Study on the Relationships between Microscopic Cross-Linked Network Structure and Properties of Cyanate Ester Self-Reinforced Composites
Abstract
1. Introduction
2. Materials and Methods
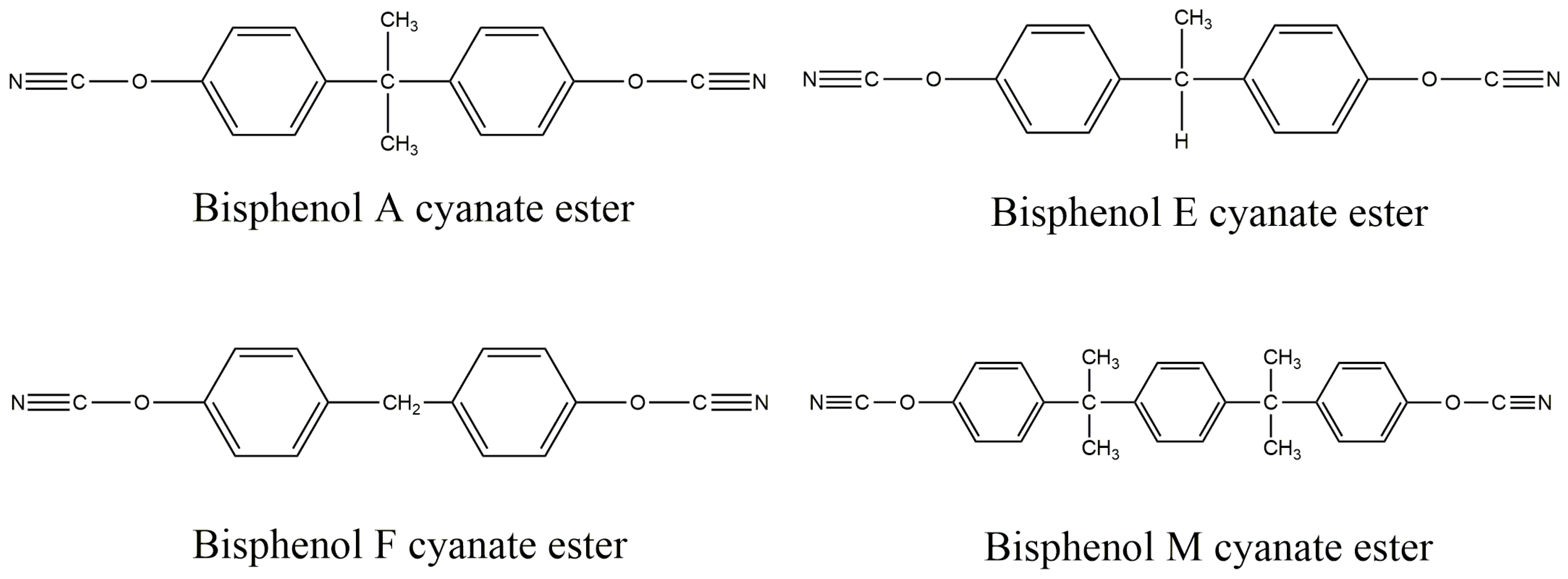
2.1. Materials
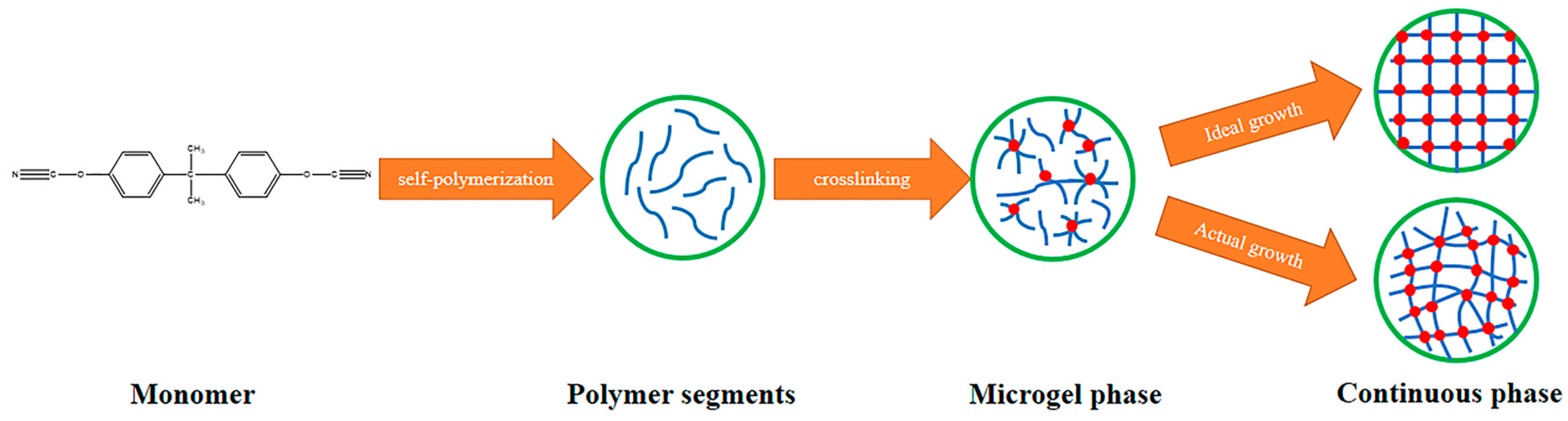
2.2. Preparation of BADCy Resin Microparticle
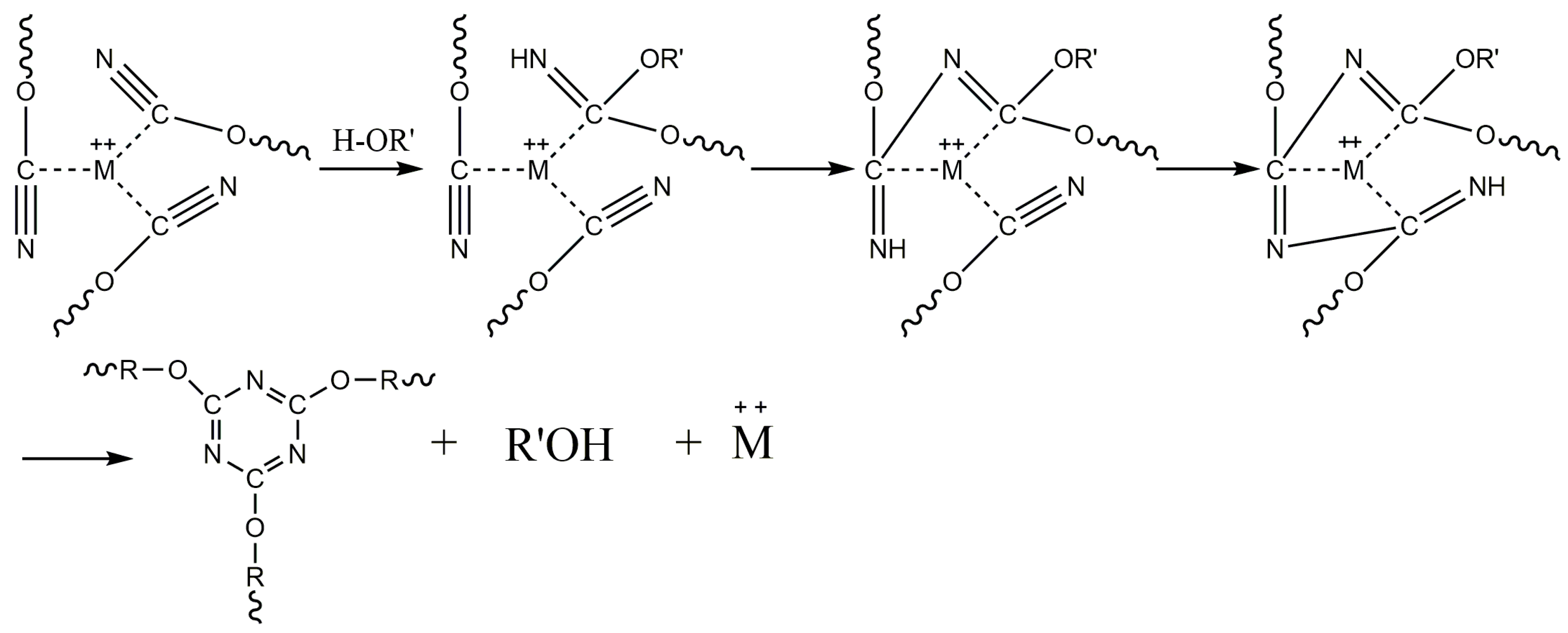
2.3. Preparation of BADCy Prepolymer Resin
2.4. Preparation of Self-Reinforced Composite Materials
2.5. Characterization and Measurements
3. Results and Discussion
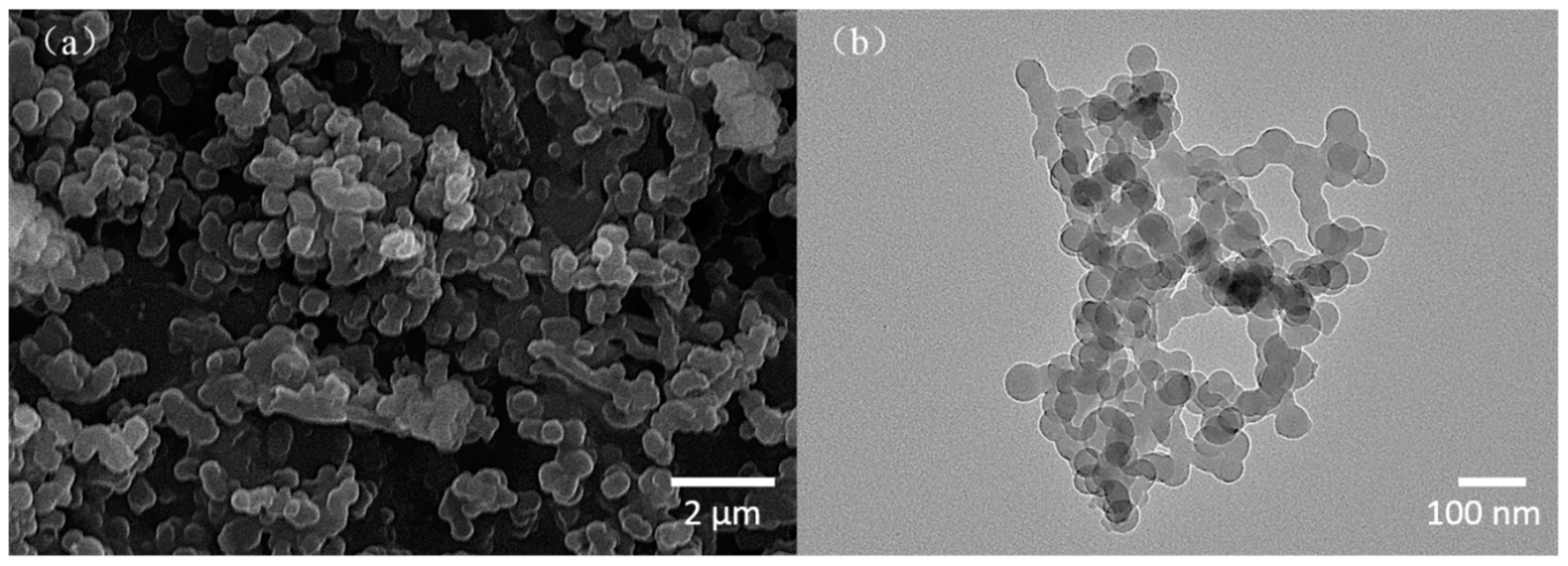
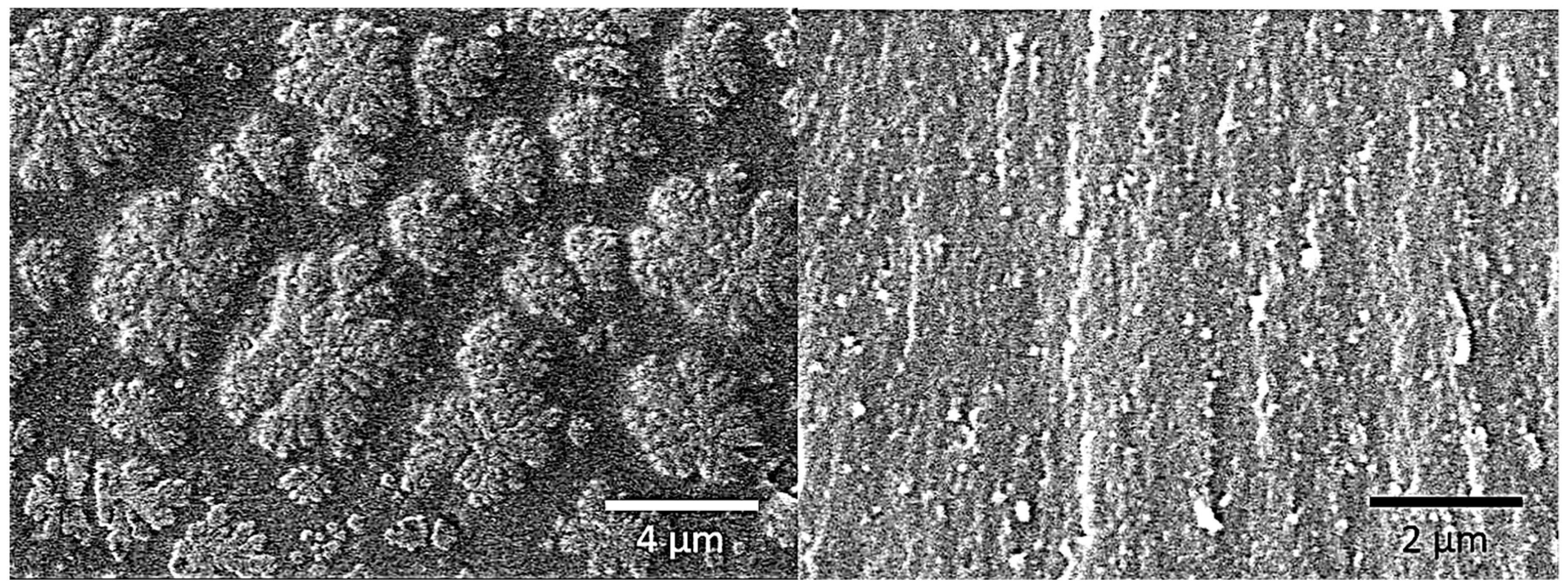
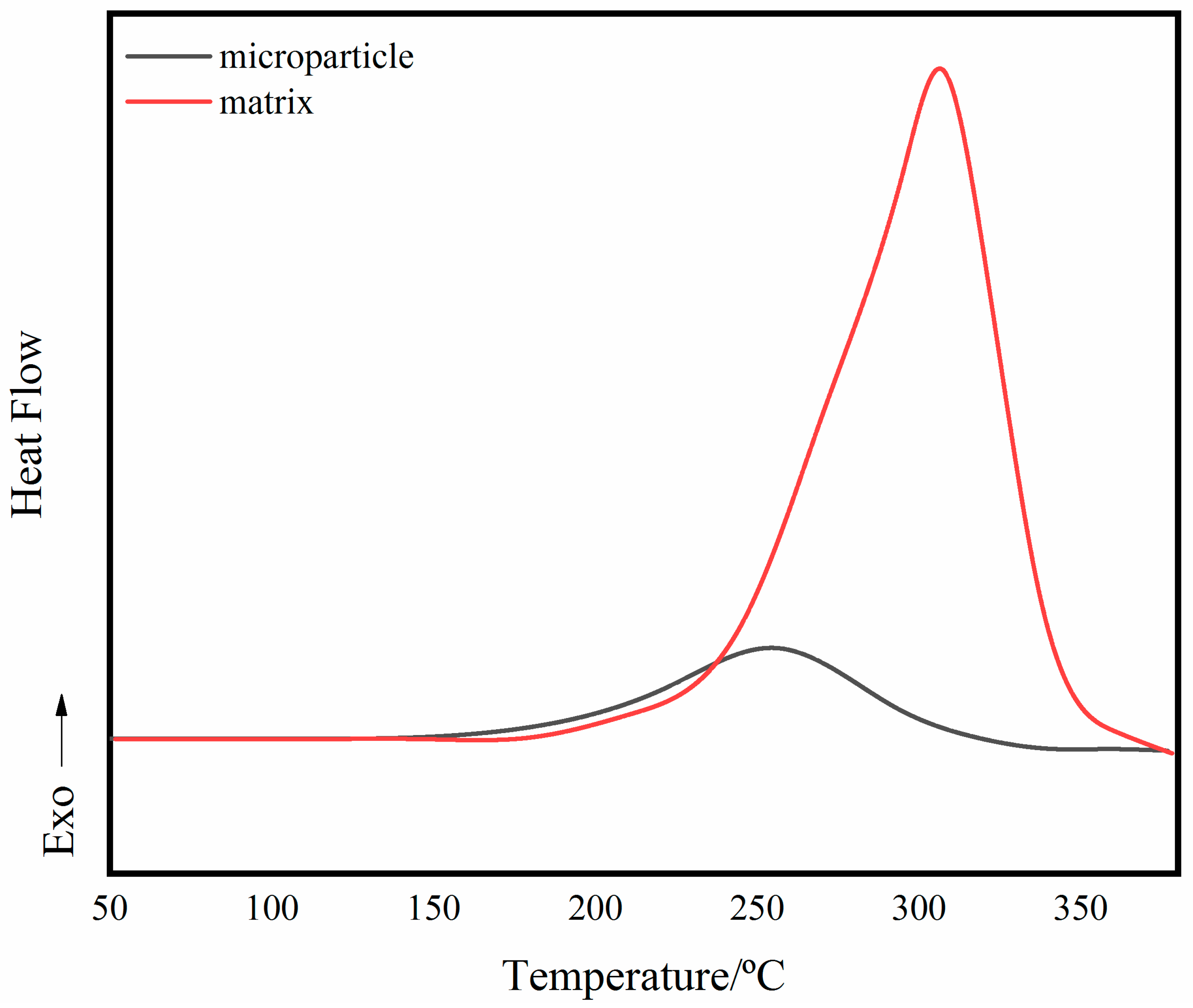
3.1. Characterization of BADCy Resin Microparticles
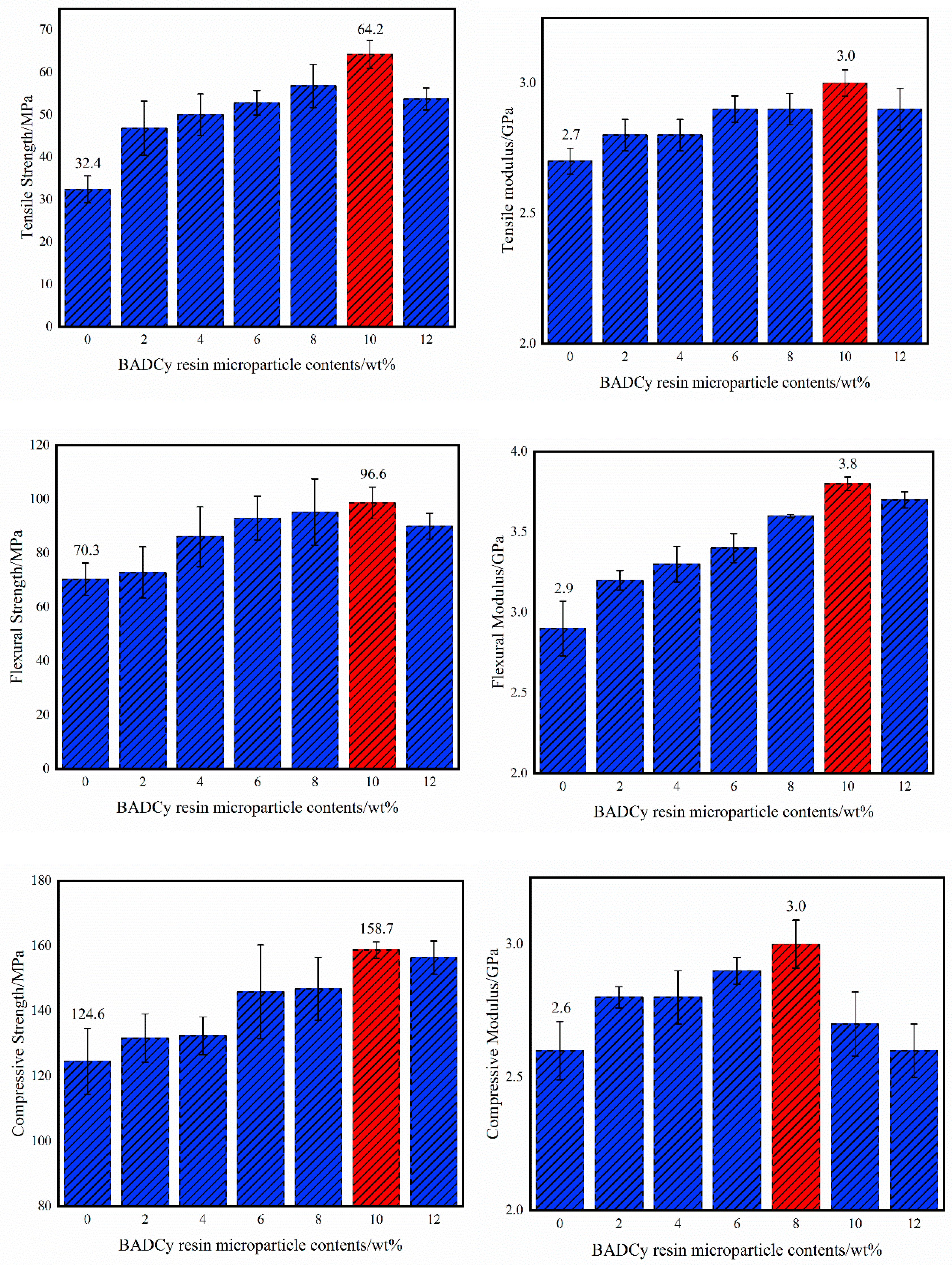
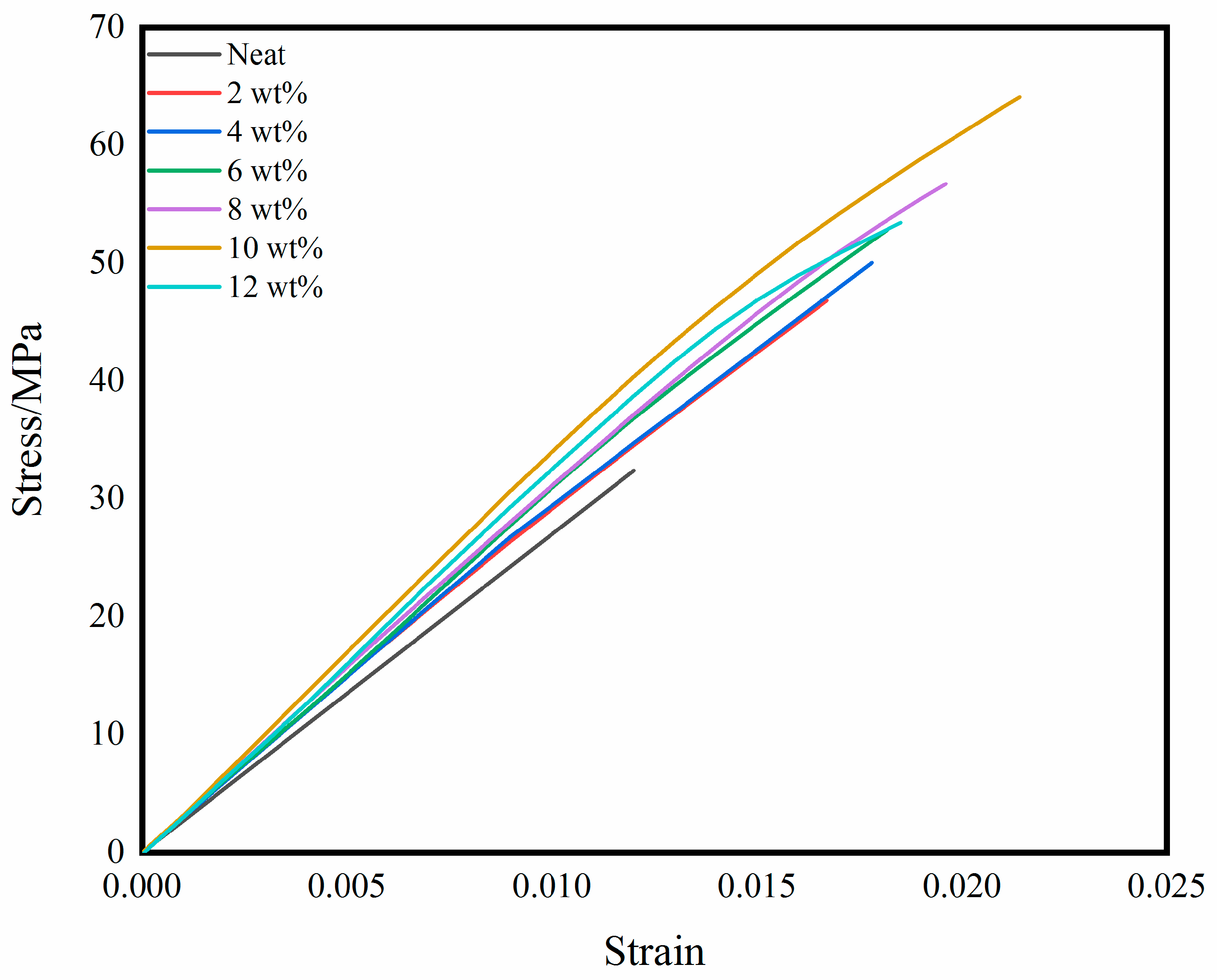
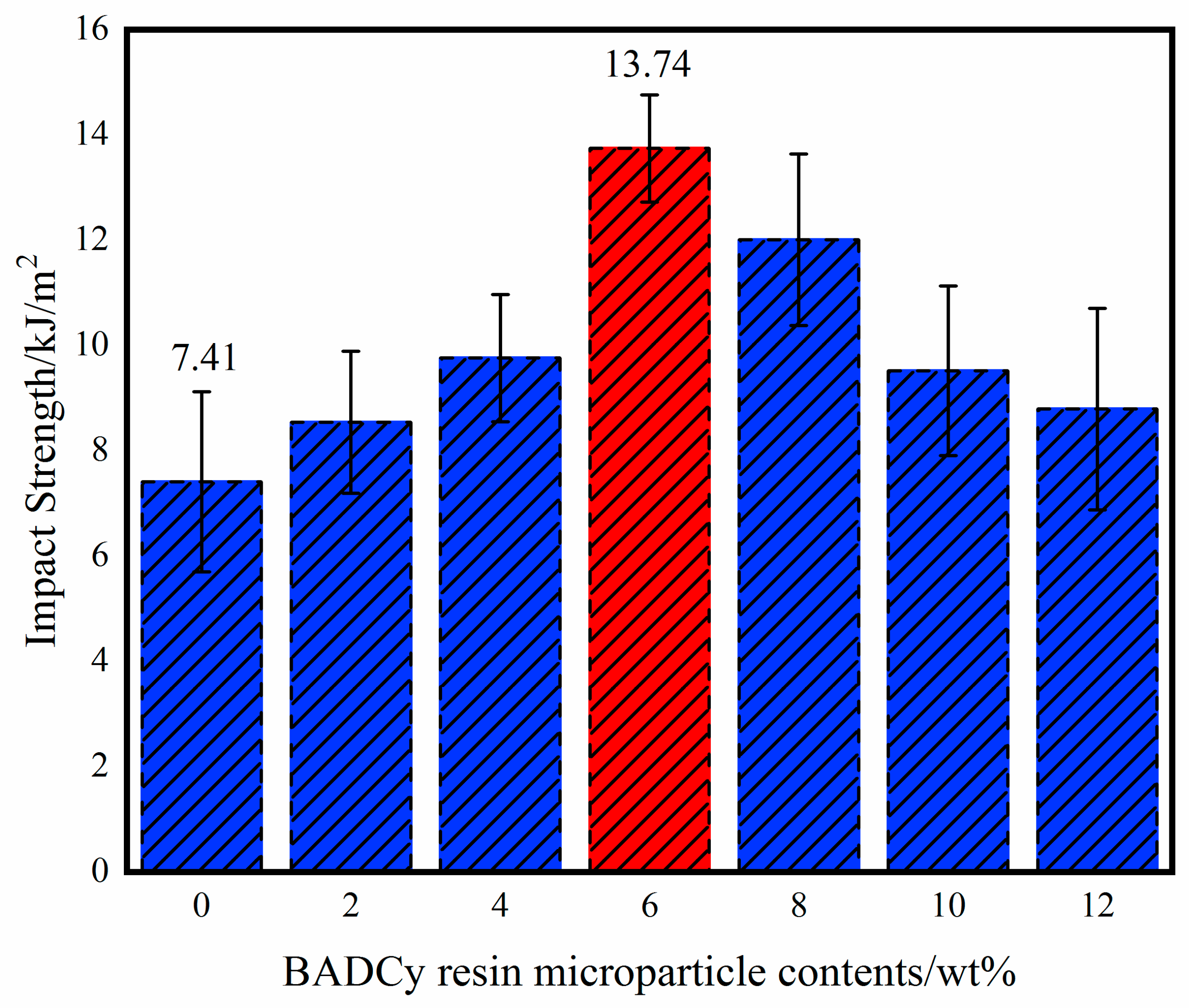
3.2. Mechanical Properties of Self-Reinforced Composites
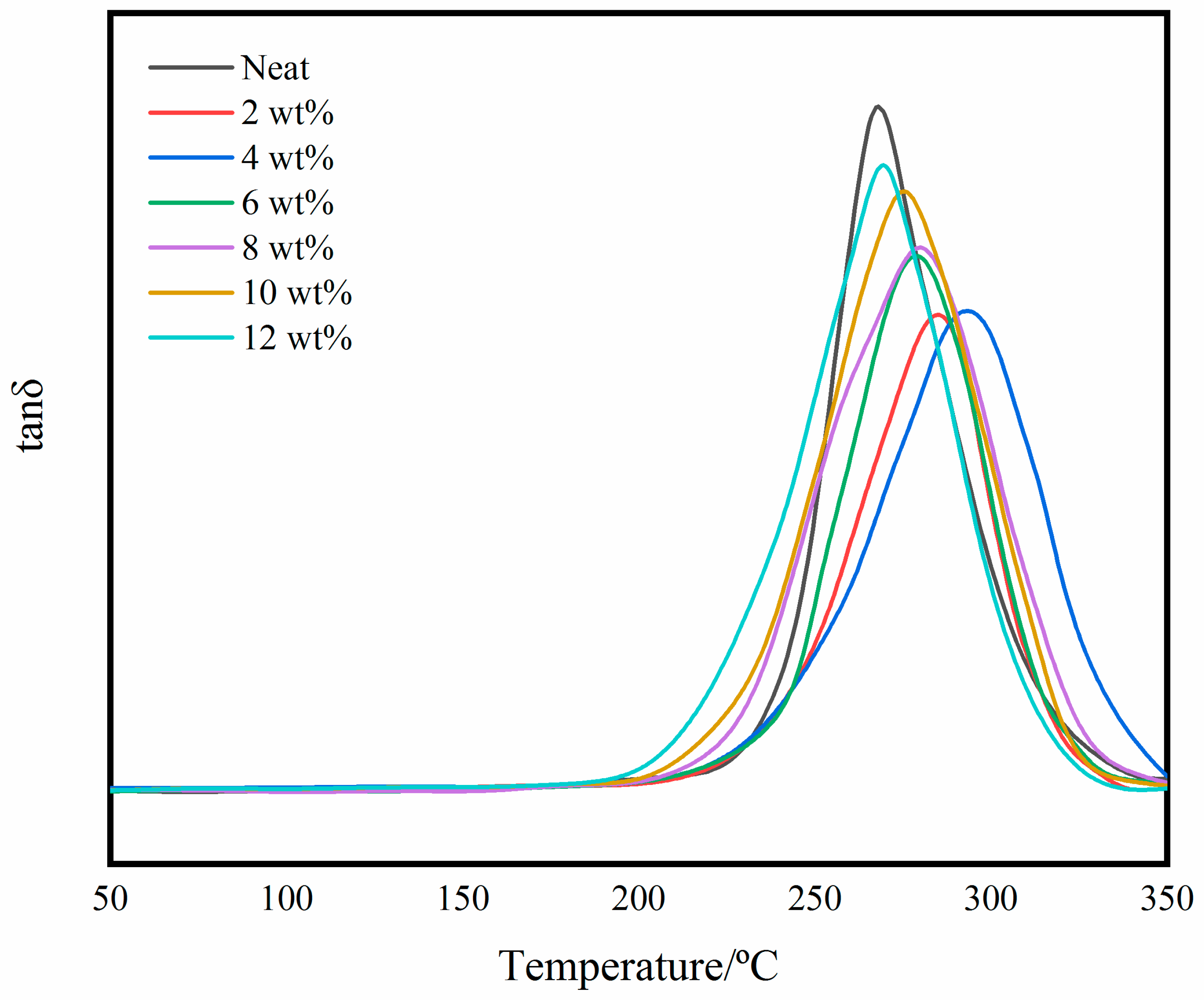
3.3. Thermal Properties of Self-Reinforced Composites
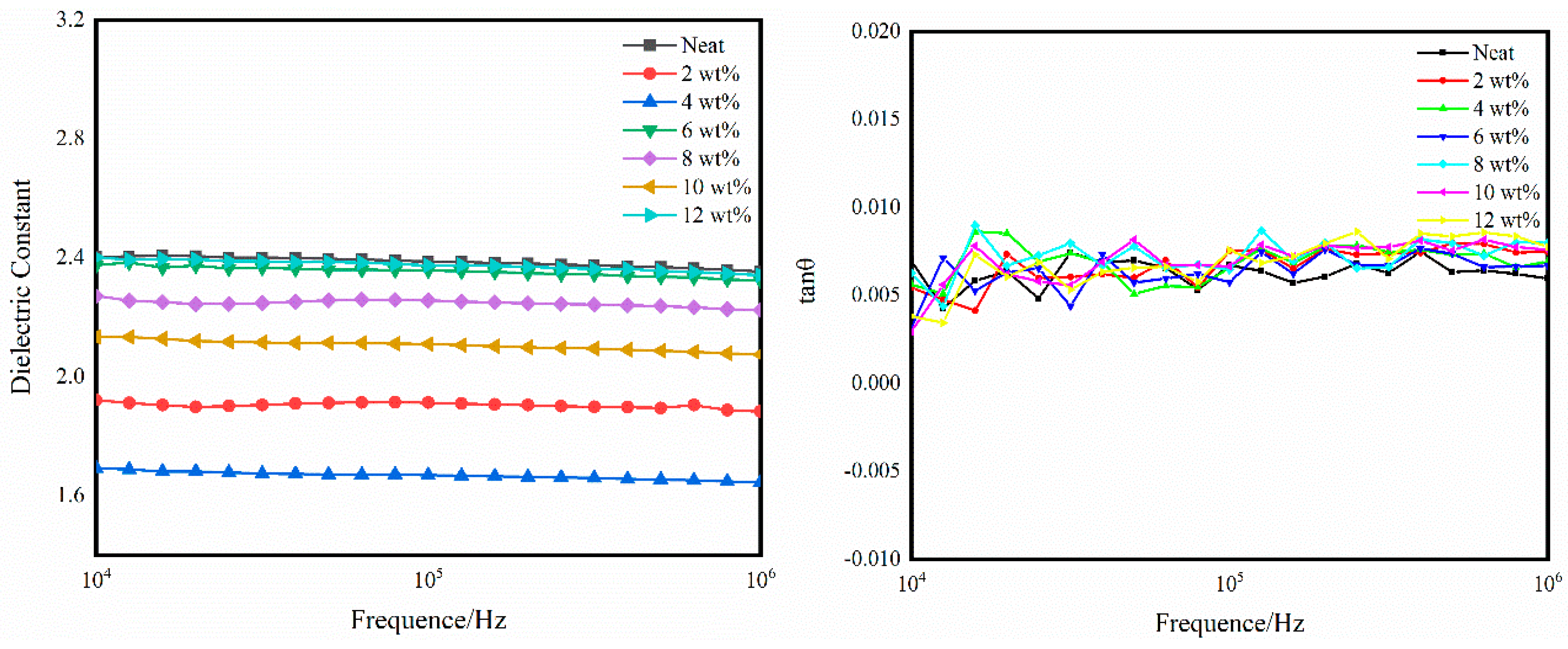
3.4. Dielectric Properties of Self-Reinforced Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capiati, N.J.; Porter, R.S. The concept of one polymer composites modelled with high density polyethylene. J. Mater. Sci. 1975, 10, 1671–1677. [Google Scholar] [CrossRef]
- Kmetty, Á.; Bárány, T.; Karger-Kocsis, J. Self-reinforced polymeric materials: A review. Prog. Polym. Sci. 2010, 35, 1288–1310. [Google Scholar] [CrossRef]
- Suuronen, R.; Wessman, L.; Mero, M.; Törmälä, P.; Vasenius, J.; Partio, E.; Vihtonen, K.; Vainionpää, S. Comparison of shear strength of osteotomies fixed with absorbable self-reinforced poly-L-lactide and metallic screws. J. Mater. Sci. Mater. Med. 1992, 3, 288–292. [Google Scholar] [CrossRef]
