Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Size Exclusion Chromatography (SEC)
2.3. Potentiometric Titration
2.4. Dynamic Light Scattering (DLS) of the Polycarboxylate/BMP-2 Complexes
2.5. Cell Culture
2.6. Cytotoxicity of Polycarboxylates
2.7. Anticoagulant Activity of Polycarboxylates
2.8. Osteogenic Differentiation and Alkaline Phosphatase (ALP) Activity in MC3T3-E1 Cells
2.9. Mineralization of MC3T3-E1 Cells
2.10. Statistical Analysis
3. Results and Discussion
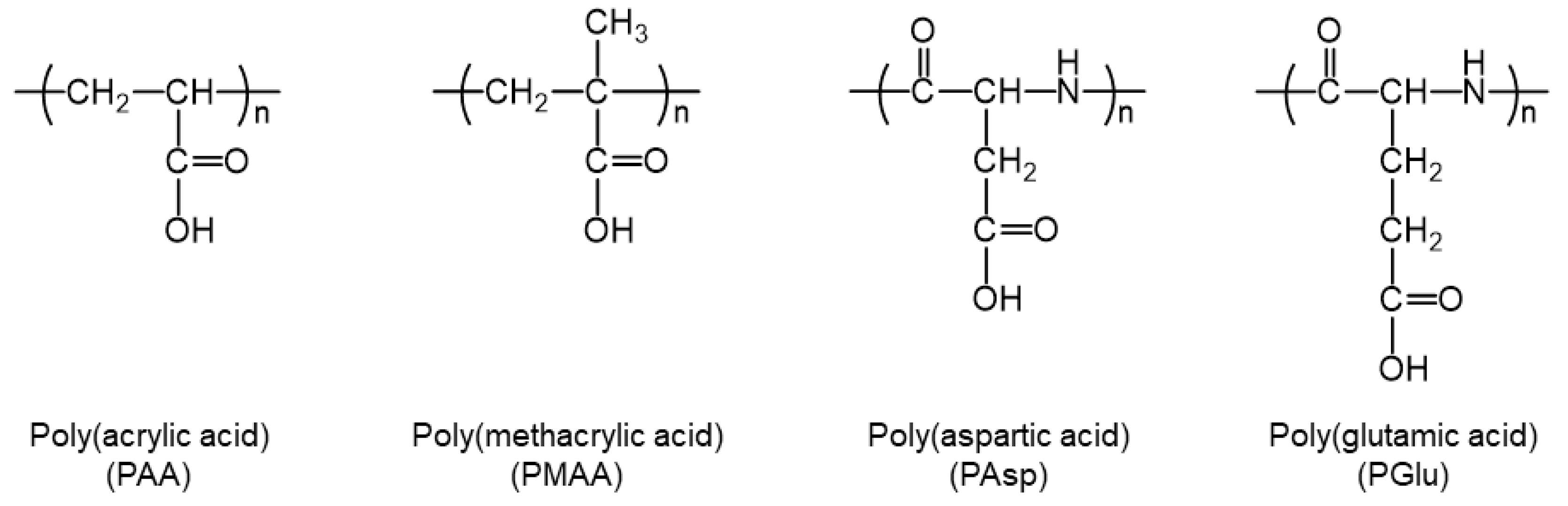
3.1. Chemical Characterizations of Polycarboxylates
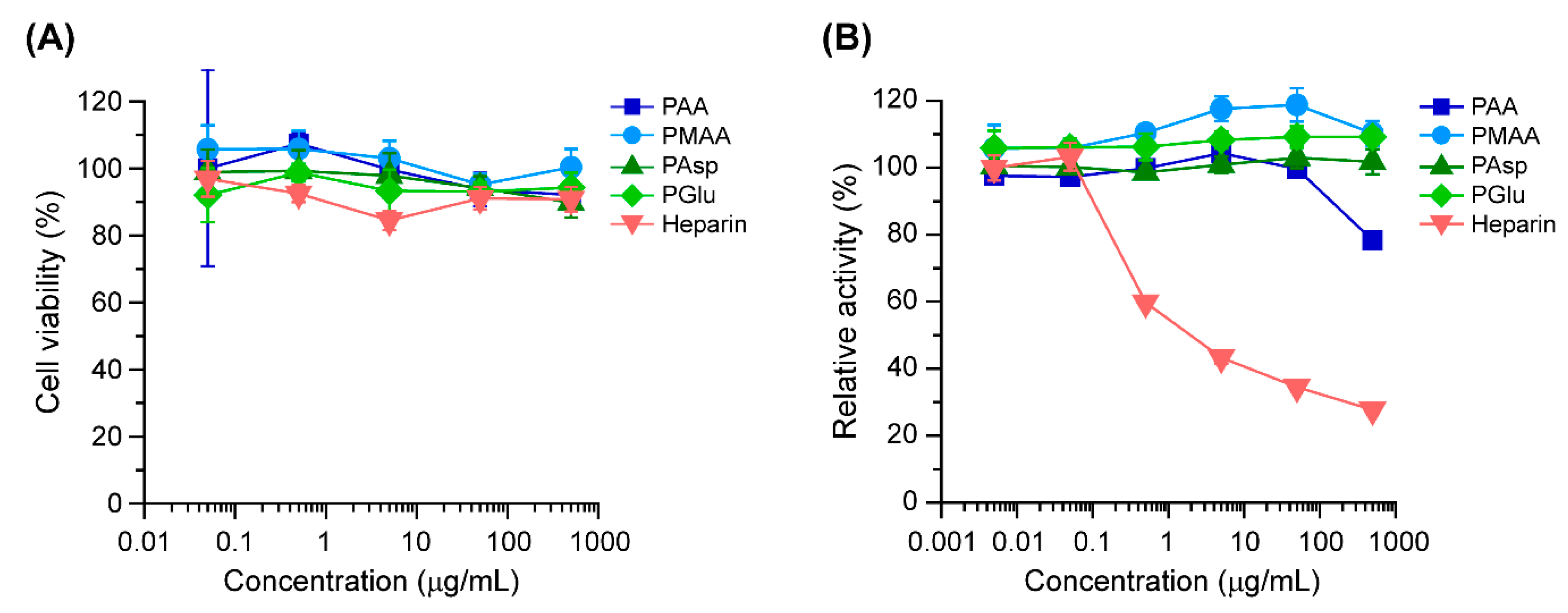
3.2. Biological Characterizations of Polycarboxylates
3.3. ALP Activity in MC3T3-E1 Cells Treated with the Polycarboxylate/BMP-2 Complexes
3.4. Mineralization of MC3T3-E1 Cells Treated with Polycarboxylate/BMP-2 Complexes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Block, M.S.; Kent, J.N. Sinus Augmentation for Dental Implants: The Use of Autogenous Bone. J. Oral Maxillofac Surg. 1997, 55, 1281–1286. [Google Scholar] [CrossRef]
- Ahlmann, E.; Patzakis, M.; Roidis, N.; Shepherd, L.; Holtom, P. Comparison of Anterior and Posterior Iliac Crest Bone Grafts in Terms of Harvest-Site Morbidity and Functional Outcomes. J. Bone Joint Surg. Am. 2002, 84, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Kaban, L.B.; Murray, J.E.; Folkman, J.; Mulliken, J.B. Application of the Biological Principle of Induced Osteogenesis for Craniofacial Defects. Lancet 1981, 1, 959–962. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/Polyglycolic Acid Copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- LeGeros, R.Z. PROPERTIES of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Ikeda, G.; Nishida, K.; Tamura, A.; Yamaguchi, S.; Harada, K.; Yui, N. Supramolecular Polyelectrolyte Complexes of Bone Morphogenetic Protein-2 with Sulfonated Polyrotaxanes to Induce Enhanced Osteogenic Differentiation. Macromol. Biosci. 