Molecular Design of Microcapsule Shells for Visible Light-Triggered Release
Abstract
1. Introduction
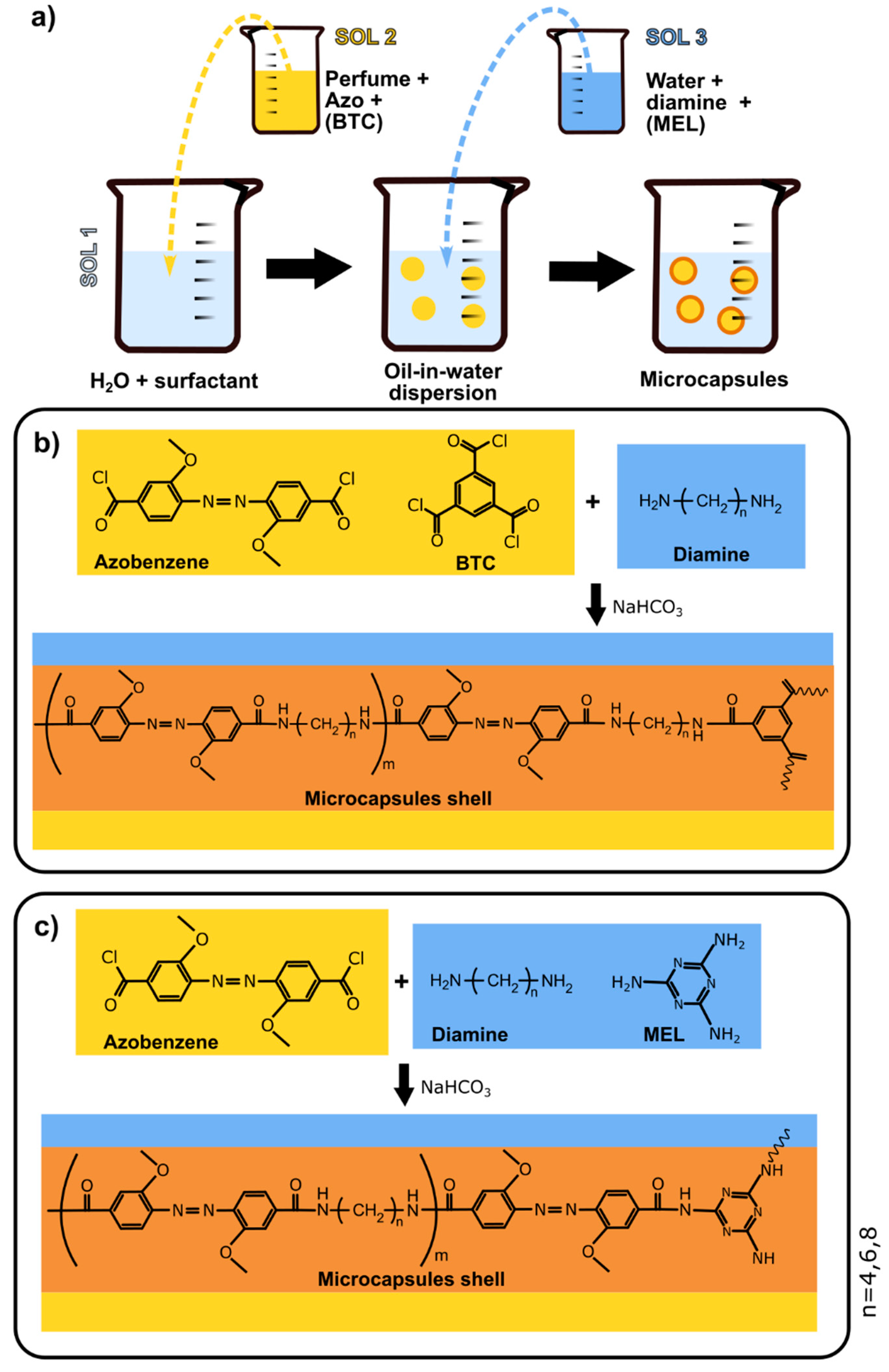
2. Materials and Methods
- Solution 1 (Sol1)—50 mL of 1% M18-88 aqueous solution;
- Solution 2 (Sol2)—0.6 g (1.63 mmol) of azo and, for samples MC1-MC3, 0,067 g (0.25 mmol) of BTC were dissolved in 25 mL of perfume oil (P).
- Solution 3 (Sol3)—the listed quantities (see Table 1) of diamines (DA), 0.29 g (3.45 mmol) of sodium hydrogen carbonate and, for samples MC4–MC6 0.032 g (0.25 mmol) of MEL cross-linker were dissolved under gentle stirring in 25 mL of 1 wt. % M18-88 solution.
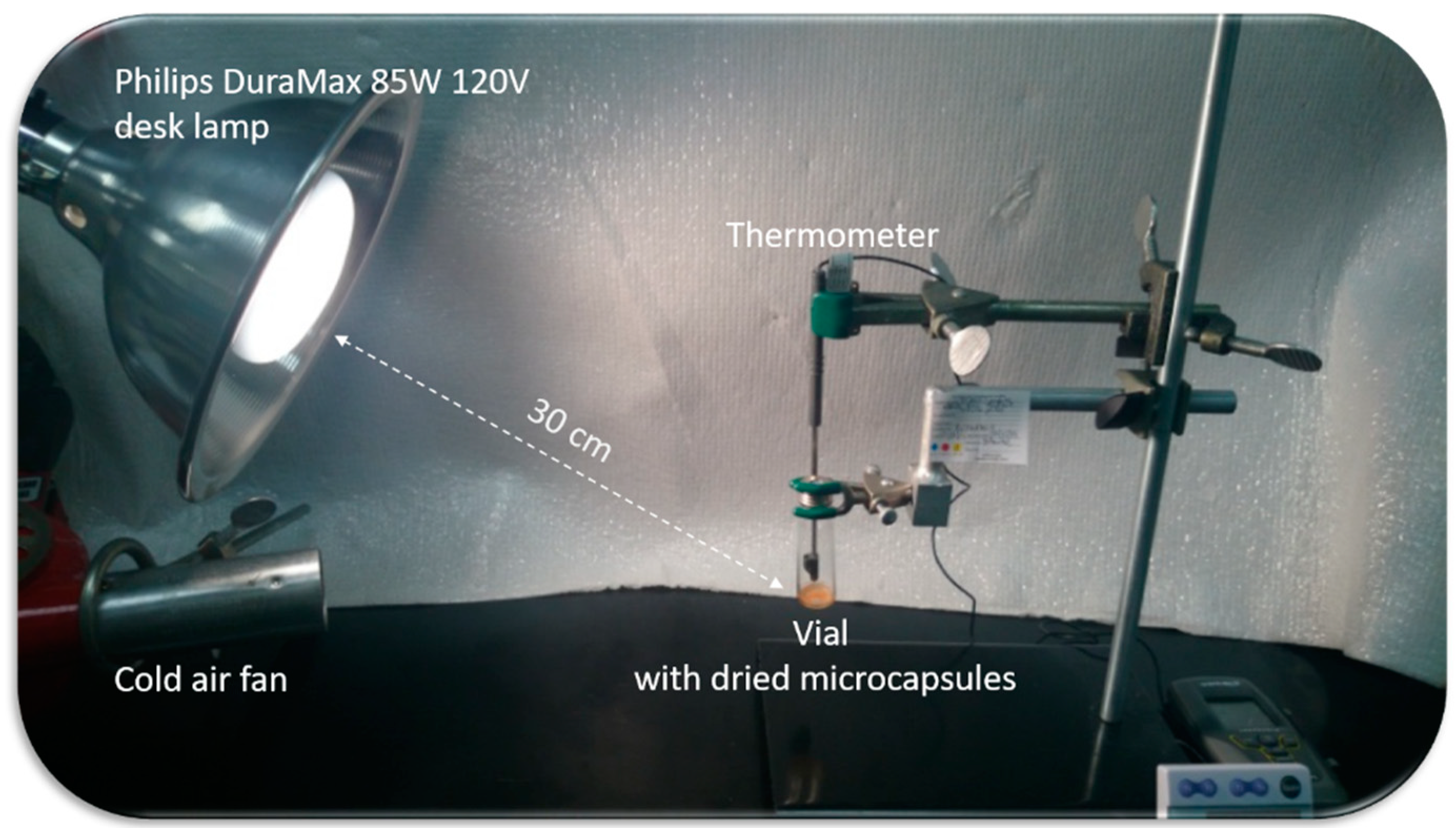
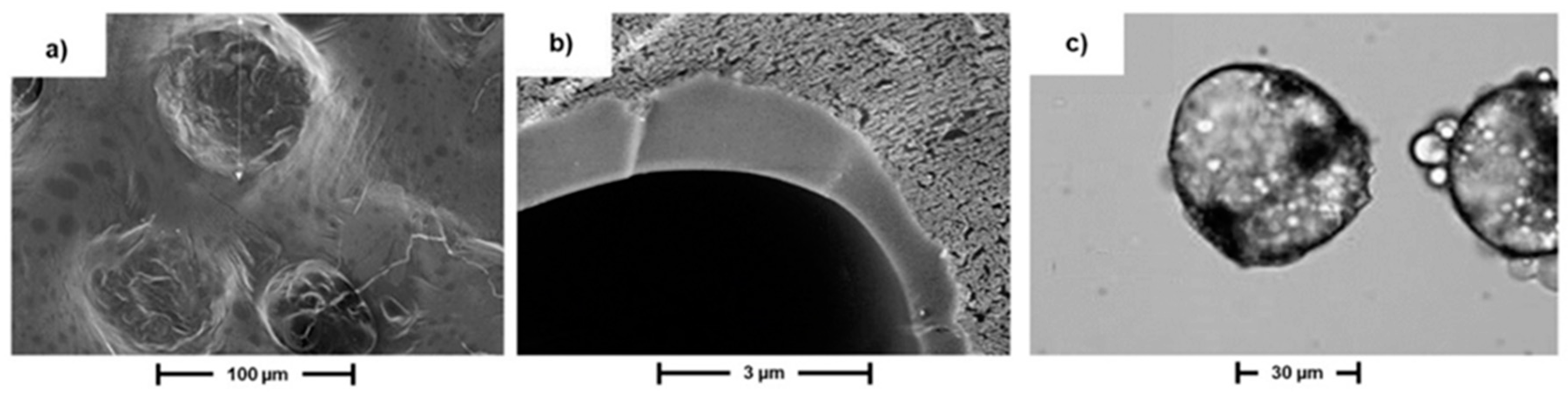
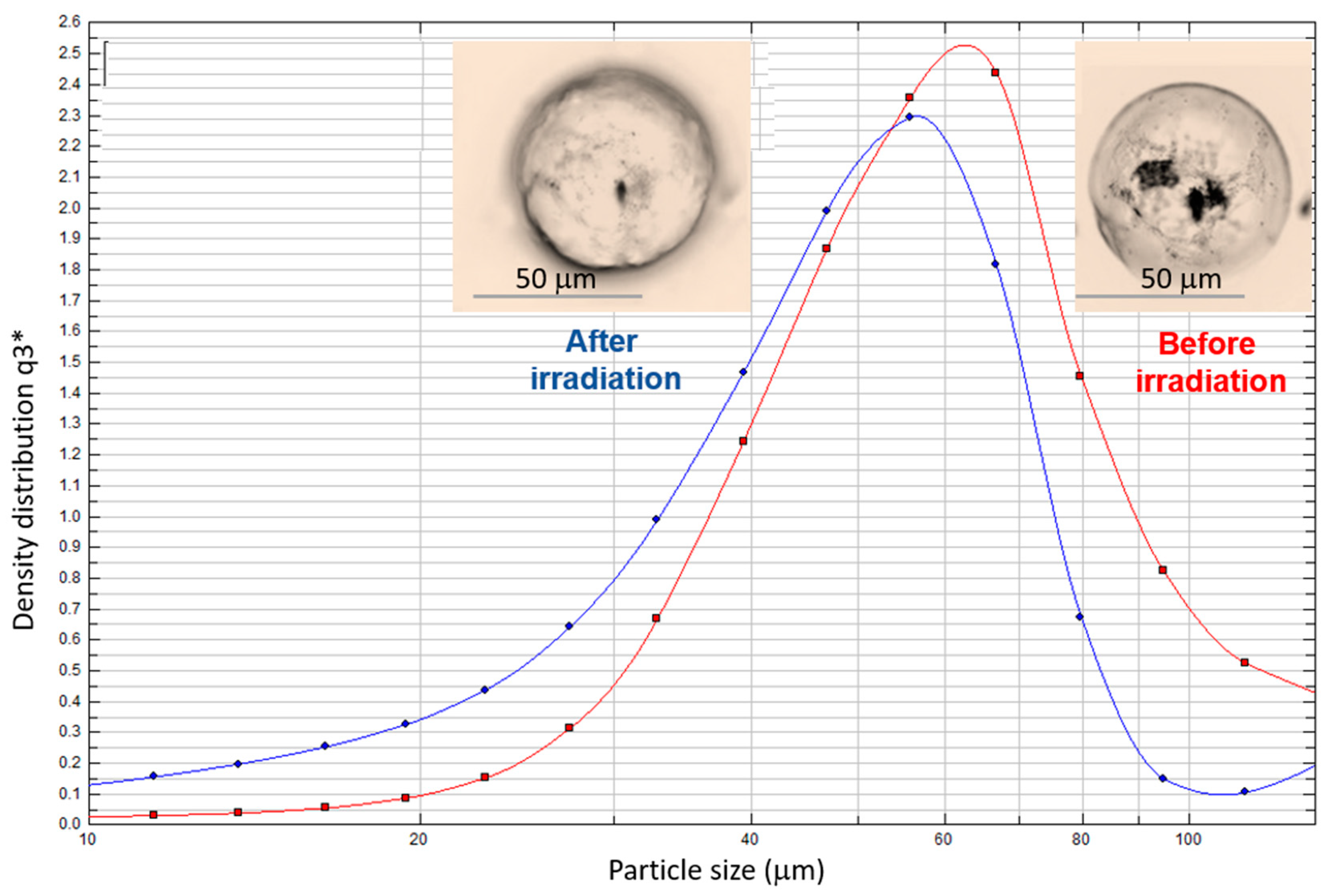
3. Results and Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Coulston, R.J.; Jones, S.T.; Geng, J.; Scherman, O.A.; Abell, C. One-Step Fabrication of Supramolecular Microcapsules from Microfluidic Droplets. Science 2012, 335, 690–694. [Google Scholar] [CrossRef]
- Marturano, V.; Bizzarro, V.; De Luise, A.; Calarco, A.; Ambrogi, V.; Giamberini, M.; Tylkowski, B.; Cerruti, P. Essential oils as solvents and core materials for the preparation of photo-responsive polymer nanocapsules. Nano Res. 2018, 11, 2783–2795. [Google Scholar] [CrossRef]
- Petzold, G.; Gianelli, M.P.; Bugueño, G.; Celan, R.; Pavez, C.; Orellana, P. Encapsulation of liquid smoke flavoring in ca-alginate and ca-alginate-chitosan beads. J. Food Sci. Technol. 2014, 51, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Potroz, M.G.; Mundargi, R.C.; Gillissen, J.J.; Tan, E.-L.; Meker, S.; Park, J.H.; Jung, H.; Park, S.; Cho, D.; Bang, S.-I.; et al. Plant-Based Hollow Microcapsules for Oral Delivery Applications: Toward Optimized Loading and Controlled Release. Adv. Funct. Mater. 2017, 27, 1700270. [Google Scholar] [CrossRef]
- Vasani, R.B.; Losic, D.; Cavallaro, A.; Voelcker, N.H. Fabrication of stimulus-responsive diatom biosilica microcapsules for antibiotic drug delivery. J. Mater. Chem. B 2015, 3, 4325–4329. [Google Scholar] [CrossRef]
- Behnke, T.; Würth, C.; Hoffmann, K.; Hübner, M.; Panne, U.; Resch-Genger, U. Encapsulation of hydrophobic dyes in polystyrene micro- and nanoparticles via swelling procedures. J. Fluoresc. 2011, 21, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Neisiany, R.E.; Lee, J.K.Y.; Khorasani, S.N.; Ramakrishna, S. Towards the development of self-healing carbon/epoxy composites with improved potential provided by efficient encapsulation of healing agents in core-shell nanofibers. Polym. Test. 2017, 62, 79–87. [Google Scholar] [CrossRef]
