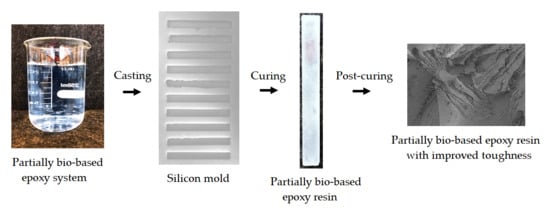
Optimization of the Curing and Post-Curing Conditions for the Manufacturing of Partially Bio-Based Epoxy Resins with Improved Toughness
Abstract
1. Introduction
2. Experimental
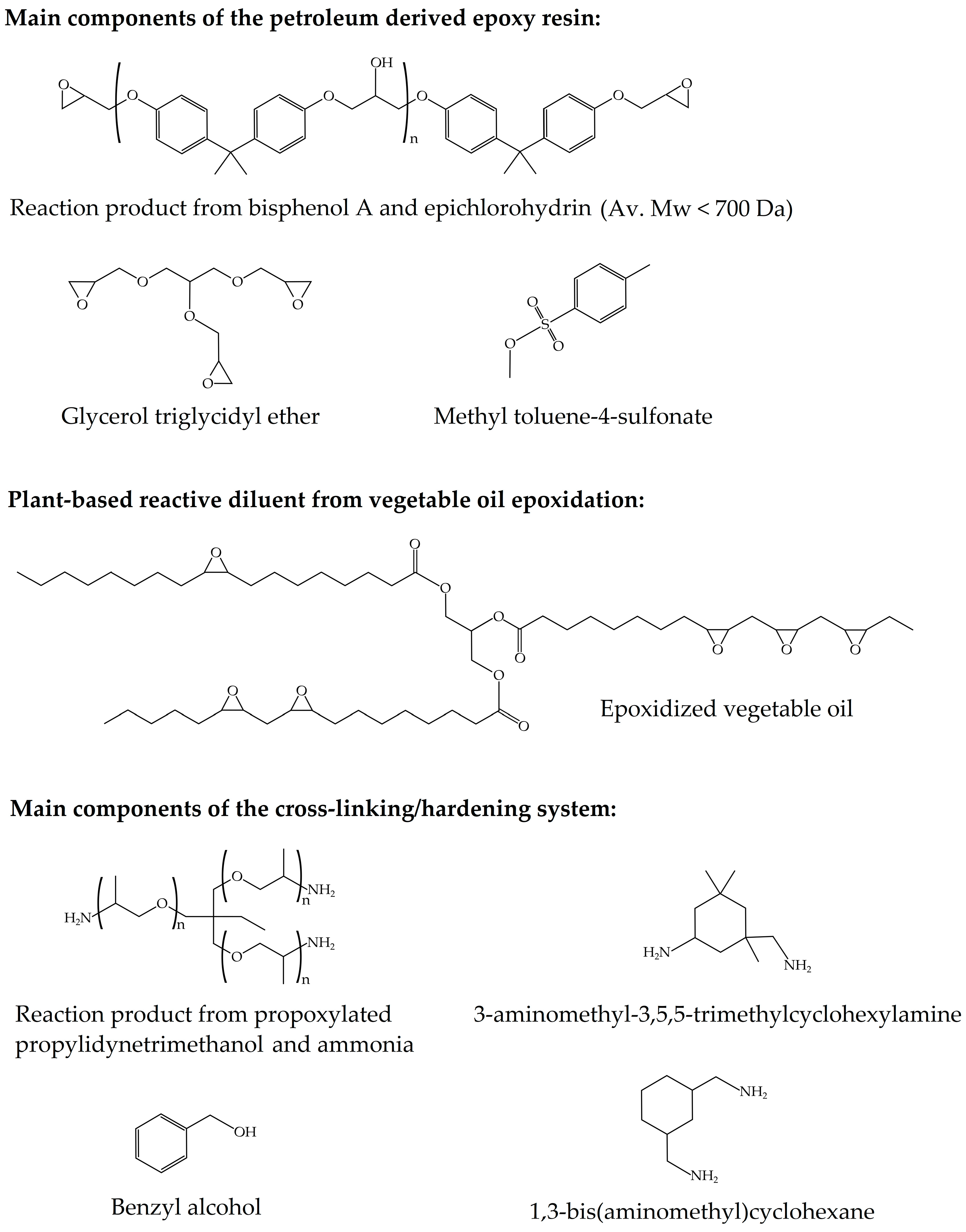
2.1. Materials
2.2. Resin Manufacturing
2.3. Mechanical Tests
2.4. Microscopy
2.5. Thermomechanical and Rheological Tests
3. Results and Discussion
3.1. Mechanical Properties of Partially Bio-Based Epoxy Resins
3.2. Morphology and Density of Partially Bio-Based Epoxy Resins
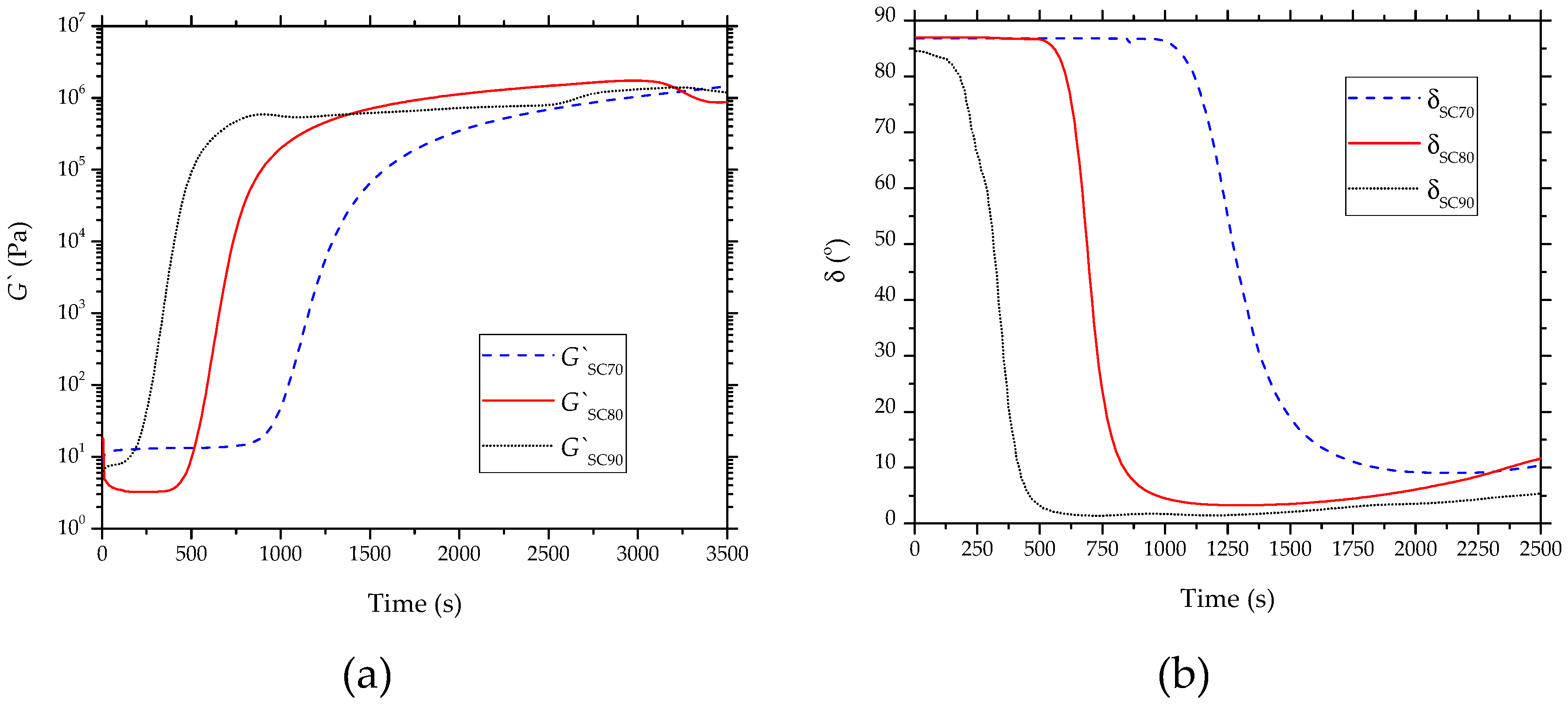
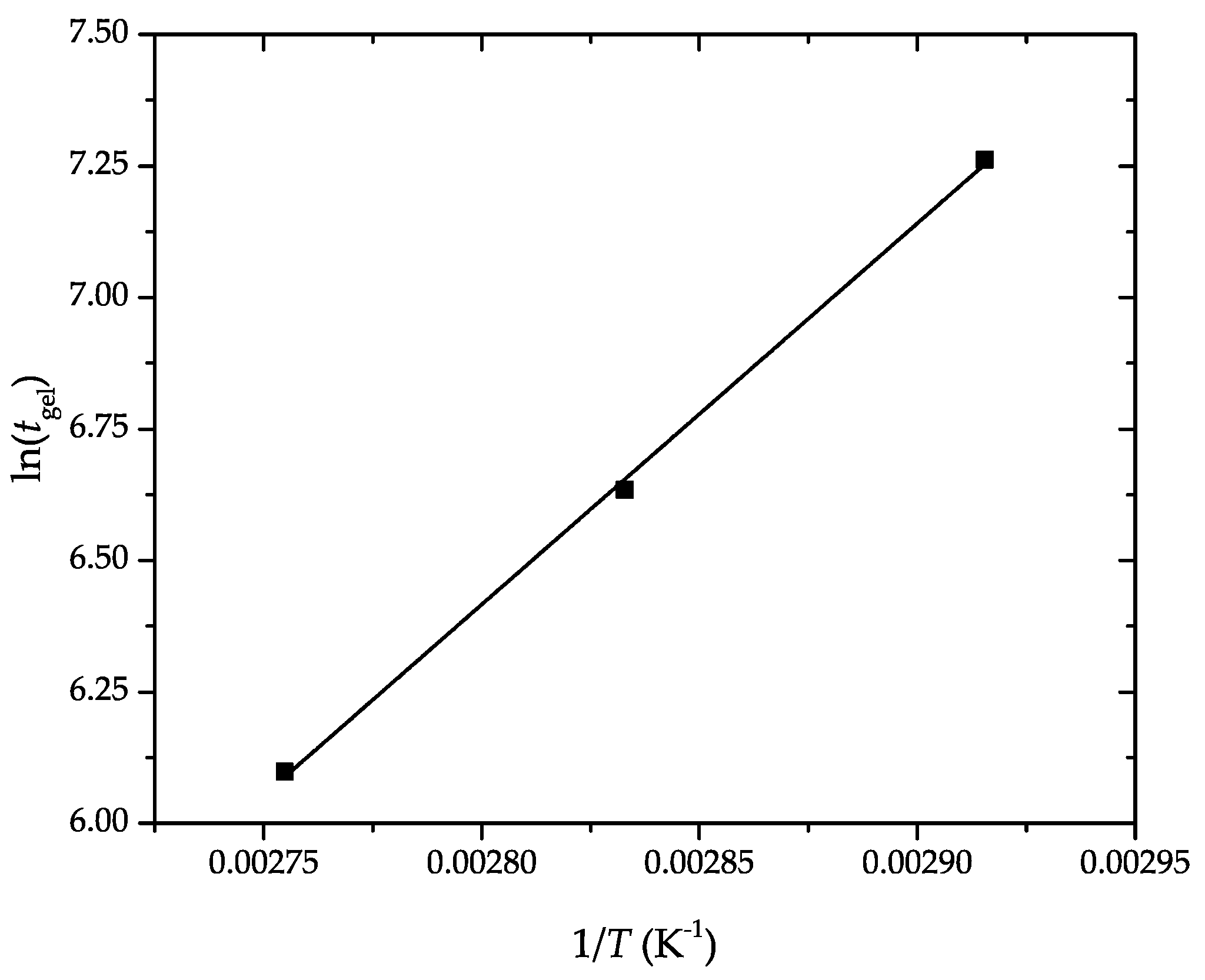
3.3. Rheological Properties of Partially Bio-Based Epoxy Resins
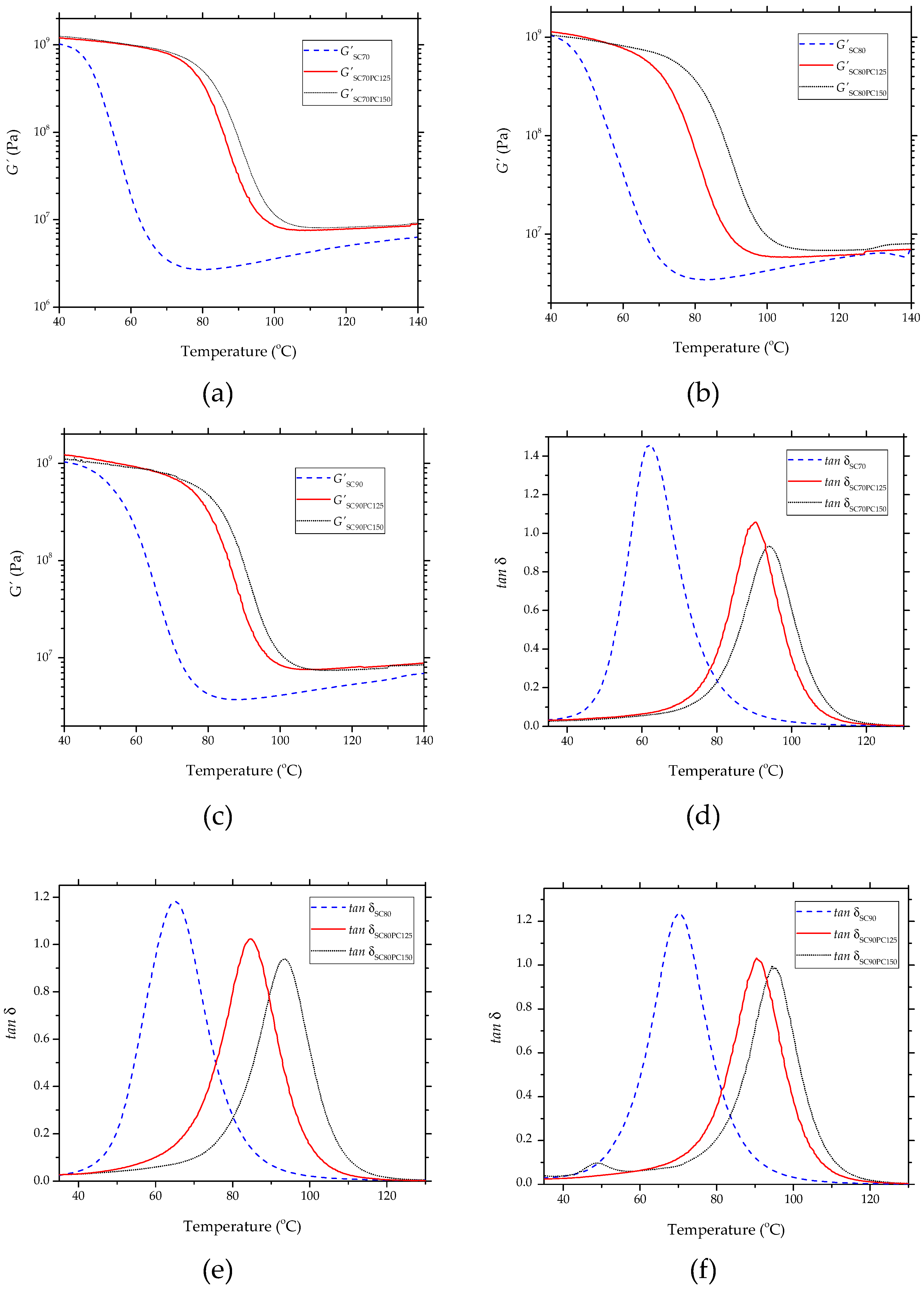
3.4. Thermomechanical Properties of Partially Bio-Based Epoxy Resins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Yu, S.; Li, X.; Guo, X.; Li, Z.; Zou, M. Curing and Characteristics of N,N,N′,N′-Tetraepoxypropyl-4,4′-Diaminodiphenylmethane Epoxy Resin-Based Buoyancy Material. Polymers 2019, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Njuguna, J.; Pielichowski, K.; Alcock, J.R. Epoxy-based fibre reinforced nanocomposites. Adv. Eng. Mater. 2007, 9, 835–847. [Google Scholar] [CrossRef]
- Holbery, J.; Houston, D. Natural-Fiber-Reinforced Polymer Composites in Automotive Applications. JOM 2006, 58, 80–86. [Google Scholar] [CrossRef]
- Jin, N.J.; Seung, I.; Choi, Y.S.; Yeon, J. Prediction of early-age compressive strength of epoxy resin concrete using the maturity method. Constr. Build. Mater. 2017, 152, 990–998. [Google Scholar] [CrossRef]
- Yin, Y.B.; Yang, Q.S.; Wang, S.L.; Gao, H.D.; He, Y.W.; Li, X.L. Formation of CO2 bubbles in epoxy resin coatings: A DFT study. J. Mol. Gr. Model. 2019, 86, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.L.; Park, S.J. Thermomechanical behavior of epoxy resins modified with epoxidized vegetable oils. Polym. Int. 2008, 57, 577–583. [Google Scholar] [CrossRef]
