Functionalized Halloysite Nanotubes–Silica Hybrid for Enhanced Curing and Mechanical Properties of Elastomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HS and HS-s-M
2.3. Preparation of SBR/HS and SBR/HS-s-M composites
2.4. Characterization of HS-s-M and SBR Composites
3. Results and Discussion
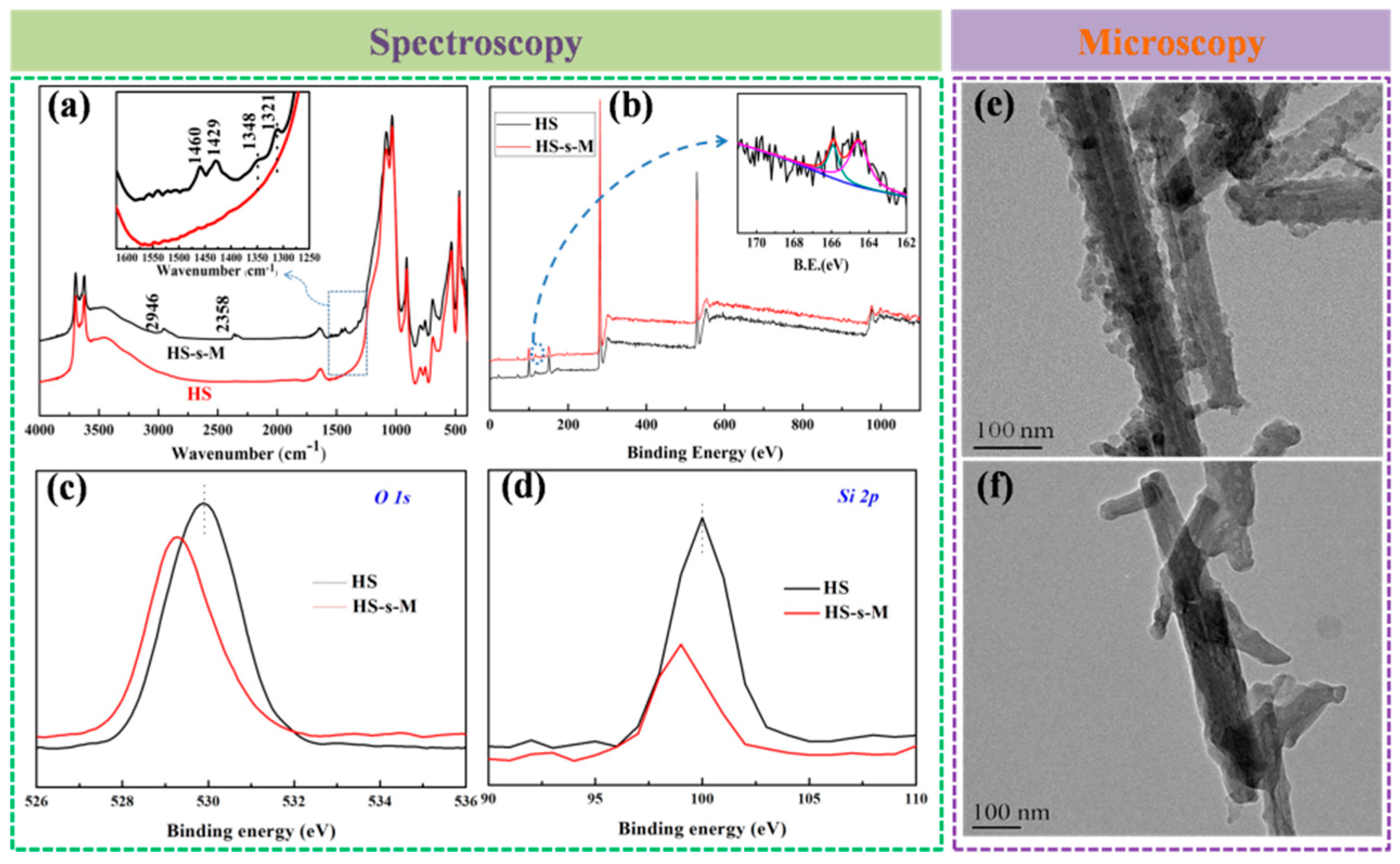
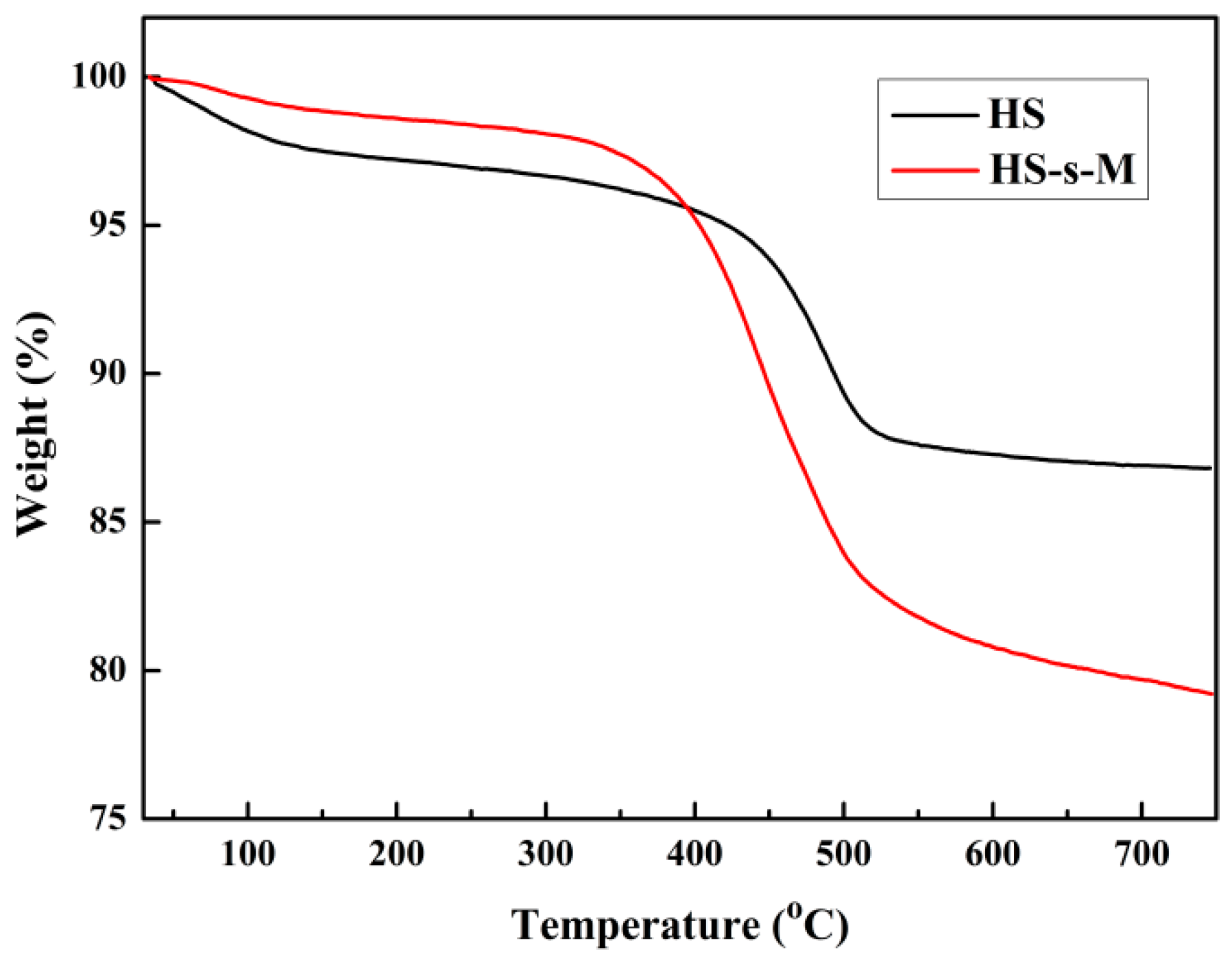
3.1. Characterization of HS-s-M
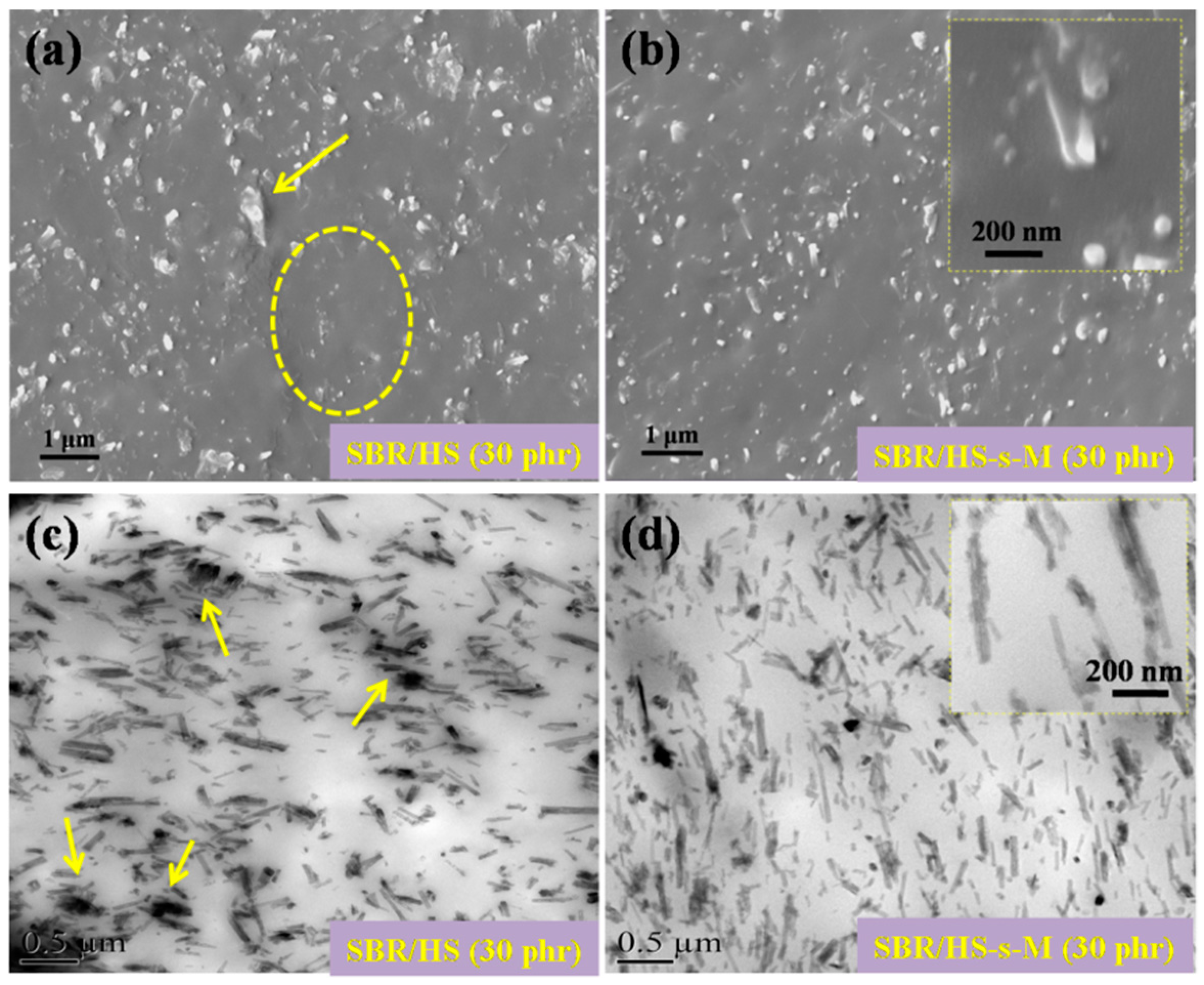
3.2. Morphologies of SBR Composites Filled with HS and HS-s-M
3.3. Curing Properties of SBR Compounds
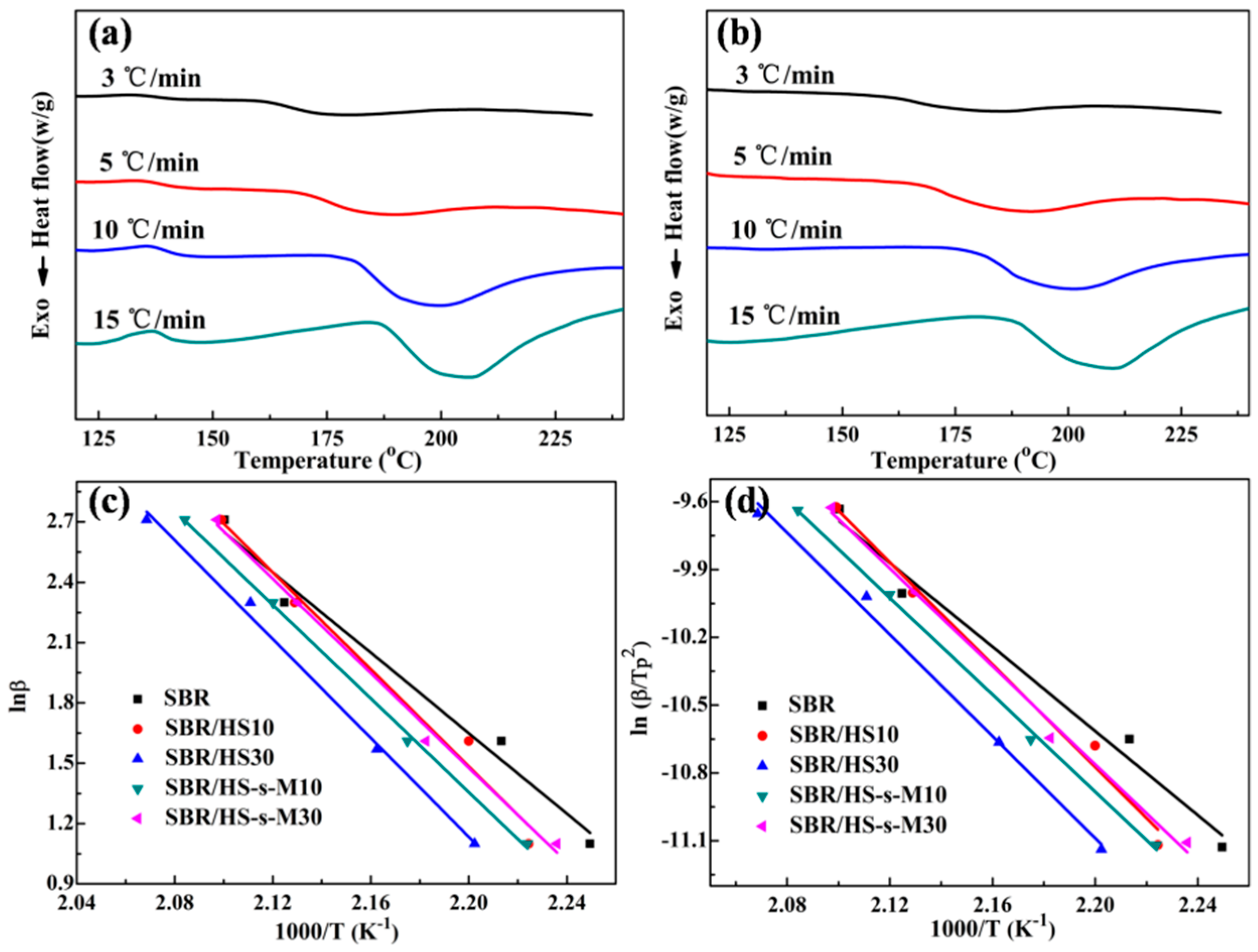
3.4. Curing Kinetics of SBR Compounds
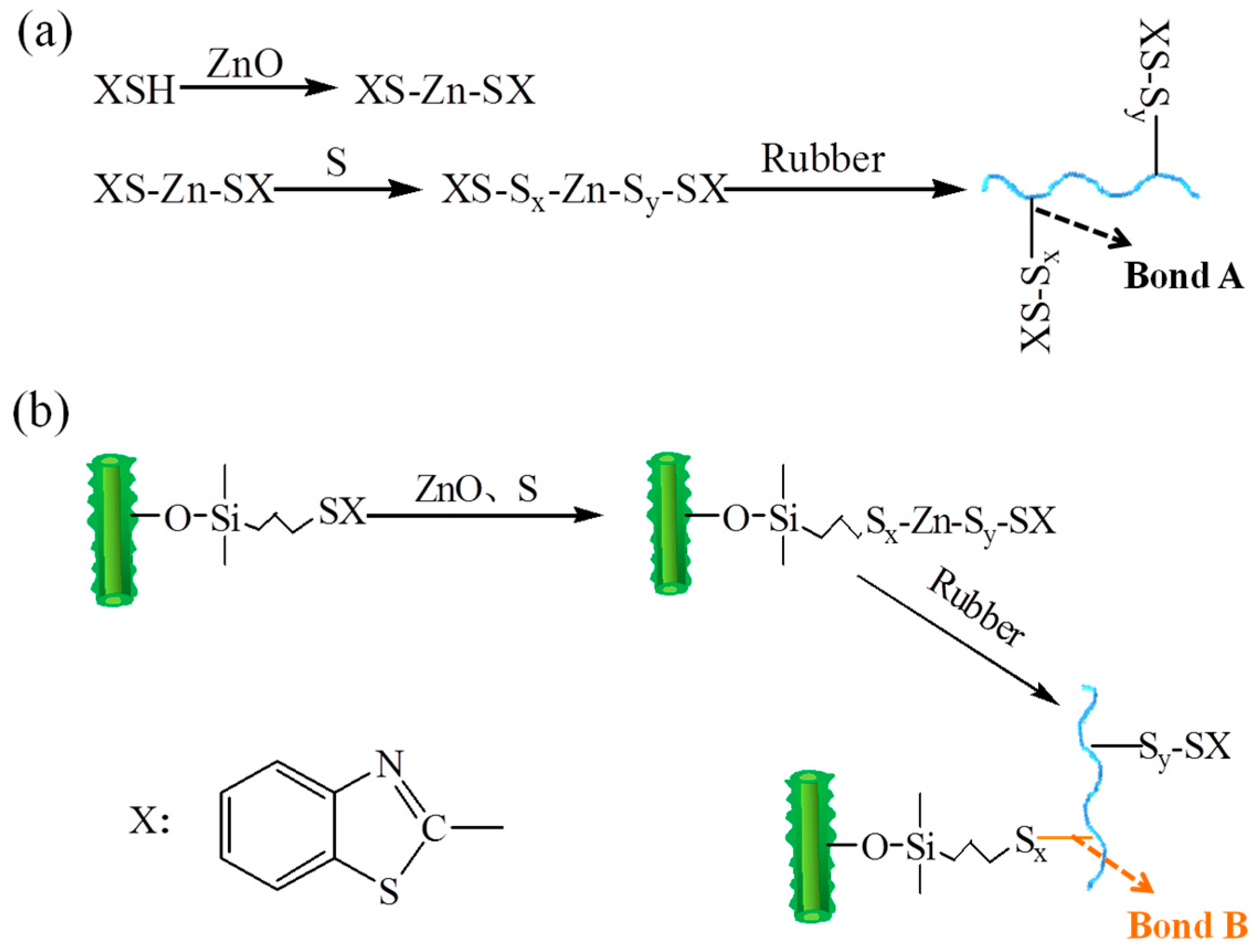
3.5. Proposed Vulcanization Mechanisms of SBR Compounds
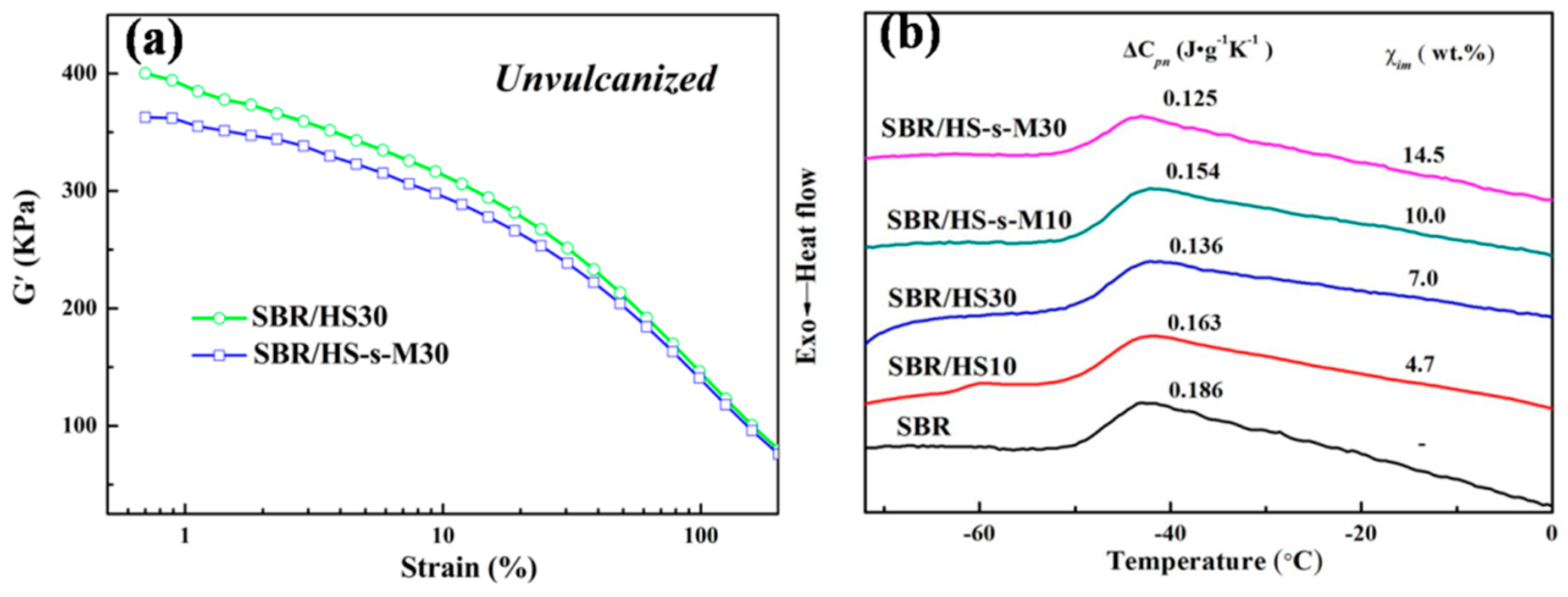
3.6. Interfacial Interaction between SBR Matrix and Filler
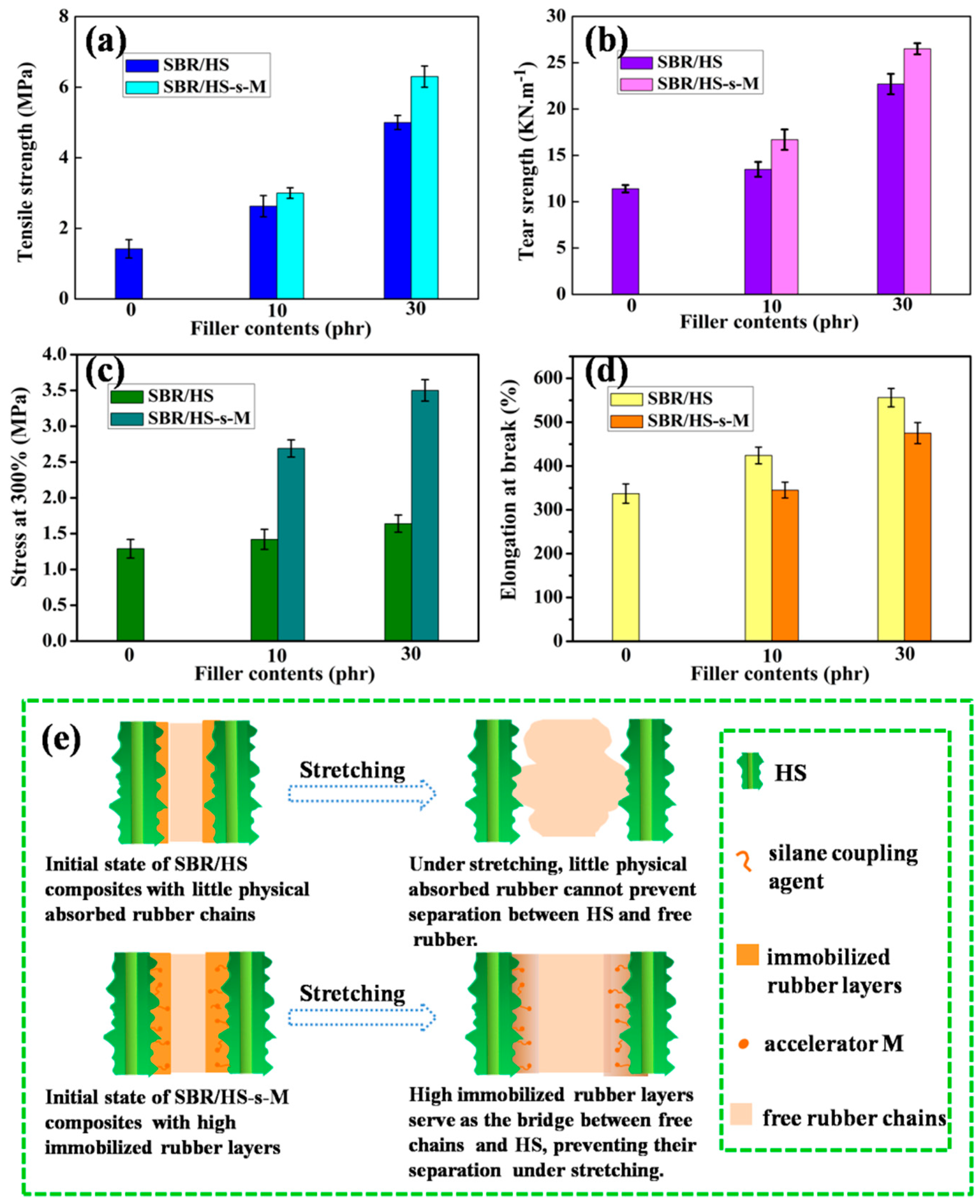
3.7. Mechanical Properties and Reinforcing Mechanism of SBR Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davris, T.; Mermet-Guyennet, M.R.B.; Bonn, D.; Lyulin, A.V. Filler Size Effects on Reinforcement in Elastomer-Based Nanocomposites: Experimental and Simulational Insights into Physical Mechanisms. Macromolecules 2016, 49, 7077–7087. [Google Scholar] [CrossRef]
