Assessing the Radiological Density and Accuracy of Mandible Polymer Anatomical Structures Manufactured Using 3D Printing Technologies
Abstract
1. Introduction
2. Materials and Methods
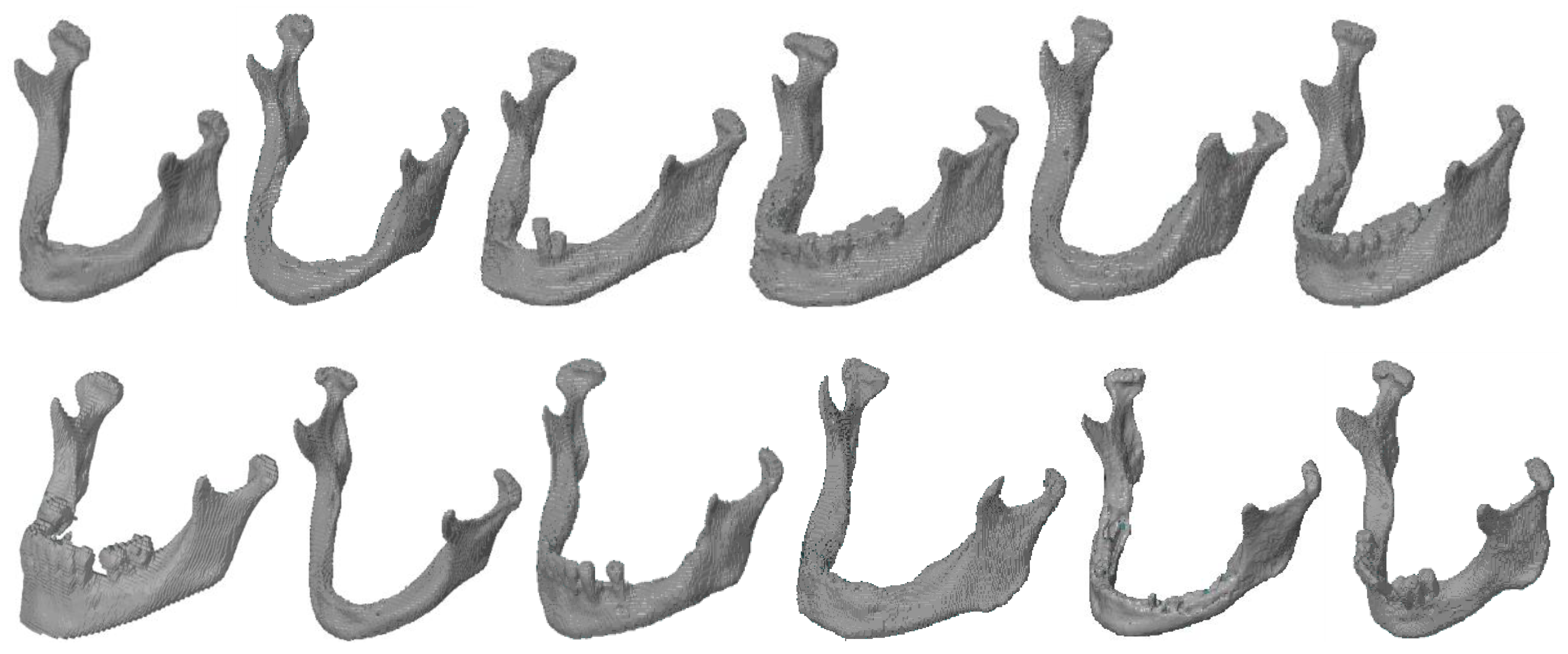
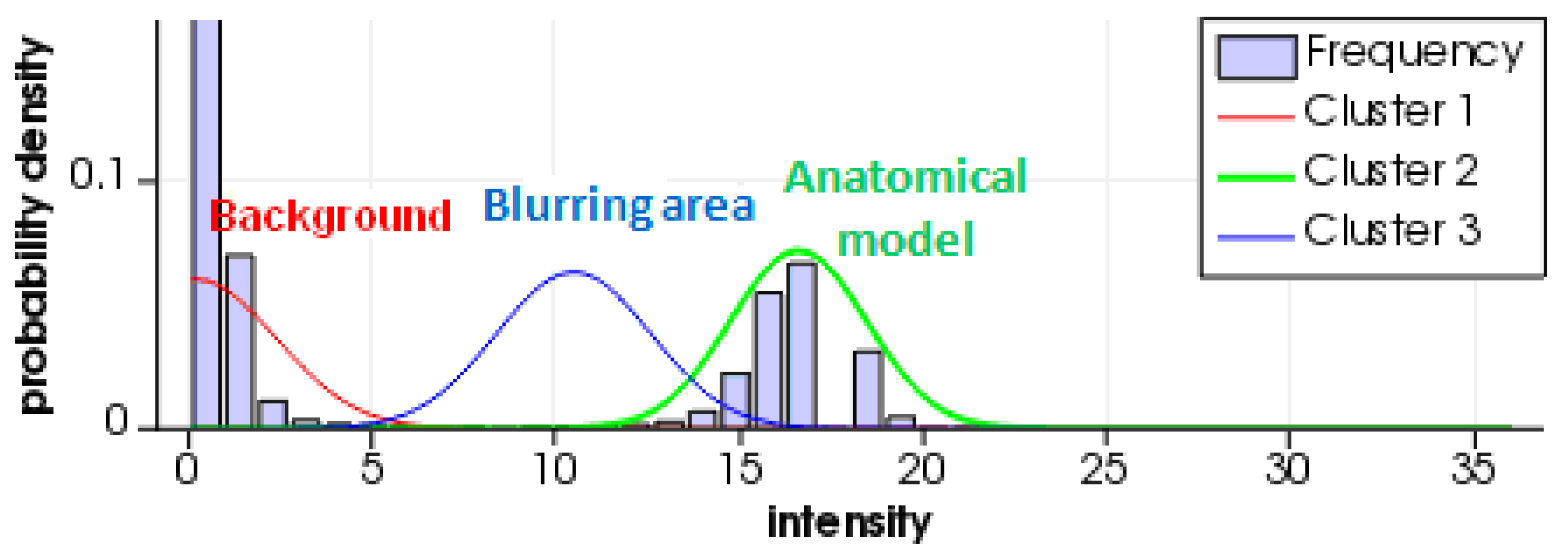
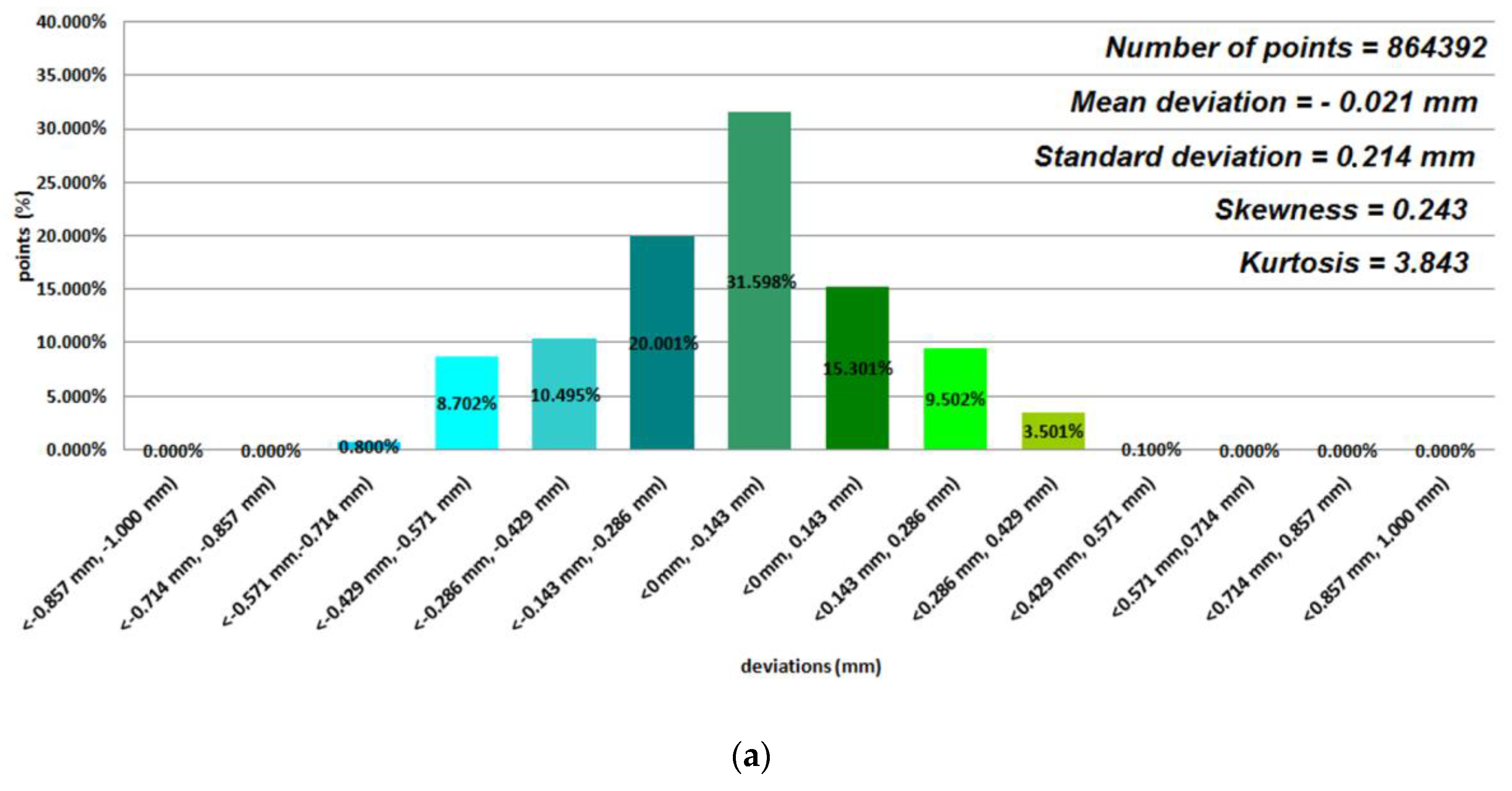
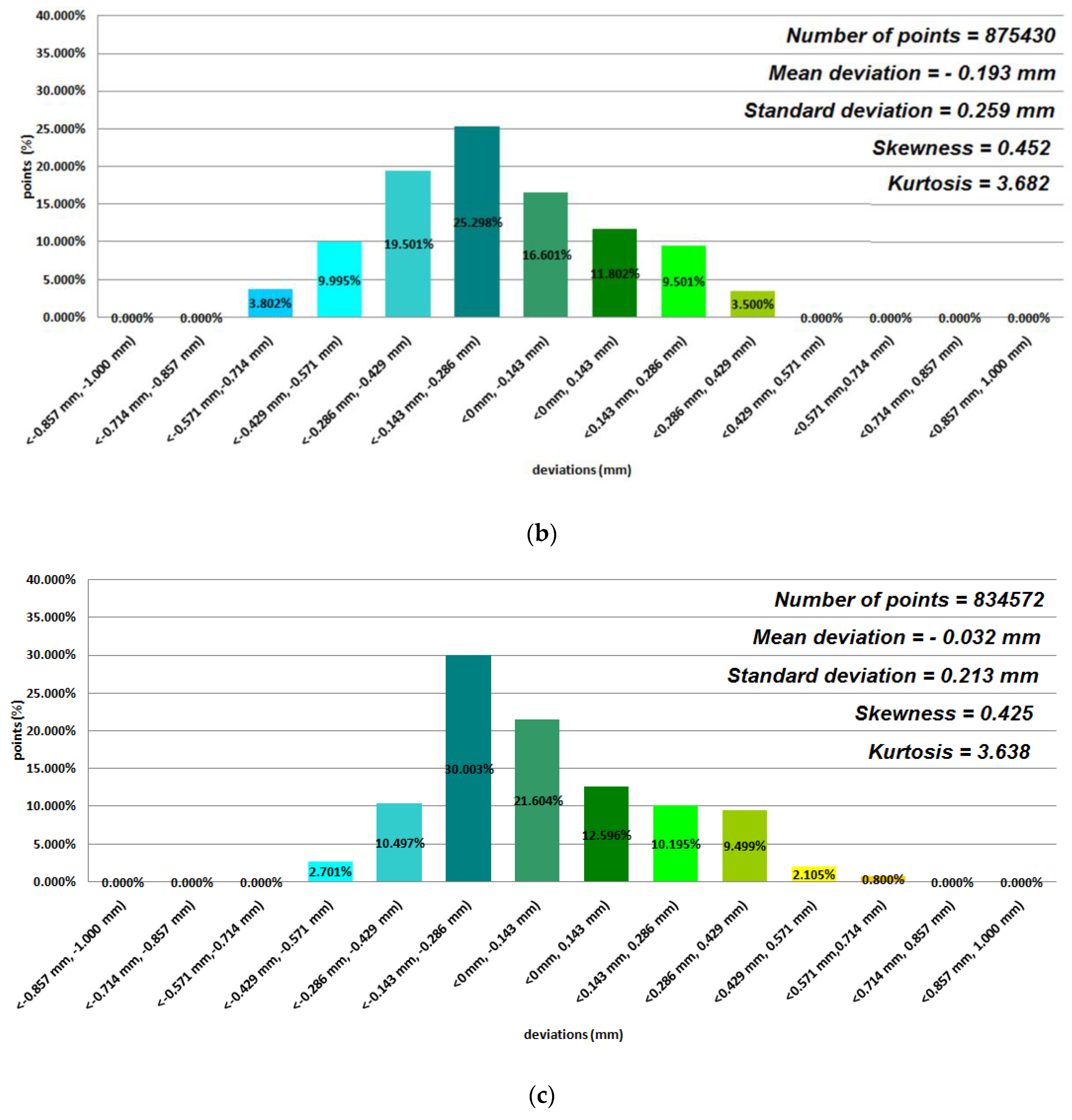
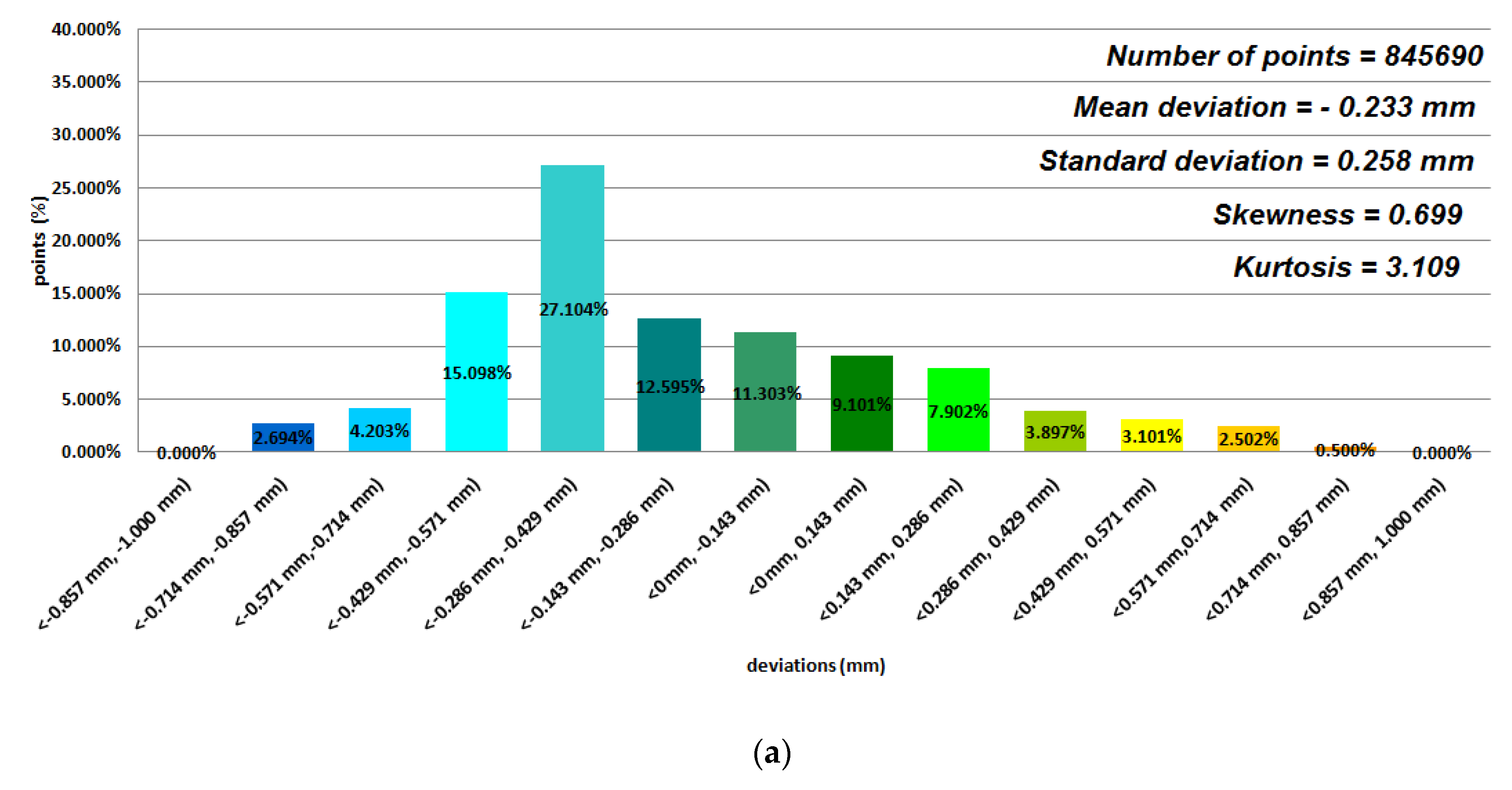
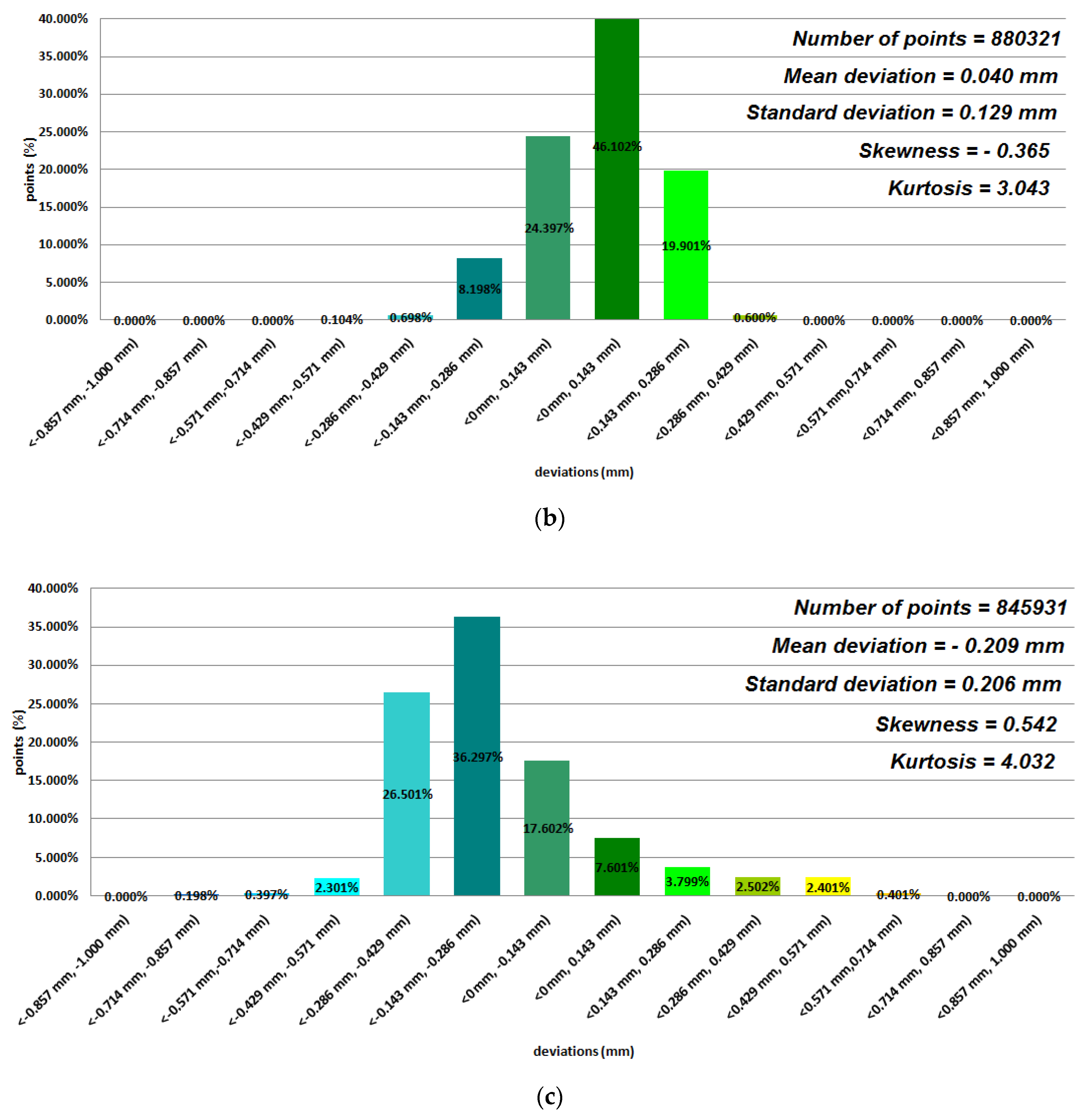
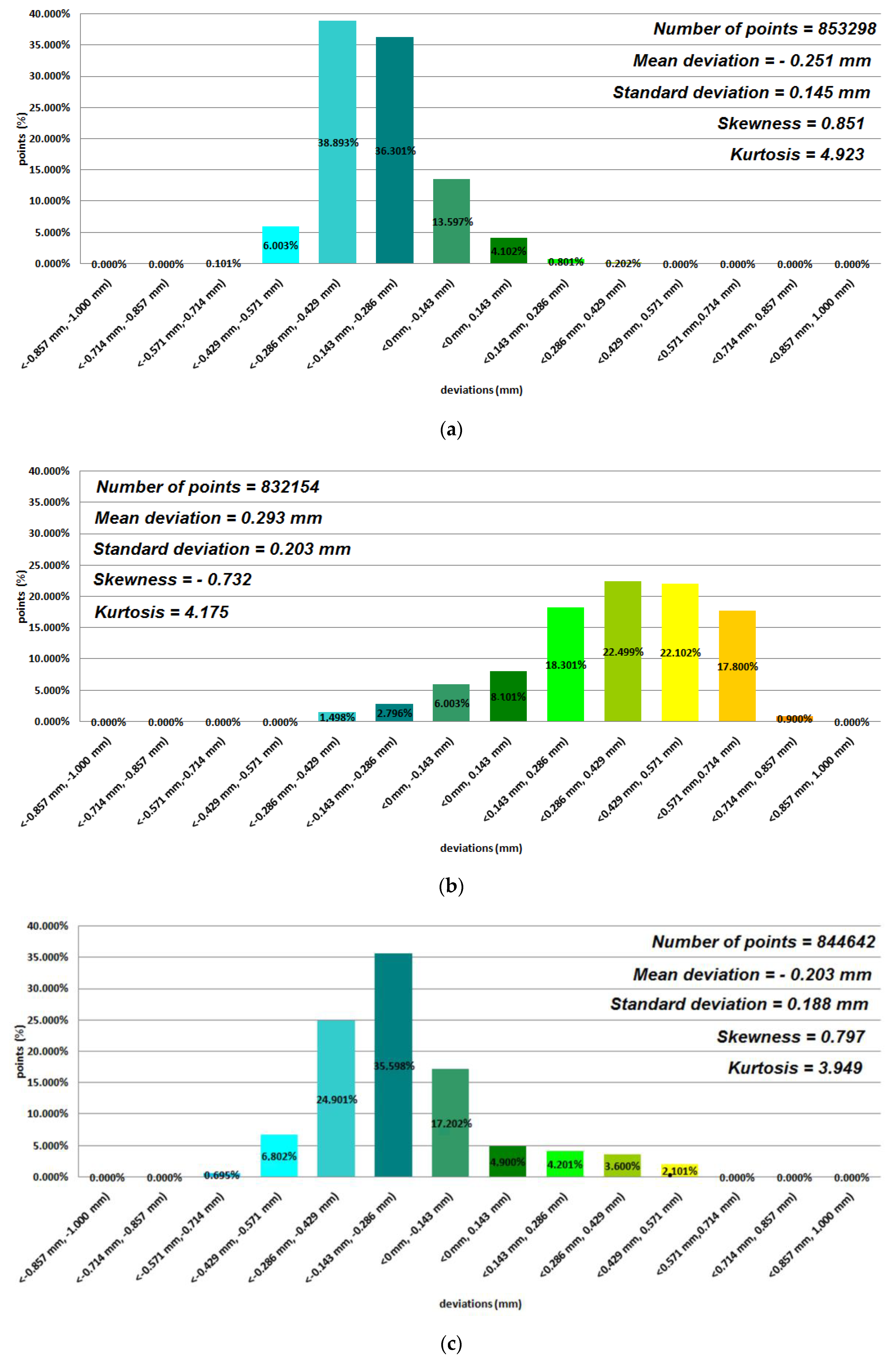
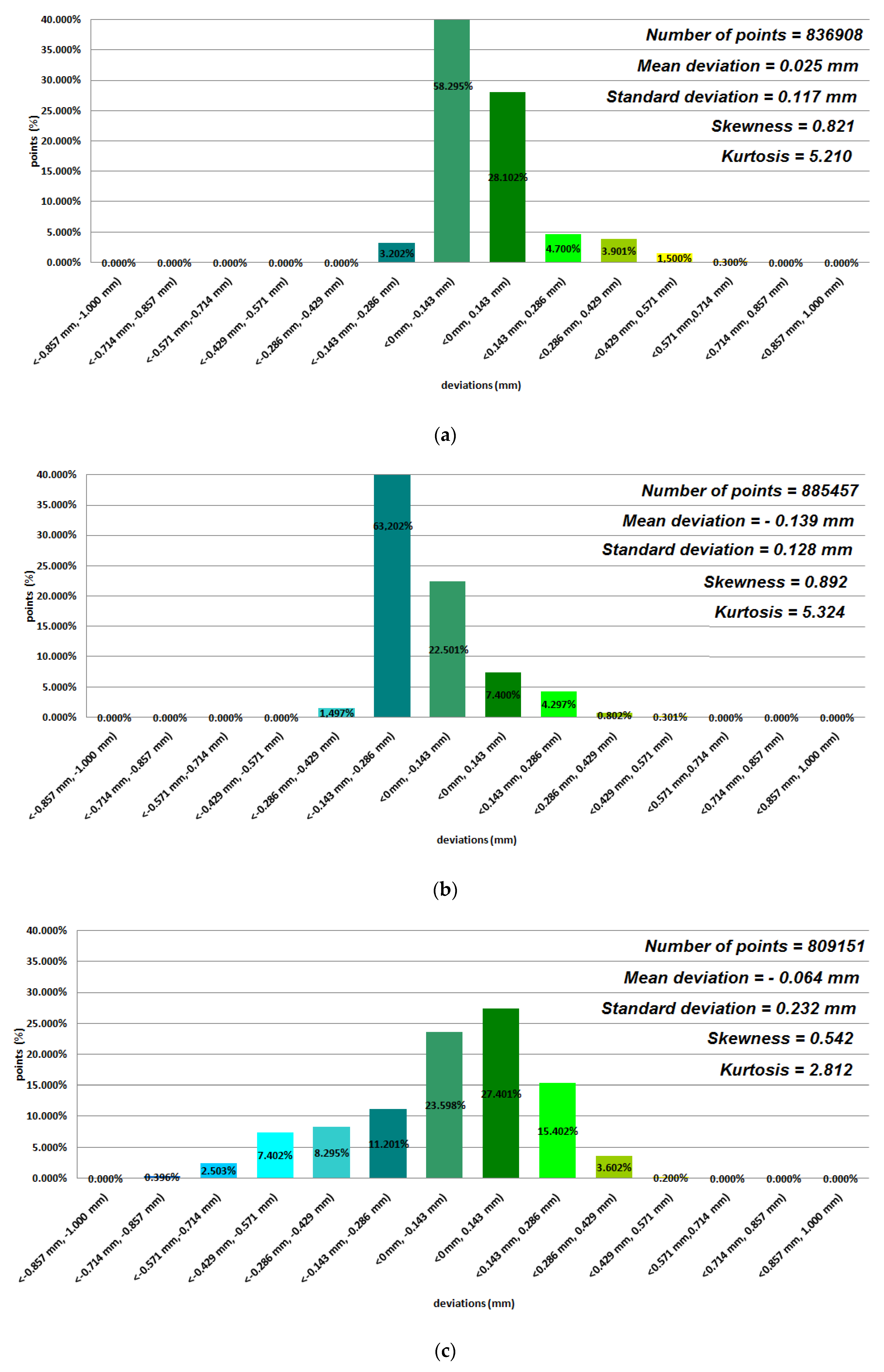
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gebhard, A. Rapid Prototyping; Hanser: Munich, Germany, 2003. [Google Scholar]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies; Springer: New York, NY, USA, 2014. [Google Scholar]
- Boboulos, M. CAD-CAM and Rapid Prototyping Application Evaluation. 2010. Available online: http://sietm.com/wp-content/uploads/2015/03/cad-cam-rapid-prototyping-application-evaluation.pdf (accessed on 19 October 2020).
- Thompson, M.K.; Moroni, G.; Vaneker, T.; Fadel, G.; Campbell, R.I.; Gibson, I.; Bernard, A.; Schulz, J.; Graf, P.; Ahuja, B.; et al. Design for Additive Manufacturing: Trends, opportunities, considerations, and constraints. CIRP Ann. Manuf. Technol. 2016, 65, 737–760. [Google Scholar] [CrossRef]
- Raja, V.; Kiran, J.F. Reverse Engineering—An Industrial Perspective; Springer: New York, NY, USA, 2010. [Google Scholar]
- Bidanda, B.; Bartolo, P. Virtual Prototyping & Bio Manufacturing in Medical Applications; Springer: New York, NY, USA, 2008. [Google Scholar]
- Ford, S.; Despeisse, M. Additive manufacturing and sustainability: An exploratory study of the advantages and challenges. J. Clean. Prod. 2016, 137, 1573–1587. [Google Scholar] [CrossRef]
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput. Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B-Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Gisario, A.; Kazarian, M.; Martina, F.; Mehrpouya, M. Metal additive manufacturing in the commercial aviation industry: A review. J. Manuf. Syst. 2019, 53, 124–149. [Google Scholar] [CrossRef]
- Chu, M.Q.; Wang, L.; Ding, H.Y.; Sun, Z.G. Additive manufacturing for aerospace application. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2015; pp. 457–461. [Google Scholar]
- Rokicki, P.; Budzik, G.; Kubiak, K.; Dziubek, T.; Zaborniak, M.; Kozik, B.; Bernaczek, J.; Przeszlowski, L.; Nowotnik, A. The assessment of geometric accuracy of aircraft engine blades with the use of an optical coordinate scanner. Aircr. Eng. Aerosp. Tec. 2016, 88, 374–381. [Google Scholar] [CrossRef]