- Ellis, B.; Ashcroft, W.R.; Shaw, S.J.; Cantwell, W.J.; Kausch, H.H.; Johari, G.P.; Jones, F.R.; Chen, X.M. Chemistry and Technology of Epoxy Resins; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Kim, R.-W.; Kim, C.-M.; Hwang, K.-H.; Kim, S.-R. Embedded Based Real-Time Monitoring in the High-Pressure Resin Transfer Molding Process for CFRP. Appl. Sci. 2019, 9, 1795. [Google Scholar] [CrossRef]
- Rudawska, A. The Impact of the Seasoning Conditions on Mechanical Properties of Modified and Unmodified Epoxy Adhesive Compounds. Polymers 2019, 11, 804. [Google Scholar] [CrossRef]
- Enns, J.B.; Gillham, J.K. Effect of the extent of cure on the modulus, glass transition, water absorptio, and density of an amine-cured epoxy. J. Appl. Polym. Sci. 1983, 28, 2831–2846. [Google Scholar] [CrossRef]
- Ivankovic, M.; Incarnato, L.; Kenny, J.M.; Nicolais, L. Curing kinetics and chemorheology of epoxy/anhydride system. J. Appl. Polym. Sci. 2003, 90, 3012–3019. [Google Scholar] [CrossRef]
- Zilg, C.; Mülhaupt, R.; Finter, J. Morphology and toughness/stiffness balance of nanocomposites based upon anhydride-cured epoxy resins and layered silicates. Macromol. Chem. Phys. 1999, 200, 661–670. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, X.; Lu, C.; Zhang, X.; Ji, Y.; Bai, C.; Chen, Y.; Qiao, Y. Studies on Curing Kinetics and Tensile Properties of Silica-Filled Phenolic Amine/Epoxy Resin Nanocomposite. Polymers 2019, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, N.; Haddar, N.; Elleuch, K.; Ayedi, H.F. Investigations on the fabrication and the characterization of glass/epoxy, carbon/epoxy and hybrid composites used in the reinforcement and the repair of aeronautic structures. Mater. Des. 2014, 56, 714–724. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, M.K.; Lee, J.R. Isothermal cure kinetics of epoxy/phenol-novolac resin blend system initiated by cationic latent thermal catalyst. J. Polym. Sci. A Polym. Chem. 2000, 38, 2945–2956. [Google Scholar] [CrossRef]
- Mostovoy, S.; Ripling, E.J. Fracture toughness of an epoxy system. J. Appl. Polym. Sci. 1966, 10, 1351–1371. [Google Scholar] [CrossRef]
- Mohammadi, B.; Nokken, M.R. Influence of moisture content on water absorption in concrete. In Proceedings of the Canadian Society for Civil Engineering. Annual Conference, Montreal, QC, Canada, 29 May–1 June 2013; pp. 4092–4100. [Google Scholar]
- Fu, K.; Xie, Q.; Lu, F.; Duan, Q.; Wang, X.; Zhu, Q.; Huang, Z. Molecular Dynamics Simulation and Experimental Studies on the Thermomechanical Properties of Epoxy Resin with Different Anhydride Curing Agents. Polymers 2019, 11, 975. [Google Scholar] [CrossRef]
- Kenyon, A.S.; Nielsen, L.E. Characterization of Network Structure of Epoxy Resins by Dynamic Mechanical and Liquid Swelling Tests. J. Macromol. Sci. A Chem. 1969, 3, 275–295. [Google Scholar] [CrossRef]
- Czub, P. Application of modified natural oils as reactive diluents for epoxy resins. Macromol. Symp. 2006, 242, 60–64. [Google Scholar] [CrossRef]
- Park, Y.T.; Qian, Y.; Chan, C.; Suh, T.; Nejhad, M.G.; Macosko, C.W.; Stein, A. Epoxy toughening with low graphene loading. Adv. Funct. Mater. 2015, 25, 575–585. [Google Scholar] [CrossRef]
