Curing and Characteristics of N,N,N′,N′-Tetraepoxypropyl-4,4′-Diaminodiphenylmethane Epoxy Resin-Based Buoyancy Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Procedure
2.2. Differential Scanning Calorimeter (DSC)
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Mechanical Performance
2.5. Saturated Water Absorption
2.6. Scanning Electron Microscopy (SEM)
2.7. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Nonisothermal Curing of Epoxy Resin
3.2. The Comprehensive Performance of Buoyancy Material
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geng, H.T.; Hu, X.X.; Zhou, J.Y.; Xu, X.Q.; Wang, M.C.; Guo, A.R.; Du, H.Y.; Liu, J.C. Fabrication and compressive properties of closed-cell alumina ceramics by binding hollow alumina spheres with high-temperature binder. Ceram. Int. 2016, 42, 16071–16076. [Google Scholar] [CrossRef]
- Ren, S.; Guo, A.R.; Dong, X.; Tao, X.; Xu, X.Q.; Zhang, J.; Geng, H.T.; Liu, J.C. Preparation and characteristic of a temperature resistance buoyancy material through a gelcasting process. Chem. Eng. J. 2016, 288, 59–69. [Google Scholar] [CrossRef]
- Zheng, B.; Zhuang, M.M.; Guo, A.R.; Wang, M.C.; Ren, S.E.; Geng, H.T.; Liu, J.C. Buoyancy materials of aluminium borosilicate glass for high temperature resistance. Mater. Res. Innov. 2015, 19, 49–53. [Google Scholar] [CrossRef]
