Complex Permittivity and Microwave Absorption Properties of OPEFB Fiber–Polycaprolactone Composites Filled with Recycled Hematite (α-Fe2O3) Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of α-Fe2O3 Nanoparticles from Mill Scale
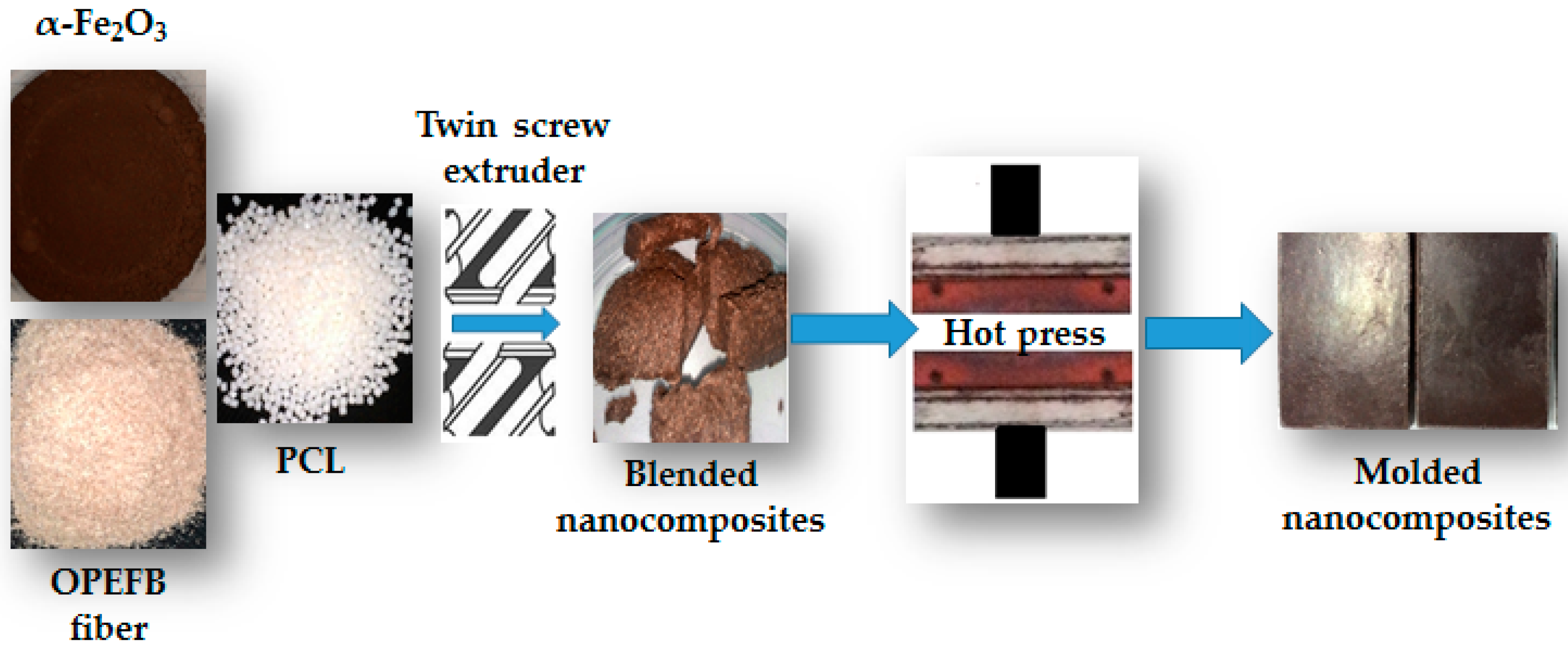
2.3. Fabrication of Recycled α-Fe2O3/OPEFB/PCL Nanocomposites
2.4. Characterizations
2.4.1. Structure and Composition
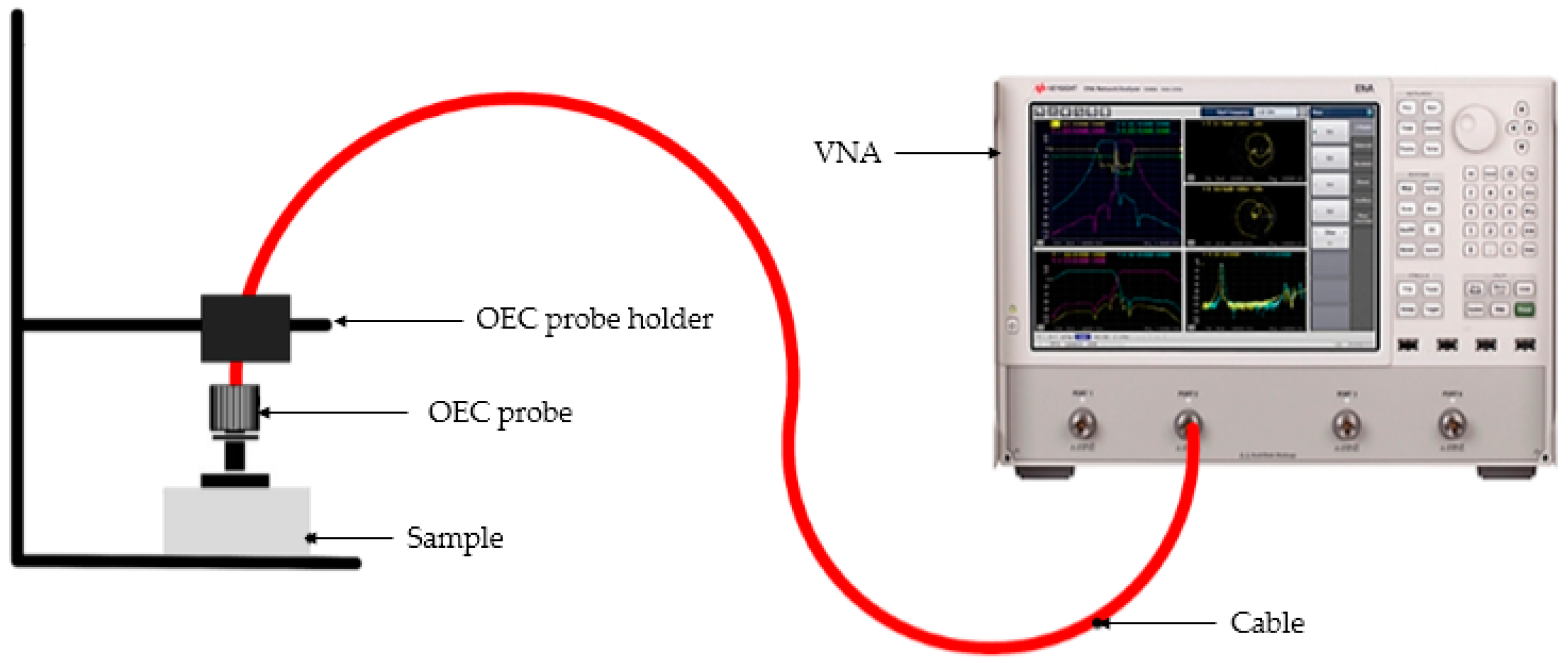
2.4.2. Complex Permittivity
2.4.3. Microwave Absorption
3. Results and Discussion
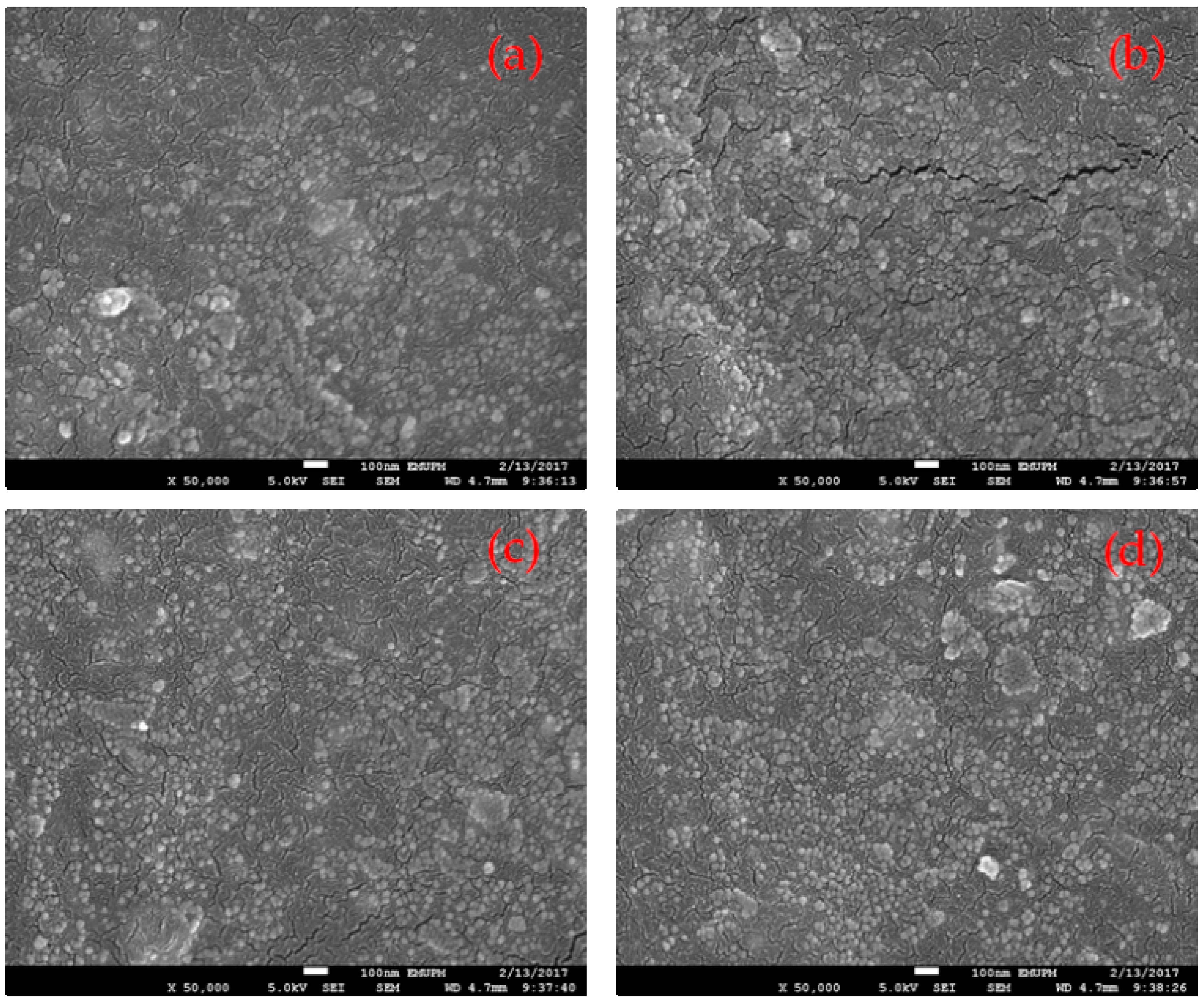
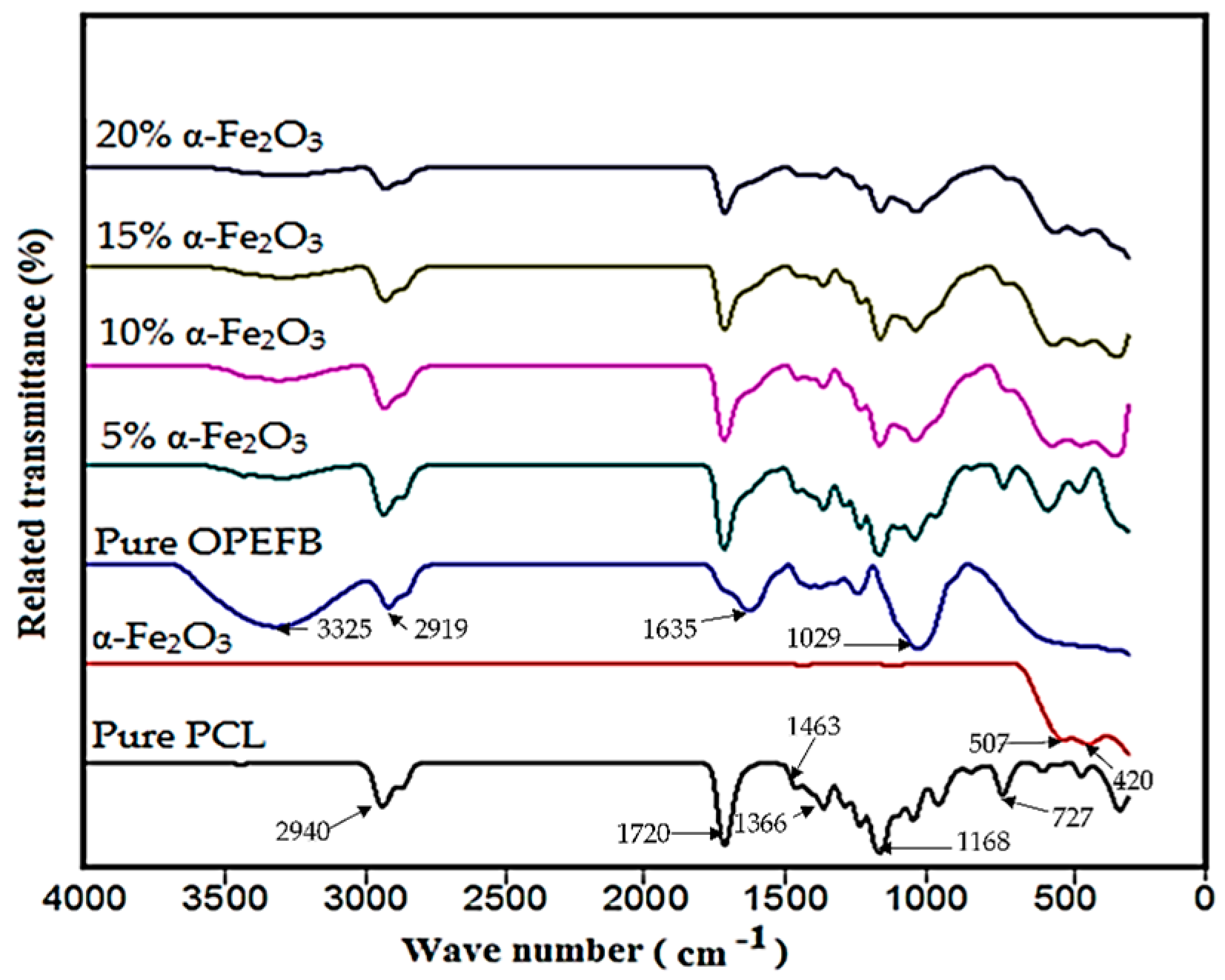
3.1. Structure and Composition
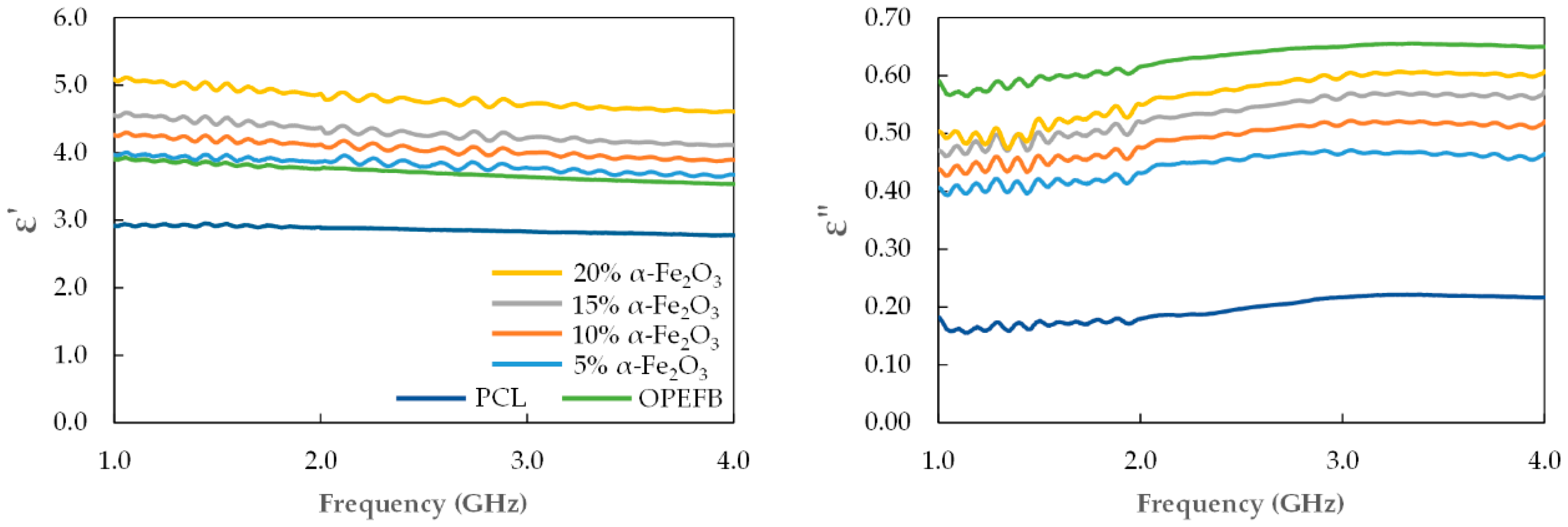
3.2. Complex Permittivity
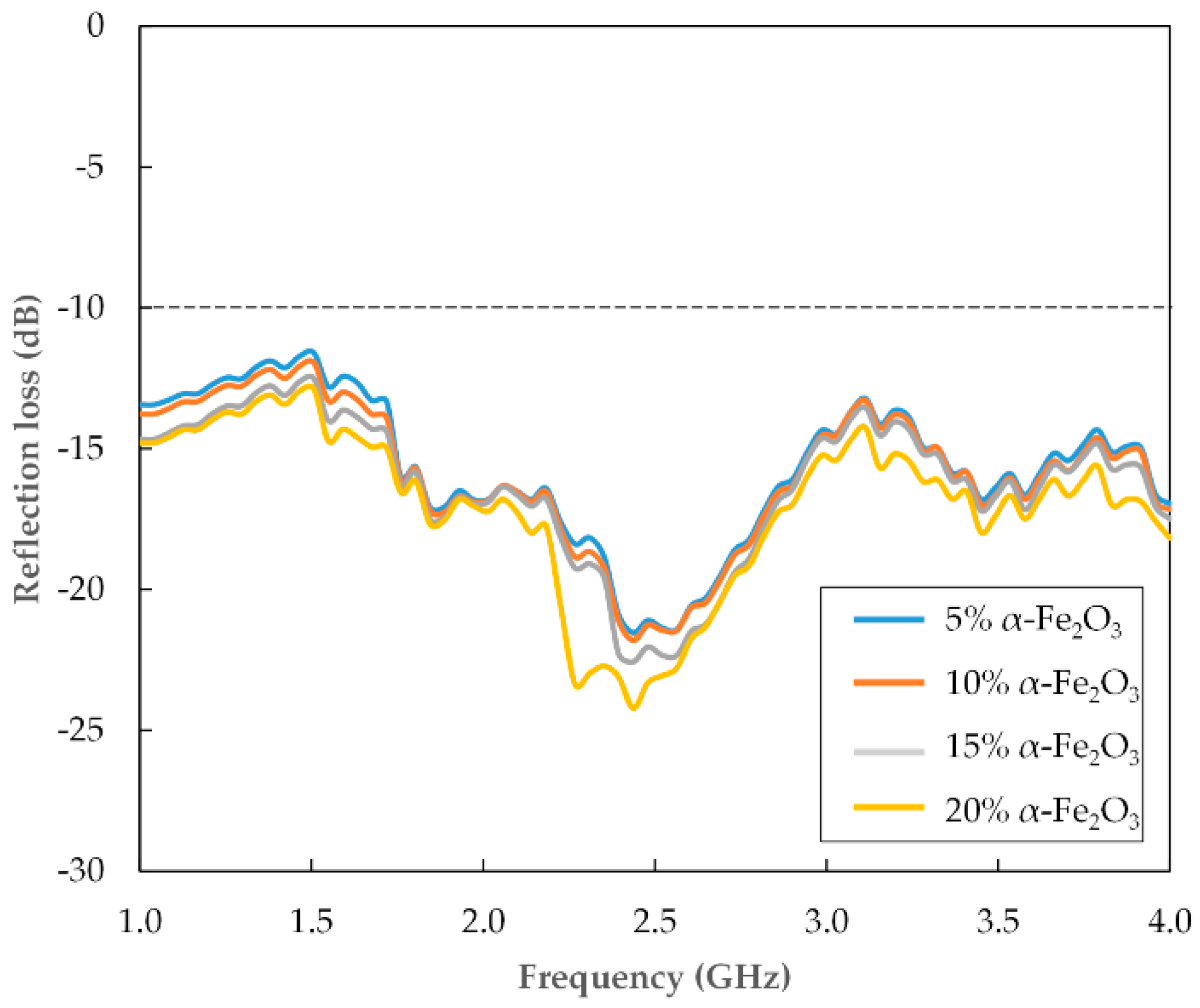
3.3. Microwave Absorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rostami, M.; Ara, M.H.M. The dielectric, magnetic and microwave absorption properties of Cu-substituted Mg-Ni spinel ferrite-MWCNT nanocomposites. Ceram. Int. 2019, 45, 7606–7613. [Google Scholar] [CrossRef]
- Pratap, V.; Soni, A.K.; Dayal, S.; Abbas, S.M.; Siddiqui, A.M.; Prasad, N.E. Electromagnetic and absorption properties of U-type barium hexaferrite-epoxy composites. J. Magn. Magn. Mater. 2018, 465, 540–545. [Google Scholar] [CrossRef]
