Molecular Dynamics Simulation and Experimental Studies on the Thermomechanical Properties of Epoxy Resin with Different Anhydride Curing Agents
Abstract
1. Introduction
2. Methodology
2.1. Simulation
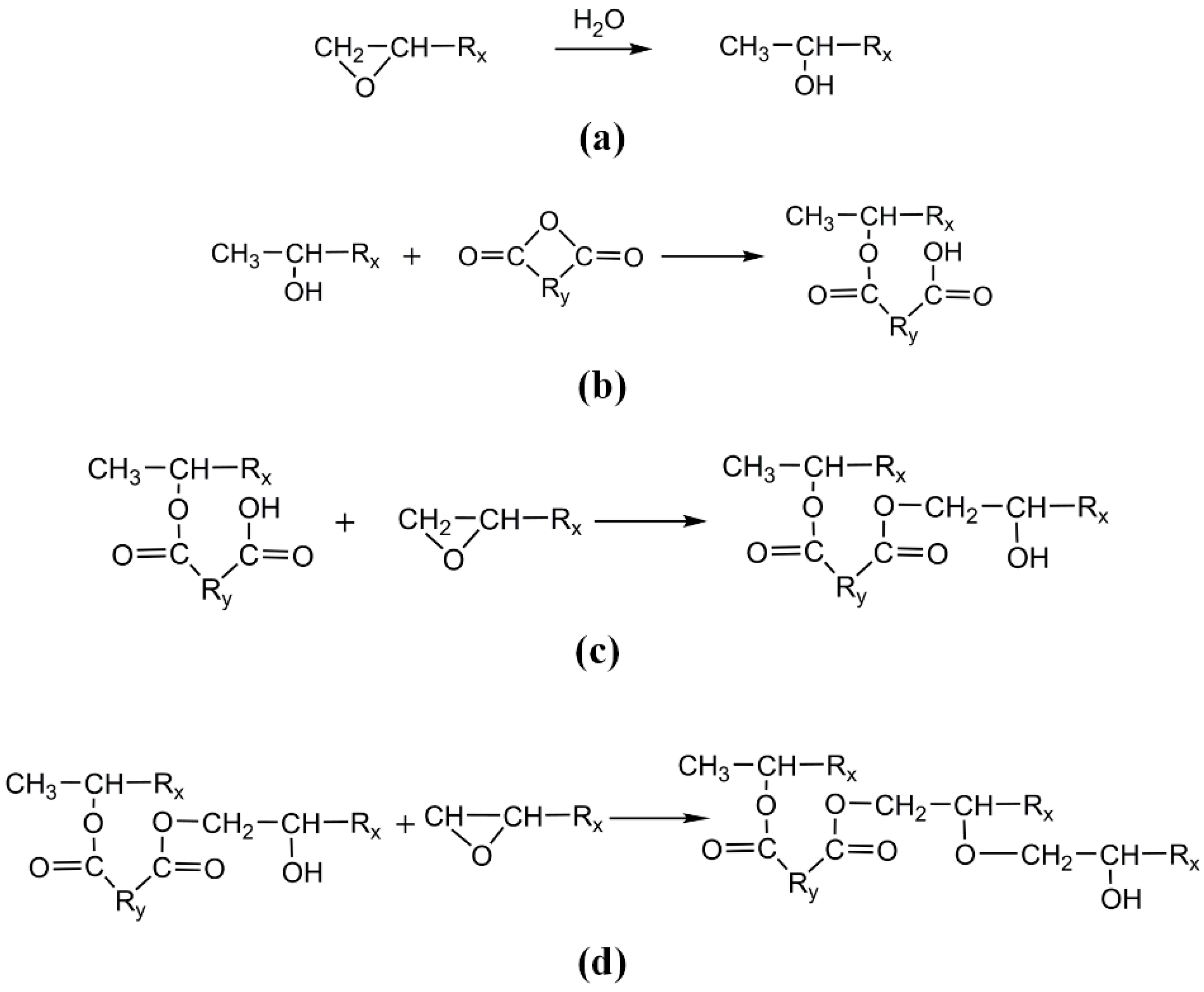
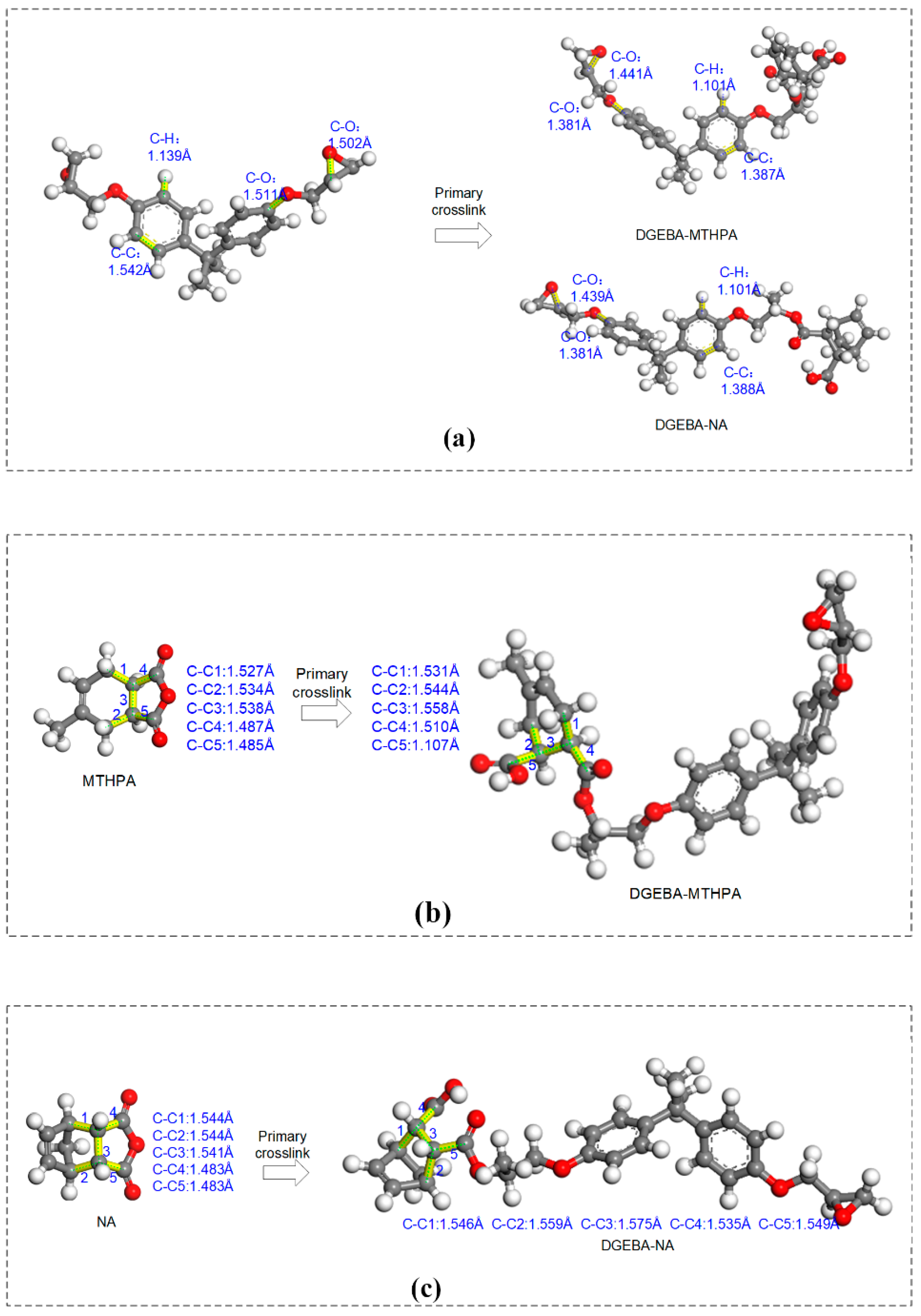
2.1.1. Crosslinking Mechanism of Epoxy Resin and Anhydride
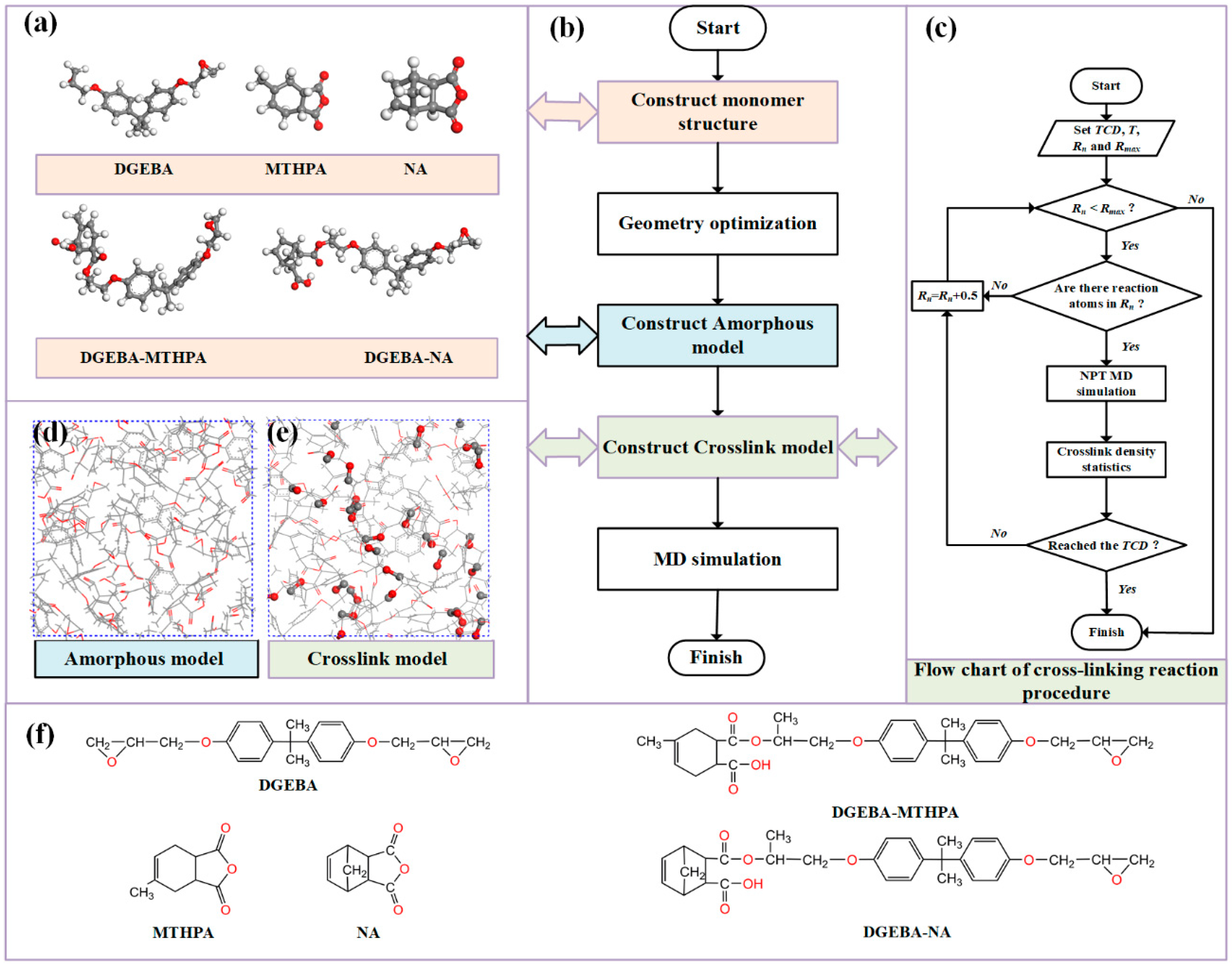
2.1.2. Establishment of Models
2.1.3. Simulation Details
2.2. Experimental
3. Results and Discussions
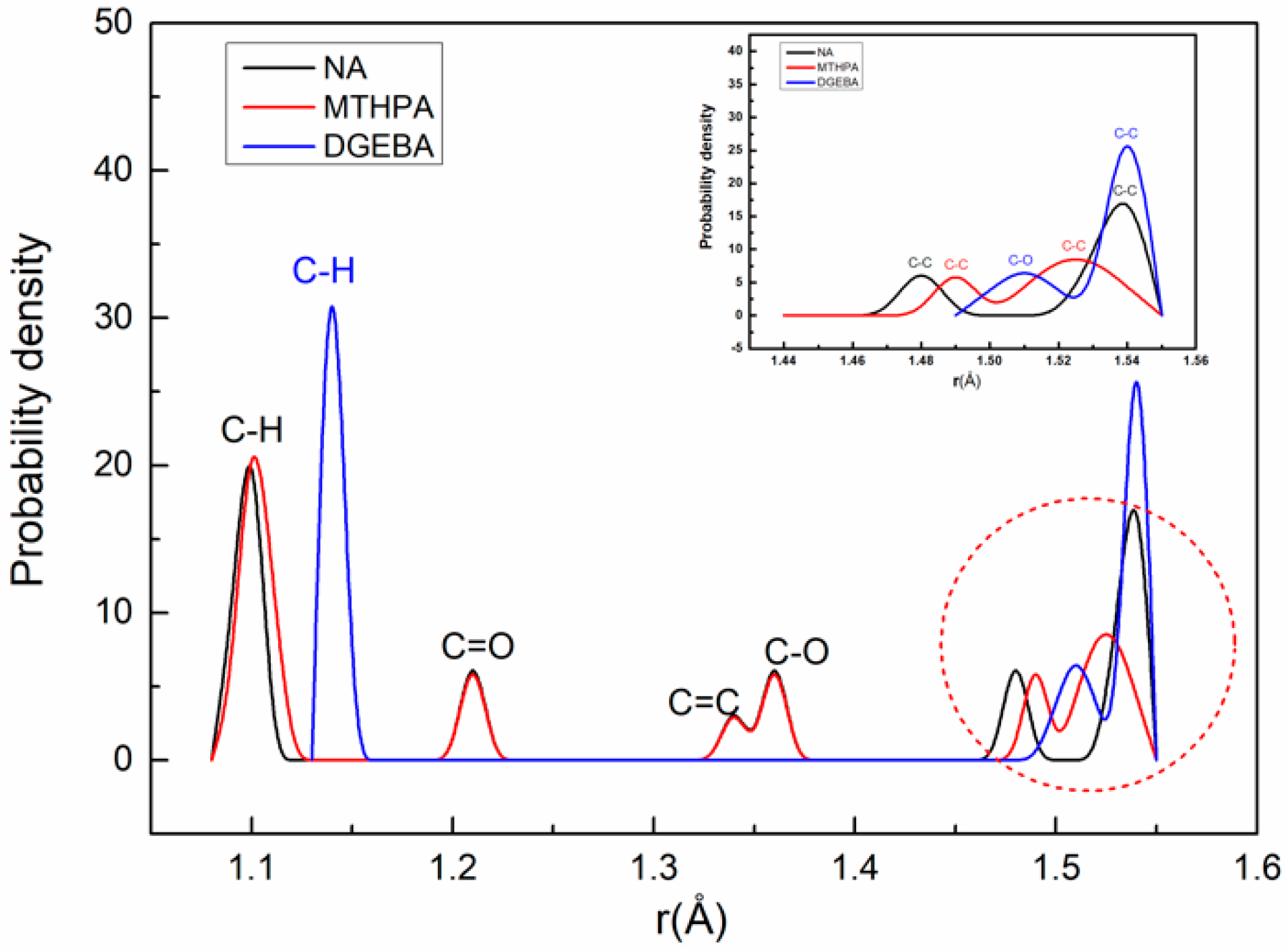
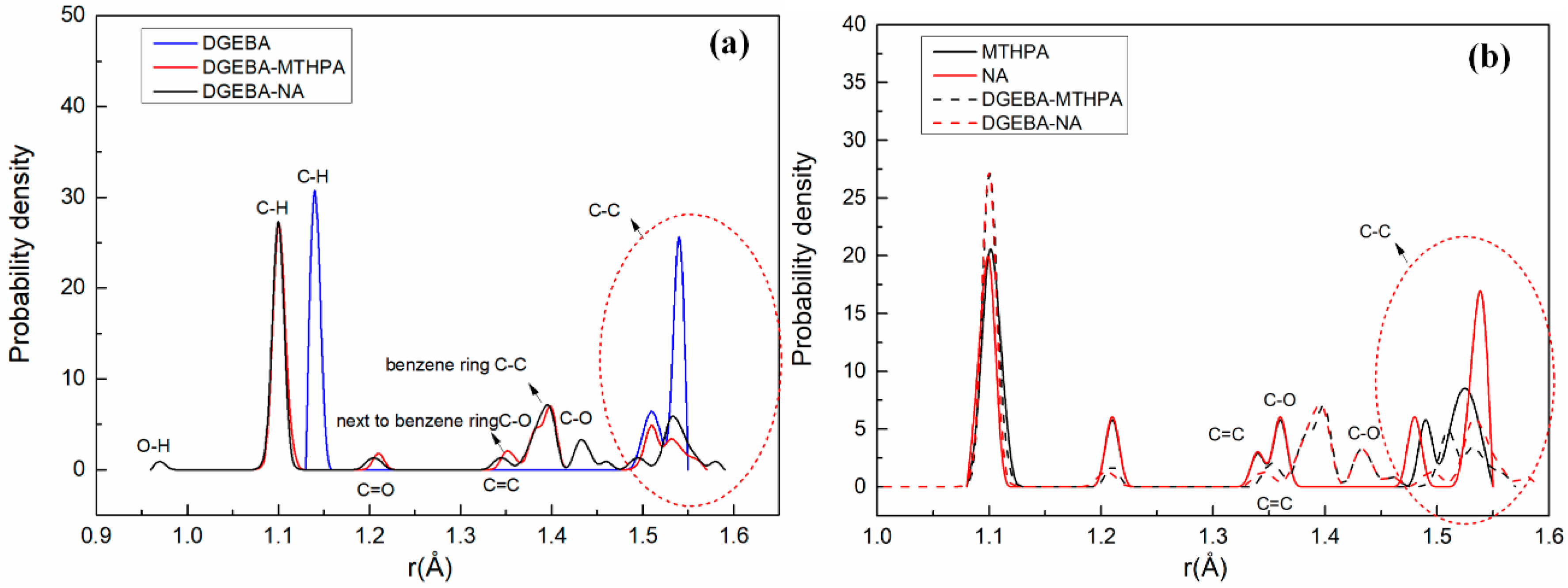
3.1. Bond-Length Distribution Analysis
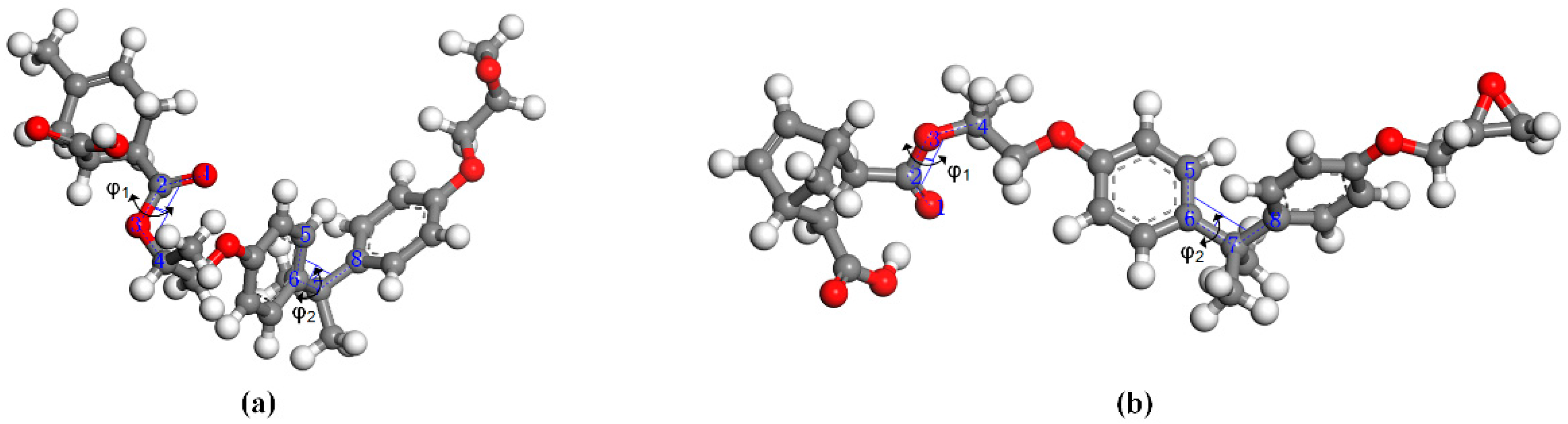
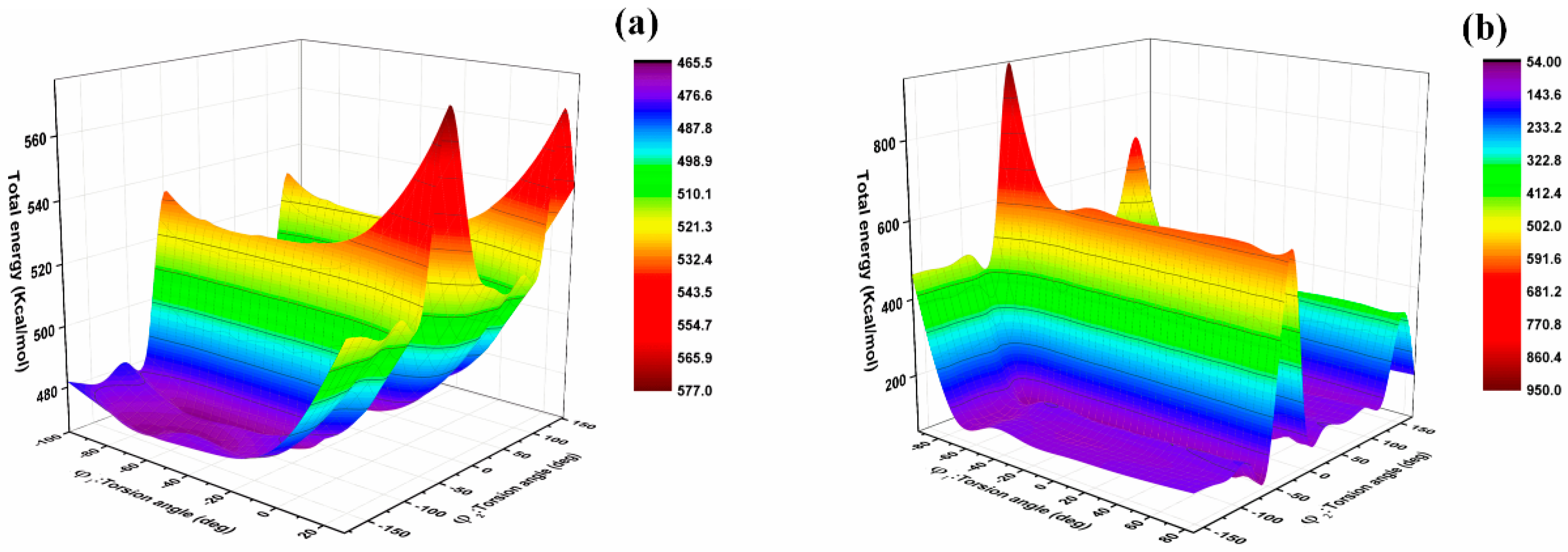
3.2. Conformation and Cohesive Energy Analysis
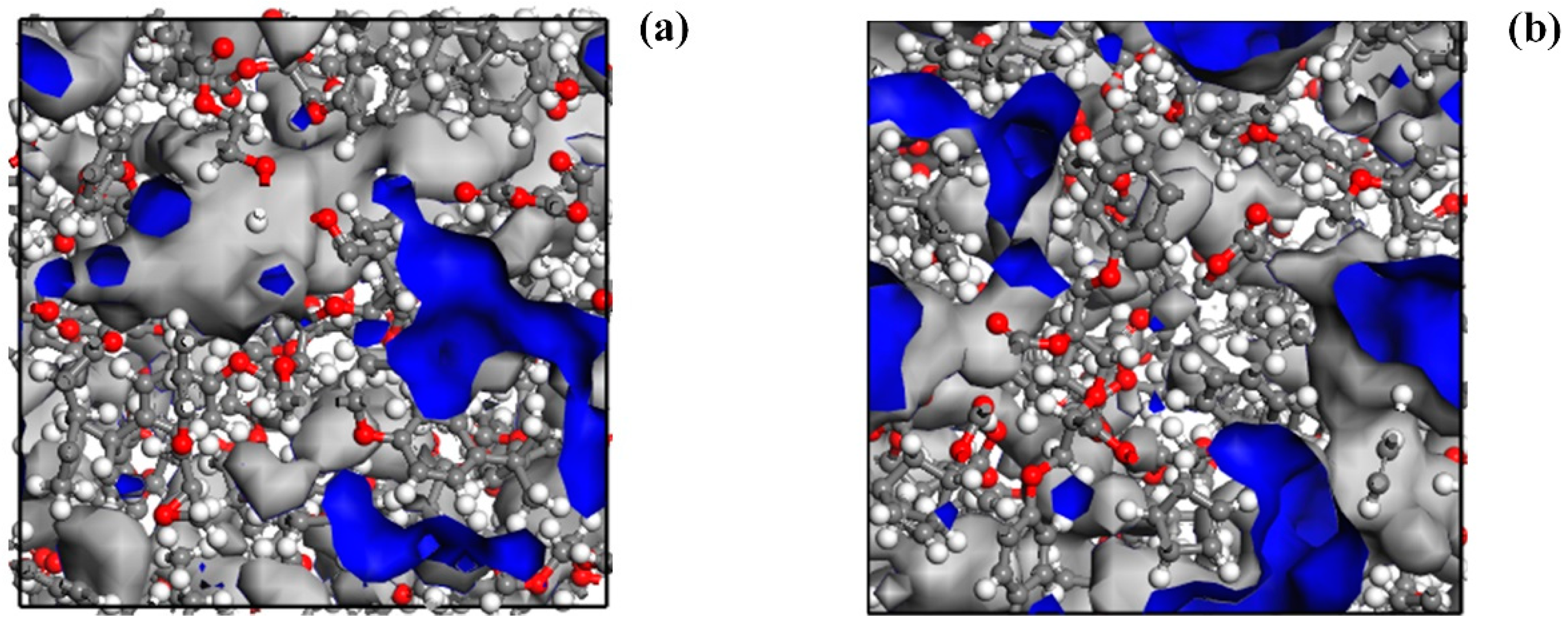
3.3. Free Volume Analysis
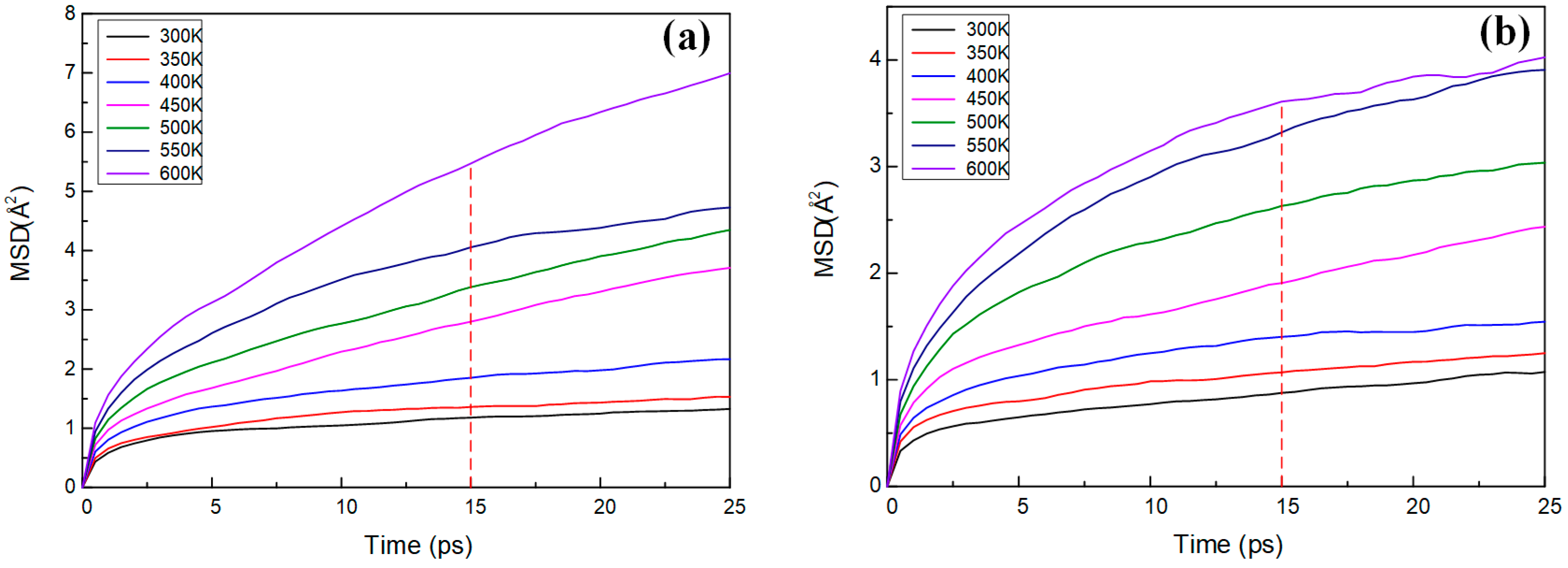
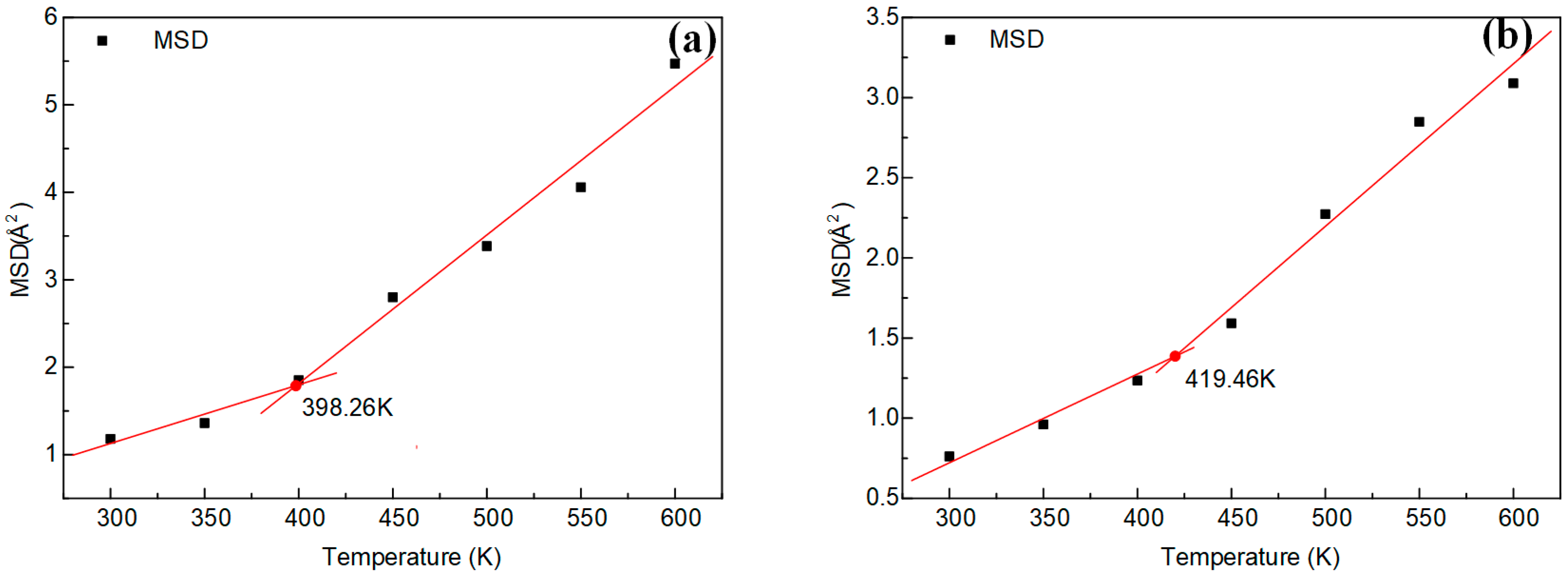
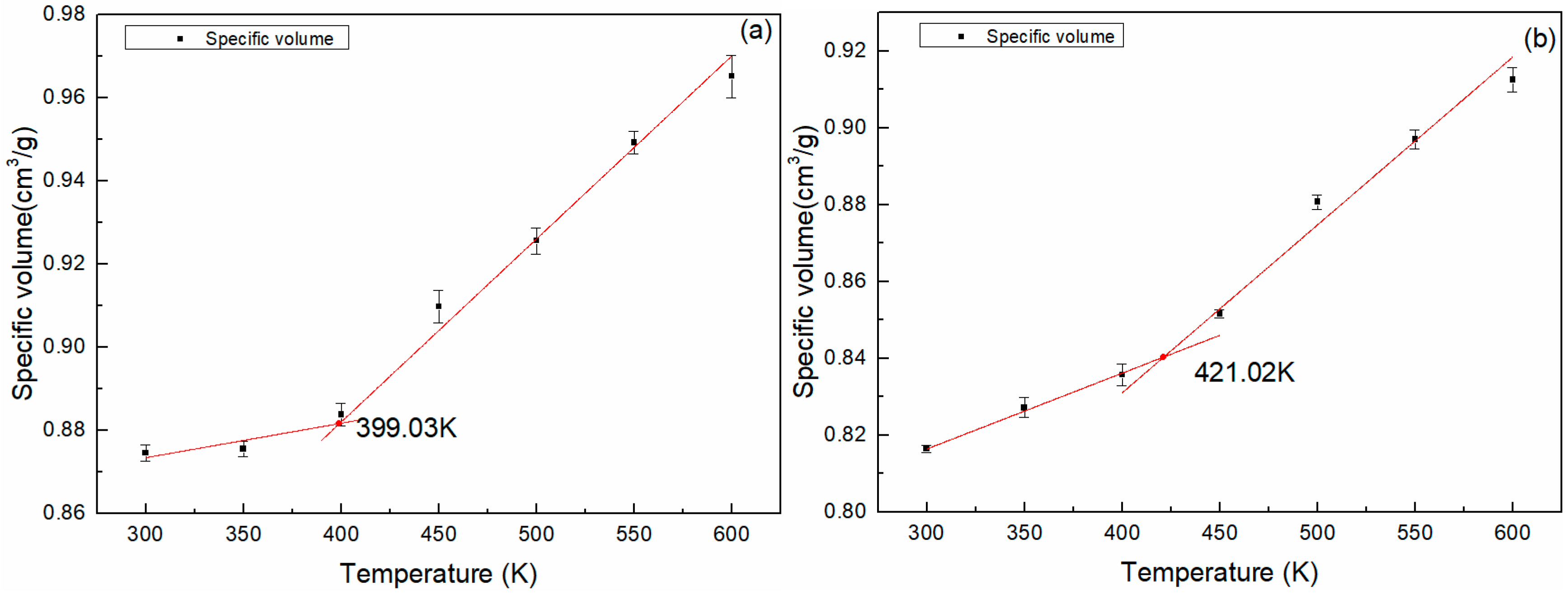
3.4. Thermal Properties Analysis
3.5. Mechanical Properties Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jeyranpour, F.; Alahyarizadeh, G.; Minuchehr, A. The thermo-mechanical properties estimation of fullerene-reinforced resin epoxy composites by molecular dynamics simulation—A comparative study. Polymer 2016, 88, 9–18. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Arab, B. The effect of cross linking density on the mechanical properties and structure of the epoxy polymers: molecular dynamics simulation. J. Mol. Model. 2013, 19, 3719–3731. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Chen, L.; Cui, L.; Zhang, Y.W.; Du, X.Z. Molecular dynamics simulation of cross-linked epoxy resin and its interaction energy with graphene under two typical force fields. Comput. Mater. Sci. 2018, 143, 240–247. [Google Scholar] [CrossRef]
- Xie, Q.; Fu, K.; Liang, S.; Liu, B.; Lu, L.; Yang, X.; Huang, Z.; Lu, F. Micro-Structure and Thermomechanical Properties of Crosslinked Epoxy Composite Modified by Nano-SiO2: A Molecular Dynamics Simulation. Polymers 2018, 10, 801. [Google Scholar] [CrossRef]
- Wang, D. Production and Application of Epoxy Resin; Chemical Industry Press: Beijing, China, 2001. [Google Scholar]
- LÜ, F.; Yin, K.; Fu, K.; Lin, H.; Xie, Q.; Hou, Z.; Zhang, C. Effect of Fluorination and Exfoliation Filler on the Surface Flashover Voltage of Boron Nitride/Epoxy Resin Composites. High Voltage Eng. 2017, 43, 2800–2807. [Google Scholar]
- Qi, B.; Gao, C.J.; Lv, Y.Z.; Li, C.R.; Tu, Y.P.; Xiong, J. The impact of nano-coating on surface charge accumulation of epoxy resin insulator: characteristic and mechanism. J. Phys. D Appl. Phys. 2018, 51, 11. [Google Scholar] [CrossRef]
- Du, B.X.; Liang, H.C.; Li, J.; Zhang, C. Temperature Dependent Surface Potential Decay and Flashover Characteristics of Epoxy/SiC Composites. IEEE Trns. Dielectr. Electr. Insul. 