- Ren, S.; Li, X.T.; Zhang, X.J.; Xu, X.Q.; Dong, X.; Liu, J.C.; Du, H.Y.; Guo, A.R. Mechanical properties and high-temperature resistance of the hollow glass microspheres/borosilicate glass composite with different particle size. J. Alloys Compd. 2017, 722, 321–329. [Google Scholar] [CrossRef]
- Narkis, M.; Puterman, M.; Boneh, H.; Kenig, S. Rotational molding of thermosetting three-phase syntactic foams. Polym. Eng. Sci. 1982, 22, 417–421. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.H.; Pei, J.H.; Wang, Z.; Wu, Z.J. Fabrication and mechanical properties of a novel epoxy-hollow glass microsphere composite. J. Compos. Mater. 2018, 52, 1627–1632. [Google Scholar] [CrossRef]
- Colombo, P.; Modesti, M. Silicon oxycarbide ceramic foams from a preceramic polymer. J. Am. Ceram. Soc. 1999, 82, 573–578. [Google Scholar] [CrossRef]
- Li, Y.; Badrinarayanan, P.; Kessler, M.R. Liquid crystalline epoxy resin based on biphenyl mesogen: Thermal characterization. Polymer 2013, 54, 3017–3025. [Google Scholar] [CrossRef]
- Ha, S.R.; Ryu, S.H.; Park, S.J.; Rhee, K.Y. Effect of clay surface modification and concentration on the tensile performance of clay/epoxy nanocomposites. Mater. Sci. Eng. A 2007, 448, 264–268. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, F.L.; Park, S.J. Thermo-mechanical behaviors of epoxy resins reinforced with nano-Al2O3 particles. J. Ind. Eng. Chem. 2012, 18, 594–596. [Google Scholar] [CrossRef]