- Hine, P.J.; Olley, R.H.; Ward, I.M. The use of interleaved films for optimising the production and properties of hot compacted, self reinforced polymer composites. Compos. Sci. Technol. 2008, 68, 1413–1421. [Google Scholar] [CrossRef]
- Critchley, J.P.; Knight, G.J.; Wright, W.W. Thermosetting Polymers; Springer: Boston, MA, USA, 1983. [Google Scholar]
- Pham, H.Q.; Marks, M.J. Epoxy Resins. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Hubbard, J.W.; Orange, F.; Guinel, M.J.; Guenthner, A.J.; Mabry, J.M.; Sahagun, C.M.; Rinaldi, C. Curing of a bisphenol E based cyanate ester using magnetic nanoparticles as an internal heat source through induction heating. ACS Appl. Mater. Interfaces 2013, 5, 11329–11335. [Google Scholar] [CrossRef]
- Throckmorton, J.; Palmese, G. Acceleration of cyanate ester trimerization by dicyanamide RTILs. Polymer 2016, 91, 7–13. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Bank, L.C. A Critical Review of Research on Reuse of Mechanically Recycled FRP Production and End-of-Life Waste for Construction. Polymers 2014, 6, 1810–1826. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Zhang, G. Synthesis and properties of thermosetting resin based on urushiol. RSC Adv. 2012, 2, 2768. [Google Scholar] [CrossRef]
- Castellon, J.; Nguyen, H.N.; Agnel, S.; Toureille, A.; Frechette, M.; Savoie, S.; Krivda, A.; Schmidt, L.E. Electrical Properties Analysis of Micro and Nano Composite Epoxy Resin Materials. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 651–658. [Google Scholar] [CrossRef]