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Li, L.; Yan, C.; Xu, H.; Liu, D.; Shi, P.; Zhu, Y.; Chen, G.; Wu, X.; Liu, W. Improving the interfacial properties of carbon fiber-epoxy resin composites with a graphene-modified sizing agent. J. Appl. Polym. Sci. 2019, 136, 47122. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, M.; Jin, L.; Liu, L.; Li, N.; Shang, L.; Li, M.; Xiao, L.; Ao, Y. Enhancing interfacial properties of carbon fibers reinforced epoxy composites via Layer-by-Layer self assembly GO/SiO2 multilayers films on carbon fibers surface. Appl. Surf. Sci. 2019, 470, 543–554. [Google Scholar] [CrossRef]
- Koda, T.; Toyoshima, T.; Komatsu, T.; Takezawa, Y.; Nishioka, A.; Miyata, K. Ordering simulation of high thermal conductivity epoxy resins. Polym. J. 2013, 45, 444–448. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Yu, X.; Bao, H.; Yang, N. High Thermal Conductivity of Bulk Epoxy Resin by Bottom-Up Parallel-Linking and Strain: A Molecular Dynamics Study. J. Phys. Chem. C 2018, 122, 13140–13147. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X.; Shi, L.; Yang, X.; Sui, G. The thermal characteristics of epoxy resin: Design and predict by using molecular simulation method. Polymer 2013, 54, 6447–6454. [Google Scholar] [CrossRef]
- Li, C.; Coons, E.; Strachan, A. Material property prediction of thermoset polymers by molecular dynamics simulations. Acta Mech. 2014, 225, 1187–1196. [Google Scholar] [CrossRef]
- Fu, Y.; Michopoulos, J.G.; Song, J.H. On Investigating the Thermomechanical Properties of Cross-linked Epoxy Via Molecular Dynamics Analysis. Nanosc. Microsc. Therm. 2017, 21, 8–25. [Google Scholar] [CrossRef]
