High Performance Attapulgite/Polypyrrole Nanocomposite Reinforced Polystyrene (PS) Foam Based on Supercritical CO2 Foaming
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Foam Extrusion of ATP/PPy Nanocomposite Reinforced PS
2.3. Thermal and Mechanical Properties
3. Results and Discussion
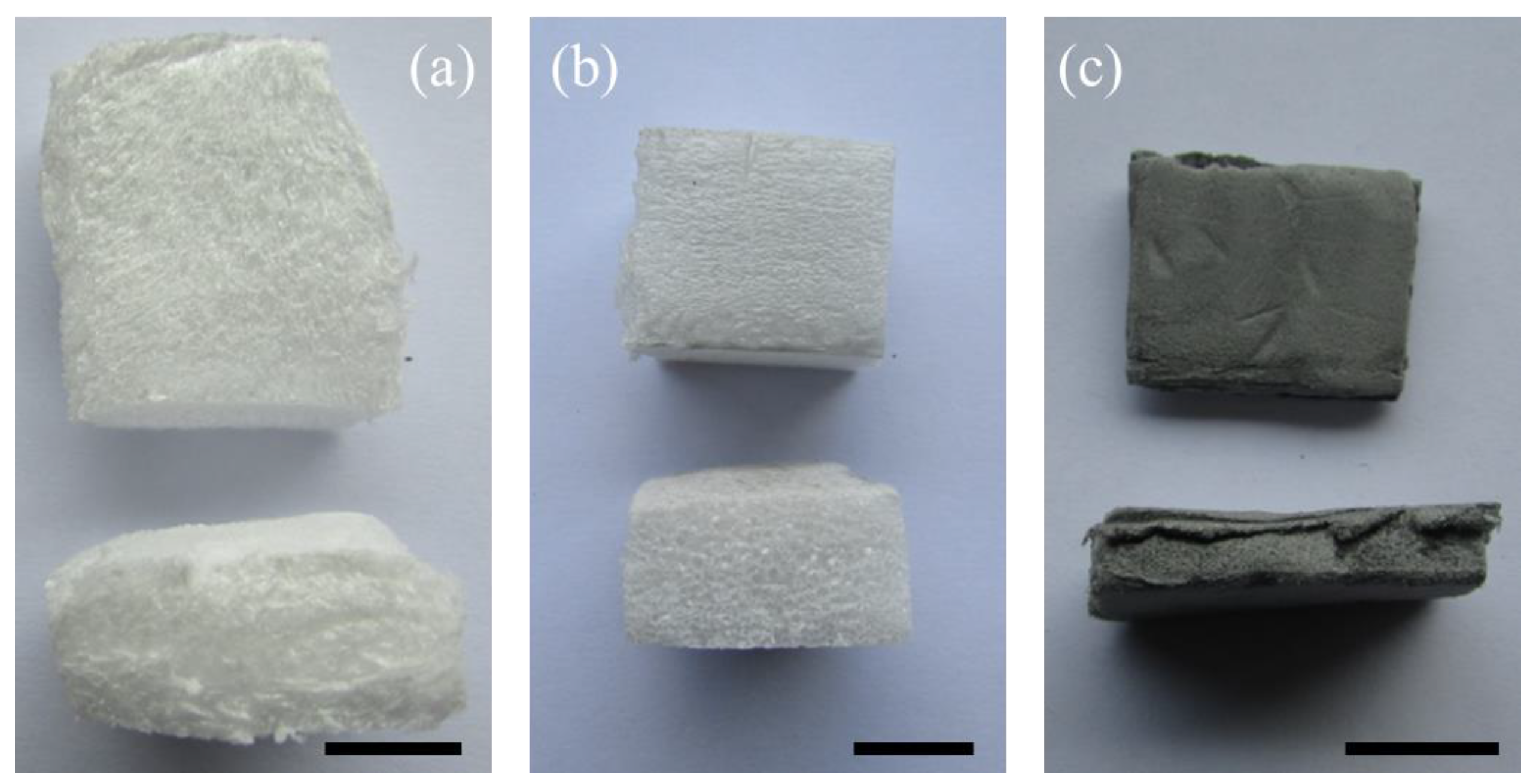
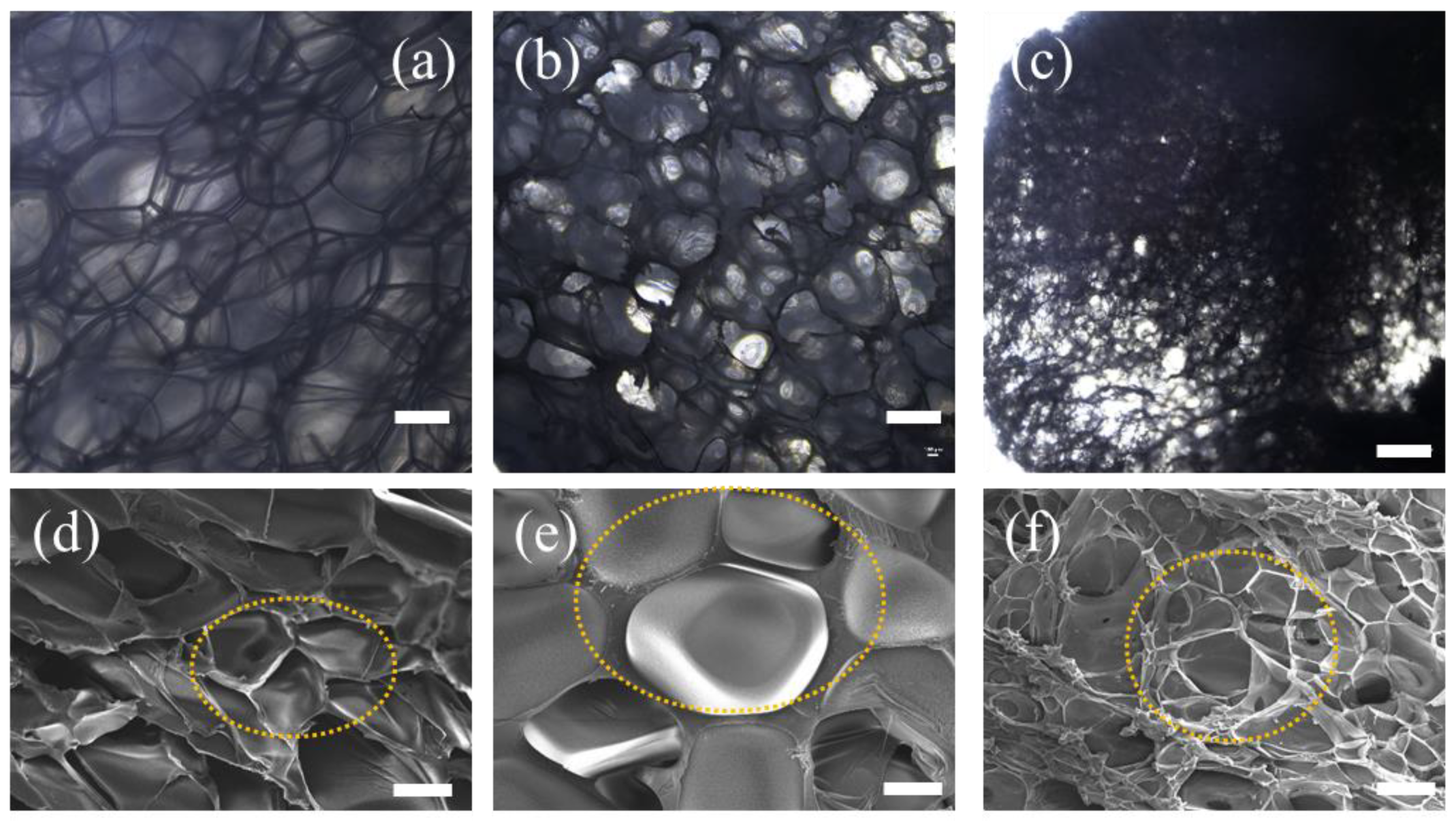
3.1. Foam Morphology
3.2. Thermal and Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, M.; Gu, X.; Zhang, S.; Li, H.; Jiang, P. Effects of Acidic Sites in HA Zeolite on the fire performance of polystyrene composite. Ind. Eng. Chem. Res. 2013, 52, 9145–9154. [Google Scholar] [CrossRef]
- Demirel, B. Optimization of the composite brick composed of expanded polystyrene and pumice blocks. Constr. Build. Mater. 2013, 40, 306–313. [Google Scholar] [CrossRef]
- Chen, W.; Hao, H.; Hughes, D.; Shi, Y.; Cui, J.; Li, Z.-X. Static and dynamic mechanical properties of expanded polystyrene. Mater. Des. 2015, 69, 170–180. [Google Scholar] [CrossRef]
- Min, Z.; Yang, H.; Chen, F.; Kuang, T. Scale-up production of lightweight high-strength polystyrene/carbonaceous filler composite foams with high-performance electromagnetic interference shielding. Mater. Lett. 2018, 230, 157–160. [Google Scholar] [CrossRef]
- Jing, X.; Peng, X.-F.; Mi, H.-Y.; Wang, Y.-S.; Zhang, S.; Chen, B.-Y.; Zhou, H.-M.; Mou, W.-J. Cell evolution and compressive properties of styrene–butadiene–styrene toughened and calcium carbonate reinforced polystyrene extrusion foams with supercritical carbon dioxide. J. Appl. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Ji, W.-Y.; Feng, L.-F.; Zhang, C.-L.; Hoppe, S.; Hu, G.-H.; Dumas, D. A concept of reactive compatibilizer-tracer for studying reactive polymer blending processes. AIChE J. 2016, 62, 359–366. [Google Scholar] [CrossRef]