- Leal, R.; Barreiros, F.M.; Alves, L.; Romeiro, F.; Vasco, J.C.; Santos, M.; Marto, C. Additive manufacturing tooling for the automotive industry. Int. J. Adv. Manuf. Technol. 2017, 92, 1671–1676. [Google Scholar] [CrossRef]
- Lecklider, T. 3D printing drives automotive innovation. Eval. Eng. 2017, 56, 16–20. [Google Scholar]
- Ciocca, L.; Mazzoni, S.; Fantini, M.; Persiani, F.; Baldissara, P.; Marchetti, C.; Scotti, R. A CAD/CAM-prototyped anatomical condylar prosthesis connected to a custom-made bone plate to support a fibula free lap. Med. Biol. Eng. Comput. 2012, 50, 743–749. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Ke, C.-J.; Yen, K.-C.; Hsieh, H.-C.; Sun, J.-S.; Lin, F.-H. 3D porous calcium-alginate scaffolds cell culture system improved human osteoblast cell clusters for cell therapy. Theranostics 2015, 5, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Turek, P.; Budzik, G.; Oleksy, M.; Bulanda, K. Polymer materials used in medicine processed by additive techniques. Polimery 2020, 65, 510–515. [Google Scholar] [CrossRef]
- Faber, J.; Berto, P.M.; Quaresma, M. Rapid prototyping as a tool for diagnosis and treatment planning for maxillary canine impaction. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 583–589. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Kim, Y.; Ahn, H.-W.; Kim, K.-B.; Chung, K.-R.; Sunny, S.-H.K. Computer-Aided Designing and Manufacturing of Lingual Fixed Orthodontic Appliance Using 2D/3D Registration Software and Rapid Prototyping. Int. J. Dent. 2014, 2014, 164164. [Google Scholar] [CrossRef]
- Martorelli, M.; Gerbino, S.; Giudice, M.; Ausiello, P. A comparison between customized clear and removable orthodontic appliances manufactured using RP and CNC techniques. Dent. Mater. 2013, 29, e1–e10. [Google Scholar] [CrossRef]
- Gibson, I.; Kvan, T.; Ming, L.W. Rapid prototyping for architectural models. Rapid Prototyp. J. 2002, 8, 91–95. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Using additive manufacturing applications for design and development of food and agricultural equipments. Int. J. Mater. Prod. Technol. 2019, 58, 225–238. [Google Scholar] [CrossRef]
- Niu, X.; Singh, S.; Garg, A.; Singh, H.; Panda, B.; Peng, X.; Zhang, Q. Review of materials used in laser-aided additive manufacturing processes to produce metallic products. Front. Mech. Eng. 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Joshi, S.C.; Sheikh, A.A. 3D printing in aerospace and its long-term sustainability. Virtual Phys. Prototy. 2015, 10, 175–185. [Google Scholar] [CrossRef]
- Dziubek, T.; Oleksy, M. Application of ATOS II optical system in the techniques of rapid prototyping of epoxy resin-based gear models. Polimery 2017, 62, 44–52. [Google Scholar] [CrossRef]