- Fu, Y.; Michopoulos, J.; Song, J.H. Coarse-grained molecular dynamics simulations of epoxy resin during the curing process. Comput. Mater. Sci. 2015, 107, 24–32. [Google Scholar] [CrossRef]
- Jeyranpour, F.; Alahyarizadeh, G.; Arab, B. Comparative investigation of thermal and mechanical properties of cross-linked epoxy polymers with different curing agents by molecular dynamics simulation. J. Mol. Graph. Model. 2015, 62, 157–164. [Google Scholar] [CrossRef]
- Zhang, W.; Qing, Y.; Zhong, W.; Sui, G.; Yang, X. Mechanism of modulus improvement for epoxy resin matrices: A molecular dynamics simulation. React. Funct. Polym. 2017, 111, 60–67. [Google Scholar] [CrossRef]
- Wu, C.F.; Xu, W.J. Atomistic molecular modelling of crosslinked epoxy resin. Polymer 2006, 47, 6004–6009. [Google Scholar] [CrossRef]
- Yang, S.; Qu, J. Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations. Polymer 2012, 53, 4806–4817. [Google Scholar] [CrossRef]
- Liu, J.; Shen, J.X.; Cao, D.P.; Zhang, L.Q. Computer Simulation of Dispersion and Interface in Polymer Nanocomposites. Acta Polym. Sin. 2016, 8, 1048–1061. [Google Scholar]
- Zhang, W.; Li, H.; Gao, L.; Zhang, Q.; Zhong, W.; Sui, G.; Yang, X. Molecular simulation and experimental analysis on thermal and mechanical properties of carbon nanotube/epoxy resin composites with different curing agents at high-low temperature. Polym. Compos. 2018, 39, E945–E954. [Google Scholar] [CrossRef]
