysia Forest Records.no 41; Forest Research Institute Malaysia: Kepong, Malaysia, 1995. [Google Scholar]
- Hermawan, D.; Lai, T.K.; Jafarzadeh, S.; Gopakumar, D.A.; Hasan, M.; Owolabi, F.T.; Aprilia, N.S.; Rizal, S.; Abdul Khalil, H.P.S. Development of seaweed-based bamboo microcrystalline cellulose films intended for sustainable food packaging applications. BioResources 2019, 14, 3389–3410. [Google Scholar]
- Izzati, A.N.A.; John, W.; Fazita, M.N.; Najieha, N.; Azniwati, A.; Abdul Khalil, H.P.S. Effect of empty fruit bunches microcrystalline cellulose (MCC) on the thermal, mechanical and morphological properties of biodegradable poly (lactic acid)(PLA) and polybutylene adipate terephthalate (PBAT) composites. Mater. Res. Express 2020, 7, 015336. [Google Scholar] [CrossRef]
- Zakaria, Z.; Islam, M.; Hassan, A.; Mohamad Haafiz, M.; Arjmandi, R.; Inuwa, I.; Hasan, M. Mechanical properties and morphological characterization of PLA/chitosan/epoxidized natural rubber composites. Adv. Mater. Sci. Eng. 2013, 2013. [Google Scholar] [CrossRef]
- Rizal, S.; Fizree, H.; Hossain, M.S.; Gopakumar, D.A.; Ni, E.C.W.; Abdul Khalil, H.P.S. The role of silica-containing agro-industrial waste as reinforcement on physicochemical and thermal properties of polymer composites. Heliyon 2020, 6, e03550. [Google Scholar] [CrossRef]
- Pawar, R.; Tekale, S.U.; Shisodia, S.; Totre, J.T.; Domb, A.J. Biomedical applications of poly (lactic acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Jawaid, M.; Abdul Khalil, H.P.S. Cellulosic/synthetic fibre reinforced polymer hybrid composites: A review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Pathak, V.M. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Zarina, S.; Ahmad, I. Biodegradable composite films based on κ-carrageenan reinforced by cellulose nanocrystal from kenaf fibers. BioResources 2015, 10, 256–271. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Torres-Hernández, Y.G.; Ortega-Díaz, G.M.; Téllez-Jurado, L.; Castrejón-Jiménez, N.S.; Altamirano-Torres, A.; García-Pérez, B.E.; Balmori-Ramírez, H. Biological compatibility of a polylactic acid composite reinforced with natural chitosan obtained from shrimp waste. Materials 2018, 11, 1465. [Google Scholar] [CrossRef]
- Suvachittanont, S.; Ratanapan, P. Optimization of micro crystalline cellulose production from corn cob for pharmaceutical industry investment. J. Chem. Chem. Eng. 2013, 7, 1136. [Google Scholar]
- Pachuau, L.; Vanlalfakawma, D.C.; Tripathi, S.K.; Lalhlenmawia, H. Muli bamboo (Melocanna baccifera) as a new source of microcrystalline cellulose. J. Appl. Pharm. Sci. 2014, 4, 87–94. [Google Scholar]
- Chuayjuljit, S.; Su-uthai, S.; Charuchinda, S. Poly (vinyl chloride) film filled with microcrystalline cellulose prepared from cotton fabric waste: Properties and biodegradability study. Waste Manag. Res. 2010, 28, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Almasi, H. Physical properties of edible emulsified films based on carboxymethyl cellulose and oleic acid. Int. J. Biol. Macromol. 2011, 48, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yi, Y.; Wang, H.; Zhou, W.; Yang, Y.; Wang, C. Physical and degradable properties of mulching films prepared from natural fibers and biodegradable polymers. Appl. Sci. 2016, 6, 147. [Google Scholar] [CrossRef]