- Zhou, H.; Feng, L.; Zhang, Y.; Fan, W.; Liu, J.; Zhen, W.; Tong, Z. Novel Acetylene-Terminated Polyisoimides with Excellent Processability and Properties Comparison with Corresponding Polyimides. J. Appl. Polym. Sci. 2011, 122, 3493–3503. [Google Scholar] [CrossRef]
- Sun, W.; Sun, W.; Kessler, M.R.; Bowler, N.; Dennis, K.W.; McCallum, R.W.; Li, Q.; Tan, X. Multifunctional properties of cyanate ester composites with SiO2 coated Fe3O4 fillers. ACS Appl. Mater. Interfaces 2013, 5, 1636–1642. [Google Scholar] [CrossRef]
- Yan, H.; Li, P.; Zhang, J.; Ning, R. Mechanical Properties of Novel Bismaleimide Nanocomposites with Si3N4 Nanoparticles. J. Reinf. Plast. Compos. 2009, 29, 1515–1522. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Enhancement of fracture toughness, mechanical and thermal properties of rubber/epoxy composites by incorporation of graphene nanoplatelets. Compos. Part A Appl. Sci. Manuf. 2016, 87, 10–22. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Huang, X.; Chen, Y.; Lin, B.; Fu, L. Self-Healing Natural Rubber with Tailorable Mechanical Properties Based on Ionic Supramolecular Hybrid Network. ACS Appl. Mater. Interfaces 2017, 9, 29363–29373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Q.; Zhu, H.; Lin, Q.; Wang, C. Impact Resistance Enhancement by Adding Core-Shell Particle to Epoxy Resin Modified with Hyperbranched Polymer. Polymers 2017, 9, 684. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liang, Y.; Cheng, J.; Chen, S.; Zhang, A.; Miao, M.; Zhang, D. Synthesis of a Degradable High-Performance Epoxy-Ended Hyperbranched Polyester. ACS Omega 2017, 2, 1350–1359. [Google Scholar] [CrossRef]