2015, 15, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Inada, T.; Kanemaru, T.; Ikeda, G.; Tonegawa, A.; Nishida, K.; Arisaka, Y.; Tamura, A.; Yamaguchi, S.; Yui, N. Potentiating Bioactivity of BMP-2 by Polyelectrolyte Complexation with Sulfonated Polyrotaxanes to Induce Rapid Bone Regeneration in A Mouse Calvarial Defect. J. Biomed. Mater. Res. A 2017, 105, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Tamura, A.; Terauchi, M.; Yamaguchi, S.; Yui, N. A Silencing-Mediated Enhancement of Osteogenic Differentiation by Supramolecular Ternary siRNA Polyplexes Comprising Biocleavable Cationic Polyrotaxanes and Anionic Fusogenic Peptides. Biomater. Sci. 2018, 6, 440–450. [Google Scholar] [CrossRef]
- Terauchi, M.; Inada, T.; Tonegawa, A.; Tamura, A.; Yamaguchi, S.; Harada, K.; Yui, N. Supramolecular Inclusion Complexation of Simvastatin with Methylated β-cyclodextrins for Promoting Osteogenic Differentiation. Int. J. Biol. Macromol. 2016, 93, 1492–1498. [Google Scholar] [CrossRef]
- Terauchi, M.; Tamura, A.; Yamaguchi, S.; Yui, N. Enhanced Cellular Uptake and Osteogenic Differentiation Efficiency of Melatonin by Inclusion Complexation with 2-Hydroxypropyl β-Cyclodextrin. Int. J. Pharm. 2018, 547, 53–60. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The Role of Growth Factors in the Repair of Bone. Biology and Clinical Applications. J. Bone Joint Surg. Am. 2002, 84, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Sun, M.H.; Cheng, H.; Peng, Y.; Montag, A.G.; Deyrup, A.T.; Jiang, W.; Luu, H.H.; Luo, J.; Szatkowski, J.P.; et al. Characterization of the Distinct Orthotopic Bone-Forming Activity of 14 BMPs Using Recombinant Adenovirus-Mediated Gene Delivery. Gene Ther. 2004, 11, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.D.; Kang, J.; Sandhu, H.; Heller, J.G. Use of Recombinant Human Bone Morphogenetic Protein-2 to Achieve Posterolateral Lumbar Spine Fusion in Humans: A Prospective, Randomized Clinical Pilot Trial: 2002 Volvo Award in Clinical Studies. Spine 2002, 27, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G.; et al. Recombinant Human Bone Morphogenetic Protein-2 for Treatment of Open Tibial Fractures: A Prospective, Controlled, Randomized Study of Four Hundred and Fifty Patients. J. Bone Joint Surg. Am. 2002, 84, 2123–2134. [Google Scholar]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, M.; Sugimoto, T.; Kaji, H.; Kobayashi, T.; Nishiyama, K.; Fukase, M.; Kumegawa, M.; Chihara, K. Stimulatory