- Liu, M.; Qi, P.; Luo, B.; Zhou, C. The improvement of mechanical performance and water-response of carboxylated SBR by chitin nanocrystals. Eur. Polym. J. 2015, 68, 190–206. [Google Scholar] [CrossRef]
- Kong, J.; Sun, J.; Tong, Y.; Dou, Q.; Wei, Y.; Thitsartarn, W.; Chee Chuan Yeo, J.; He, C. Carbon nanotubes-bridged-fumed silica as an effective binary nanofillers for reinforcement of silicone elastomers. Compos. Sci. Technol. 2019, 169, 232–241. [Google Scholar] [CrossRef]
- Varghese, T.V.; Ajith Kumar, H.; Anitha, S.; Ratheesh, S.; Rajeev, R.S.; Lakshmana Rao, V. Reinforcement of acrylonitrile butadiene rubber using pristine few layer graphene and its hybrid fillers. Carbon 2013, 61, 476–486. [Google Scholar] [CrossRef]
- Kong, L.; Li, F.; Wang, F.; Miao, Y.; Huang, X.; Zhu, H. In situ assembly of SiO2 nanodots/layered double hydroxide nanocomposite for the reinforcement of solution-polymerized butadiene styrene rubber/butadiene rubber. Compos. Sci. Technol. 2018, 158, 9–18. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M. Conductive carboxylated styrene butadiene rubber composites by incorporation of polypyrrole-wrapped halloysite nanotubes. Compos. Sci. Technol. 2017, 143, 56–66. [Google Scholar]
- Yong, L.; Liu, S.; Jian, P.; Lan, L. The filler–rubber interface and reinforcement in styrene butadiene rubber composites with graphene/silica hybrids: A quantitative correlation with the constrained region. Compos. Part A Appl. S 2016, 86, 19–30. [Google Scholar]
- Wu, S.; Zhang, L.; Weng, P.; Yang, Z.; Tang, Z.; Guo, B. Correlating synergistic reinforcement with chain motion in elastomer/nanocarbon hybrids composites. Soft Matter 2016, 12, 6893–6901. [Google Scholar] [CrossRef]
- Kong, L.; Feng, L.; Wang, F.; Ye, M.; Huang, X.; Hong, Z.; Lu, Y. High-performing multi-walled carbon nanotube/silica nanocomposites for elastomer application. Compos. Sci. Technol. 2018, 162, 23–32. [Google Scholar] [CrossRef]
- Ling, J.; Chao, Z.; Liu, M.; Zhe, Y.; Weng, W.T.; Liu, T. Simultaneous reinforcement and toughening of polyurethane composites with carbon nanotube/halloysite nanotube hybrids. Compos. Sci. Technol. 2014, 91, 98–103. [Google Scholar]