- Chaudhary, S.; Surekha, P.; Kumar, D.; Rajagopal, C.; Roy, P.K. Amine-functionalized poly(styrene) microspheres as thermoplastic toughener for epoxy resin. Polym. Compos. 2015, 36, 174–183. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, A.; Liang, G.; Zhuo, D.; Yuan, L. Liquid crystalline epoxy resin modified cyanate ester for high performance electronic packaging. J. Polym. Res. 2010, 18, 1441–1450. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Interfaces in Polymer Matrix Composites; Elsevier: Amsterdam, The Netherlands, 1974. [Google Scholar]
- Reams, J.T.; Guenthner, A.J.; Lamison, K.R.; Vij, V.; Lubin, L.M.; Mabry, J.M. Effect of chemical structure and network formation on physical properties of di(cyanate ester) thermosets. ACS Appl. Mater. Interfaces 2012, 4, 527–535. [Google Scholar] [CrossRef]
- Hamerton, I. Chemistry and Technology of Cyanate Ester Resins; Springer: New York, NY, USA, 1994. [Google Scholar]
- Fang, T.; Shimp, D.A. Polycyanate esters: Science and applications. Prog. Polym. 1995, 20, 61–118. [Google Scholar] [CrossRef]
- Georjon, O.; Galy, J.; Pascault, J.P. Isothermal curing of an uncatalyzed dicyanate ester monomer: Kinetics and modeling. J. Appl. Polym. Sci. 2010, 49, 1441–1452. [Google Scholar] [CrossRef]
- Gómez, C.M.; Recalde, I.B.; Mondragon, I. Kinetic parameters of a cyanate ester resin catalyzed with different proportions of nonylphenol and cobalt acetylacetonate catalyst. Eur. Polym. J. 2005, 41, 2734–2741. [Google Scholar] [CrossRef]
- Harismendy, I.; Gómez, C.M.; Río, M.D.; Mondragon, I. Cure monitoring of catalysed cyanate ester resins. Polym. Int. 2015, 49, 735–742. [Google Scholar] [CrossRef]
