The Preparation of Green Fluorescence-Emissioned Carbon Dots/Poly(N-Isopropylacrylamide) Temperature-Sensitive Hydrogels and Research on Their Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Apparatus
2.2. Synthesis of G-CDs
2.3. Synthesis of PNIPAM Microgels
2.4. Synthesis of CDs/PNIPAM Microgels
2.5. Cytotoxicity Assay
3. Results and Discussion
3.1. The Preparation of CDs/PNIPAM Complex Hydrogel
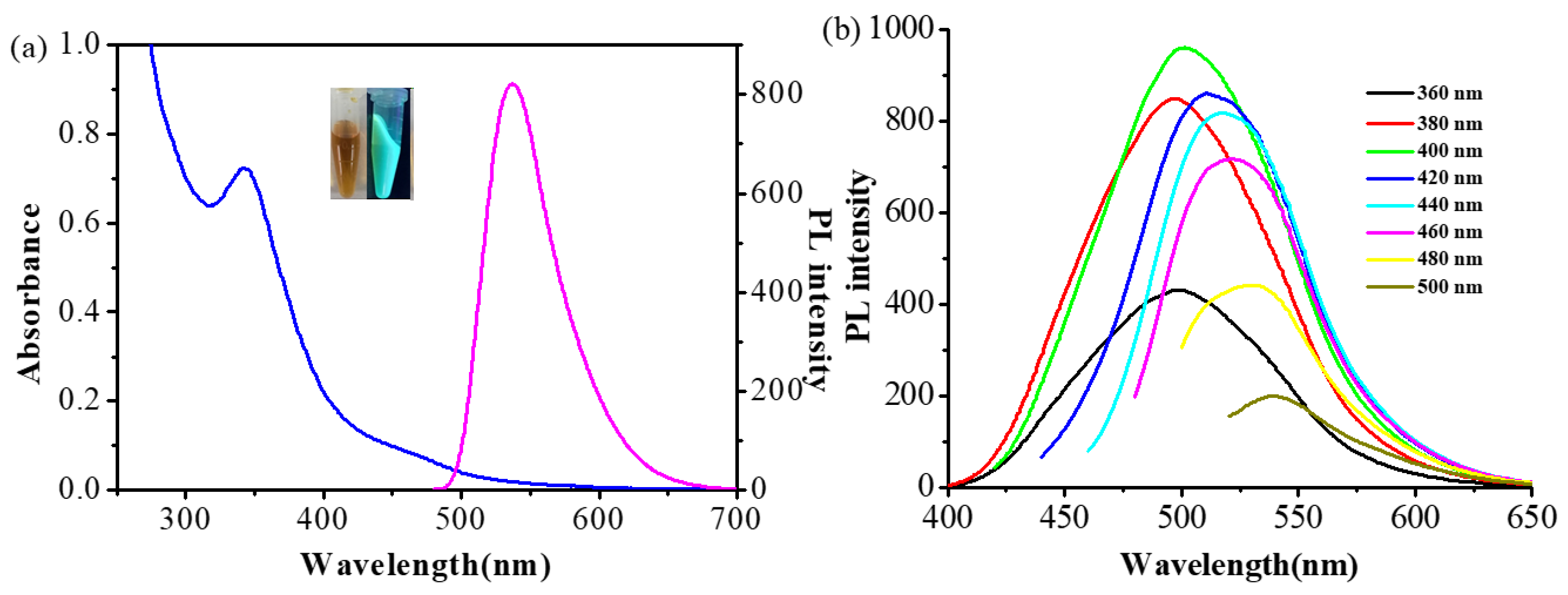
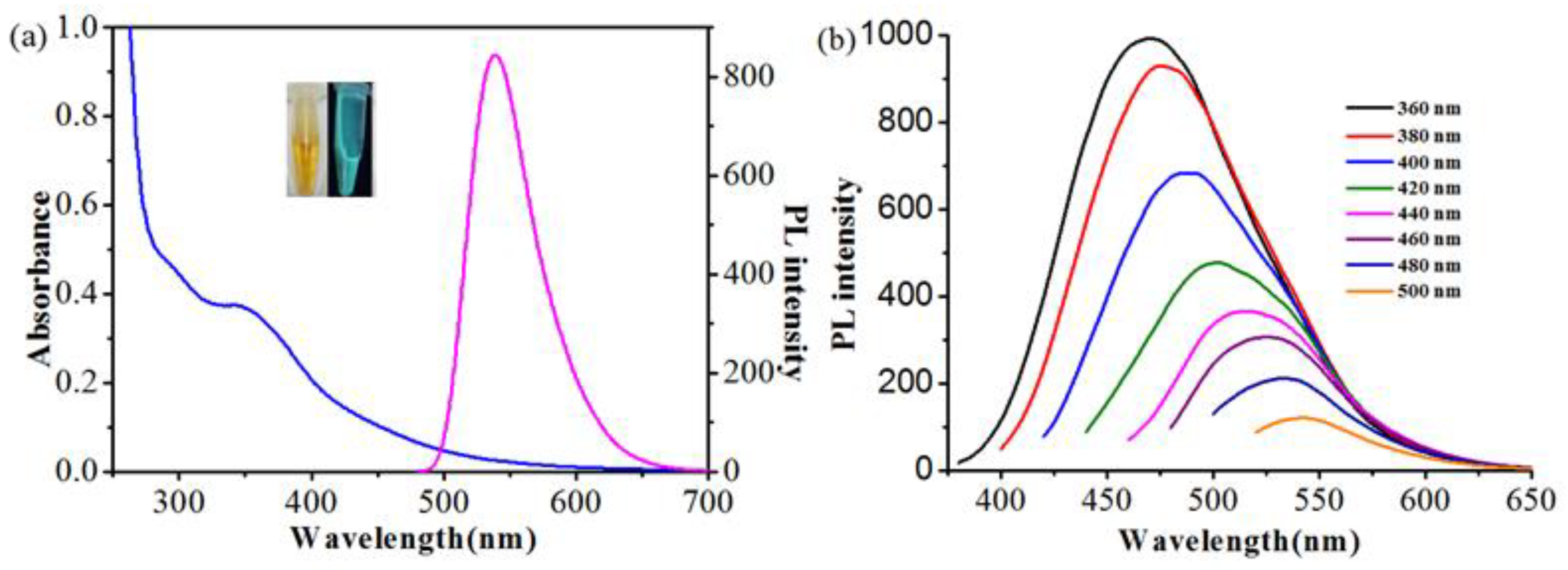
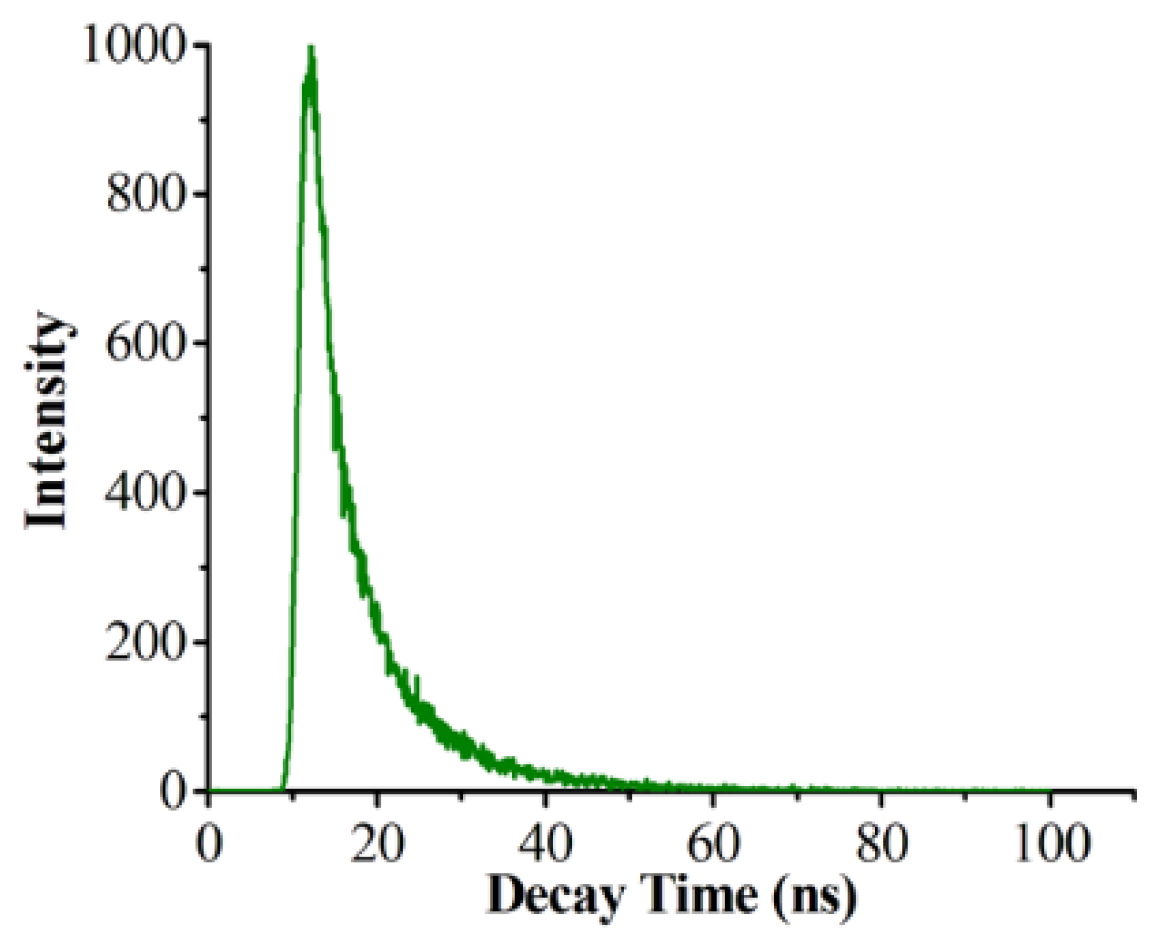
3.2. Optical Properties
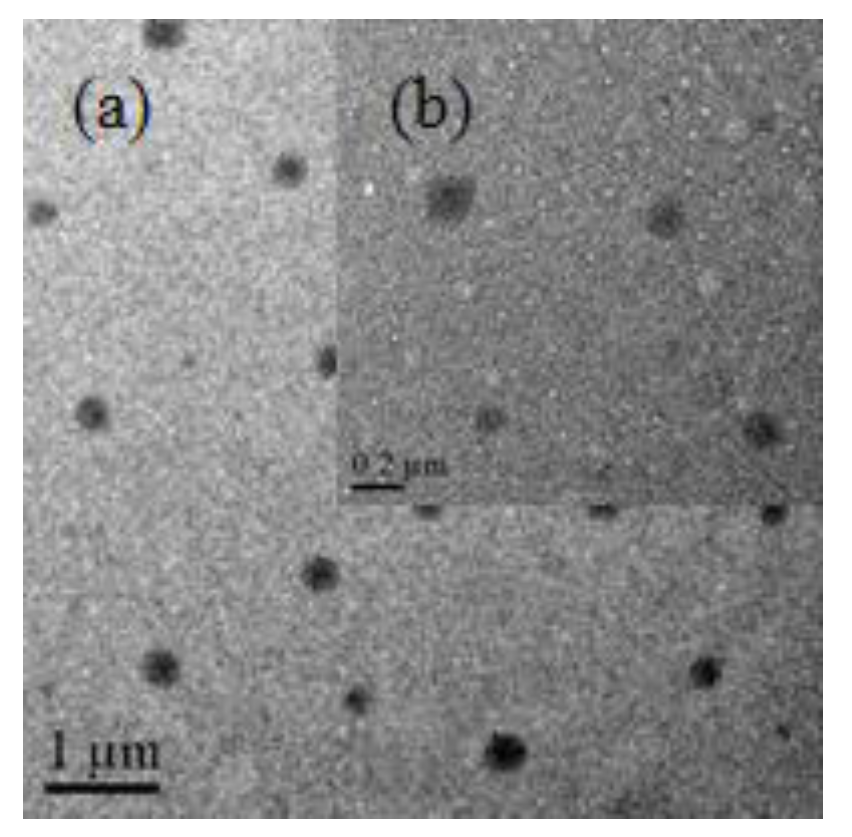
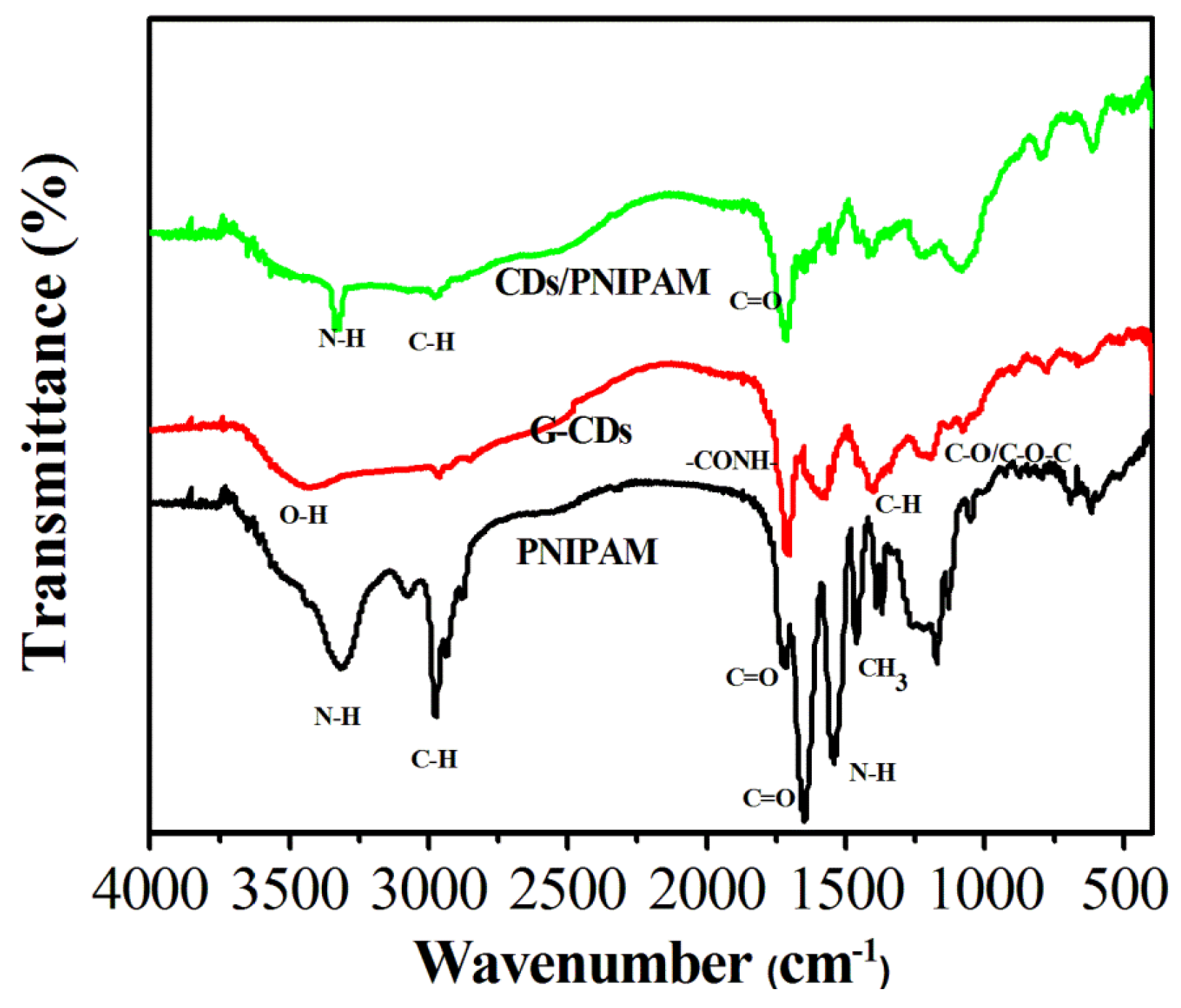
3.3. The Analysis of TEM, Zeta Potential and FTIR Spectroscopy
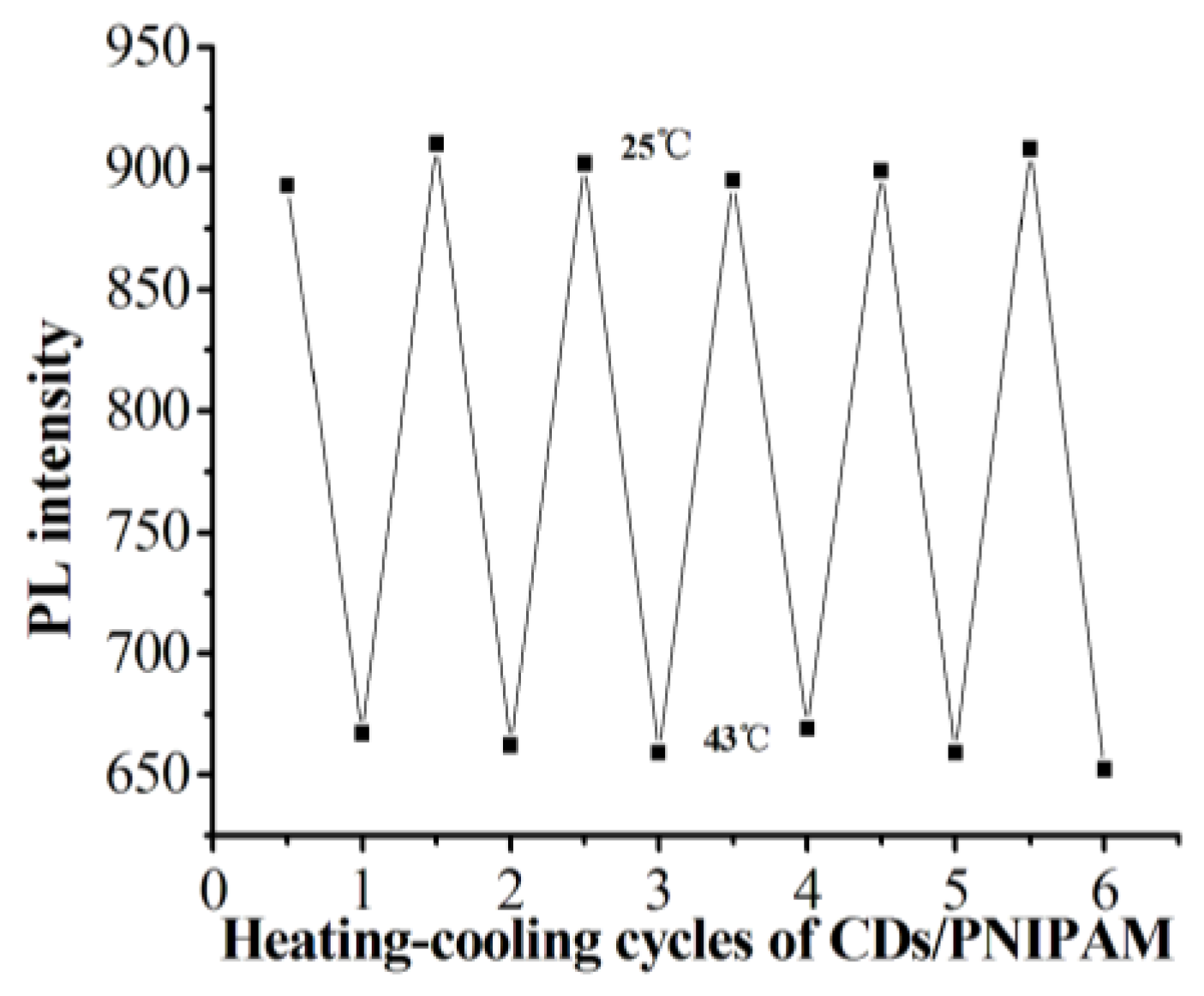
3.4. The Temperature-Sensitive Performance
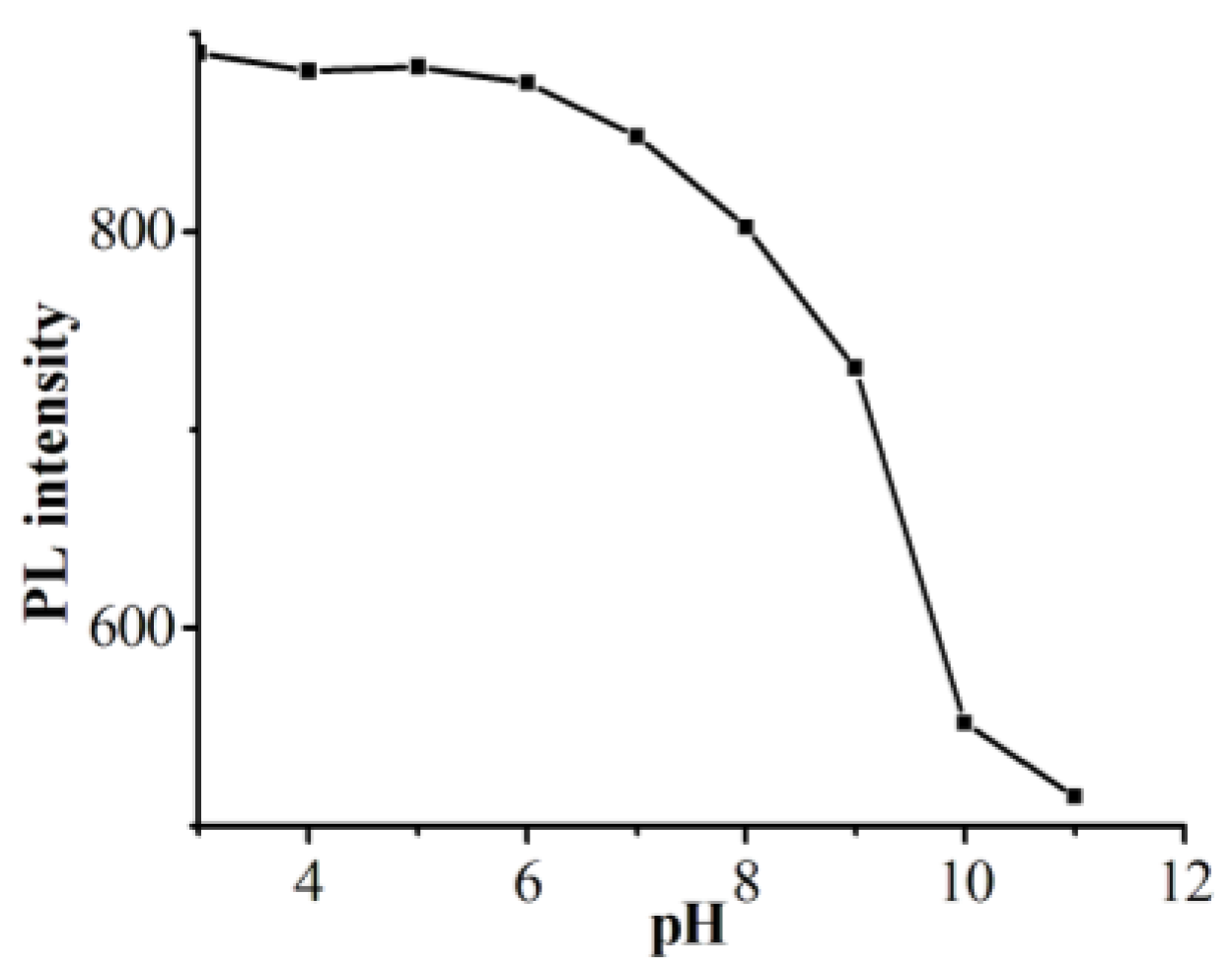
3.5. pH-Sensitive Performance
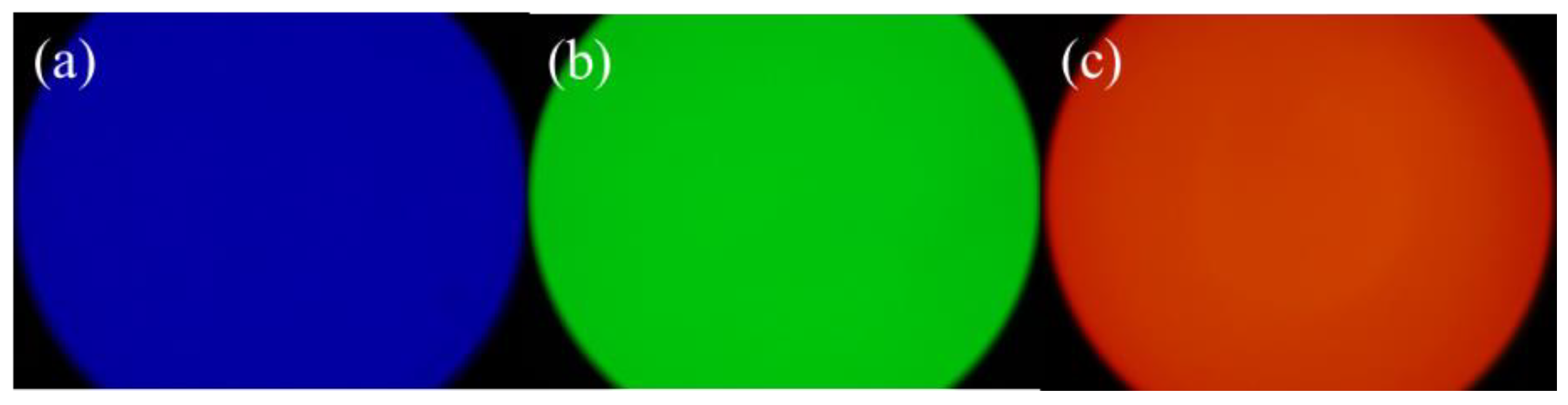
3.6. The Application of CDs/PNIPAM
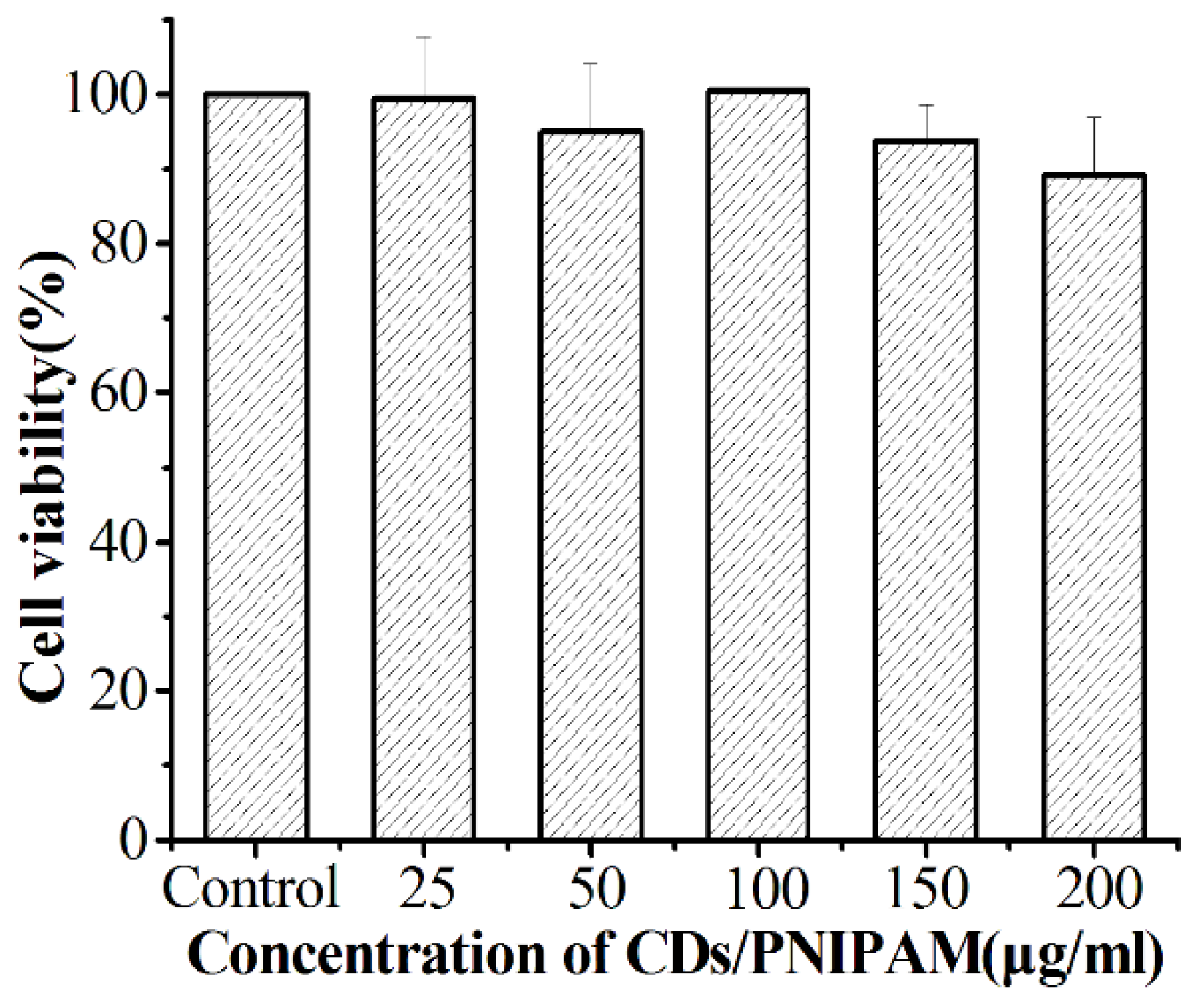
3.6.1. Biocompatibility of CDs/PNIPAM
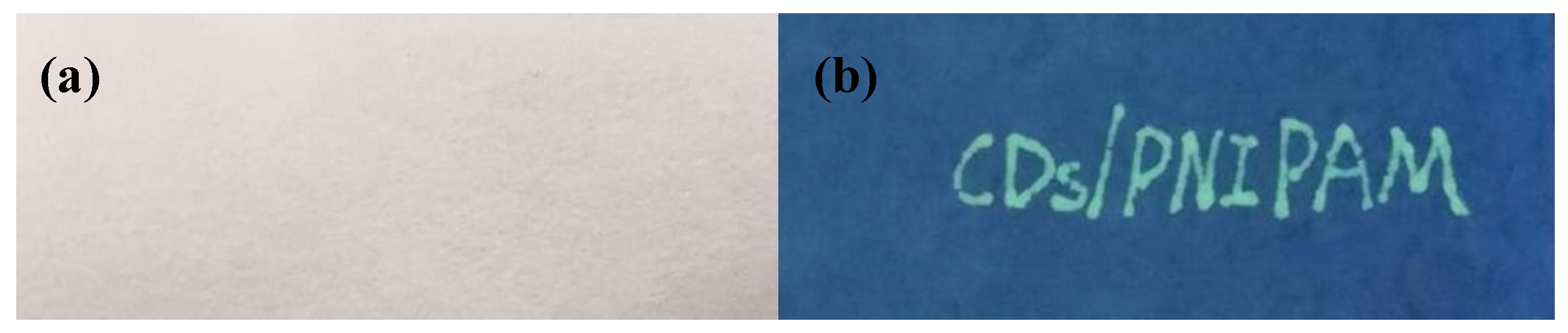
3.6.2. Fluorescent Ink