- Dosoudil, R.; Ušáková, M.; Franek, J.; Grusková, A.; Sláma, J. Dispersion of complex permeability and EM-wave absorbing characteristics of polymer-based composites with dual ferrite filler. J. Magne. Magn. Mater. 2008, 320, e849–e852. [Google Scholar] [CrossRef]
- Ali, N.N.; Atassi, Y.; Salloum, A.; Charba, A.; Malki, A.; Jafarian, M. Comparative study of microwave absorption characteristics of (polyaniline/NiZn ferrite) nanocomposites with different ferrite percentages. Mater. Chem. Phys. 2018, 211, 79–87. [Google Scholar] [CrossRef]
- Yao, Z.; Lin, H.; Haidry, A.A.; Zhou, J.; Liu, P. Synthesis and high-performance microwave absorption of reduced graphene oxide/Co-doped ZnNi ferrite/polyaniline composites. Mater. Lett. 2019, 236, 456–459. [Google Scholar]
- Almessiere, M.A.; Slimani, Y.; El Sayed, H.S.; Baykal, A.; Ercan, I. Microstructural and magnetic investigation of vanadium-substituted Sr- nanohexaferrite. J. Magn. Magn. Mater. 2019, 471, 124–132. [Google Scholar] [CrossRef]
- Pawar, R.C.; Um, J.H.; Kang, S.; Yoon, W.-S.; Choe, H.; Lee, C.S. Solvent-polarity-induced hematite (α-Fe2O3) nanostructures for lithium-ion battery and photoelectrochemical applications. Electrochimica Acta 2017, 245, 643–653. [Google Scholar] [CrossRef]
- Daud, N.; Azis, R.A.S.; Hashim, M.; Matori, K.A.; Jumiah, H.; Saiden, N.M.; Mariana, N. Preparation and characterization of Sr1−xNdxFe12O19 derived from steel-waste product via mechanical alloying. Mater. Sci. Forum 2016, 846, 403–409. [Google Scholar] [CrossRef]
- Shahrani, M.; Mariana, N.; Azis, R.A.S.; Hashim, M.; Jumiah, H.; Azmi, Z.; Daud, N. Effect of variation sintering temperature on magnetic permeability and grain sizes of Y3Fe5O12 via mechanical alloying technique. Mater. Sci. Forum 2016, 846, 395–402. [Google Scholar] [CrossRef]
- Yang, H.; Ye, T.; Lin, Y.; Zhu, J.; Wang, F. Microwave absorbing properties of the ferrite composites based on grapheme. J. Alloys Compd. 2016, 683, 567–574. [Google Scholar] [CrossRef]
- Abdalhadi, D.M.; Abbas, Z.; Ahmad, A.F.; Ibrahim, N.A. Determining the complex permittivity of oil palm empty fruit bunch fibre material by open-ended coaxial probe technique for microwave applications. BioResources 2017, 12, 3976–3991. [Google Scholar] [CrossRef][Green Version]