- Zeng, C.; Han, X.; Lee, L.J.; Koelling, K.W.; Tomasko, D.L. Polymer–clay nanocomposite foams prepared using carbon dioxide. Adv. Mater. 2003, 15, 1743–1747. [Google Scholar] [CrossRef]
- Han, X.; Zeng, C.; Lee, L.J.; Koelling, K.W.; Tomasko, D.L. Extrusion of polystyrene nanocomposite foams with supercritical CO2. Polym. Eng. Sci. 2003, 43, 1261–1275. [Google Scholar] [CrossRef]
- Yu, P.; Liu, G.; Li, K.; Huang, A.; Chen, B.; Mi, H.; Zhang, S.; Peng, X. Fabrication of polystyrene/nano-CaCO3 foams with unimodal or bimodal cell structure from extrusion foaming using supercritical carbon dioxide. Polym. Compos. 2016, 37, 1864–1873. [Google Scholar] [CrossRef]
- Cabrera, E.D.; Mulyana, R.; Castro, J.M.; Lee, L.J.; Min, Y. Pressurized water pellets and supercritical nitrogen in injection molding. J. Appl. Polym. Sci. 2013, 127, 3760–3767. [Google Scholar] [CrossRef]
- Han, X.; Koelling, K.W.; Tomasko, D.L.; Lee, L.J. Continuous microcellular polystyrene foam extrusion with supercritical CO2. Polym. Eng. Sci. 2002, 42, 2094–2106. [Google Scholar] [CrossRef]
- Xu, L.-Q.; Huang, H.-X. Formation mechanism and tuning for bi-modal cell structure in polystyrene foams by synergistic effect of temperature rising and depressurization with supercritical CO2. J. Supercrit. Fluids 2016, 109, 177–185. [Google Scholar] [CrossRef]
- Sovova, H.; Nistor, A.; Topiar, M.; Kosek, J. Vitrification conditions and porosity prediction of CO2 blown polystyrene foams. J. Supercrit. Fluids 2017, 127, 1–8. [Google Scholar] [CrossRef]
- Sauceau, M.; Nikitine, C.; Rodier, E.; Fages, J. Effect of supercritical carbon dioxide on polystyrene extrusion. J. Supercrit. Fluids 2007, 43, 367–373. [Google Scholar] [CrossRef]