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; Ke, F.Y.; Mararenko, A.; Wei, Z.Y.; Banerjee, P.; Zhou, S.Q. Responsive polymer-fluorescent carbon nanoparticle hybrid nanogels for optical temperature sensing, near-infrared light-responsive drug release, and tumor cell imaging. Nanoscale 2014, 6, 7443–7452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, C.; Yang, X.D.; Shen, B.W.; Sun, Y.Q.; Chen, Y.; Xu, X.W.; Sun, H.C.; Yu, K.; Yang, B.; et al. pH- and temperature-sensitive hydrogel nanoparticles with dual photoluminescence for bioprobes. ACS Nano 2016, 10, 5856–5863. [Google Scholar] [CrossRef]
- Li, J.Z.; Liu, J.H.; Xu, L.Q.; Chen, J.C. Preparation of thermoresponsive fluorescent carbon dots for cellular imaging. Polym. Int. 2017, 66, 92–97. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, W.S.; Yu, X.Q.; Zhang, G.H.; Su, Z.Q. Synthesis and biomedical applications of fluorescent nanogels. Polym. Chem. 2016, 7, 5749–5762. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, F.A. Thermo-sensitive and photoluminescent hydrogels: Synthesis, characterization, and their drug-release property. Mater. Sci. Eng. C 2011, 31, 1429–1435. [Google Scholar] [CrossRef]