Effect of Bone Morphogenetic Protein-2 on Osteoclast-Like Cell Formation and Bone-Resorbing Activity. J. Bone Miner. Res. 1995, 10, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Feldman, G.J.; Billings, P.C.; Patel, R.V.; Caron, R.J.; Guenther, C.; Kingsley, D.M.; Kaplan, F.S.; Shore, E.M. Over-expression of BMP4 and BMP5 in a Child with Axial Skeletal Malformations and Heterotopic Ossification: A New Syndrome. Am. J. Med. Genet. A 2007, 143A, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation in Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef]
- Takada, T.; Katagiri, T.; Ifuku, M.; Morimura, N.; Kobayashi, M.; Hasegawa, K.; Ogamo, A.; Kamijo, R. Sulfated Polysaccharides Enhance the Biological Activities of Bone Morphogenetic Proteins. J. Biol. Chem. 2003, 278, 43229–43235. [Google Scholar] [CrossRef]
- Zhao, B.; Katagiri, T.; Toyoda, H.; Takada, T.; Yanai, T.; Fukuda, T.; Chung, U.I.; Koike, T.; Takaoka, K.; Kamijo, R. Heparin Potentiates the in Vivo Ectopic Bone Formation Induced by Bone Morphogenetic protein-2. J. Biol. Chem. 2006, 281, 23246–23253. [Google Scholar] [CrossRef]
- Kanzaki, S.; Ariyoshi, W.; Takahashi, T.; Okinaga, T.; Kaneuji, T.; Mitsugi, S.; Nakashima, K.; Tsujisawa, T.; Nishihara, T. Dual Effects of Heparin on BMP-2-Induced Osteogenic Activity in MC3T3-E1 Cells. Pharmacol. Rep. 2011, 63, 1222–12230. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyauchi, S.; Tawada, A.; Anada, T.; Matsuzaka, S.; Suzuki, O. Oversulfated Chondroitin Sulfate-E Binds to BMP-4 and Enhances Osteoblast Differentiation. J. Cell Physiol. 2008, 217, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Bramono, D.S.; Murali, S.; Rai, B.; Ling, L.; Poh, W.T.; Lim, Z.X.; Stein, G.S.; Nurcombe, V.; van Wijnen, A.J.; Cool, S.M. Bone Marrow-Derived Heparan Sulfate Potentiates the Osteogenic Activity of Bone Morphogenetic Protein-2 (BMP-2). Bone 2012, 50, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.L.; Samuel, R.E.; Shah, N.J.; Padera, R.F.; Beben, Y.M.; Hammond, P.T. Tissue Integration of Growth Factor-eluting Layer-by-Layer Polyelectrolyte Multilayer Coated Implants. Biomaterials 2011, 32, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Damus, P.S.; Hicks, M.; Rosenberg, R.D. Anticoagulant Action of Heparin. Nature 1973, 246, 355–357. [Google Scholar] [CrossRef]