- García-Martínez, H.; Ávila-Navarro, E.; Torregrosa-Penalva, G.; Rodriguez-Martinez, A.; Blanco-Angulo, C.; de la Casa-Lillo, M. Low-Cost Additive Manufacturing Techniques Applied to the Design of Planar Microwave Circuits by Fused Deposition Modeling. Polymers 2020, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
- Zanjanijam, A.R.; Major, I.; Lyons, J.G.; Lafont, U.; Devine, D.M. Fused Filament Fabrication of PEEK: A Review of Process-Structure-Property Relationships. Polymers 2020, 12, 1665. [Google Scholar] [CrossRef] [PubMed]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive manufacturing of ceramic-based materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- Deckers, J.; Vleugels, J.; Kruth, J.-P. Additive manufacturing of ceramics: A review. J. Ceram. Sci. Technol. 2014, 5, 245–260. [Google Scholar]
- Parandoush, P.; Lin, D. A review on additive manufacturing of polymer-fiber composites. Compos. Struct. 2017, 182, 36–53. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D printing of polymer nanocomposites via stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Berretta, S.; Evans, K.; Ghita, O. Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance. Mater. Des. 2018, 139, 141–152. [Google Scholar] [CrossRef]
- Chen, P.; Cai, H.; Li, Z.; Li, M.; Wu, H.; Su, J.; Wen, S.; Zhou, Y.; Liu, J.; Wang, C.; et al. Crystallization Kinetics of Polyetheretherketone during High Temperature-Selective Laser Sintering. Addit. Manuf. 2020, 36, 101615. [Google Scholar] [CrossRef]
- Kozior, T.; Mamun, A.; Trabelsi, M.; Sabantina, L.; Ehrmann, A. Quality of the Surface Texture and Mechanical Properties of FDM Printed Samples after Thermal and Chemical Treatment. Stroj. Vestn.-J. Mech. Eng. 2020, 66, 105–113. [Google Scholar]
- Dizon, J.R.C.; Espera, A.H., Jr.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel materials for 3D printing by photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Sakhaei, A.H.; Layani, M.; Zhang, B.; Ge, Q.; Magdassi, S. Highly stretchable and UV curable elastomers for digital light processing based 3D printing. Adv. Mater. 2017, 29, 1606000. [Google Scholar] [CrossRef] [PubMed]
- Budzik, G.; Burek, J.; Bazan, A.; Turek, P. Analysis of the accuracy of reconstructed two teeth models manufactured using the 3DP and FDM technologies. Stroj. Vestn.-J. Mech. Eng. 2016, 62, 11–20. [Google Scholar] [CrossRef][Green Version]
- Budzik, G.; Turek, P.; Dziubek, T.; Gdula, M. Elaboration of the measuring procedure facilitating precision assessment of the geometry of mandible anatomical model manufactured using additive methods. Meas. Control. 2020, 53, 181–191. [Google Scholar] [CrossRef]