- Zhou, L.; He, B.Z.; Huang, J.C. Amphibious fluorescent carbon dots: One-step green synthesis and application for light-emitting polymer nanocomposites. Chem. Commun. 2013, 49, 8078–8080. [Google Scholar] [CrossRef]
- Liu, J.Z.; Shu, T.; Su, L.; Zhang, X.J.; Serpe, M.J. Synthesis of poly (N-isopropylacrylamide)-co-(acrylic acid) microgel-entrapped CdS quantum dots and their photocatalytic degradation of an organic dye. RSC Adv. 2018, 8, 16850–16857. [Google Scholar] [CrossRef]
- Kavitha, T.; Kim, J.O.; Jang, S.; Kim, D.P.; Kang, I.K.; Park, S.Y. Multifaceted thermoresponsive poly(N-vinylcaprolactam) coupled with carbon dots for biomedical applications. Mater. Sci. Eng. C 2016, 61, 492–498. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Li, C.Y.; He, C.C.; Dong, Y.Q.; Liu, Q.S.; Qin, P.F.; Zeng, C.; Wang, H.L. Luminescent hydrogels based on di(4-propoxyphenyl)-dibenzofulvene exhibiting four emission colours and organic solvents/thermal dual-responsive properties. J. Mater. Chem. C 2014, 2, 5829–5835. [Google Scholar] [CrossRef]