- Wang, L.; Feng, L.-F.; Gu, X.-P.; Zhang, C.-L. Influences of the viscosity ratio and processing conditions on the formation of highly oriented ribbons in polymer blends by tape extrusion. Ind. Eng. Chem. Res. 2015, 54, 11080–11086. [Google Scholar] [CrossRef]
- Ji, W.-Y.; Feng, L.-F.; Zhang, C.-L.; Hoppe, S.; Hu, G.-H. Development of a reactive compatibilizer-tracer for studying reactive polymer blends in a twin-screw extruder. Ind. Eng. Chem. Res. 2015, 54, 10698–10706. [Google Scholar] [CrossRef]
- Kwag, C.; Manke, C.W.; Gulari, E. Rheology of molten polystyrene with dissolved supercritical and near-critical gases. J. Polym. Sci. Part B 1999, 37, 2771–2781. [Google Scholar] [CrossRef]
- Wang, K.; Pang, Y.; Liu, W.; Wu, F.; Zheng, W. A new approach designed for improving in situ compatibilization of polypropylene/polystyrene blends via reactive extrusion with supercritical CO2 as the processing medium. J. Supercrit. Fluids 2016, 118, 203–209. [Google Scholar] [CrossRef]
- Han, X.; Shen, J.; Huang, H.; Tomasko, D.L.; Lee, L.J. CO2 foaming based on polystyrene/poly(methyl methacrylate) blend and nanoclay. Polym. Eng. Sci. 2007, 47, 103–111. [Google Scholar] [CrossRef]
- Xiao, W.; Liao, X.; Li, S.; Xiong, J.; Yang, Q.; Li, G. The distinctive nucleation of polystyrene composites with differently shaped carbon-based nanoparticles as nucleating agent in the supercritical CO2 foaming process. Polym. Int. 2018, 67, 1488–1501. [Google Scholar] [CrossRef]
- Wan, H.; Que, Y.; Chen, C.; Wu, Z.; Gu, Z.; Meng, J.; Wang, L.; Guan, G. Preparation of metal-organic framework/attapulgite hybrid material for CO2 capture. Mater. Lett. 2017, 194, 107–109. [Google Scholar] [CrossRef]