- Turek, P. Automatic the process of designing and manufacturing polymeric models of anatomical structures of mandible with Industry 4.0 convention. Polimery 2019, 64, 522–529. [Google Scholar] [CrossRef]
- Pietruski, P.; Majak, M.; Swiatek-Najwer, E.; Popek, M.; Szram, D.; Jaworowski, J. Accuracy of experimental mandibular osteotomy using the image-guided sagittal saw. Int J. Oral Maxillofac. Surg. 2016, 45, 793–800. [Google Scholar] [CrossRef]
- Farias, T.P.; Dias, F.L.; Sousa, B.A.; Galvão, M.S.; Bispo, D.; Pastl, A.C. Prototyping: Major Advance in Surgical Planning and Customizing Prostheses in Patients with Bone Tumors of the Head and Neck. Int. J. Clin. Med. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C. 3D printing of polyether-ether-ketone for biomedical application. Eur. Polym. J. 2019, 114, 234–248. [Google Scholar] [CrossRef]
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef]
- Kozakiewicz, M. Computer-aided orbital wall defects treatment by individual design ultrahigh molecular weight polyethylene implants. J. Craniomaxillofac. Surg. 2014, 42, 283–289. [Google Scholar] [CrossRef]
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Tutak, J.S. Design of ELISE robot for the paretic upper limb of stroke survivors. J. Vibroeng. 2016, 18, 4069–4085. [Google Scholar] [CrossRef]
- Barrios-Muriel, J.; Romero-Sánchez, F.; Alonso-Sánchez, F.J.; Rodríguez Salgado, D. Advances in orthotic and prosthetic manufacturing: A technology review. Materials 2020, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Paloheimo, K.-S.; Tuomi, J.; Wolff, J.; Mäkitie, A. Accuracy of medical models made by additive manufacturing (rapid manufacturing). J. Craniomaxillofac. Surg. 2013, 41, 603–609. [Google Scholar] [CrossRef] [PubMed]
- El-Katatny, I.; Masood, S.H.; Morsi, Y.S. Error analysis of FDM fabricated medical replicas. Rapid Prototyp. J. 2010, 16, 36–43. [Google Scholar] [CrossRef]
- Ibrahim, D.; Broilo, T.L.; Heitz, C.; de Oliveira, M.G.; de Oliveira, H.W.; Nobre, S.M.W.; Gomes dos Santos Filho, J.H.; Silva, D.N. Dimensional error of selective laser sintering, threedimensional printing and PolyJet™ models in the reproduction of mandibular anatomy. J. Craniomaxillofac. Surg. 2009, 37, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Nizam, A.; Gopal, R.N.; Naing, L.; Hakim, A.B.; Samsudin, A.R. Dimensional accuracy of the skull models produced by rapid prototyping technology using stereolithography apparatus. Arch. Orofac Sci. 2006, 1, 60–66. [Google Scholar]