- Lü, J.H.; Fu, Y.Q.; Wang, D.M.; Lü, C.L. A facile synthesis of thermo-responsive copolymer stabilized fluorescent silver nanoclusters and their application in pH sensing. Sens. Actuators B 2018, 254, 996–1004. [Google Scholar] [CrossRef]
- Shen, X.R.; Yang, X.D.; Su, C.Y.; Yang, J.H.; Zhang, L.; Liu, B.H.; Gao, S.Y.; Gai, F.Y.; Shao, Z.B.; Gao, G.H. Thermo-responsive photoluminescent silver clusters/hydrogel nanocomposites for highly sensitive and selective detection of Cr(VI). J. Mater. Chem. C 2018, 6, 2088–2094. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, T.S. Thermoresponsive, and reversibly emissive, core–shell nanogel composed of PNIPAM and carbon nanodots. Polym. Bull. 2016, 73, 2615–2625. [Google Scholar] [CrossRef]
- Cai, Y.T.; Wu, X.Q.; Liu, Q.H.; Liu, H.Y. Alternative procedure for the incorporation of quantum dots into poly (N-isopropyl acrylamide-co-acrylic acid) microgels based on multiple interactions. J. Appl. Polym. Sci. 2016, 133, 43227. [Google Scholar] [CrossRef]