- Wana, J.T.; Li, B.; Gan, C.; Jon, M.A.; Kalali, E.N.; Wang, X.; Wang, D.Y. A sustainable, eugenol-derived epoxy resin with high biobased content, modulus, hardness and low flammability: Synthesis, curing kinetics and structure–property relationship. Chem. Eng. J. 2016, 284, 1080–1093. [Google Scholar] [CrossRef]
- John, H.; Luft, M.D. Improvements in epoxy resin embedding methods. J. Biophys. Biochem. Cytol. 1961, 9, 409–414. [Google Scholar]
- Jeon, H.R.; Park, J.H.; Shon, M.Y. Corrosion protection by epoxy coating containing multi-walled carbon nanotubes. J. Ind. Eng. Chem. 2013, 19, 849–853. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Hong, I.K.; Yoon, Y.S.; Lee, S.B. Selection of thinner for epoxy type resins for neon transformer housing. J. Ind. Eng. Chem. 2012, 18, 1997–2003. [Google Scholar] [CrossRef]
- Park, S.J.; Park, B.J. Electrochemically Modified PAN Carbon Fibers and Interfacial Adhesion in Epoxy-resin Composites. J. Mater. Sci. Lett. 1999, 18, 47–49. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]
- Wei, K.; Ma, B.; Wang, H.N.; Li, N. Effect of a tetra functional epoxy monomer on the thermomechanical properties of shape-memory epoxy resin. Fibers Polym. 2015, 16, 2343–2348. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, S.L.; Dong, S.L.; Yang, D.Z. Effect of 120 keV proton irradiation on mass loss and chemical structure of AG-80 epoxy resin. Radiat. Eff. Defects Solids 2010, 165, 857–867. [Google Scholar] [CrossRef]