- Nallamuthu, I.; Khanum, F.; Fathima, S.J.; Patil, M.M.; Anand, T. 16—Enhanced nutrient delivery through nanoencapsulation techniques: The current trend in food industry. In Nutrient Delivery; Grumezescu, A.M., Ed.; Nanotechnology in the Agri-Food Industry; Elsevier: London, UK, 2017; pp. 619–651. ISBN 978-0-12-804304-2. [Google Scholar]
- Han, J.; Bong, J.; Lim, T.; Lee, K.-H.; Yang, H.; Ju, S. Water repellent spray-type encapsulation of quantum dot light-emitting diodes using super-hydrophobic self-assembled nanoparticles. Appl. Surf. Sci. 2015, 353, 338–341. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Paseta, L.; Simón-Gaudó, E.; Gracia-Gorría, F.; Coronas, J. Encapsulation of essential oils in porous silica and MOFs for trichloroisocyanuric acid tablets used for water treatment in swimming pools. Chem. Eng. J. 2016, 292, 28–34. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Ding, Z.-Q.; Zhang, F.; Ye, W.-B. Recent development in cell encapsulations and their therapeutic applications. Mater. Sci. Eng. C 2017, 77, 1247–1260. [Google Scholar] [CrossRef]
- Saha, R.; Verbanic, S.; Chen, I.A. Lipid vesicles chaperone an encapsulated RNA aptamer. Nat. Commun. 2018, 9, 2313. [Google Scholar] [CrossRef]
- Hu, C.; Ma, N.; Li, F.; Fang, Y.; Liu, Y.; Zhao, L.; Qiao, S.; Li, X.; Jiang, X.; Li, T.; et al. Cucurbit[8]uril-Based Giant Supramolecular Vesicles: Highly Stable, Versatile Carriers for Photoresponsive and Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 4603–4613. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-Responsive Polymer Micro- and Nano-Capsules. Polymers 2017, 9, 8. [Google Scholar] [CrossRef]
- Mak, W.C.; Olesen, K.; Sivlér, P.; Lee, C.J.; Moreno-Jimenez, I.; Edin, J.; Courtman, D.; Skog, M.; Griffith, M. Controlled Delivery of Human Cells by Temperature Responsive Microcapsules. J. Funct. Biomater. 2015, 6, 439–453. [Google Scholar] [CrossRef]
- Ping, Y.; Guo, J.; Ejima, H.; Chen, X.; Richardson, J.J.; Sun, H.; Caruso, F. pH-Responsive Capsules Engineered from Metal-Phenolic Networks for Anticancer Drug Delivery. Small Weinh. Bergstr. Ger. 2015, 11, 2032–2036. [Google Scholar] [CrossRef]
- Guo, W.; Jia, Y.; Tian, K.; Xu, Z.; Jiao, J.; Li, R.; Wu, Y.; Cao, L.; Wang, H. UV-Triggered Self-Healing of a Single Robust SiO2 Microcapsule Based on Cationic Polymerization for Potential Application in Aerospace Coatings. ACS Appl. Mater. Interfaces 2016, 8, 21046–21054. [Google Scholar] [CrossRef]
- Ott, A.; Yu, X.; Hartmann, R.; Rejman, J.; Schütz, A.; Ochs, M.; Parak, W.J.; Carregal-Romero, S. Light-Addressable and Degradable Silica Capsules for Delivery of Molecular Cargo to the Cytosol of Cells. Chem. Mater. 2015, 27, 1929–1942. [Google Scholar] [CrossRef]