- Hu, D.; Zhong, B.; Jia, Z.; Lin, J.; Liu, M.; Luo, Y.; Jia, D. A novel hybrid filler of halloysite nanotubes/silica fabricated by electrostatic self-assembly. Mater. Lett. 2017, 188, 327–330. [Google Scholar] [CrossRef]
- Lin, J.; Zhong, B.; Jia, Z.; Hu, D.; Ding, Y.; Luo, Y.; Jia, D. In-situ fabrication of halloysite nanotubes/silica nano hybrid and its application in unsaturated polyester resin. Appl. Surf. Sci. 2017, 407, 130–136. [Google Scholar] [CrossRef]
- Jing, L.; Zhong, B.; Luo, Y.; Jia, Z.; Hu, D.; Xu, T.; Jia, D. Enhancing interfacial and mechanical strength of styrene-butadiene rubber composites via in situ fabricated halloysite nanotubes/silica nano hybrid. Polym. Compos. 2019, 40, 677–684. [Google Scholar]
- Rabiei, S.; Shojaei, A. Vulcanization kinetics and reversion behavior of natural rubber/styrene-butadiene rubber blend filled with nanodiamond—The role of sulfur curing system. Eur. Polym. J. 2016, 81, 98–113. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Luo, Y.; Jia, Z.; Jie, B.; Chen, Y.; Jia, D. Effect of novel supported vulcanizing agent on the interfacial interaction and strain-induced crystallization properties of natural rubber nanocomposites. Polymer 2018, 148, 390–399. [Google Scholar] [CrossRef]