- Okabe, H.; Nishimura, H.; Hara, K.; Kai, S. Gelation and glass transition in thermosetting process of epoxy resin. Prog. Theor. Phys. Suppl. 1997. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. On the use of acrylated epoxidized soybean oil as a reactive compatibilizer in injection-molded compostable pieces consisting of polylactide filled with orange peel flour. Polym. Int. 2018, 67, 1341–1351. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Fenollar, O.; García-Sanoguera, D.; Balart, R. Development and optimization of renewable vinyl plastisol/wood flour composites exposed to ultraviolet radiation. Mater. Des. 2016, 108, 648–658. [Google Scholar] [CrossRef]
- Khot, S.N.; Lascala, J.J.; Can, E.; Morye, S.S.; Williams, G.I.; Palmese, G.R.; Kusefoglu, S.H.; Wool, R.P. Development and application of triglyceride-based polymers and composites. J. Appl. Polym. Sci. 2001, 82, 703–723. [Google Scholar] [CrossRef]
- Jaillet, F.; Desroches, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New biobased carboxylic acid hardeners for epoxy resins. Eur. J. Lipid Sci. Technol. 2013, 115, 698–708. [Google Scholar] [CrossRef]
- Stemmelen, M.; Lapinte, V.; Habas, J.P.; Robin, J.J. Plant oil-based epoxy resins from fatty diamines and epoxidized vegetable oil. Eur. Polym. J. 2015, 68, 536–545. [Google Scholar] [CrossRef]
- Pethrick, R.A.; Hollins, E.A.; McEwan, I.; Pollock, E.A.; Hayward, D.; Johncock, P. Effect of Cure Temperature on the Structure and Water Absorption of Epoxy/Amine Thermosets. Polym. Int. 1996, 39, 275–288. [Google Scholar] [CrossRef]
- Tucker, S.J.; Fu, B.; Kar, S.; Heinz, S.; Wiggins, J.S. Ambient cure POSS-epoxy matrices for marine composites. Compos. A Appl. Sci. Manuf. 2010, 41, 1441–1446. [Google Scholar] [CrossRef]
- Gupta, V.B.; Rich, J.; Drazal, L.T.; Lee, C.Y.C. The Temperature-Dependence of Some Mechanical Properties of a Cured Epoxy Resin System. Polym. Eng. Sci. 1985, 25. [Google Scholar] [CrossRef]