- Ahmad, A.; Abbas, Z.; Obaiys, S.; Abdalhadi, D. Improvement of dielectric, magnetic and thermal properties of OPEFB fibre–polycaprolactone composite by adding Ni–Zn ferrite. Polymers 2017, 9, 12. [Google Scholar] [CrossRef]
- Yakovenko, O.S.; Matzui, L.Y.; Vovchenko, L.L.; Trukhanov, A.V.; Kazakevich, I.S.; Trukhanov, S.V.; Prylutskyy, Y.I.; Ritter, U. Magnetic anisotropy of the graphite nanoplatelet-epoxy and MWCNT-epoxy composites with aligned barium ferrite filler. J. Mater. Sci. 2017, 52, 5345–5358. [Google Scholar] [CrossRef]
- Zdujić, M.; Jovalekić, Č.; Karanović, L.; Mitrić, M. The ball milling induced transformation of α-Fe2O3 powder in air and oxygen atmosphere. Mater. Sci. Eng. A 1999, 262, 204–213. [Google Scholar] [CrossRef]
- Azis, R.S.; Hashim, M.; Saiden, N.M.; Daud, N.; Shahrani, N.M.M. Study the iron environments of the steel waste product and its possible potential applications in ferrites. Adv. Mater. Res. 2015, 1109, 295–299. [Google Scholar] [CrossRef]