- Oommen, C.; Amanulla, S.; Jain, S.R. Characterization of diglycidylamine epoxy resins based on bis-hydrazones. Eur. Polym. J. 2000, 36, 779–782. [Google Scholar] [CrossRef]
- Matejka, L.; Dusek, K. Influence of the reaction mechanism on network formation in amine-cured N, N-diglycidylamine epoxy resins. Polymer 1991, 32, 3195–3200. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L. Synthesis and characterization of UV-curable acrylic resin containing fluorine groups. Polym. Int. 2005, 54, 705–709. [Google Scholar] [CrossRef]
- Jin, F.L.; Park, S.J. Interfacial toughness properties of trifunctional epoxy resins/calcium carbonate nanocomposites. Mater. Sci. Eng. A 2008, 475, 190–193. [Google Scholar] [CrossRef]
- Roşu, D.; Caşcaval, C.N.; Mustatǎ, F.; Ciobanu, C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim. Acta 2002, 383, 119–127. [Google Scholar] [CrossRef]
- Ferdosiana, F.; Yuana, Z.S.; Andersonb, M.; Xua, C.B. Sustainable lignin-based epoxy resins cured with aromatic and aliphatic amine curing agents: Curing kinetics and thermal properties. Thermochim. Acta 2015, 618, 48–55. [Google Scholar] [CrossRef]
- Cole, K.C. A new approach to modeling the cure kinetics of epoxy/amine thermosetting resins. 1. Mathematical development. Macromolecules 1991, 24, 3093–3097. [Google Scholar] [CrossRef]
- Patel, R.D.; Patel, R.G.; Patel, V.S. Cure kinetics of epoxy resins by differential scanning calorimetry technique. Br. Polym. J. 2015, 19, 37–41. [Google Scholar] [CrossRef]