- Jin, L.; Abrahams, J.P.; Skinner, R.; Petitou, M.; Pike, R.N.; Carrell, R.W. The Anticoagulant Activation of Antithrombin by Heparin. Proc. Natl. Acad. Sci. USA 1997, 94, 14683–14688. [Google Scholar] [CrossRef]
- Cao, L.; Werkmeister, J.A.; Wang, J.; Glattauer, V.; McLean, M.M.; Liu, C. Bone Regeneration Using Photocrosslinked Hydrogel Incorporating rhBMP-2 Loaded 2-N, 6-O-Sulfated Chitosan Nanoparticles. Biomaterials 2014, 35, 2730–2742. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, J.; Wang, J.; Yao, W.; Liu, C.; Chen, J.; Cao, X. Enhanced Bioactivity of Bone Morphogenetic Protein-2 with Low Dose of 2-N, 6-O-Sulfated Chitosan in Vitro and in Vivo. Biomaterials 2009, 30, 1715–1724. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Paluck, S.J.; McGahran, A.J.; Heather, D.; Maynard, H.D. Poly(vinyl sulfonate) Facilitates bFGF-Induced Cell Proliferation. Biomacromolecules 2015, 16, 2684–2692. [Google Scholar] [CrossRef]
- Yang, D.K.; Moon, S.W.; Lee, D. Surface Modification of Titanium with BMP-2/GDF-5 by a Heparin Linker and Its Efficacy as A Dental Implant. Int. J. Mol. Sci. 2017, 18, 229. [Google Scholar] [CrossRef]
- Tan, H.; Wang, H.; Chai, Y.; Yu, Y.; Hong, H.; Yang, F.; Qu, X.; Liu, C. Engineering A Favourable Osteogenic Microenvironment by Heparin Mediated Hybrid Coating Assembly and rhBMP-2 Loading. RSC Adv. 2017, 7, 11439–11447. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Rouse, T.; Chou, C.; Krishnan, L.; Stevens, H.Y.; Li, M.A.; McDevitt, T.C.; Guldberg, R.E. Enhanced in Vivo Retention of Low Dose BMP-2 via Heparin Microparticle Delivery Does Not Accelerate Bone Healing in A Critically Sized Femoral Defect. Acta Biomater. 2017, 59, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.D.; Damus, P.S. The Purification and Mechanism of Action of Human Antithrombin-Heparin Cofactor. J. Biol. Chem. 1973, 248, 6490–6505. [Google Scholar] [PubMed]