- Uthaya Kumar, U.S.; Abdulmadjid, S.; Olaiya, N.; Amirul, A.; Rizal, S.; Rahman, A.; Alfatah, T.; Mistar, E.; Abdul Khalil, H.P.S. Extracted Compounds from Neem Leaves as Antimicrobial Agent on the Physico-Chemical Properties of Seaweed-Based Biopolymer Films. Polymers 2020, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Achor, M.; Oyeniyi, Y.; Yahaya, A. Extraction and characterization of microcrystalline cellulose obtained from the back of the fruit of Lageriana siceraria (water gourd). J. Appl. Pharm. Sci. 2014, 4, 57–60. [Google Scholar]
- Ohwoavworhua, F.; Adelakun, T. Some physical characteristics of microcrystalline cellulose obtained from raw cotton of Cochlospermum planchonii. Trop. J. Pharm. Res. 2005, 4, 501–507. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Cao, J.; Wei, S.; Tan, H.; Zhang, Y. Effect of starch/polylactic acid ratio on the interdependence of two-phase and the properties of composites. J. Wuhan Univ. of Technol. Mater. Sci. Ed. 2015, 30, 1108–1114. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose. Carbohydr. Polym. 2014, 110, 480–488. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Characteristics of Eucheuma cottonii waste from East Malaysia: Physical, thermal and chemical composition. Eur. J. Phycol. 2017, 52, 200–207. [Google Scholar] [CrossRef]
- Whatley, B.R.; Wen, X. Intervertebral disc (IVD): Structure, degeneration, repair and regeneration. Mater. Sci. Eng. C 2012, 32, 61–77. [Google Scholar] [CrossRef]
- dos Santos, F.A.; Iulianelli, G.C.; Tavares, M.I. Effect of microcrystalline and nanocrystals cellulose fillers in materials based on PLA matrix. Polym. Test. 2017, 61, 280–288. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Li, Y. Structure–property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Pothan, L.A.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable nanocomposite films based on sodium alginate and cellulose nanofibrils. Materials 2016, 9, 50. [Google Scholar] [CrossRef]




| Film | MC (%) | WSC (%) | Thickness (µm) |
|---|---|---|---|
| Plain-SW | 38.17 ± 0.6 d | 180 ± 3.0 b | 76 ± 0.003 a |
| SW/2% GL-MCC | 31.43 ± 1.8 c | 140 ± 4.0 a | 93 ± 0.004 a b |
| SW/4% GL-MCC | 23.83 ± 1.6 b | 136 ± 3.0 a | 102 ± 0.003 ab |
| SW/6% GL-MCC | 25.30 ± 0.9 a | 146 ± 3.0 a | 105 ± 0.005 ab |
| SW/8% GL-MCC | 27.50 ± 0.2 a | 158 ± 5.0 a | 108 ± 0.006 b |
| Samples | Droplet Image | Contact Angle (°) | CA ± SD |
|---|---|---|---|
| Plain-SW | 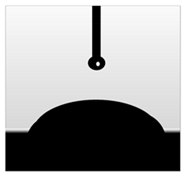 |  | 42.00 ± 0.53 a |
| SW/2% GL-MCC | 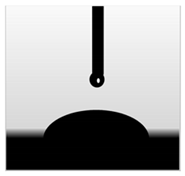 |  | 49.11 ± 0.24 b |
| SW/4% GL-MCC |  | 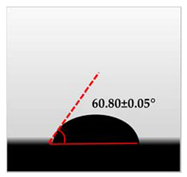 | 60.80 ± 0.05 d |
| SW/6% GL-MCC |  |  | 54.76 ± 0.50 c |
| SW/8% GL-MCC |  |  | 50.12 ± 0.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizal, S.; Lai, T.K.; Muksin, U.; Olaiya, N.G.; Abdullah, C.K.; Ikramullah; Yahya, E.B.; Chong, E.W.N.; Abdul Khalil, H.P.S. Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers 2020, 12, 2554. https://doi.org/10.3390/polym12112554
Rizal S, Lai TK, Muksin U, Olaiya NG, Abdullah CK, Ikramullah, Yahya EB, Chong EWN, Abdul Khalil HPS. Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers. 2020; 12(11):2554. https://doi.org/10.3390/polym12112554
Chicago/Turabian StyleRizal, Samsul, Tze Kiat Lai, Umar Muksin, N.G. Olaiya, C.K. Abdullah, Ikramullah, Esam Bashir Yahya, E.W.N. Chong, and H.P.S. Abdul Khalil. 2020. "Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre" Polymers 12, no. 11: 2554. https://doi.org/10.3390/polym12112554
APA StyleRizal, S., Lai, T. K., Muksin, U., Olaiya, N. G., Abdullah, C. K., Ikramullah, Yahya, E. B., Chong, E. W. N., & Abdul Khalil, H. P. S. (2020). Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers, 12(11), 2554. https://doi.org/10.3390/polym12112554