- Liu, H.; George, G.A. Determination of thermal cure kinetics of thin films of photocatalysed dicyanate ester by FTIR emission spectroscopy. Polym. Int. 2015, 49, 1505–1512. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Chailan, J.F.; Vernet, J.L. Curing of cyanate ester resin: A novel approach based on FTIR spectroscopy and comparison with other techniques. Eur. Polym. J. 2001, 37, 659–670. [Google Scholar] [CrossRef]
- Mathew, D.; Nair, C.P.R.; Krishnan, K.; Ninan, K.N. Catalysis of the cure reaction of Bisphenol A dicyanate. A DSC study. J. Polym. Sci. Part A Polym. Chem. 2015, 37, 1103–1114. [Google Scholar] [CrossRef]
- Li, W.; Liang, G.; Xin, W. Triazine reaction of cyanate ester resin systems catalyzed by organic tin compound: Kinetics and mechanism. Polym. Int. 2004, 53, 869–876. [Google Scholar] [CrossRef]
- Osei-Owusu, A.; Martin, G.C.; Gotro, J.T. Analysis of the curing behavior of cyanate ester systems. Polym. Eng. Sci. 2010, 31, 1604–1609. [Google Scholar] [CrossRef]
- Grenier-Loustalot, M.F.; Lartigau, C.; Grenier, P. A study of the mechanisms and kinetics of the molten state reaction of non-catalyzed cyanate and epoxy-cyanate systems. Eur. Polym. J. 1995, 31, 1139–1153. [Google Scholar] [CrossRef]
- Pollard, M.; Kardos, J.L. Analysis of epoxy resin curing kinetics using the Avrami theory of phase change. Polym. Eng. Sci. 1987, 27, 829–836. [Google Scholar] [CrossRef]
- Peng, L.; Yang, X.; Yu, Y.; Yu, D. Cure kinetics, microheterogeneity, and mechanical properties of the high-temperature cure of vinyl ester resins. J. Appl. Polym. Sci. 2010, 92, 1124–1133. [Google Scholar]