- Barton, J.M.; Harnerton, I.; Howlin, B.J.; Jones, J.R.; Liu, S. Studies of cure schedule and final property relationships of a commercial epoxy resin using modified imidazole curing agents. Polymer 1998, 39, 1929–1937. [Google Scholar] [CrossRef]
- Russo, C.; Fernandez-Francos, X.; De la Flor, S. Rheological and Mechanical Characterization of Dual-Curing Thiol-Acrylate-Epoxy Thermosets for Advanced Applications. Polymers 2019, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Kotnarowska, D. Influence of ultraviolet radiation and aggressive media on epoxy coating degradation. Prog. Org. Coat. 1999, 37, 149–159. [Google Scholar] [CrossRef]
- Imanaka, M.; Liu, X.; Kimoto, M. Comparison of fracture behavior between acrylic and epoxy adhesives. Int. J. Adhes. Adhes. 2017, 75, 31–39. [Google Scholar] [CrossRef]
- Rahman, R.; Putra, S.Z.F.S. Tensile properties of natural and synthetic fiber-reinforced polymer composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 81–102. [Google Scholar]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Kinetic Analysis of the Curing of a Partially Biobased Epoxy Resin Using Dynamic Differential Scanning Calorimetry. Polymers 2019, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Larroque, M.; Subirats, J.T.; Gérard, J.F. Food-contact epoxy resin: Co-variation between migration and degree of cross-linking. Part II. Food Addit. Contam. 1998, 15, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Bueche, F. Tensile strength of rubbers. J. Polym. Sci. 1957, 24, 189–200. [Google Scholar] [CrossRef]
- Levita, G.; De Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink density and fracture toughness of epoxy resins. J. Mater. Sci. 1991, 26, 2348–2352. [Google Scholar] [CrossRef]