- Stöckelhuber, K.W.; Svistkov, A.S.; Pelevin, A.G.; Heinrich, G. Impact of Filler Surface Modification on Large Scale Mechanics of Styrene Butadiene/Silica Rubber Composites. Macromolecules 2011, 44, 4366–4381. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Stöckelhuber, K.W.; Wang, D.Y.; Galiatsatos, V.; Heinrich, G. Understanding the reinforcing behavior of expanded clay particles in natural rubber compounds. Soft Matter 2013, 9, 3798–3808. [Google Scholar] [CrossRef]
- Zhang, X.; Loo, L.S. Study of Glass Transition and Reinforcement Mechanism in Polymer/Layered Silicate Nanocomposites. Macromolecules 2009, 42, 5196–5207. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Razzaghi-Kashani, M. Vulcanization kinetics of nano-silica filled styrene butadiene rubber. Polymer 2014, 55, 6426–6434. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Hu, D.; Luo, Y.; Jia, D.; Fang, L. Enhancing interfacial interaction and mechanical properties of styrene-butadiene rubber composites via silica-supported vulcanization accelerator. Compos. Part A Appl. S 2017, 96, 129–136. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Helmuth, M.H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef]
- Jie, L.; Li, X.; Xu, L.; Zhang, P. Investigation of aging behavior and mechanism of nitrile-butadiene rubber (NBR) in the accelerated thermal aging environment. Polym. Test. 2016, 54, 59–66. [Google Scholar]
- Wu, J.; Wang, X.; Huang, G.; Hui, L.; Tang, M.; Wu, S.; Liu, Y. Vulcanization kinetics of graphene/natural rubber nanocomposites. Polymer 2013, 54, 3314–3323. [Google Scholar] [CrossRef]
- Sargsyan, A.; Tonoyan, A.; Davtyan, S.; Schick, C. The amount of immobilized polymer in PMMA SiO2 nanocomposites determined from calorimetric data. Eur. Polym. J. 2007, 43, 3113–3127. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P. Glass transition and segmental dynamics in poly(dimethylsiloxane)/silica nanocomposites studied by various techniques. J. Non-Cryst. Solids. 2007, 353, 4344–4352. [Google Scholar] [CrossRef]