- Novoselova, L.Y. Hematite nanopowder obtained from waste: Iron-removal sludge. Powder Technol. 2016, 287, 364–372. [Google Scholar] [CrossRef]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Akurati, K.K. Synthesis of TiO2 based Nanoparticles for Photocatalytic Applications; Cuvillier Verlag: Göttingen, Germany, 2008; p. 60. [Google Scholar]
- Kendall, K.; Kendall, M.; Rehfeldt, F. Adhesion of cells, viruses and nanoparticles. Adhes. Cells Viruses Nanoparticles 2011. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Jansi Rani, B.; Ravi, G.; Sakunthala, A.; Yuvakkumar, R. Influence of reducing agent concentration on the structure, morphology and ferromagnetic properties of hematite (α-Fe2O3) nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 8093–8100. [Google Scholar] [CrossRef]
- Airimioaei, M.; Stanculescu, R.; Preutu, V.; Ciomaga, C.; Horchidan, N.; Tascu, S.; Lutic, D.; Pui, A.; Mitoseriu, L. Effect of particle size and volume fraction of BaTiO3 powders on the functional properties of BaTiO3/poly(ε-caprolactone) composites. Mater. Chem. Phys. 2016, 182, 246–255. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Hashim, N.; Rahman, M.Z.A.; Yunus, W.M.Z.W. Mechanical properties and morphology of oil palm empty fruit bunch–polypropylene composites: effect of adding ENGAGETM 7467. J. Thermoplast. Compos. Mater. 2011, 24, 713–732. [Google Scholar] [CrossRef]
- Maleknejad, Z.; Gheisari, K.; Raouf, A.H. Structure, microstructure, magnetic, electromagnetic, and dielectric properties of nanostructured Mn–Zn ferrite synthesized by microwave-induced urea–nitrate process. J. Supercond. Nov. Magn. 2016, 29, 2523–2534. [Google Scholar] [CrossRef]
- Gözüak, F.; Köseoğlu, Y.; Baykal, A.; Kavas, H. Synthesis and characterization of CoxZn1−xFe2O4 magnetic nanoparticles via a PEG-assisted route. J. Magn. Magn. Mater. 2009, 321, 2170–2177. [Google Scholar] [CrossRef]
- Kumar, E.R.; Kamzin, A.S.; Prakash, T. Effect of particle size on structural, magnetic and dielectric properties of manganese substituted nickel ferrite nanoparticles. J. Magn. Magn. Mater. 2015, 378, 389–396. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Yadav, A.; Varshney, D. Structural and dielectric properties of copper-substituted Mg–Zn spinel ferrites. J. Supercond. Nov. Magn. 2017, 30, 1297–1302. [Google Scholar] [CrossRef]