- Min, B.G.; Hodgkin, J.H.; Stachurski, Z.H. The dependence of fracture properties on cure temperature in a DGEBA/DDS epoxy system. J. Appl. Polym. Sci. 1993, 48, 1303–1312. [Google Scholar] [CrossRef]
- Turk, M.; Hamerton, I.; Ivanov, D.S. Ductility potential of brittle epoxies: Thermomechanical behaviour of plastically-deformed fully-cured composite resins. Polymer 2017, 120, 43–51. [Google Scholar] [CrossRef]
- Gupta, V.; Brahatheeswaran, C. Molecular packing and free volume in crosslinked epoxy networks. Polymer 1991, 32, 1875–1884. [Google Scholar] [CrossRef]
- Karkanas, P.I.; Partridge, I.K. Cure modeling and monitoring of epoxy/amine resin systems. I. Cure kinetics modeling. J. Appl. Polym. Sci. 2000, 77, 1419–1431. [Google Scholar] [CrossRef]
- Woo, E.M.; Seferis, J.C. Cure kinetics of epoxy/anhydride thermosetting matrix systems. J. Appl. Polym. Sci. 1990, 40, 1237–1256. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Grishchuk, S.; Sorochynska, L.; Rong, M.Z. Curing, gelling, thermomechanical, and thermal decomposition behaviors of anhydride-cured epoxy (DGEBA)/epoxidized soybean oil compositions. Polym. Eng. Sci. 2014, 54, 747–755. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Evolution of network topology of bifunctional epoxy thermosets during cure and its relationship to thermo-mechanical properties: A molecular dynamics study. Polymer 2015, 75, 151–160. [Google Scholar] [CrossRef]
- Dyakonov, T.; Chen, Y.; Holland, K.; Drbohlav, J.; Burns, D.; Velde, D.V.; Seib, L.; Soloski, E.J.; Kuhn, J.; Mann, P.J.; et al. Thermal analysis of some aromatic amine cured model epoxy resin systems—I: Materials synthesis and characterization, cure and post-cure. Polym. Degrad. Stab. 1996, 53, 217–242. [Google Scholar] [CrossRef]