- ASTM Committee D-20 on Plastics. Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Weidner, E. Impregnation via supercritical CO2–What we know and what we need to know. J. Supercrit. Fluids 2018, 134, 220–227. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Novel carbon nanotube−polystyrene foam composites for electromagnetic interference shielding. Nano Lett. 2005, 5, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.; Diekmann, K.; van der Vegt, N.F.A.; Wessling, M. Open nanoporous morphologies from polymeric blends by carbon dioxide foaming. Macromolecules 2002, 35, 1738–1745. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Production of controlled polymeric foams by supercritical CO2. J. Supercrit. Fluids 2007, 40, 144–152. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, L.; Shan, L.; Wang, R.; Min, Y. Comparative study on the adsorption of NO2 using different clay/polyaniline composites. Ind. Eng. Chem. Res. 2018, 57, 6897–6903. [Google Scholar] [CrossRef]
- Huang, J.; Li, H.; Shan, L.; Gao, L.; Yu, Y.; Min, Y. Adsorption of sulfur dioxide in flue gas by polyamine based composite absorbent. Chin. J. Environ. Eng. 2017, 11, 3587–3593. [Google Scholar]
- Gu, J.; Li, N.; Tian, L.; Lv, Z.; Zhang, Q. High thermal conductivity graphite nanoplatelet/UHMWPE nanocomposites. RSC Adv. 2015, 5, 36334–36339. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Su, Z. Thermal stabilities of attapulgite/polystyrene (ATP/PS) nanocomposites via microwave-assisted bulk polymerization. J. Dispers. Sci. Technol. 2008, 29, 478–481. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Xue, G.; Wang, Z.; Zou, W. Magnetic and conducting particles: preparation of polypyrrole layer on Fe3O4 nanospheres. Appl. Surf. Sci. 2003, 218, 216–222. [Google Scholar] [CrossRef]
- Oh, E.J.; Jang, K.S.; MacDiarmid, A.G. High molecular weight soluble polypyrrole. Synth. Met. 2001, 125, 267–272. [Google Scholar] [CrossRef]