- Kim, B.; Choi, J.; Yang, S.; Yu, S.; Cho, M. Influence of crosslink density on the interfacial characteristics of epoxy nanocomposites. Polymer 2015, 60, 186–197. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Molecular dynamics predictions of thermal and mechanical properties of thermoset polymer EPON862/DETDA. Polymer 2011, 52, 2920–2928. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Valavala, P.K.; Clancy, T.C.; Wise, K.E.; Odegard, G.M. Molecular modeling of crosslinked epoxy polymers: The effect of crosslink density on thermomechanical properties. Polymer 2011, 52, 2445–2452. [Google Scholar] [CrossRef]
- Chen, P.; Liu, S.; Wang, D. Epoxy Resin and Its Application; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef]
- Komarov, P.V.; Chiu, Y.T.; Chen, S.M.; Khalatur, P.G.; Reineker, P. Highly cross-linked epoxy resins: An atomistic molecular dynamics simulation combined with a mapping/reverse mapping procedure. Macromolecules 2007, 40, 8104–8113. [Google Scholar] [CrossRef]
- Fan, H.B.; Yuen, M.M.F. Material properties of the cross-linked epoxy resin compound predicted by molecular dynamics simulation. Polymer 2007, 48, 2174–2178. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Wu, Y. Computational Thermomechanical Properties of Silica-Epoxy Nanocomposites by Molecular Dynamic Simulation. Polymers 2017, 9, 430. [Google Scholar] [CrossRef]