- Ambrosone, A.; Marchesano, V.; Carregal-Romero, S.; Intartaglia, D.; Parak, W.J.; Tortiglione, C. Control of Wnt/β-Catenin Signaling Pathway in Vivo via Light Responsive Capsules. ACS Nano 2016, 10, 4828–4834. [Google Scholar] [CrossRef]
- Hong, C.-S.; Park, J.H.; Lee, S.; Rhoo, K.Y.; Lee, J.T.; Paik, S.R. Fabrication of Protease-Sensitive and Light-Responsive Microcapsules Encompassed with Single Layer of Gold Nanoparticles by Using Self-Assembly Protein of α-Synuclein. ACS Appl. Mater. Interfaces 2018, 10, 26628–26640. [Google Scholar] [CrossRef]
- Budhlall, B.M.; Marquez, M.; Velev, O.D. Microwave, Photo- and Thermally Responsive PNIPAm−Gold Nanoparticle Microgels. Langmuir 2008, 24, 11959–11966. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, G.; Hu, J.; Liu, S. Photo- and Reduction-Responsive Polymersomes for Programmed Release of Small and Macromolecular Payloads. Biomacromolecules 2018, 19, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.R.; Rangadurai, P.; Antony, L.; Swaminathan, S.; de Pablo, J.J.; Thayumanavan, S. Dynamic actuation of glassy polymersomes through isomerization of a single azobenzene unit at the block copolymer interface. Nat. Chem. 2018, 10, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Döbbelin, M.; Ciesielski, A.; Haar, S.; Osella, S.; Bruna, M.; Minoia, A.; Grisanti, L.; Mosciatti, T.; Richard, F.; Prasetyanto, E.A.; et al. Light-enhanced liquid-phase exfoliation and current photoswitching in graphene-azobenzene composites. Nat. Commun. 2016, 7, 11090. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.J.; Applegate, M.B.; Bushuyev, O.S.; Omenetto, F.G.; Kaplan, D.L.; Cronin-Golomb, M.; Barrett, C.J. Photo-induced structural modification of silk gels containing azobenzene side groups. Soft Matter 2017, 13, 2903–2906. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.S. The Cis-form of Azobenzene. Nature 1937, 140, 281. [Google Scholar] [CrossRef]
- Motevalli, B.; Taherifar, N.; Wu, B.; Tang, W.; Liu, J.Z. A density functional theory computational study of adsorption of Di-Meta-Cyano Azobenzene molecules on Si (111) surfaces. Appl. Surf. Sci. 2017, 422, 557–565. [Google Scholar] [CrossRef]
- Tylkowski, B.; Trojanowska, A.; Marturano, V.; Nowak, M.; Marciniak, L.; Giamberini, M.; Ambrogi, V.; Cerruti, P. Power of light—Functional complexes based on azobenzene molecules. Coord. Chem. Rev. 2017, 351, 205–217. [Google Scholar] [CrossRef]
- Hamelmann, F.; Heinzmann, U.; Siemeling, U.; Bretthauer, F.; Vor der Brüggen, J. Light-stimulated switching of azobenzene-containing self-assembled monolayers [rapid communication]. Appl. Surf. Sci. 2004, 222, 1–5. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Angelini, G.; Campestre, C.; Scotti, L.; Gasbarri, C. Kinetics and Energetics of Thermal Cis-Trans Isomerization of a Resonance-Activated Azobenzene in BMIM-Based Ionic Liquids for PF6−/Tf2N− Comparison. Molecules 2017, 22, 1273. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Mochizuki, T.; Liang, X.; Asanuma, H.; Tanaka, S.; Suzuki, K.; Kitamura, S.; Nishikawa, A.; Ui-Tei, K.; Hagiya, M. Robust and photocontrollable DNA capsules using azobenzenes. Nano Lett. 2010, 10, 3560–3565. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.Z. Azo polymers with electronical push and pull structures prepared via RAFT polymerization and its photoinduced birefringence behavior. eXPRESS Polym. Lett. 2008, 2, 589–601. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Yang, Y.; Gong, X.; Liao, Y.; Xie, X. Photomechanically Controlled Encapsulation and Release from pH-Responsive and Photoresponsive Microcapsules. Langmuir 2015, 31, 5456–5463. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Schmidt, B.V.K.J.; Barner-Kowollik, C.; Piñol, M.; Oriol, L. A Novel Photoresponsive Azobenzene-Containing Miktoarm Star Polymer: Self-Assembly and Photoresponse Properties. Macromolecules 2014, 47, 3693–3700. [Google Scholar] [CrossRef]
- Beharry, A.A.; Sadovski, O.; Woolley, G.A. Azobenzene Photoswitching without Ultraviolet Light. J. Am. Chem. Soc. 2011, 133, 19684–19687. [Google Scholar] [CrossRef]
- Tylkowski, B.; Giamberini, M.; Underiner, T.; Prieto, S.F.; Smets, J. Photo-Triggered Microcapsules. Macromol. Symp. 2016, 360, 192–198. [Google Scholar] [CrossRef]
- Del Pezzo, R.; Bandeira, N.A.G.; Trojanowska, A.; Fernandez, P.S.; Underiner, T.; Giamberini, M.; Tylkowski, B. Ortho-substituted azobenzene: Shedding light on new benefits. Pure Appl. Chem. 2018. [Google Scholar] [CrossRef]
- Duman, M.; Neundlinger, I.; Zhu, R.; Preiner, J.; Lamprecht, C.; Chtcheglova, L.A.; Rankl, C.; Puntheeranurak, T.; Ebner, A.; Hinterdorfer, P. 2.7 Atomic Force Microscopy. In Comprehensive Biophysics; Egelman, E.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 111–143. ISBN 978-0-08-095718-0. [Google Scholar]
- Trojanowska, A.; Bandeira, N.A.G.; Nogalska, A.; Marturano, V.; Giamberini, M.; Cerruti, P.; Ambrogi, V.; Tylkowski, B. Squeezing release mechanism of encapsulated compounds from photo-sensitive microcapsules. Appl. Surf. Sci. 2019, 472, 143–149. [Google Scholar] [CrossRef]
- El Halabieh, R.H.; Mermut, O.; Barrett, C.J. Using light to control physical properties of polymers and surfaces with azobenzene chromophores. Pure Appl. Chem. 2004, 76, 1445–1465. [Google Scholar] [CrossRef]
- Marturano, V.; Marcille, H.; Cerruti, P.; Bandeira, N.A.G.; Giamberini, M.; Trojanowska, A.; Tylkowski, B.; Carfagna, C.; Ausanio, G.; Ambrogi, V. Visible-Light Responsive Nanocapsules for Wavelength-Selective Release of Natural Active Agents. ACS Appl. Nano Mater. submitted.