- Lange, F.F.; Radford, K.C. Fracture energy of an epoxy composite system. J. Mater. Sci. 1971, 6, 1197–1203. [Google Scholar] [CrossRef]
- Wetzel, B.; Rosso, P.; Haupert, F.; Friedrich, K. Epoxy nanocomposites–fracture and toughening mechanisms. Eng. Fract. Mech. 2006, 73, 2375–2398. [Google Scholar] [CrossRef]
- Changgu, L.; Xiaoding, W.; Kysar, J.W.; James, H. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar]
- Wang, Y.; Wu, G.; Kou, K.; Pan, C.; Feng, A. Mechanical, thermal conductive and dielectrical properties of organic montmorillonite reinforced benzoxazine/cyanate ester copolymer for electronic packaging. J. Mater. Sci. Mater. Electron. 2016, 27, 8279–8287. [Google Scholar] [CrossRef]
- Chen, K.; Susner, M.A.; Vyazovkin, S. Effect of the Brush Structure on the Degradation Mechanism of Polystyrene-Clay Nanocomposites. Macromol. Rapid Commun. 2005, 26, 690–695. [Google Scholar] [CrossRef]
- Tang, Y.; Lewin, M.; Pearce, E.M. Effects of Annealing on the Migration Behavior of PA6/Clay Nanocomposites. Macromol. Rapid Commun. 2006, 27, 1545–1549. [Google Scholar] [CrossRef]
- An, L.; Pan, Y.; Shen, X.; Lu, H.; Yang, Y. Rod-like attapulgite/polyimide nanocomposites with simultaneously improved strength, toughness, thermal stability and related mechanisms. J. Mater. Chem. 2008, 18, 4928. [Google Scholar] [CrossRef]
- Pan, Y.; Yue, X.; Li, A.; Lu, H.; Yang, Y.; Wei, C.; Nutt, S. Hybrid Network Structure and Mechanical Properties of Rodlike Silicate/Cyanate Ester Nanocomposites. Macromolecules 2008, 41, 9245–9258. [Google Scholar] [CrossRef]