- Chang, T.D.; Carr, S.H.; Brittain, J.O. Effect of Crosslinking on the Physical Properties of an Epoxy Resin. Ploym. Eng. Sci. 1982, 22, 1213–1220. [Google Scholar] [CrossRef]
- Wu, C.S. Influence of post-curing and temperature effects on bulk density, glass transition and stress-strain behaviour of imidazole-cured epoxy network. J. Mater. Sci. 1992, 27, 2952–2959. [Google Scholar] [CrossRef]

| Resin | ||
|---|---|---|
| SC70 | 70 | - |
| SC70PC125 | 125 | |
| SC70PC150 | 150 | |
| SC80 | 80 | - |
| SC80PC125 | 125 | |
| SC80PC150 | 150 | |
| SC90 | 90 | - |
| SC90PC125 | 125 | |
| SC90PC150 | 150 |
| Resin | Flexural Test | Shore D Hardness | Impact Strength (kJ·m−2) | |
|---|---|---|---|---|
| Ef (MPa) | ||||
| SC70 | 977 ± 127 | 77.4 ± 13.4 | 77.4 ± 2.9 | 11.9 ± 2.1 |
| SC70PC125 | 1766 ± 391 | 89.2 ± 13.1 | 81.3 ± 1.2 | 12.2 ± 1.9 |
| SC70PC150 | 1854 ± 256 | 93.2 ± 22.2 | 80.0 ± 1.2 | 12.8 ± 1.3 |
| SC80 | 1260 ± 192 | 81.1 ± 15.9 | 82.2 ± 1.8 | 11.5 ± 1.6 |
| SC80PC125 | 2379 ± 185 | 114.4 ± 22.0 | 85.3 ± 1.0 | 13.3 ± 1.7 |
| SC80PC150 | 3237 ± 377 | 123.9 ± 7.6 | 85.3 ± 0.5 | 16.8 ± 2.5 |
| SC90 | 2403 ± 210 | 105.6 ± 10.3 | 85.0 ± 1.5 | 12.0 ± 0.9 |
| SC90PC125 | 2520 ± 298 | 110.6 ± 6.3 | 84.3 ± 0.6 | 15.9 ± 3.7 |
| SC90PC150 | 2207 ± 164 | 101.1 ± 12.3 | 83.4 ± 1.7 | 12.4 ± 1.2 |
| Resin | Density (g·cm−3) |
|---|---|
| SC70 | 1.15 ± 0.02 |
| SC70PC125 | 1.11 ± 0.05 |
| SC70PC150 | 1.35 ± 0.17 |
| SC80 | 1.60 ± 0.26 |
| SC80PC125 | 1.32 ± 0.07 |
| SC80PC150 | 1.45 ± 0.19 |
| SC90 | 1.19 ± 0.01 |
| SC90PC125 | 1.07 ± 0.04 |
| SC90PC150 | 0.97 ± 0.17 |
| tgel (s) | tcuring (s) | G′max (GPa) | |
|---|---|---|---|
| 70 | 1426.1 ± 28.5 | 2250.3 ± 56.1 | 1.042 ± 0.02 |
| 80 | 760.6 ± 15.2 | 1250.0 ± 18.8 | 1.738 ± 0.03 |
| 90 | 445.2 ± 8.9 | 750.2 ± 18.7 | 1.311 ± 0.03 |