- Xie, Q.; Liang, S.; Liu, B.; Fu, K.; Zhan, Z.; Lu, L.; Yang, X.; Lu, F.; Huang, Z. Structure, microparameters and properties of crosslinked DGEBA/MTHPA: A molecular dynamics simulation. Aip. Adv. 2018, 8, 075332. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, C.; Hu, D.; Gui, Y. Effect of Aminosilane Coupling Agents with Different Chain Lengths on Thermo-Mechanical Properties of Cross-Linked Epoxy Resin. Nanomaterials 2018, 8, 951. [Google Scholar] [CrossRef]
- Aghadavoudi, F.; Golestanian, H.; Beni, Y.T. Investigating the Effects of Resin Crosslinking Ratio on Mechanical Properties of Epoxy-Based Nanocomposites Using Molecular Dynamics. Polym. Compos. 2017, 38, E433–E442. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-Order Transition Temperatures and Related Properties of Polystyrene. I. Influence of Molecular Weight. J. Appl. Phys 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.; Wang, Y.; Yang, M. A molecular dynamic simulation method to elucidate the interaction mechanism of nano-SiO2 in polymer blends. J. Mater. Sci. 2017, 52, 12889–12901. [Google Scholar] [CrossRef]
- Duek, K. Epoxy Resins and Composites III; Springer-Verlag: Berlin, Germany, 1986. [Google Scholar]
- Arab, B.; Shokuhfar, A. Molecular dynamics simulation of cross-linked urea-formaldehyde polymers for self-healing nanocomposites: Prediction of mechanical properties and glass transition temperature. J. Mol. Model. 2013, 19, 5053–5062. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Lian, Q.; Cheng, J.; Zhang, J. Influence of cross-linking density on the structure and properties of the interphase within supported ultrathin epoxy films. J. Mater. Sci. 2016, 51, 9019–9030. [Google Scholar] [CrossRef]