- Irie, M.; Suzuki, T. Photoresponsive polymers. Reversible solution viscosity change of poly(dimethylsiloxane) with azobenzene residues in the main chain. Makromol. Chem. Rapid Commun. 1987, 8, 607–610. [Google Scholar] [CrossRef]
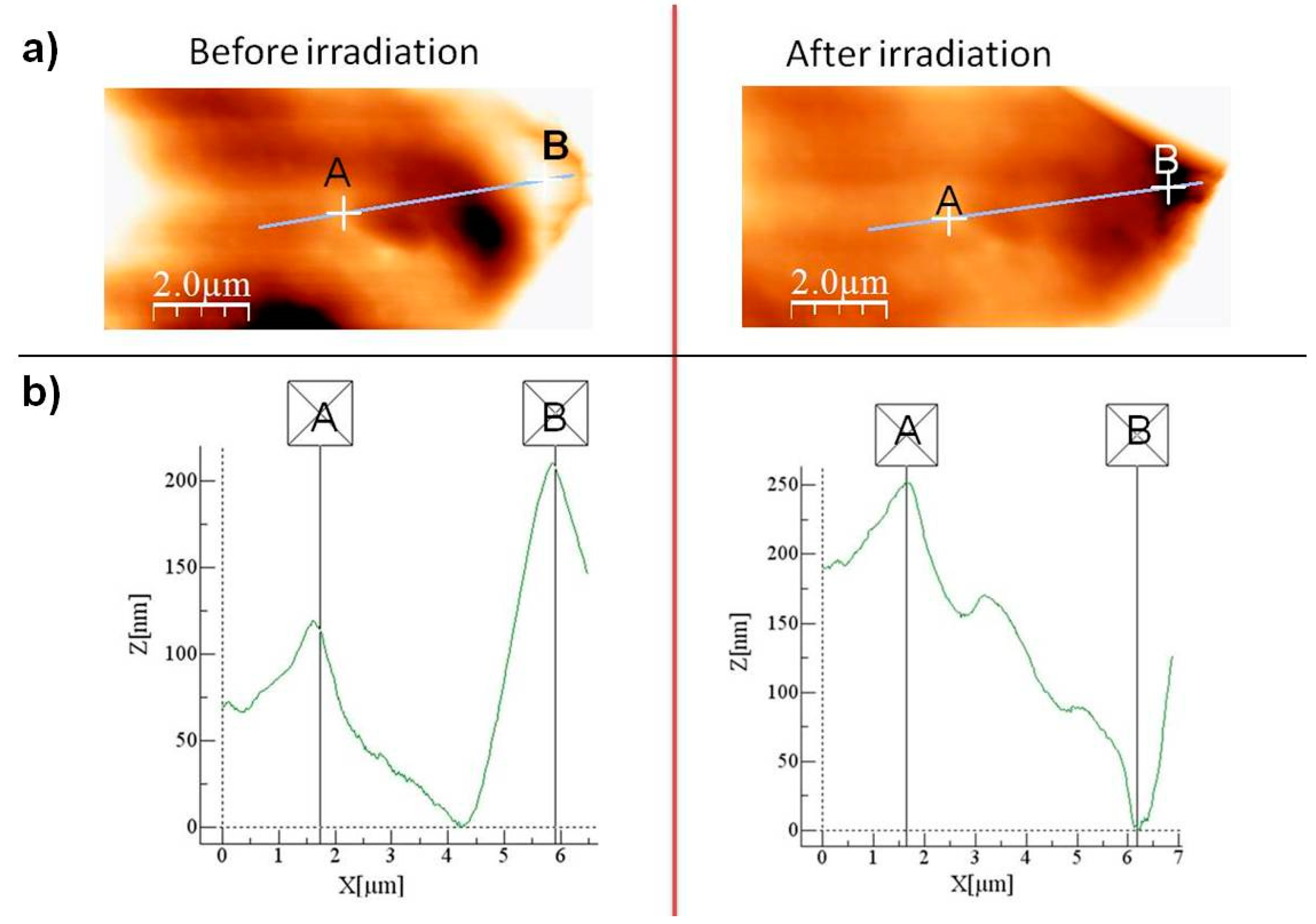
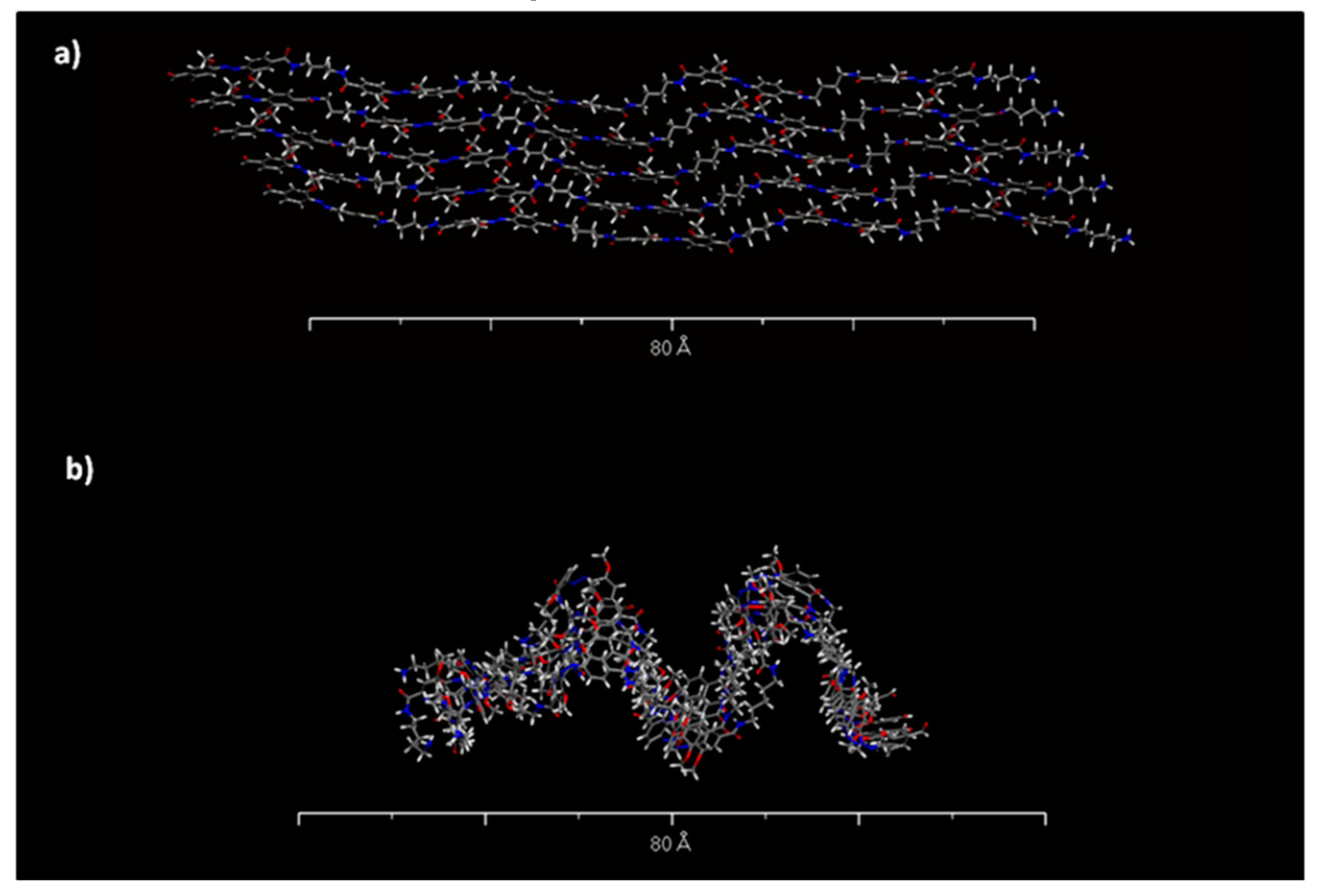
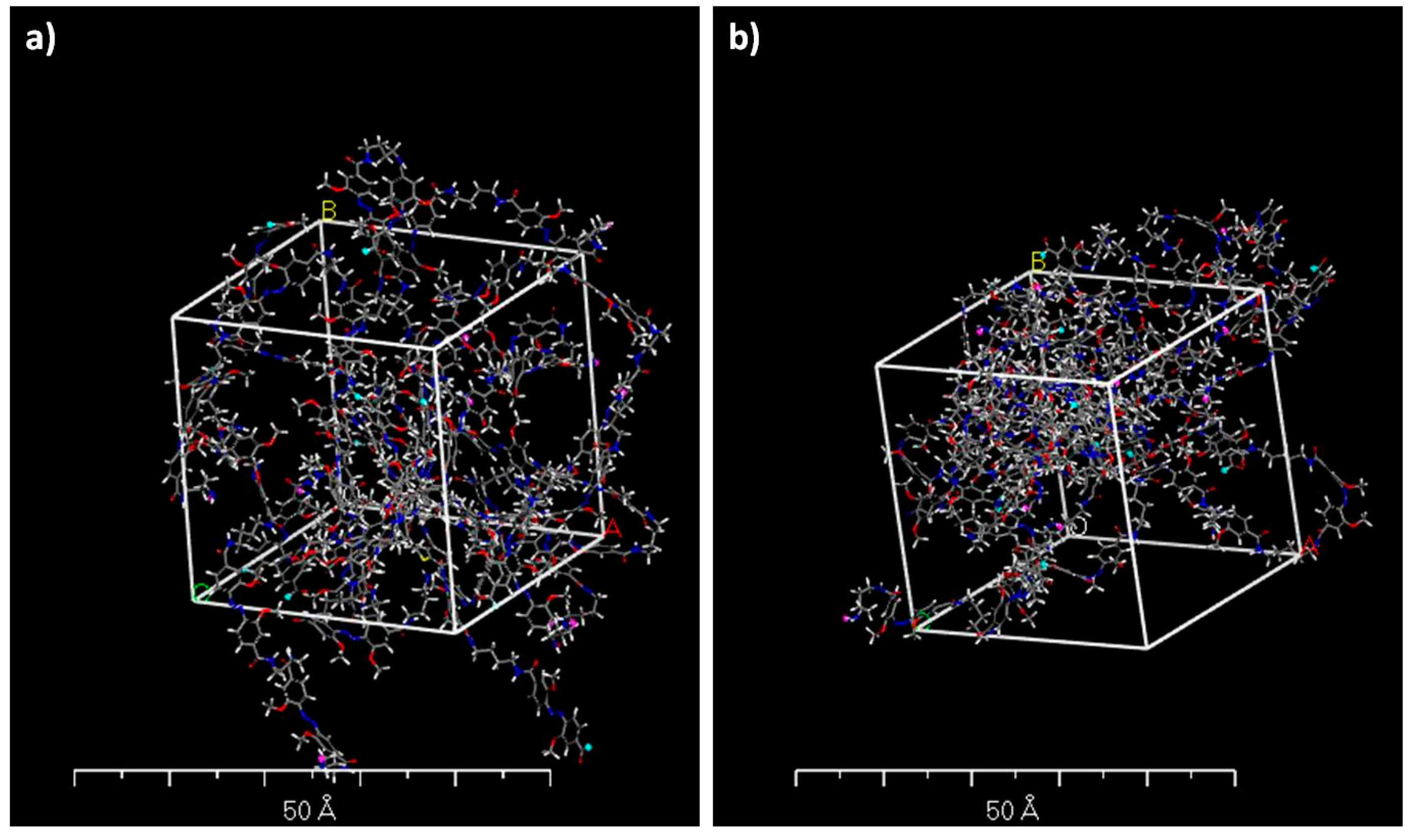
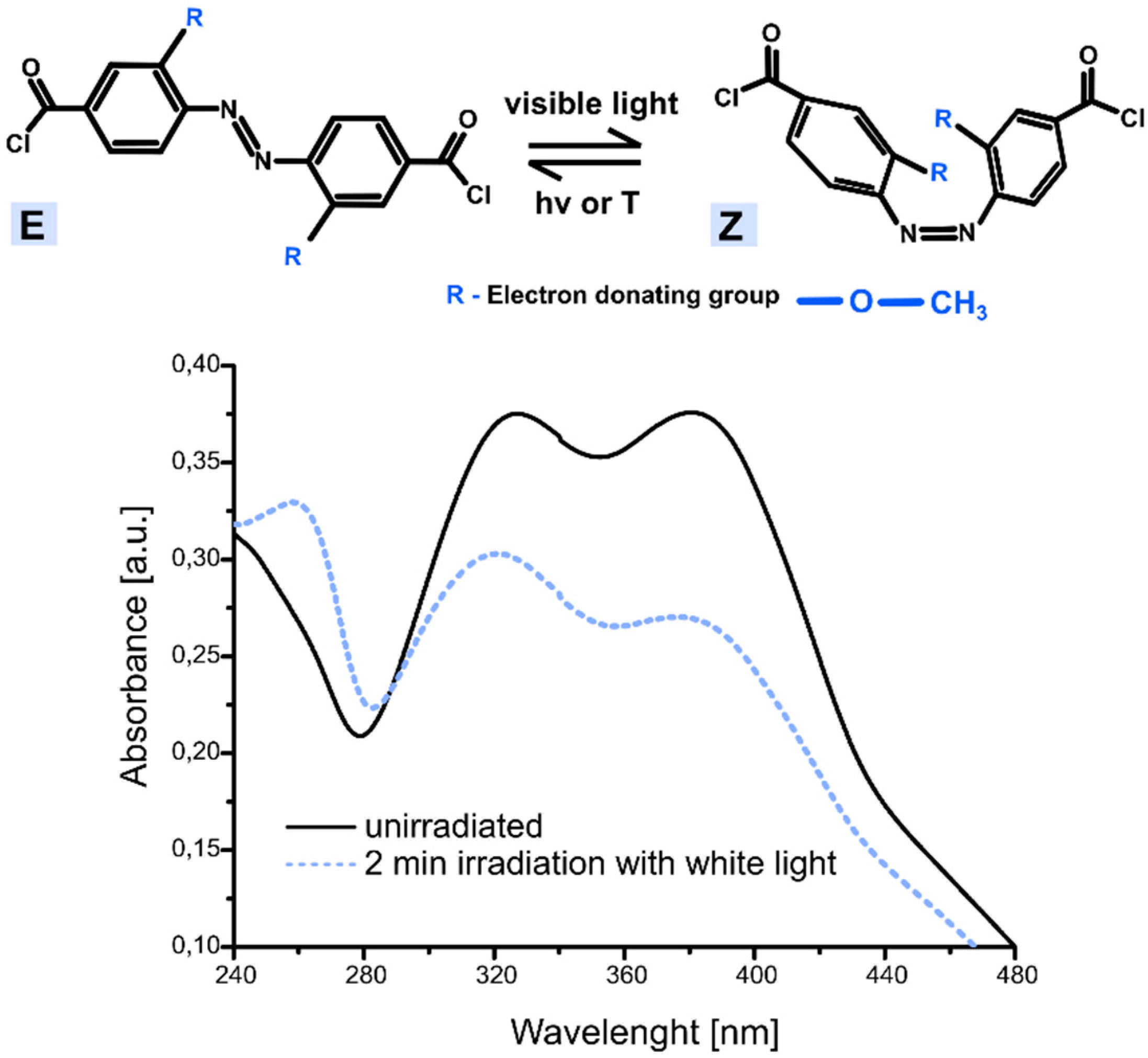
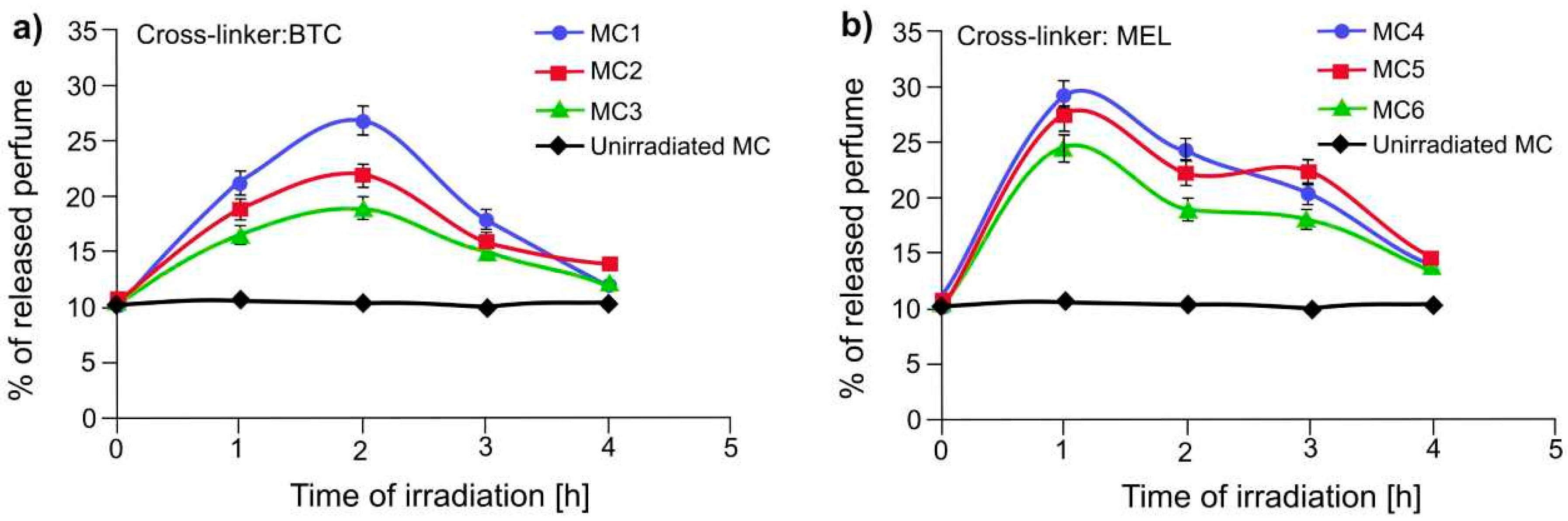
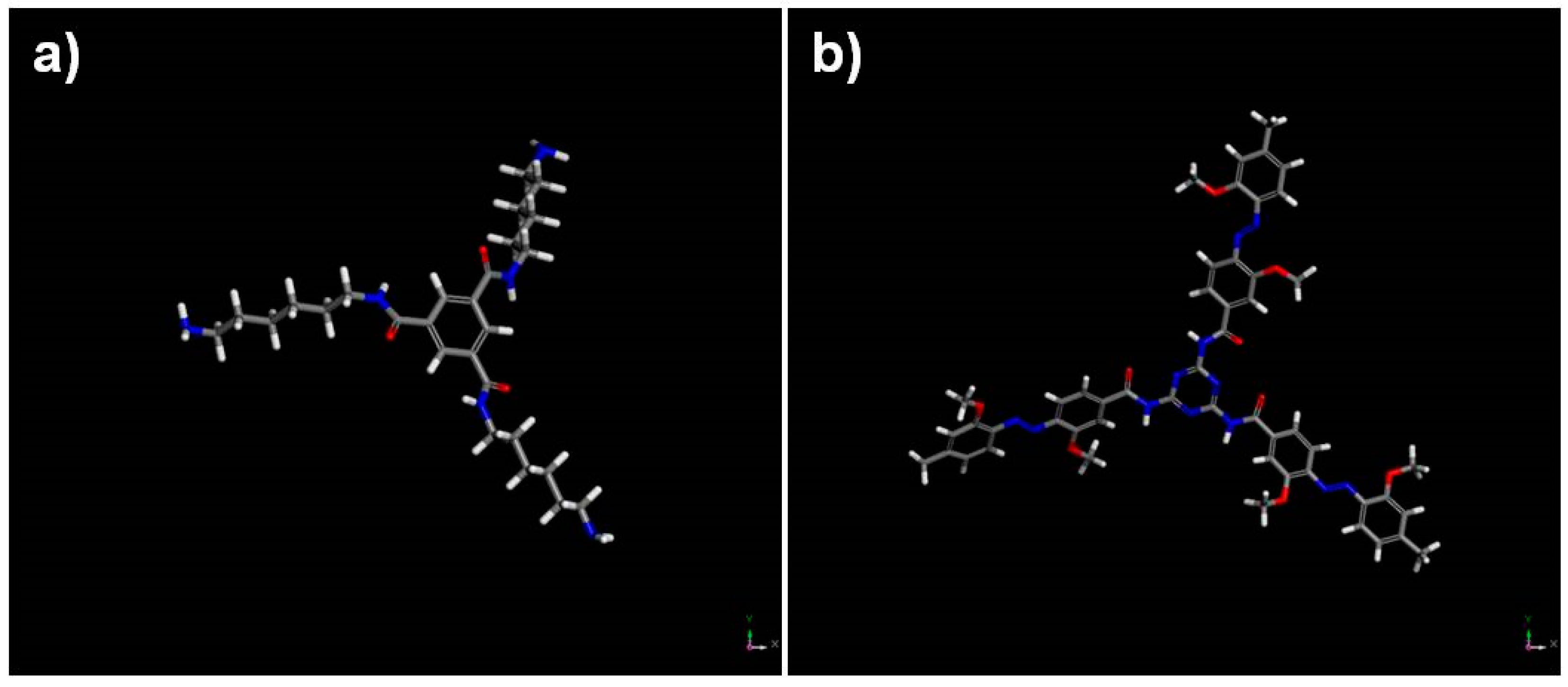
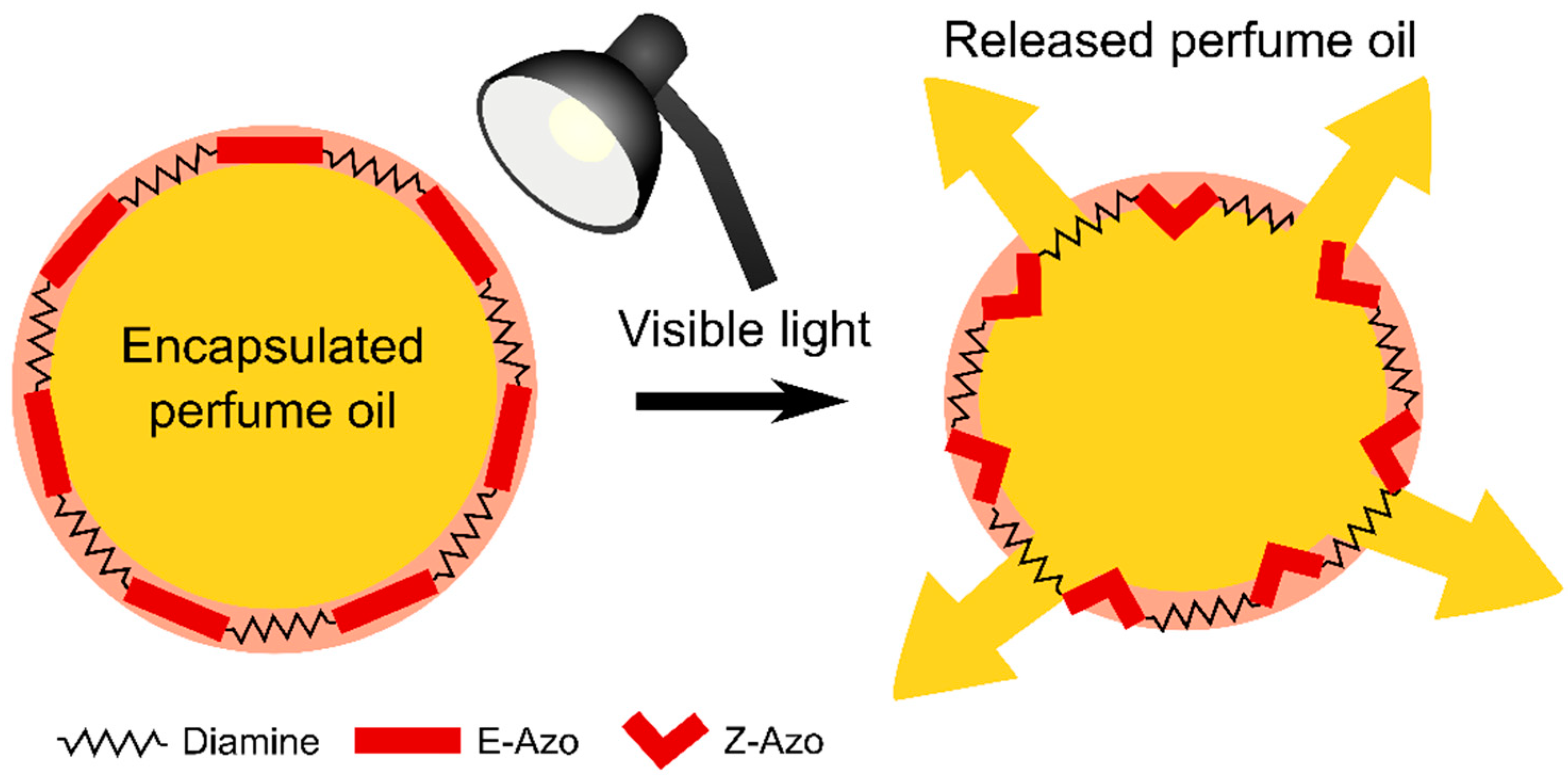
| Sample | Sol 1 | Sol 2 | Sol3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| - | Waterc (mL) | M18-88 (g) | P (mL) | Azo (g) | BTC (g) | Water (mL) | M18-88 (g) | DA Type | DA (g) | MEL (g) | NaHCO3 (g) |