- Hogg, P.J.; Jackson, C.M. Fibrin Monomer Protects Thrombin from Inactivation by Heparin-Antithrombin III: Implications for Heparin Efficacy. Proc. Natl. Acad. Sci. 1989, 86, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Raschke, R.; Warkentin, T.E.; Dalen, J.E.; Deykin, D.; Poller, L. Heparin: Mechanism of Action, Pharmacokinetics, Dosing Considerations, Monitoring, Efficacy, and Safety. Chest 1995, 108, 258S–275S. [Google Scholar] [CrossRef] [PubMed]
- Monien, B.H.; Cheang, K.I.; Desai, U.R. Mechanism of Poly(acrylic acid) Acceleration of Antithrombin Inhibition of Thrombin: Implications for the Design of Novel Heparin Mimics. J. Med. Chem. 2005, 48, 5360–5368. [Google Scholar] [CrossRef] [PubMed]
- Rawadi, G.; Vayssière, B.; Dunn, F.; Baron, R.; Roman-Roman, S. BMP-2 Controls Alkaline Phosphatase Expression and Osteoblast Mineralization by A Wnt Autocrine Loop. J. Bone Miner. Res. 2003, 18, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B. Molecular Mechanisms Mediating Proliferation/Differentiation Interrelationships During Progressive Development of the Osteoblast Phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef] [PubMed]
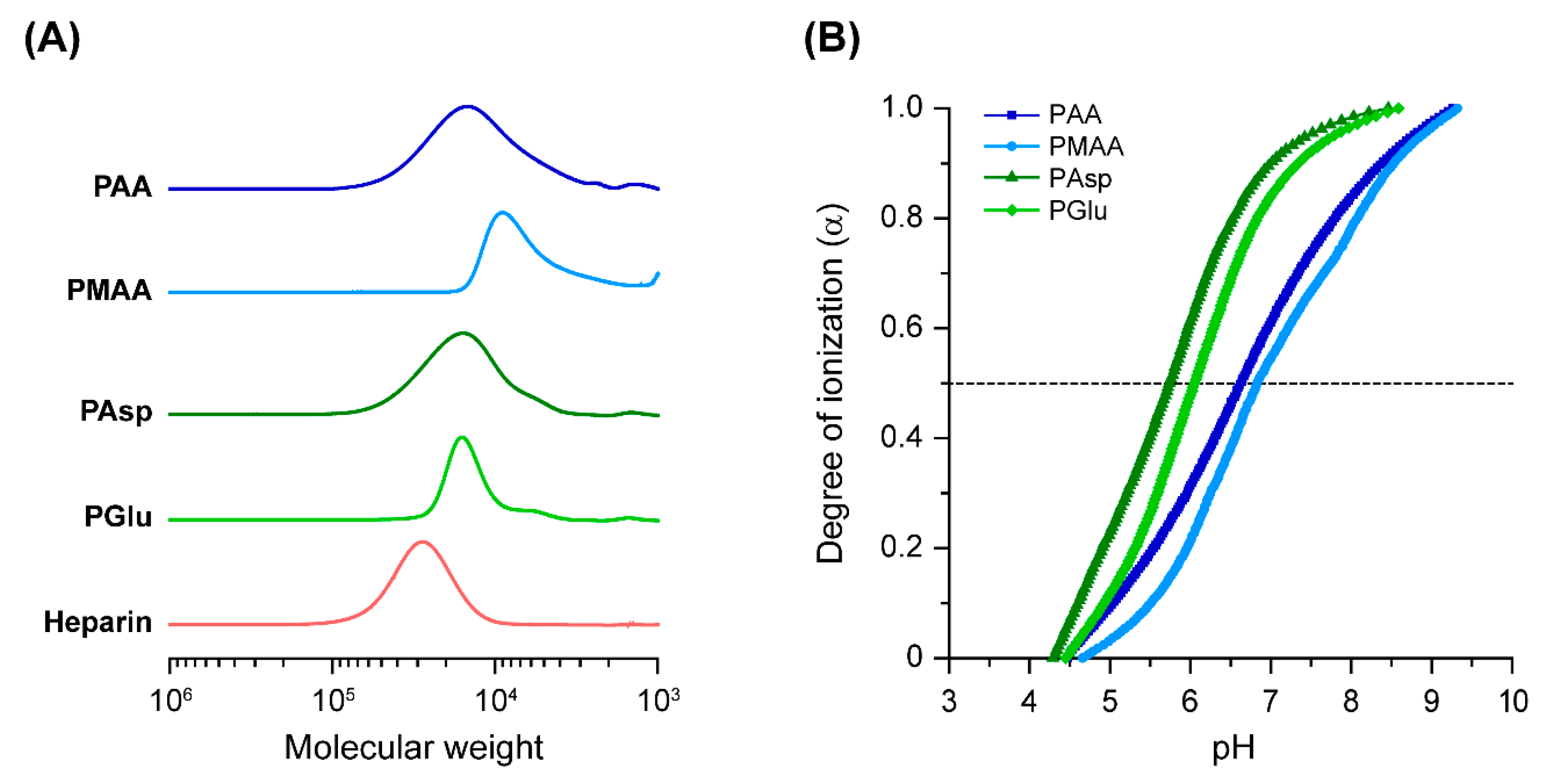



| Sample | Mn,SEC1 | Mw/Mn1 | pKa 2 | α at pH 7.4 2 |
|---|---|---|---|---|
| PAA | 10,100 | 1.56 | 6.65 | 0.71 |
| PMAA | 5100 | 1.36 | 6.84 | 0.64 |
| PAsp | 13,500 | 1.37 | 5.74 | 0.94 |
| PGlu | 12,700 | 1.16 | 6.05 | 0.90 |
| Heparin | 25,600 | 1.23 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terauchi, M.; Tamura, A.; Tonegawa, A.; Yamaguchi, S.; Yoda, T.; Yui, N. Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates. Polymers 2019, 11, 1327. https://doi.org/10.3390/polym11081327
Terauchi M, Tamura A, Tonegawa A, Yamaguchi S, Yoda T, Yui N. Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates. Polymers. 2019; 11(8):1327. https://doi.org/10.3390/polym11081327
Chicago/Turabian StyleTerauchi, Masahiko, Atsushi Tamura, Asato Tonegawa, Satoshi Yamaguchi, Tetsuya Yoda, and Nobuhiko Yui. 2019. "Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates" Polymers 11, no. 8: 1327. https://doi.org/10.3390/polym11081327
APA StyleTerauchi, M., Tamura, A., Tonegawa, A., Yamaguchi, S., Yoda, T., & Yui, N. (2019). Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates. Polymers, 11(8), 1327. https://doi.org/10.3390/polym11081327