- Liras, M.; Quijada-Garrido, I.; García, O. QDs decorated with thiol-monomer ligands as new multicrosslinkers for the synthesis of smart luminescent nanogels and hydrogels. Polym. Chem. 2017, 8, 5317–5326. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.M.; Wu, D.D.; Yao, Y.; Liang, Z.L.; Tan, H.W.; Lau, A.T.Y. Acute and chronic cadmium telluride quantum dots-exposed human bronchial epithelial cells: The effects of particle sizes on their cytotoxicity and carcinogenicity. Biochem. Biophys. Res. Commun. 2018, 495, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zeng, G.M.; Chen, G.Q.; Huang, Z.Z.; Wan, J.; Chen, A.W.; Yu, Z.G.; Yang, J.L.; He, K.; Qin, L. Bioaccumulation and toxicity of CdSe/ZnS quantum dots in Phanerochaete chrysosporium. Colloids Surf. B Biointerfaces 2017, 159, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.H.; Wu, D.; Xia, L.; Chen, X.F.; Li, G.L.; Qiu, N.N.; Chen, G.; Sun, Z.W.; You, J.M.; Wu, Y.N. Carbon dots for fluorescent detection of α-glucosidase activity using enzyme activated inner filter effect and its application to anti-diabetic drug discovery. Anal. Chim. Acta 2017, 973, 91–99. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Yan, K.; Yang, Q.L.; Liu, Y.H.; Yan, Z.Y.; Chen, J.Q. One-pot synthesis of fluorescent nitrogen-doped carbon dots with good biocompatibility for cell labeling. Luminescence 2017, 32, 1488–1493. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.Y.; Zhao, Q.D.; Liu, B.J.; Liu, S.M.; Wang, S.B. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017, 496, 425–433. [Google Scholar] [CrossRef]
- Liu, W.; Diao, H.P.; Chang, H.H.; Wang, H.J.; Li, T.T.; Wei, W.L. Green synthesis of carbon dots from rose-heart radish and application for Fe3+ detection and cell imaging. Sens. Actuators B 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Wu, Z.L.; Liu, Z.X.; Yuan, Y.H. Carbon dots: Materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J. Mater. Chem. B 2017, 5, 3794–3809. [Google Scholar] [CrossRef]
- Campos, B.B.; Mutavdžić, D.; Stanković, M.; Radotić, K.; Lázaro-Martínez, J.M.; Esteves da Silva, J.C.G.; Contreras-Cáceres, R.; Pino-González, M.S.; Rodriguez-Castellón, E.; Algarra, M. Thermo-responsive microgels based on encapsulated carbon quantum dots. New J. Chem. 2017, 41, 4835–4842. [Google Scholar] [CrossRef]