- Eskander, S.B.; Tawfik, M.E.; Tawfic, M.L. Mechanical, flammability and thermal degradation characteristics of rice straw fiber-recycled polystyrene foam hard wood composites incorporating fire retardants. J. Therm. Anal. Calorim. 2018, 132, 1115–1124. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, L.; Ma, W.; Pu, H.; Gong, F. Polystyrene/attapulgite nanocomposites prepared via in situ suspension polymerization with redox initiation system. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Minas, C.; Carnelli, D.; Tervoort, E.; Studart, A.R. 3D printing of emulsions and foams into hierarchical porous ceramics. Adv. Mater. 2016, 28, 9993–9999. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.-X.; Ren, P.-G.; Pang, H.; Fu, Q.; Yang, M.-B.; Li, Z.-M. Efficient electromagnetic interference shielding of lightweight graphene/polystyrene composite. J. Mater. Chem. 2012, 22, 18772–18774. [Google Scholar] [CrossRef]
- Li, W.; Qiu, T.; Wang, L.; Ren, S.; Zhang, J.; He, L.; Li, X. Preparation and electromagnetic properties of core/shell polystyrene@polypyrrole@nickel composite microspheres. ACS Appl. Mater. Interfaces 2013, 5, 883–891. [Google Scholar] [CrossRef]
- Liao, G.; Chen, J.; Zeng, W.; Yu, C.; Yi, C.; Xu, Z. Facile preparation of uniform nanocomposite spheres with loading silver nanoparticles on polystyrene-methyl acrylic acid spheres for catalytic reduction of 4-nitrophenol. J. Phys. Chem. C 2016, 120, 25935–25944. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, J.; Song, L.; Chen, L.; Huang, Y.; Zhang, J.; Chen, T. Underwater superoleophobic carbon nanotubes/core–shell polystyrene@Au nanoparticles composite membrane for flow-through catalytic decomposition and oil/water separation. J. Mater. Chem. A 2016, 4, 10810–10815. [Google Scholar] [CrossRef]