- Widanarto, W.; Ardenti, E.; Ghoshal, S.K.; Kurniawan, C.; Effendi, M.; Cahyanto, W.T. Significant reduction of saturation magnetization and microwave-reflection loss in barium-natural ferrite via Nd3+ substitution. J. Magn. Magn. Mater. 2018, 456, 288–291. [Google Scholar] [CrossRef]


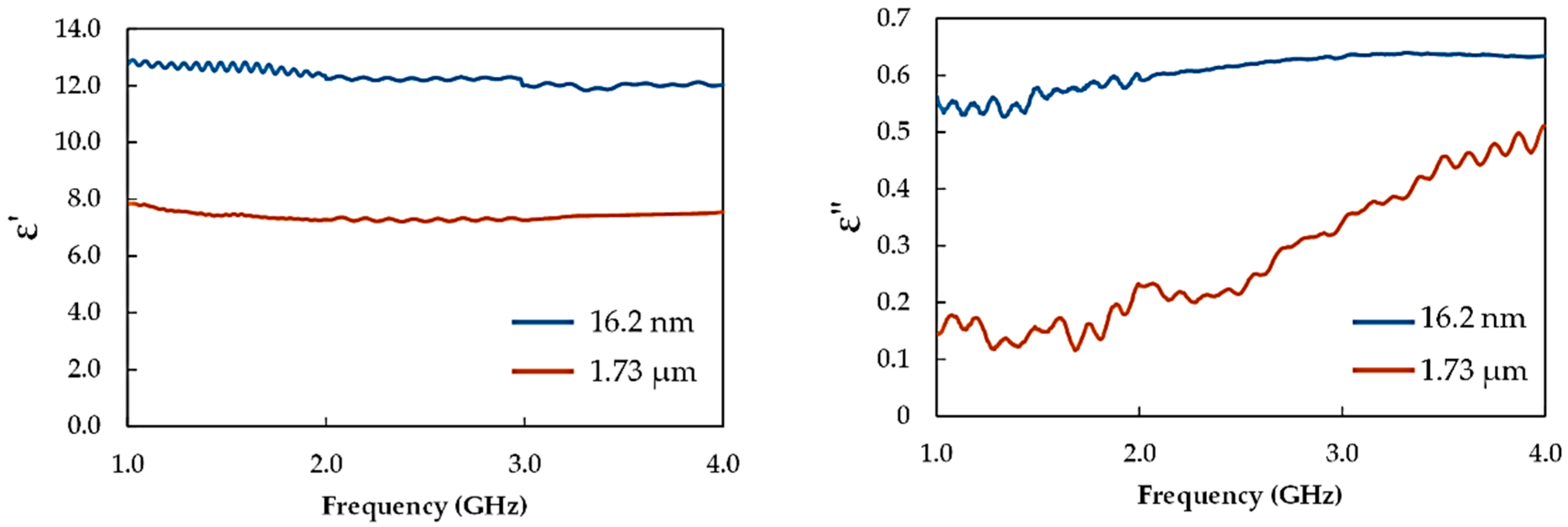
| α-Fe2O3 Sample | Crystallite Size (nm) | Particle Size | Specific Surface Area (m2/g) |
|---|---|---|---|
| Unmilled | 106.2 | 1.73 (μm) | 0.202 |
| 12 h milling | 11.1 | 16.2 (nm) | 13.159 |
| α-Fe2O3 (wt.%) | 1 GHz | 2.4 GHz | 4 GHz | |||
|---|---|---|---|---|---|---|
| ε′ | ε″ | ε′ | ε″ | ε′ | ε″ | |
| 5 | 3.97 | 0.40 | 3.88 | 0.45 | 3.67 | 0.46 |
| 10 | 4.26 | 0.43 | 4.10 | 0.49 | 3.89 | 0.52 |
| 15 | 4.56 | 0.47 | 4.32 | 0.53 | 4.11 | 0.57 |
| 20 | 5.08 | 0.50 | 4.82 | 0.57 | 4.60 | 0.60 |
| α-Fe2O3 (wt.%) | 1 GHz | 2.4 GHz | 4 GHz |
|---|---|---|---|
| 5 | −13.4 | −21.5 | −16.9 |
| 10 | −13.7 | −21.8 | −17.1 |
| 15 | −14.6 | −22.5 | −17.4 |