- Burnside, S.D.; Giannelis, E.P. Nanostructure and properties of polysiloxane-layered silicate nanocomposites. J. Polym. Sci. Part B 2015, 38, 1595–1604. [Google Scholar] [CrossRef]
- Zhuo, D.; Gu, A.; Liang, G.; Hu, J.T.; Cheng, Z.; Li, Y. Modified cyanate ester resins with lower dielectric loss, improved thermal stability, and flame retardancy. Polym. Adv. Technol. 2011, 22, 2617–2625. [Google Scholar] [CrossRef]
- Zhang, Z.; Pei, J.; Liang, G.; Li, Y. Methyl silsesquioxane/cyanate ester resin organic–inorganic hybrids with low dielectric constant. J. Appl. Polym. Sci. 2011, 121, 1004–1012. [Google Scholar] [CrossRef]
- Zhang, C.; Guang, S.; Zhu, X.; Xu, H.; Liu, X.; Jiang, M. Mechanism of Dielectric Constant Variation of POSS-Based Organic−Inorganic Molecular Hybrids. J. Phys. Chem. C 2010, 114, 22455–22461. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, G.; Wang, X.; Adhikari, S.; Pei, J. Curing behavior and dielectric properties of amino-functionalized polyhedral oligomeric silsesquioxane/cyanate ester resin hybrids. High Perform. Polym. 2013, 25, 427–435. [Google Scholar] [CrossRef]
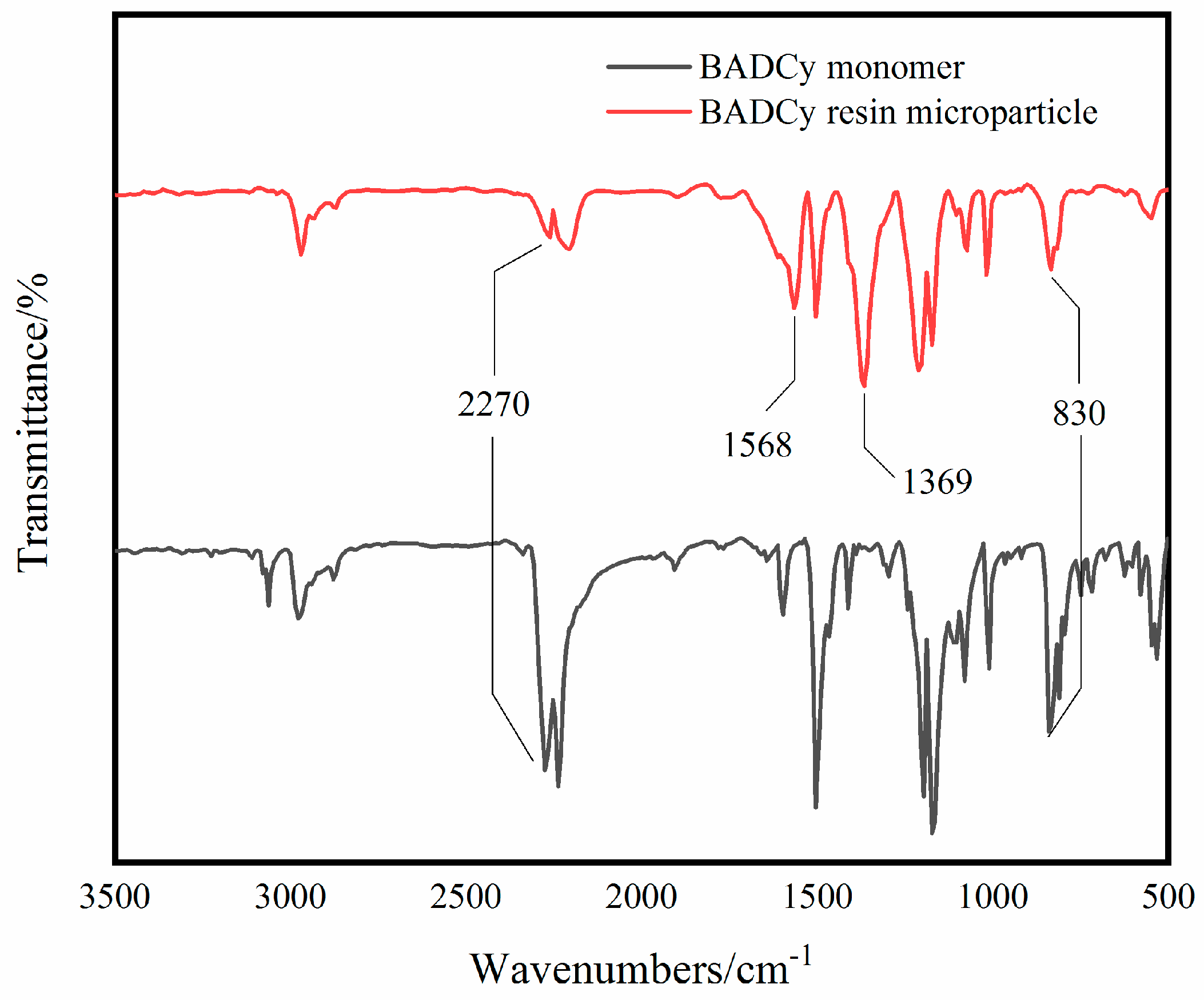




| Chemical Group | -OCN | Triazine Ring | Benzene Ring |
|---|---|---|---|
| Wavenumber/cm−1 | 2270 | 1568, 1369 | 860 |
| Contents | Neat | 2 wt% | 4 wt% | 6 wt% | 8 wt% | 10 wt% | 12 wt% | Increase |
|---|---|---|---|---|---|---|---|---|
| Tensile strength/MPa | 32.4 | 46.8 | 50.0 | 52.8 | 56.8 | 64.2 | 53.7 | 98.1% |
| Tensile modulus/GPa | 2.7 | 2.8 | 2.8 | 2.9 | 2.9 | 3.0 | 2.9 | 11.1% |
| Flexural strength/MPa | 70.3 | 72.8 | 86.1 | 94.0 | 95.2 | 98.6 | 90.7 | 40.2% |
| Flexural modulus/GPa | 2.9 | 3.2 | 3.3 | 3.4 | 3.6 | 3.8 | 3.7 | 31.0% |