- Safira, L.C.; Bastos, l.C.; Beal, V.E.; de Azevedo, R.A.; Francischone, C.E.; Sarmento, V.A. Accuracy of rapid prototyping biomodels plotted by three dimensional printing technique: Ex vivo study. Adv. Comput. Tomogr. 2013, 2, 41–45. [Google Scholar] [CrossRef]
- Silva, D.N.; de Oliveira, M.G.; Meurer, E.; Meurer, M.I.; Lopes da Silva, J.V.; Santa-Bárbara, A. Dimensional error in selective laser sintering and 3D-printing of 164 models for craniomaxillary anatomy reconstruction. J. Craniomaxillofac. Surg. 2008, 36, 443–449. [Google Scholar] [CrossRef]
- Primo, B.T.; Presotto, A.C.; de Oliveira, H.W.; Gassen, H.T.; Miguens, S.A.Q., Jr.; Silva, A.N., Jr.; Hernandez, P.A.G. Accuracy assessment of prototypes produced Rusing multi-slice and cone-beam computed tomography. Int. J. Oral Maxillofac. Surg. 2012, 41, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, J.; Jiménez, R.; Rada, M.; Loscos, F. Error compensation method for improving the accuracy of biomodels obtained from CBCT data. Med. Eng. Phys. 2014, 36, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, R.; Szymor, P.; Kozakiewicz, M. Accuracy of three-dimensional, paper-based models generated using a low-cost, three-dimensional printer. J. Craniomaxillofac. Surg. 2014, 42, 1847–1852. [Google Scholar] [CrossRef]
- Szymor, P.; Kozakiewicz, M.; Olszewski, R. Accuracy of open-source software segmentation and paper-based printed three-dimensional models. J. Craniomaxillofac. Surg. 2016, 44, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Huotilainen, E.; Jaanimets, R.; Valášek, J.; Marcián, P.; Salmi, M.; Tuomi, J.; Mäkitie, A.; Wolff, J. Inaccuracies in additive manufactured medical skull models caused by the DICOM to STL conversion process. J. Craniomaxillofac. Surg. 2014, 42, e259–e265. [Google Scholar] [CrossRef]
- Reyes, A.; Turkyilmaz, I.; Prihoda, T.J. Accuracy of surgical guides made from conventional and a combination of digital scanning and rapid prototyping techniques. J. Prosthet. Dent. 2015, 113, 295–303. [Google Scholar] [CrossRef]
- Tino, R.; Yeo, A.; Leary, M.; Brandt, M.; Kron, T. A systematic review on 3d-printed imaging and dosimetry phantoms in radiation therapy. Technol. Cancer Res. Treat. 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Bibb, R.; Thompson, D.; Winder, J. Computed tomography characterisation of additive manufacturing materials. Med. Eng. Phys. 2011, 33, 590–596. [Google Scholar] [CrossRef]
- Leary, M.; Kron, T.; Keller, C.; Franich, R.; Lonski, P.; Subic, A.; Brandt, M. Additive manufacture of custom radiation dosimetry phantoms: An automated method compatible with commercial polymer 3d printers. Mater. Design 2015, 86, 487–499. [Google Scholar] [CrossRef]