- Wei, C.J.; Li, J.; Xiao, X.C.; Yue, T.; Zhao, D. The one-step preparation of green-emission carbon dots based on the deactivator-reducing reagent synergistic effect and the study on their luminescence mechanism. RSC Adv. 2018, 8, 20016–20024. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; He, X.W.; Li, W.Y. Study on the room temperature synthesis of highly photoluminescent and temperature-sensitive CDs/PNIPAM hybrid hydrogels and their properties. RSC Adv. 2015, 5, 71030–71034. [Google Scholar] [CrossRef]
- Wu, W.T.; Aiello, M.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S.Q. In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials 2010, 31, 3023–3031. [Google Scholar] [CrossRef]


| Samples | Zeta Potential (mV) |
|---|---|
| G-CDs | +11.4 |
| PNIPAM | −14.4 |
| CDs/PNIPAM | −3.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Ma, W.; Wang, R.; Yang, X.; Li, J.; Qiu, T.; Xiao, X. The Preparation of Green Fluorescence-Emissioned Carbon Dots/Poly(N-Isopropylacrylamide) Temperature-Sensitive Hydrogels and Research on Their Properties. Polymers 2019, 11, 1171. https://doi.org/10.3390/polym11071171
Zhao D, Ma W, Wang R, Yang X, Li J, Qiu T, Xiao X. The Preparation of Green Fluorescence-Emissioned Carbon Dots/Poly(N-Isopropylacrylamide) Temperature-Sensitive Hydrogels and Research on Their Properties. Polymers. 2019; 11(7):1171. https://doi.org/10.3390/polym11071171
Chicago/Turabian StyleZhao, Dan, Wenting Ma, Rong Wang, Xinzhou Yang, Jun Li, Ting Qiu, and Xincai Xiao. 2019. "The Preparation of Green Fluorescence-Emissioned Carbon Dots/Poly(N-Isopropylacrylamide) Temperature-Sensitive Hydrogels and Research on Their Properties" Polymers 11, no. 7: 1171. https://doi.org/10.3390/polym11071171
APA StyleZhao, D., Ma, W., Wang, R., Yang, X., Li, J., Qiu, T., & Xiao, X. (2019). The Preparation of Green Fluorescence-Emissioned Carbon Dots/Poly(N-Isopropylacrylamide) Temperature-Sensitive Hydrogels and Research on Their Properties. Polymers, 11(7), 1171. https://doi.org/10.3390/polym11071171