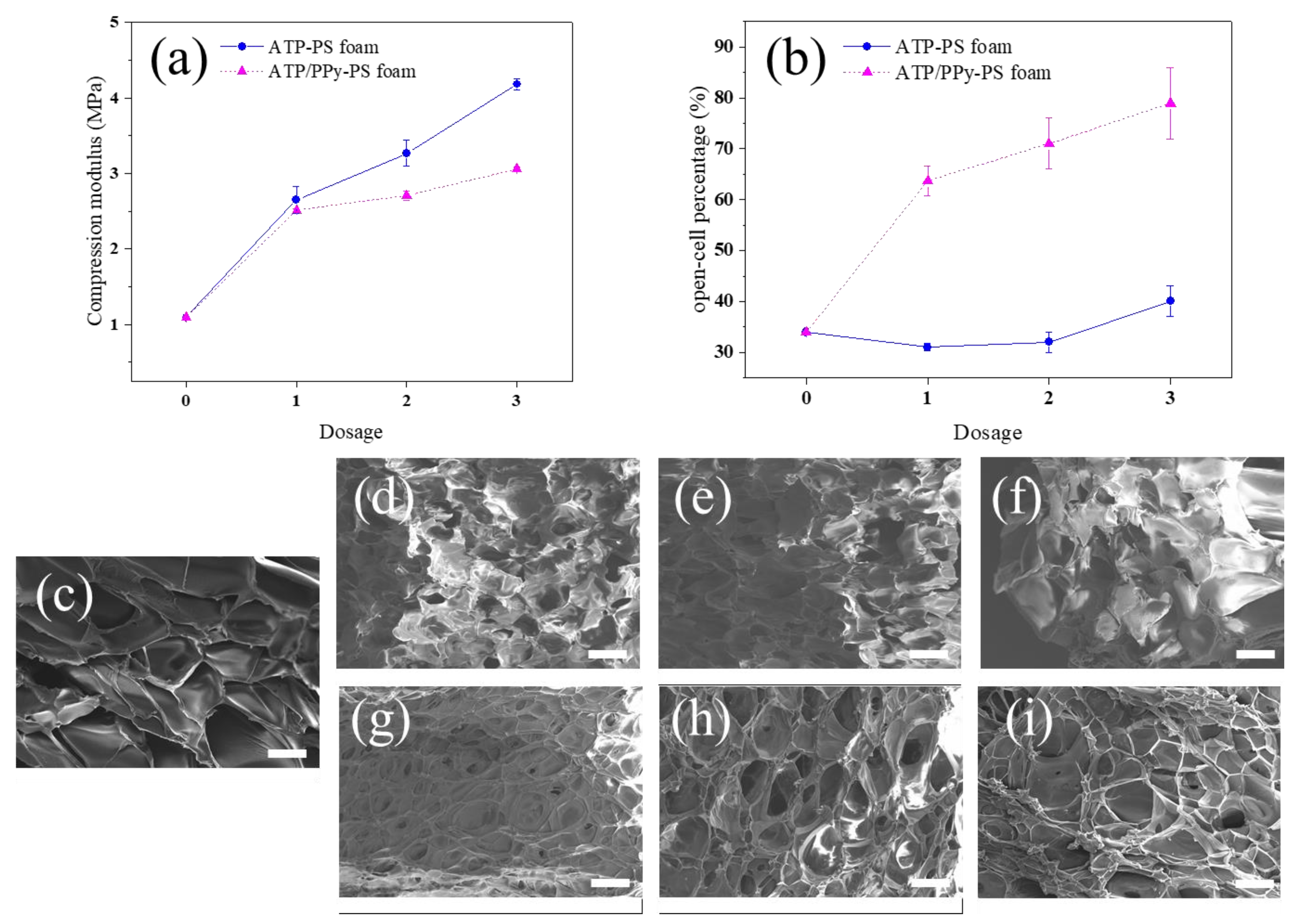
| Sample | Foam Density (g/cm3) | Cell Size (mm) | Cell Density (cells/cm3) |
|---|---|---|---|
| neat PS | 0.070 ± 0.004 | 0.52 ± 0.06 | (3.5 ± 0.2) × 103 |
| ATP-PS 1 | 0.188 ± 0.015 | 0.40 ± 0.03 | (8.3 ± 0.2) × 104 |
| ATP-PS 2 | 0.132 ± 0.008 | 0.53 ± 0.02 | (4.2 ± 0.2) × 104 |
| ATP-PS 3 | 0.159 ± 0.016 | 0.56 ± 0.04 | (1.3 ± 0.1) × 104 |
| ATP/PPy-PS 1 | 0.052 ± 0.004 | 0.19 ± 0.01 | (1.9 ± 0.1) × 106 |
| ATP/PPy-PS 2 | 0.058 ± 0.007 | 0.27 ± 0.02 | (3.7 ± 0.2) × 105 |
| ATP/PPy-PS 3 | 0.055 ± 0.003 | 0.22 ± 0.01 | (3.5 ± 0.1) × 105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jian, L.; Xiao, T.; Liu, R.; Yi, S.; Zhang, S.; Wang, L.; Wang, R.; Min, Y. High Performance Attapulgite/Polypyrrole Nanocomposite Reinforced Polystyrene (PS) Foam Based on Supercritical CO2 Foaming. Polymers 2019, 11, 985. https://doi.org/10.3390/polym11060985
Liu Y, Jian L, Xiao T, Liu R, Yi S, Zhang S, Wang L, Wang R, Min Y. High Performance Attapulgite/Polypyrrole Nanocomposite Reinforced Polystyrene (PS) Foam Based on Supercritical CO2 Foaming. Polymers. 2019; 11(6):985. https://doi.org/10.3390/polym11060985
Chicago/Turabian StyleLiu, Yidong, Lingfeng Jian, Tianhua Xiao, Rongtao Liu, Shun Yi, Shiyang Zhang, Lingzhi Wang, Ruibin Wang, and Yonggang Min. 2019. "High Performance Attapulgite/Polypyrrole Nanocomposite Reinforced Polystyrene (PS) Foam Based on Supercritical CO2 Foaming" Polymers 11, no. 6: 985. https://doi.org/10.3390/polym11060985
APA StyleLiu, Y., Jian, L., Xiao, T., Liu, R., Yi, S., Zhang, S., Wang, L., Wang, R., & Min, Y. (2019). High Performance Attapulgite/Polypyrrole Nanocomposite Reinforced Polystyrene (PS) Foam Based on Supercritical CO2 Foaming. Polymers, 11(6), 985. https://doi.org/10.3390/polym11060985