- Craft, D.F.; Kry, S.F.; Balter, P.; Salehpour, M.; Woodward, W.; Howell, R.M. Material matters: Analysis of density uncertainty in 3D printing and its consequences for radiation oncology. Med. Phys. 2018, 45, 1614–1620. [Google Scholar] [CrossRef]
- Solc, J.; Vrba, T.; Burianova, L. Tissue-equivalence of 3D-printed plastics for medical phantoms in radiology. J. Instrum. 2018, 13, P09018. [Google Scholar] [CrossRef]
- Kamomae, T.; Shimizu, H.; Nakaya, T.; Okudaira, K.; Aoyama, T.; Oguchi, H.; Komori, M.; Kawamura, M.; Ohtakara, K.; Monzen, H.; et al. Three-dimensional printer-generated patient-specific phantom for artifical in vivo dosimetry in radiotherapy quality assurance. Phys. Med. 2017, 44, 205–2011. [Google Scholar] [CrossRef] [PubMed]
- Alssabbagh, M.; Tajuddin, A.A.; Abdulmanap, M.; Zainon, R. Evaluation of 3D printing materials for fabrication of a novel multifunctional 3D thyroid phantom for medical dosimetry and image quality. Radiat. Phys. Chem. 2017, 135, 106–112. [Google Scholar] [CrossRef]
- Gear, J.I.; Long, C.; Rushforth, D.; Chittenden, S.J.; Cummings, C.; Flux, G.D. Development of patient-specific molecular imaging phantoms using a 3D printer. Med. Phys. 2014, 41, 082502. [Google Scholar] [CrossRef] [PubMed]
- Jasiuk, I.; Abueidda, D.W.; Kozuch, C.; Pang, S.; Su, F.Y.; McKittrick, J. An overview on additive manufacturing of polymers. JOM 2018, 70, 275–283. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. 3D printed medical parts with different materials using additive manufacturing. Clin. Epidemiol. Glob. Health 2020, 8, 215–223. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Champion, A.F.; Curiel, T.M.; Diecidue, R.J. Analysis of computed tomography Hounsfield units of benign lesions in the maxillofacial region: Is there a correlation? Eur. J. Oral Maxillofac. Surg. 2020, 4, 45–48. [Google Scholar] [CrossRef]
- Budzik, G.; Turek, P.; Traciak, J. The influence of change in slice thickness on the accuracy of reconstruction of cranium geometry. Proc. Inst. Mech. Eng. H 2017, 231, 197–202. [Google Scholar] [CrossRef]
- Alsleem, H.; Davidson, R. Factors affecting contrast-detail performance in computed tomography: A review. J. Med. Imaging Radiat Sci. 2013, 44, 62–70. [Google Scholar] [CrossRef]
- Ford, J.M.; Decker, S.J. Computed tomography slice thickness and its effects on three-dimensional reconstruction of anatomical structures. J. Forensic Radiol. Imaging 2016, 4, 43–46. [Google Scholar] [CrossRef]
- Romans, L. Computed Tomography for Technologists: A Comprehensive Text; Wolters Kluwer: Baltimore, MD, USA, 2011. [Google Scholar]