| Compressive strength/MPa | 124.6 | 131.7 | 132.4 | 145.9 | 146.8 | 158.7 | 156.4 | 27.4% |
| Compressive modulus/GPa | 2.6 | 2.8 | 2.8 | 2.9 | 3.0 | 2.7 | 2.6 | 15.4% |
| Impact strength/kJ/m2 | 7.41 | 8.54 | 9.76 | 13.74 | 12.01 | 9.52 | 8.79 | 85.4% |
| Contents | Neat | 2 wt% | 4 wt% | 6 wt% | 8 wt% | 10 wt% | 12 wt% |
|---|---|---|---|---|---|---|---|
| Td5% (°C) | 400 | 403 | 402 | 401 | 410 | 402 | 408 |
| Td10% (°C) | 427 | 429 | 429 | 428 | 433 | 429 | 432 |
| Char yield (%) | 34.25 | 34.74 | 35.17 | 35.18 | 36.32 | 35.93 | 36.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Liu, B.; Ye, Y.; Liu, Y.; Li, P. Study on the Relationships between Microscopic Cross-Linked Network Structure and Properties of Cyanate Ester Self-Reinforced Composites. Polymers 2019, 11, 950. https://doi.org/10.3390/polym11060950
Cao H, Liu B, Ye Y, Liu Y, Li P. Study on the Relationships between Microscopic Cross-Linked Network Structure and Properties of Cyanate Ester Self-Reinforced Composites. Polymers. 2019; 11(6):950. https://doi.org/10.3390/polym11060950
Chicago/Turabian StyleCao, Hongtao, Beijun Liu, Yiwen Ye, Yunfang Liu, and Peng Li. 2019. "Study on the Relationships between Microscopic Cross-Linked Network Structure and Properties of Cyanate Ester Self-Reinforced Composites" Polymers 11, no. 6: 950. https://doi.org/10.3390/polym11060950
APA StyleCao, H., Liu, B., Ye, Y., Liu, Y., & Li, P. (2019). Study on the Relationships between Microscopic Cross-Linked Network Structure and Properties of Cyanate Ester Self-Reinforced Composites. Polymers, 11(6), 950. https://doi.org/10.3390/polym11060950