- Yang, J.; Tian, M.; Jia, Q.; Shi, J.; Zhang, L.; Lim, S. Improved mechanical and functional properties of elastomer/graphite nanocomposites prepared by latex compounding. Acta Mater. 2007, 55, 6372–6382. [Google Scholar] [CrossRef]
- Shih, R.S.; Kuo, S.W.; Chang, F.C. Thermal and mechanical properties of microcellular thermoplastic SBS/PS/SBR blend: Effect of crosslinking. Polymer 2011, 52, 752–759. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Susanna, A.; D’Arienzo, M.; Credico, B.D.; Giannini, L.; Hanel, T.; Grandori, R.; Morazzoni, F.; Mostoni, S.; Santambrogio, C.; Scotti, R. Catalytic effect of ZnO anchored silica nanoparticles on rubber vulcanization and cross-link formation. Eur. Polym. J. 2017, 93, 63–74. [Google Scholar] [CrossRef]
- Liavitskaya, T.; Vyazovkin, S. Kinetics of Thermal Polymerization Can Be Studied during Continuous Cooling. Macromol. Rapid Commun. 2017, 39, 1700624. [Google Scholar] [CrossRef]
- Susanna, A.; Armelao, L.; Callone, E.; Dirè, S.; D’Arienzo, M.; Credico, B.D.; Giannini, L.; Hanel, T.; Morazzoni, F.; Scotti, R. ZnO nanoparticles anchored to silica filler. A curing accelerator for isoprene rubber composites. Chem. Eng. J. 2015, 275, 245–252. [Google Scholar] [CrossRef]