| MC1 | 50 | 0.5 | 25 | 0.6 | 0.067 | 25 | 0.25 | DAO | 0.25 | - | 0.29 |
| MC2 | 50 | 0.5 | 25 | 0.6 | 0.067 | 25 | 0.25 | DAE | 0.20 | - | 0.29 |
| MC3 | 50 | 0.5 | 25 | 0.6 | 0.067 | 25 | 0.25 | DAB | 0.15 | - | 0.29 |
| MC4 | 50 | 0.5 | 25 | 0.6 | - | 25 | 0.25 | DAO | 0.15 | 0.032 | 0.29 |
| MC5 | 50 | 0.5 | 25 | 0.6 | - | 25 | 0.25 | DAE | 0.12 | 0.032 | 0.29 |
| MC6 | 50 | 0.5 | 25 | 0.6 | - | 25 | 0.25 | DAB | 0.09 | 0.032 | 0.29 |
| Sample | EE (%) | MC Diameter (µm) | MC Diameter (µm) (after 3 h Irradiation with Visible Light) |
|---|---|---|---|
| MC1 | 90.8 ± 0.9 | 63.7 ± 2.0 | 52.7 ± 2.0 |
| MC2 | 93.2 ± 0.9 | 62.5 ± 2.0 | 53.1 ± 2.0 |
| MC3 | 96.0 ± 0.7 | 68.7 ± 2.0 | 59.8 ± 2.0 |
| MC4 | 99.5 ± 0.2 | 63.3 ± 2.0 | 52.6 ± 2.0 |
| MC5 | 99.5 ± 0.2 | 63.4 ± 2.0 | 51.9 ± 2.0 |
| MC6 | 99.6 ± 0.2 | 62.6 ± 2.0 | 51.6 ± 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirone, D.; Marturano, V.; Del Pezzo, R.; Fernández Prieto, S.; Underiner, T.; Giamberini, M.; Tylkowski, B. Molecular Design of Microcapsule Shells for Visible Light-Triggered Release. Polymers 2019, 11, 904. https://doi.org/10.3390/polym11050904
Pirone D, Marturano V, Del Pezzo R, Fernández Prieto S, Underiner T, Giamberini M, Tylkowski B. Molecular Design of Microcapsule Shells for Visible Light-Triggered Release. Polymers. 2019; 11(5):904. https://doi.org/10.3390/polym11050904
Chicago/Turabian StylePirone, Domenico, Valentina Marturano, Rita Del Pezzo, Susana Fernández Prieto, Todd Underiner, Marta Giamberini, and Bartosz Tylkowski. 2019. "Molecular Design of Microcapsule Shells for Visible Light-Triggered Release" Polymers 11, no. 5: 904. https://doi.org/10.3390/polym11050904
APA StylePirone, D., Marturano, V., Del Pezzo, R., Fernández Prieto, S., Underiner, T., Giamberini, M., & Tylkowski, B. (2019). Molecular Design of Microcapsule Shells for Visible Light-Triggered Release. Polymers, 11(5), 904. https://doi.org/10.3390/polym11050904