| Resin | (GPa) | (MPa) | |
|---|---|---|---|
| SC70 | 1.029 ± 0.021 | 4.29 ± 0.09 | 62.5 ± 1.25 |
| SC70PC125 | 1.203 ± 0.024 | 7.56 ± 0.15 | 90.5 ± 1.71 |
| SC70PC150 | 1.255 ± 0.027 | 8.15 ± 0.16 | 94.1 ± 1.97 |
| SC80 | 1.039 ± 0.025 | 4.99 ± 0.09 | 65.1 ± 1.63 |
| SC80PC125 | 1.132 ± 0.019 | 5.89 ± 0.11 | 84.9 ± 1.95 |
| SC80PC150 | 1.048± 0.022 | 6.96 ± 0.17 | 93.4 ± 1.87 |
| SC90 | 1.037 ± 0.024 | 4.68 ± 0.09 | 70.4 ± 1.47 |
| SC90PC125 | 1.230 ± 0.025 | 7.57 ± 0.15 | 90.7 ± 2.35 |
| SC90PC150 | 1.121 ± 0.02 | 7.47 ± 0.14 | 95.3 ± 3.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lascano, D.; Quiles-Carrillo, L.; Torres-Giner, S.; Boronat, T.; Montanes, N. Optimization of the Curing and Post-Curing Conditions for the Manufacturing of Partially Bio-Based Epoxy Resins with Improved Toughness. Polymers 2019, 11, 1354. https://doi.org/10.3390/polym11081354
Lascano D, Quiles-Carrillo L, Torres-Giner S, Boronat T, Montanes N. Optimization of the Curing and Post-Curing Conditions for the Manufacturing of Partially Bio-Based Epoxy Resins with Improved Toughness. Polymers. 2019; 11(8):1354. https://doi.org/10.3390/polym11081354
Chicago/Turabian StyleLascano, Diego, Luis Quiles-Carrillo, Sergio Torres-Giner, Teodomiro Boronat, and Nestor Montanes. 2019. "Optimization of the Curing and Post-Curing Conditions for the Manufacturing of Partially Bio-Based Epoxy Resins with Improved Toughness" Polymers 11, no. 8: 1354. https://doi.org/10.3390/polym11081354
APA StyleLascano, D., Quiles-Carrillo, L., Torres-Giner, S., Boronat, T., & Montanes, N. (2019). Optimization of the Curing and Post-Curing Conditions for the Manufacturing of Partially Bio-Based Epoxy Resins with Improved Toughness. Polymers, 11(8), 1354. https://doi.org/10.3390/polym11081354