- Choi, J.; Yu, S.; Yang, S.; Cho, M. The glass transition and thermoelastic behavior of epoxy-based nanocomposites: A molecular dynamics study. Polymer 2011, 52, 5197–5203. [Google Scholar] [CrossRef]
- Gavrilov, A.A.; Komarov, P.V.; Khalatur, P.G. Thermal Properties and Topology of Epoxy Networks: A Multiscale Simulation Methodology. Macromolecules 2015, 48, 206–212. [Google Scholar] [CrossRef]

| Structure Models | Molecular Number of | Amorphous Models | Crosslinked Models | Volumetric Shrinkage | ||||
|---|---|---|---|---|---|---|---|---|
| Volume | Density | Volume | Density | |||||
| DGEBA | MTHPA | DGEBA-MTHPA/NA | Å3 | g/cm3 | Å3 | g/cm3 | % | |
| DGEBA/MTHPA | 8 | 18 | 2 | 9782.613 | 1.143 | 9693.979 | 1.153 | 0.91% |
| DGEBA/NA | 8 | 18 | 2 | 9387.993 | 1.184 | 9224.818 | 1.205 | 1.74% |
| Epoxy System | DGEBA/MTHPA | DGEBA/NA |
|---|---|---|
| CED (J·cm−3) | 464.12 ± 21.43 | 475.50 ± 24.33 |
| Epoxy System | Vf (Å3) | Vtotal (Å3) | FFV (%) |
|---|---|---|---|
| DGEBA/MTHPA | 1616.96 ± 67.88 | 9697.1 ± 66.52 | 16.67 ± 0.59 |
| DGEBA/NA | 1285.69 ± 42.66 | 9067.72 ± 35.84 | 14.18 ± 0.43 |
| Method | DGEBA-MTHPA | DGEBA-NA | ||
|---|---|---|---|---|
| Tg (K) | Tg (K) | |||
| Simulation | SV | 399.03 | SV | 421.02 |
| M | 398.26 | M | 419.46 | |