- Lee, J.Y.; Shim, M.J.; Kim, S.W. Thermal decomposition kinetics of an epoxy resin with rubber-modified curing agent. J. Appl. Polym. Sci. 2000, 81, 479–485. [Google Scholar] [CrossRef]
- Zabihi, O.; Khayyam, H.; Foxa, L.B.; Naebe, M. Enhanced thermal stability and lifetime of epoxy nanocomposites using covalently functionalized clay: Experimental and modelling. New J. Chem. 2015, 39, 2269–2278. [Google Scholar] [CrossRef]
- Wan, J.T.; Li, C.; Bu, Z.Y.; Xu, C.J.; Li, B.G.; Fan, H. A comparative study of epoxy resin cured with a linear diamine and a branched polyamine. Chem. Eng. J. 2012, 188, 160–172. [Google Scholar] [CrossRef]
- Flynn, J.H. Thermal analysis kinetics-past, present and future. Thermochim. Acta 1992, 203, 519–526. [Google Scholar] [CrossRef]
- Chen, Y.X. Synthesis and Curing Kinetics of Epoxy Resin Tough Curing Agent. Master’s Thesis, Sichuan University, Chengdu, China, 2007. [Google Scholar]
- Lin, L.; Song, W.; Li, C.Q. Curing kinetics and thermal stability of low viscosity epoxy resin systems. China Surf. Eng. 2011, 24, 73–77. [Google Scholar]
- Horie, K.; Hiura, H.; Sawada, M.; Mita, I.; Kambe, H. Calorimetric investigation of polymerization reactions. III. Curing reaction of epoxides with amines. J. Polym. Sci. Part A Polym. Chem. 1970, 8, 1357–1372. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick method for determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 5, 323–328. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules 1996, 29, 1867–1873. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Yousefi, A.; Lafleur, P.G. Kinetics studies of the thermoset cure reactions: A review. Polym. Compos. 1997, 18, 157–168. [Google Scholar] [CrossRef]
- Hojjati, M.; Johnson, A.; Cole, K.C. Cure kinetics of hexcel W3T282-42/F155 graphite/epoxy prepreg. Sci. Eng. Compos. Mater. 2000, 3, 111–122. [Google Scholar] [CrossRef]
- Vyazovkin, S. A unified approach to kinetic processing of nonisothermal data. Int. J. Chem. Kinet. 1996, 28, 95–101. [Google Scholar] [CrossRef]
- Turcsanyi, B.; Pukanszky, B.; Tudos, F. Composition dependence of tensile yield stress in filled polymers. Mater. Sci. Lett. 1988, 7, 160–162. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Singh Tomer, N.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization-Trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and flameretardancy of epoxy resins-a review of the recent literature. Polym. Int. 2004, 53, 1901–1929. [Google Scholar] [CrossRef]
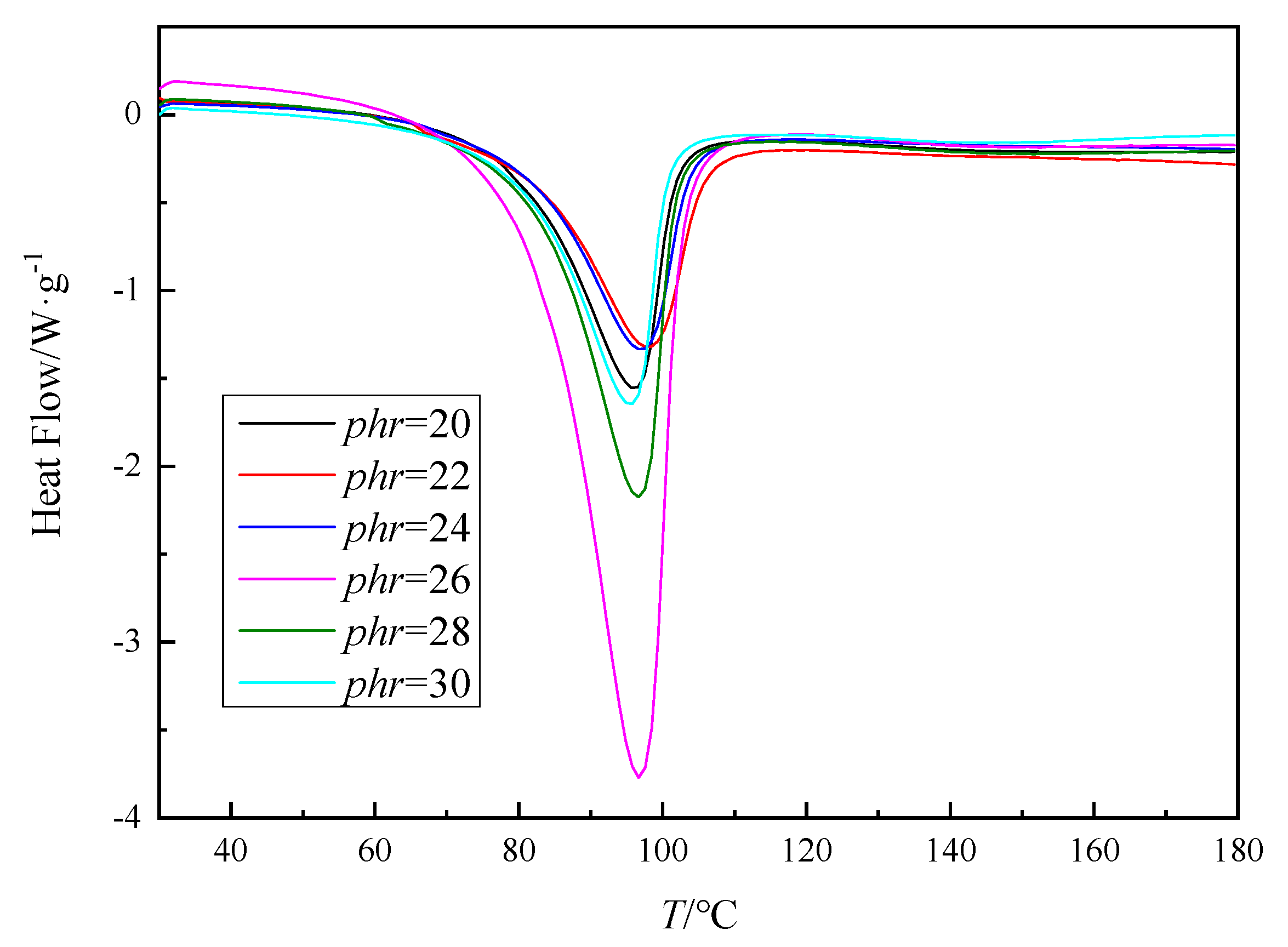
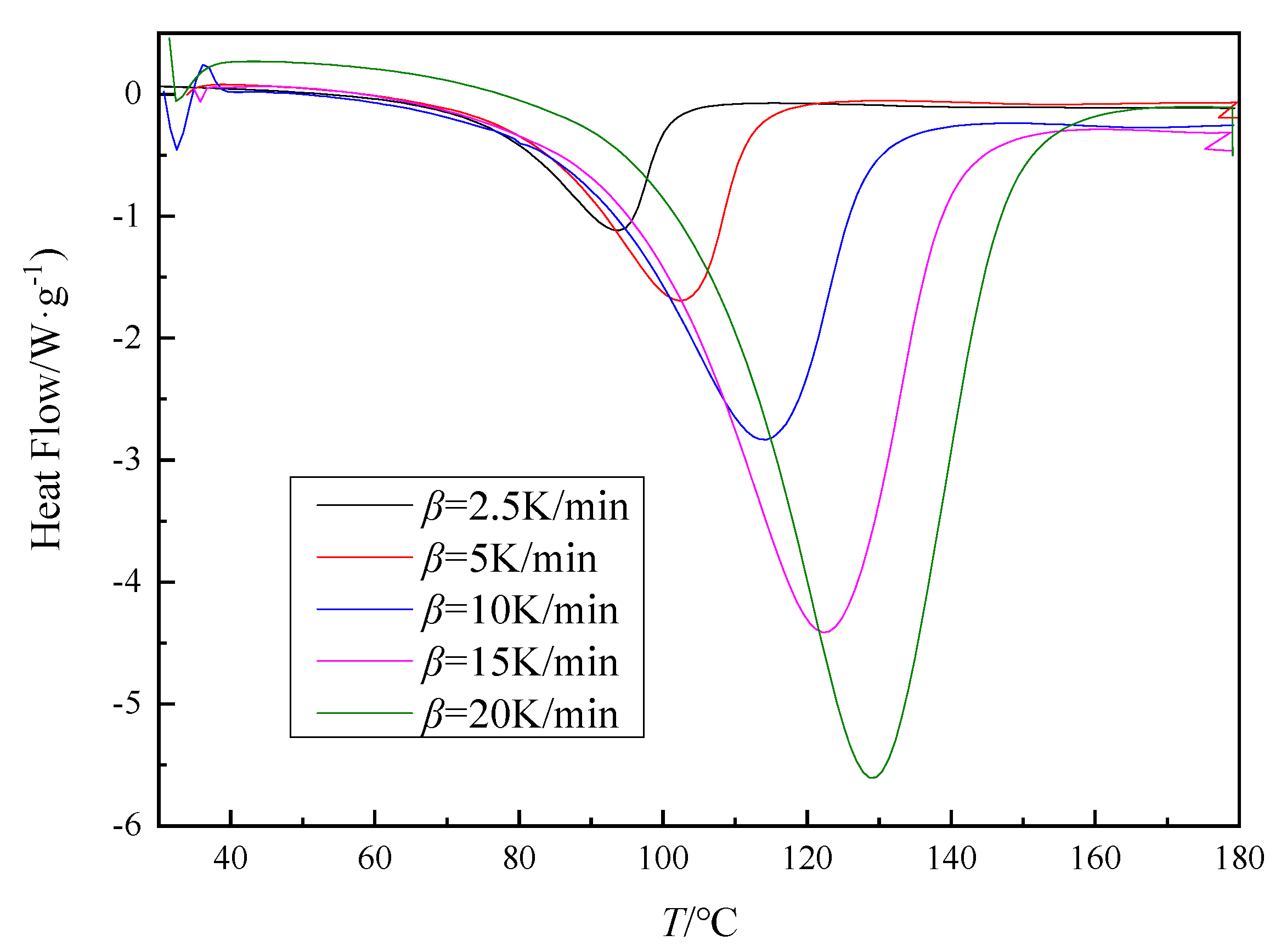

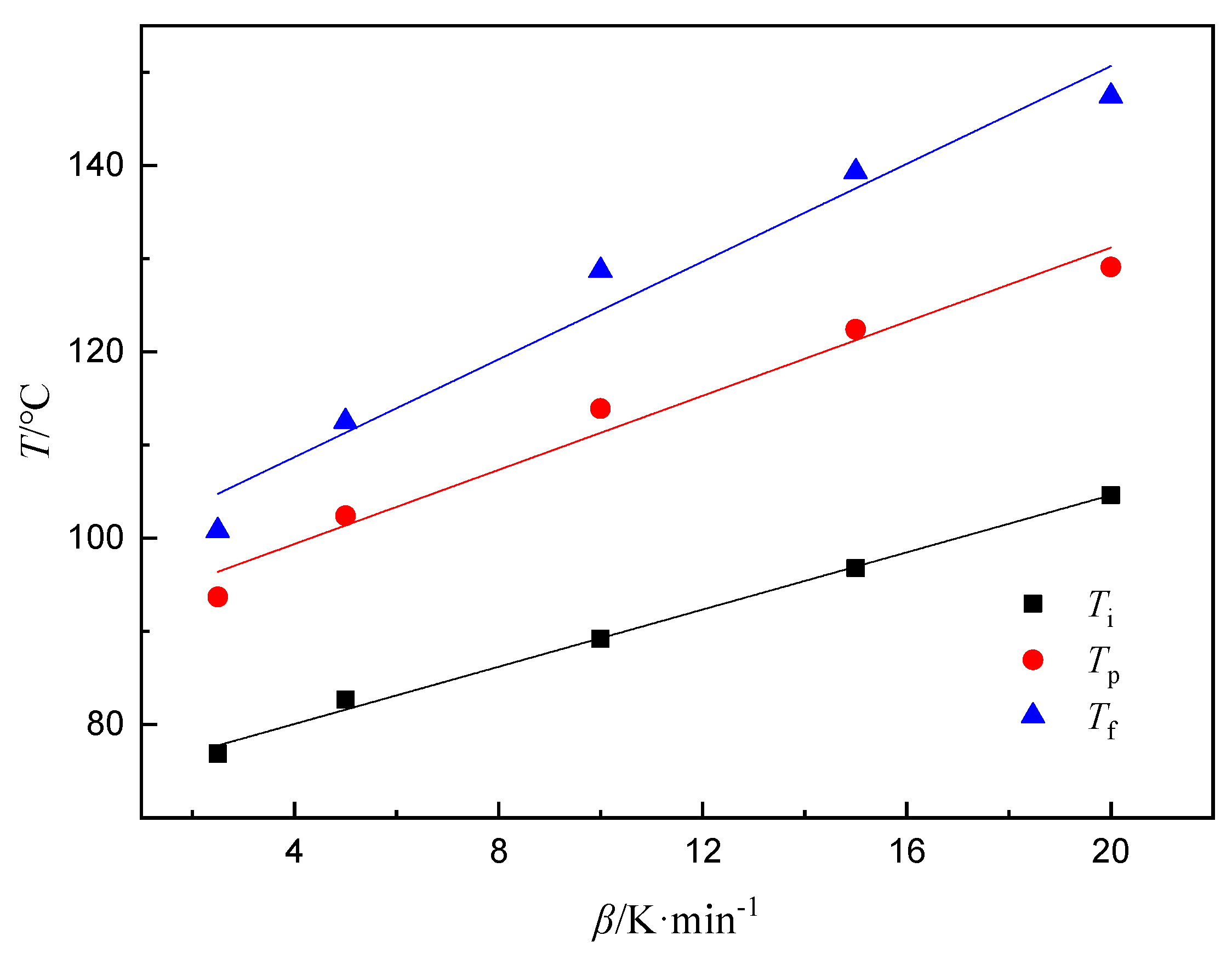
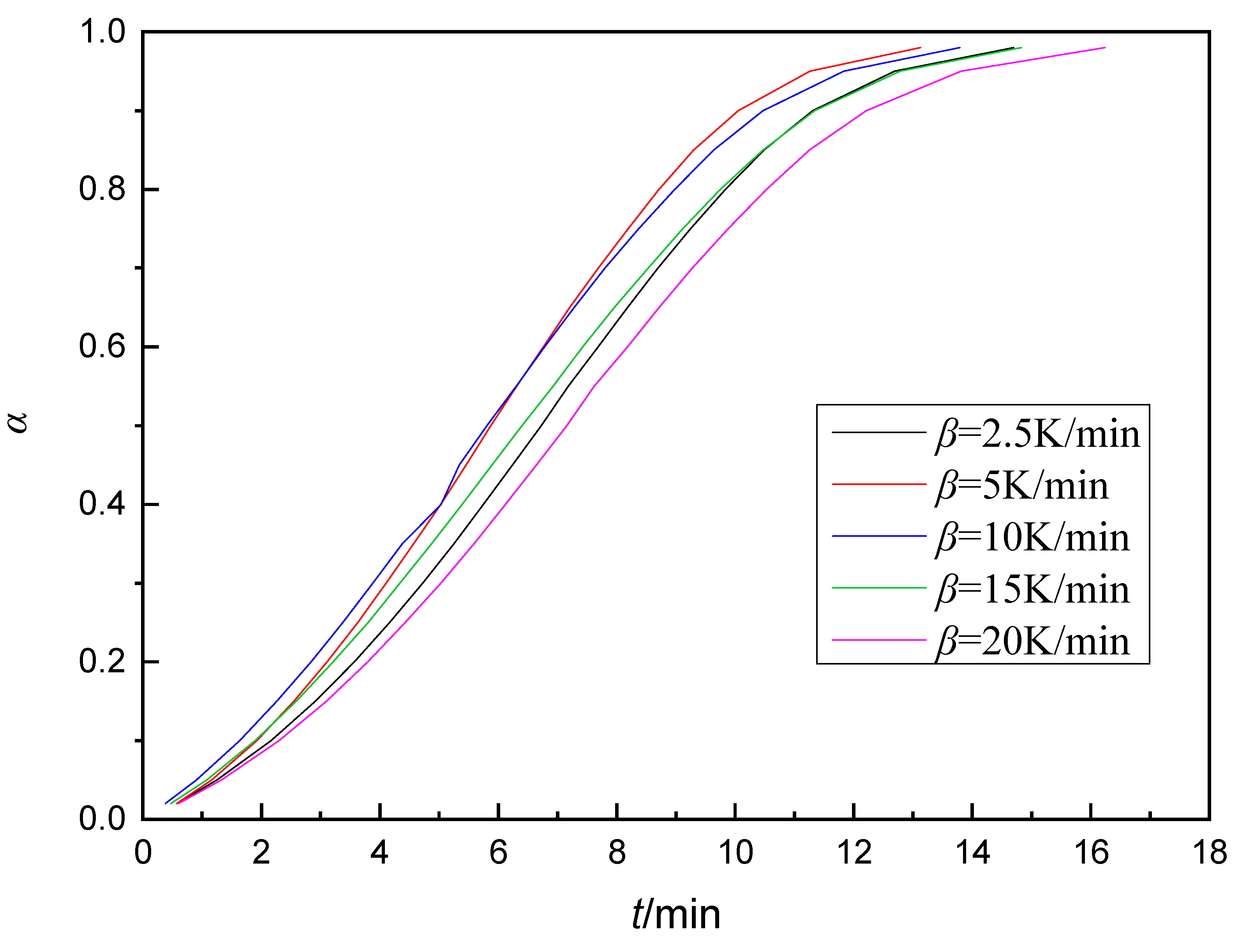
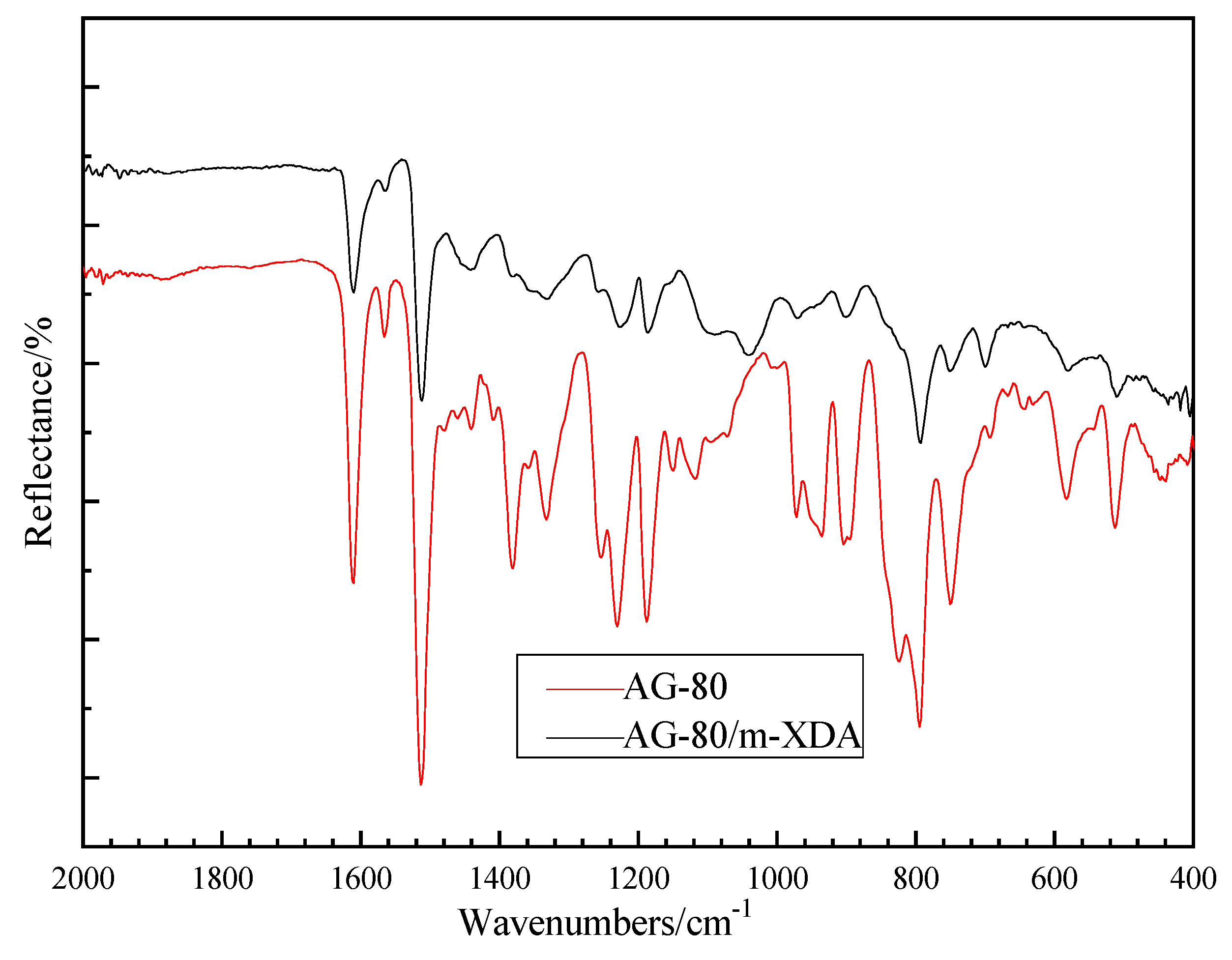

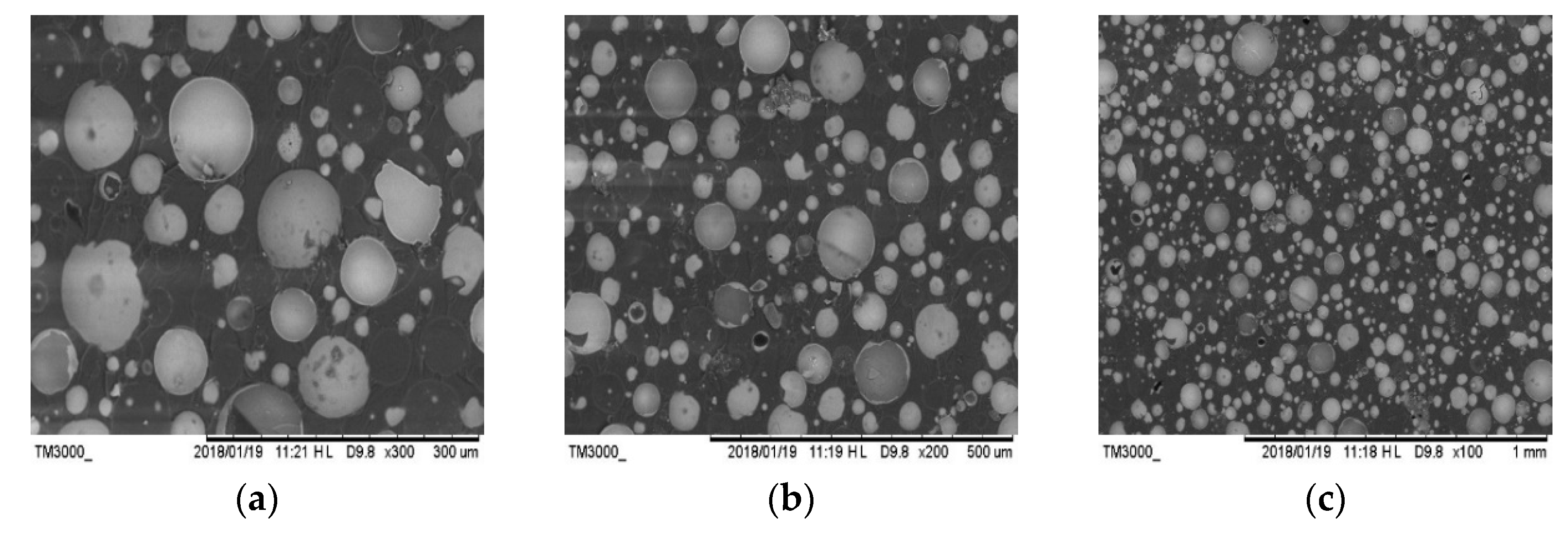
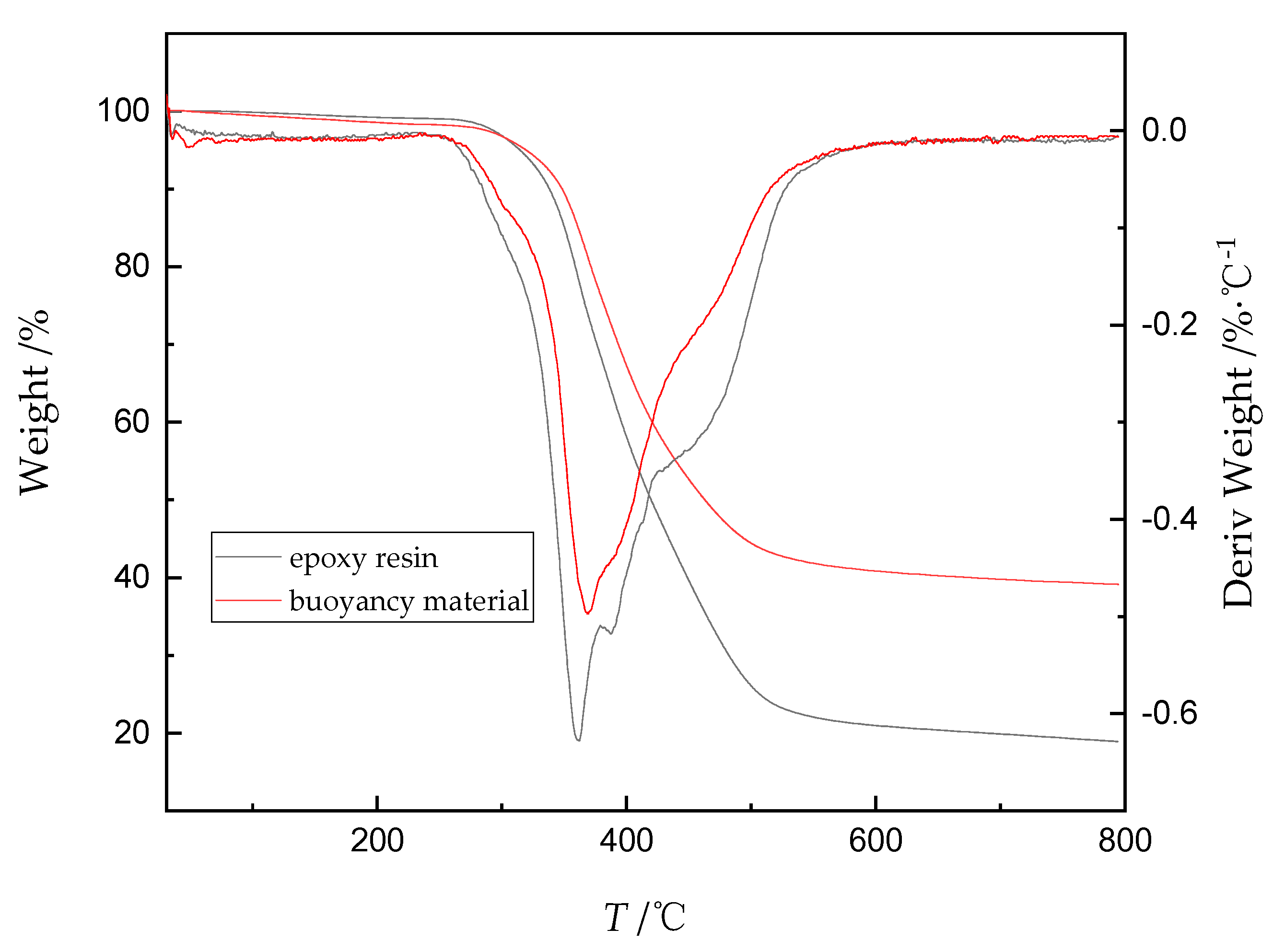
| Curing System | phr | Q/J·g−1 |
|---|---|---|
| AG-80/m-XDA | 20 | 544.7 |