| 3D Printing Processes | Description | AM Technologies | Application |
|---|---|---|---|
| Vat Polymerization | Selective curing of photo-curable material in a liquid container | Stereolithography (SLA); Digital Light Processing (DLP); Scan, Spin, and Selectively Photocure (3SP); Continuous Digital Light Processing (CDLP) | This technology is most suitable for applications in injection molding [21], jewelry [1], dental [20,21], and medical industries [6], where a smooth surface finish and high accuracy are required |
| Powder Bed Fusion | Fusing of powder in a bed by melting the selected region | Multi Jet Fusion (MJF), Selective Laser Sintering (SLS), Direct Metal Laser Sintering (DMLS)/Selective Laser Melting (SLM), Electron Beam Melting (EBM) | Powder bed fusion builds functional prototypes with good mechanical properties and is used in aerospace [12,13], automotive [7,25], and medical industries [17,25] |
| Material Extrusion | Layer-by-layer deposition of molten material | Fused Deposition Modeling (FDM)/Fused Filament Fabrication (FFF)/Melted Extruded Modeling (MEM), 3D Bioprinting | Material extrusion has dimensional accuracy limitations, so it is mainly used in low-cost prototyping [17,19,22]. Industrial systems can also produce functional prototypes from engineering materials [2,28].3D bioprinting focuses on building scaffolds [6,18] |
| Directed Energy Deposition | Direct fusion of the material | Laser Engineering Net Shape (LENS), Electron Beam Additive Manufacturing (EBAM) | Directed energy deposition technology can be used for repairing or adding material to existing components. This technology is most suitable for applications in aerospace [12,13], automotive [15,16], and medical industries [6,25] |
| Sheet Lamination | Bonding of individual sheets of material | Laminated Object Manufactured (LOM) | Sheet lamination technology can be used only in ergonomic manufacturing studies [1,7], for visualizing topography [2,3], or for creating architecture models [23] with paper-made objects |
| Material Jetting | Material deposition and subsequent curing | Material Jetting (MJ), Nanoparticle Jetting (NPJ), Drop On Demand (DOD) | Material jetting is used in lost wax casting and investment casting applications [14], as well as dental [22] and medical industries [6], because it has high accuracy and gives a smooth surface finish |
| Binder Jetting | Selective dispensing of binder for joining powder in a bed | Binder Jetting (BJ) | Ceramic-based binder jetting can be used typically for manufacturing visual or light-duty functional prototypes (e.g., architectural models) [23]. This technology is not intended for functional applications [1,3,7] |