| 20 | −14.7 | −24.2 | −18.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, E.E.; Abbas, Z.; Azis, R.S.; Ibrahim, N.A.; Khamis, A.M. Complex Permittivity and Microwave Absorption Properties of OPEFB Fiber–Polycaprolactone Composites Filled with Recycled Hematite (α-Fe2O3) Nanoparticles. Polymers 2019, 11, 918. https://doi.org/10.3390/polym11050918
Mensah EE, Abbas Z, Azis RS, Ibrahim NA, Khamis AM. Complex Permittivity and Microwave Absorption Properties of OPEFB Fiber–Polycaprolactone Composites Filled with Recycled Hematite (α-Fe2O3) Nanoparticles. Polymers. 2019; 11(5):918. https://doi.org/10.3390/polym11050918
Chicago/Turabian StyleMensah, Ebenezer Ekow, Zulkifly Abbas, Raba’ah Syahidah Azis, Nor Azowa Ibrahim, and Ahmad Mamoun Khamis. 2019. "Complex Permittivity and Microwave Absorption Properties of OPEFB Fiber–Polycaprolactone Composites Filled with Recycled Hematite (α-Fe2O3) Nanoparticles" Polymers 11, no. 5: 918. https://doi.org/10.3390/polym11050918
APA StyleMensah, E. E., Abbas, Z., Azis, R. S., Ibrahim, N. A., & Khamis, A. M. (2019). Complex Permittivity and Microwave Absorption Properties of OPEFB Fiber–Polycaprolactone Composites Filled with Recycled Hematite (α-Fe2O3) Nanoparticles. Polymers, 11(5), 918. https://doi.org/10.3390/polym11050918