| 22 | 534.3 | |
| 24 | 512.5 | |
| 26 | 1383.1 | |
| 28 | 732.6 | |
| 30 | 525.5 |
| β/K·min−1 | Ti/°C | Tp/°C | Tf/°C | △H/J·g−1 |
|---|---|---|---|---|
| 2.5 | 76.9 | 93.7 | 100.8 | −450.2 |
| 5 | 82.7 | 102.4 | 112.5 | −432.9 |
| 10 | 89.2 | 113.9 | 128.7 | −423.8 |
| 15 | 96.8 | 122.4 | 139.3 | −473.5 |
| 20 | 104.6 | 129.1 | 147.4 | −484.1 |
| α | Slope | Intercept | r2 | Ea/kJ·mol−1 |
|---|---|---|---|---|
| 0.02 | −5147.41 | 15.58 | 0.9316 | 93.71 |
| 0.05 | −4506.62 | 13.51 | 0.9585 | 82.04 |
| 0.10 | −4142.63 | 12.30 | 0.9682 | 75.41 |
| 0.15 | −3975.30 | 11.72 | 0.9730 | 72.37 |
| 0.20 | −3877.87 | 11.37 | 0.9766 | 70.59 |
| 0.25 | −3803.00 | 11.10 | 0.9794 | 69.23 |
| 0.30 | −3745.06 | 10.89 | 0.9810 | 68.18 |
| 0.35 | −3705.35 | 10.74 | 0.9830 | 67.45 |
| 0.40 | −3663.82 | 10.59 | 0.9877 | 66.70 |
| 0.45 | −3624.80 | 10.45 | 0.9861 | 65.99 |
| 0.50 | −3590.99 | 10.33 | 0.9870 | 65.37 |
| 0.55 | −3569.71 | 10.24 | 0.9890 | 64.98 |
| 0.60 | −3522.66 | 10.09 | 0.9892 | 64.13 |
| 0.65 | −3484.29 | 9.96 | 0.9899 | 63.43 |
| 0.70 | −3443.85 | 9.82 | 0.9907 | 62.69 |
| 0.75 | −3399.63 | 9.68 | 0.9913 | 61.89 |
| 0.80 | −3343.24 | 9.50 | 0.9918 | 60.86 |
| 0.85 | −3275.40 | 9.29 | 0.9920 | 59.63 |
| 0.90 | −3195.53 | 9.05 | 0.9923 | 58.17 |
| 0.95 | −3093.60 | 8.73 | 0.9922 | 56.32 |
| 0.98 | −2999.04 | 8.42 | 0.9921 | 54.60 |
| Extrapolated Temperatures | T/°C | r2 |
|---|---|---|
| Ti | 73.92 | 0.997 |