| Experiment | 401.62 | 427.48 | ||
| Epoxy System | Young’s Modulus (GPa) | Bulk Modulus (GPa) | Shear Modulus (GPa) | Poisson’s Ratio |
|---|---|---|---|---|
| DGEBA/MTHPA | 4.14 ± 0.18 | 3.5 ± 0.86 | 1.6 ± 0.06 | 0.29 ± 0.06 |
| DGEBA/NA | 4.87 ± 0.41 | 3.89 ± 0.47 | 1.7 ± 0.46 | 0.3 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Xie, Q.; LÜ, F.; Duan, Q.; Wang, X.; Zhu, Q.; Huang, Z. Molecular Dynamics Simulation and Experimental Studies on the Thermomechanical Properties of Epoxy Resin with Different Anhydride Curing Agents. Polymers 2019, 11, 975. https://doi.org/10.3390/polym11060975
Fu K, Xie Q, LÜ F, Duan Q, Wang X, Zhu Q, Huang Z. Molecular Dynamics Simulation and Experimental Studies on the Thermomechanical Properties of Epoxy Resin with Different Anhydride Curing Agents. Polymers. 2019; 11(6):975. https://doi.org/10.3390/polym11060975
Chicago/Turabian StyleFu, Kexin, Qing Xie, Fangcheng LÜ, Qijun Duan, Xinjie Wang, Quansheng Zhu, and Zhengyong Huang. 2019. "Molecular Dynamics Simulation and Experimental Studies on the Thermomechanical Properties of Epoxy Resin with Different Anhydride Curing Agents" Polymers 11, no. 6: 975. https://doi.org/10.3390/polym11060975
APA StyleFu, K., Xie, Q., LÜ, F., Duan, Q., Wang, X., Zhu, Q., & Huang, Z. (2019). Molecular Dynamics Simulation and Experimental Studies on the Thermomechanical Properties of Epoxy Resin with Different Anhydride Curing Agents. Polymers, 11(6), 975. https://doi.org/10.3390/polym11060975