| AM Processes | AM Technology | 3D Printer | Commercial Material Name | Generic Name | Status of Material |
|---|---|---|---|---|---|
| Material Extrusion | Fused Deposition Modeling (FDM) | Fortus 360-mc | ABS-M30 | Acrylonitrile Butadiene Styrene | Solid-Based |
| PC-10 | Polycarbonate | ||||
| Fused Filament Fabrication (FFF) | Prusa MK3s | PLA | Polylactic acid | ||
| PET | Polyethylene terephthalate | ||||
| Vat Polymerization | Digital Light Processing (DLP) | Perfactory Vida | E-Partial | Acrylic | Liquid-Based |
| Scan, Spin, and Selectively Photocure (3SP) | 3Dent–3SP | E-Denstone | Acrylic | ||
| Xtreme 3SP | E-Model | Acrylic | |||
| Powder Bed Fusion | Selective Laser Sintering (SLS) | TMP Elite 3600 | Precimid 1170 | Polyamide 11 | Powder-Based |
| Material Jetting | Material Jetting (MJ) | Eden 260V | FullCure 830 | Acrylic | Liquid-Based |
| Objet350 Connex 3 | Digital ABS-Ivory | Acrylic | |||
| VeroClear | Acrylic | ||||
| RGD720 | Acrylic |
| Polymer Material | Mean Deviation (HU) | Standard Deviation (SD) (HU) |
|---|---|---|
| ABS-M30 | 98.041 | 5.481 |
| PC-10 | 57.287 | 5.576 |
| PLA | 48.662 | 2.995 |
| PET | 47.406 | 8.547 |
| E-Partial | 30.126 | 8.279 |
| E-Denstone | 28.594 | 9.748 |
| E-Model | 28.759 | 10.610 |
| Precimid 1170 | 16.091 | 4.348 |
| FullCure 830 | 29.409 | 4.775 |
| Digital ABS-Ivory | 30.430 | 3.814 |
| VeroClear | 29.055 | 3.206 |
| RGD720 | 28.860 | 6.525 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turek, P.; Budzik, G.; Przeszłowski, Ł. Assessing the Radiological Density and Accuracy of Mandible Polymer Anatomical Structures Manufactured Using 3D Printing Technologies. Polymers 2020, 12, 2444. https://doi.org/10.3390/polym12112444
Turek P, Budzik G, Przeszłowski Ł. Assessing the Radiological Density and Accuracy of Mandible Polymer Anatomical Structures Manufactured Using 3D Printing Technologies. Polymers. 2020; 12(11):2444. https://doi.org/10.3390/polym12112444
Chicago/Turabian StyleTurek, Paweł, Grzegorz Budzik, and Łukasz Przeszłowski. 2020. "Assessing the Radiological Density and Accuracy of Mandible Polymer Anatomical Structures Manufactured Using 3D Printing Technologies" Polymers 12, no. 11: 2444. https://doi.org/10.3390/polym12112444
APA StyleTurek, P., Budzik, G., & Przeszłowski, Ł. (2020). Assessing the Radiological Density and Accuracy of Mandible Polymer Anatomical Structures Manufactured Using 3D Printing Technologies. Polymers, 12(11), 2444. https://doi.org/10.3390/polym12112444