| Tp | 91.43 | 0.987 |
| Tf | 98.20 | 0.983 |
| Sample No. | VH/% | ρCAL/g·cm−3 | ρ/g·cm−3 | ECAL/MPa | E/MPa | E/ρ |
|---|---|---|---|---|---|---|
| 1 | 0 | —— | 1.208 | —— | 126.14 | 104.42 |
| 2 | 40 | 0.849 | 0.844 | 115.98 | 120.69 | 143.00 |
| 3 | 45 | 0.804 | 0.812 | 115.10 | 116.65 | 143.66 |
| 4 | 50 | 0.759 | 0.764 | 113.67 | 112.07 | 146.69 |
| 5 | 55 | 0.714 | 0.729 | 111.48 | 108.78 | 149.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Li, X.; Guo, X.; Li, Z.; Zou, M. Curing and Characteristics of N,N,N′,N′-Tetraepoxypropyl-4,4′-Diaminodiphenylmethane Epoxy Resin-Based Buoyancy Material. Polymers 2019, 11, 1137. https://doi.org/10.3390/polym11071137
Yu S, Li X, Guo X, Li Z, Zou M. Curing and Characteristics of N,N,N′,N′-Tetraepoxypropyl-4,4′-Diaminodiphenylmethane Epoxy Resin-Based Buoyancy Material. Polymers. 2019; 11(7):1137. https://doi.org/10.3390/polym11071137
Chicago/Turabian StyleYu, Sizhu, Xiaodong Li, Xiaoyan Guo, Zhiren Li, and Meishuai Zou. 2019. "Curing and Characteristics of N,N,N′,N′-Tetraepoxypropyl-4,4′-Diaminodiphenylmethane Epoxy Resin-Based Buoyancy Material" Polymers 11, no. 7: 1137. https://doi.org/10.3390/polym11071137
APA StyleYu, S., Li, X., Guo, X., Li, Z., & Zou, M. (2019). Curing and Characteristics of N,N,N′,N′-Tetraepoxypropyl-4,4′-Diaminodiphenylmethane Epoxy Resin-Based Buoyancy Material. Polymers, 11(7), 1137. https://doi.org/10.3390/polym11071137