- Pielichowski, K.; Czub, P.; Pielichowski, J. The kinetics of cure of epoxides and related sulphur compounds studied by dynamic DSC. Polymer 2000, 41, 4381–4388. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, Y.; He, J.; Tang, Z.; Zhu, L.; Luo, Y.; Liu, F. Enhancing mechanical properties of styrene–butadiene rubber/silica nanocomposites by in situ interfacial modification with a novel rare-earth complex. Compos. Part A Appl. S 2016, 87, 297–309. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Sizhu, W.U.; Wang, W.; Zhang, L. Novel percolation phenomena and mechanism of strengthening elastomers by nanofillers. Phys. Chem. Chem. Phys. 2010, 12, 3014–3030. [Google Scholar] [CrossRef]


| Samples | SBR | HS | HS-s-M | CZ | M | 4010NA | SA | ZnO | S |
|---|---|---|---|---|---|---|---|---|---|
| SBR | 100 | - | - | 1 | 1 | 2 | 2 | 5 | 1.6 |
| SBR/HS10 | 100 | 10 | - | 1 | 1 | 2 | 2 | 5 | 1.6 |
| SBR/HS30 | 100 | 30 | - | 1 | 1 | 2 | 2 | 5 | 1.6 |
| SBR/HS-s-M10 | 100 | - | 10 | 1 | 0.8 | 2 | 2 | 5 | 1.6 |
| SBR/HS-s-M30 | 100 | - | 30 | 1 | 0.4 | 2 | 2 | 5 | 1.6 |
| Samples | β (°C/min) | Tonset (°C) | Tpeak (°C) | ΔHr (J/g) | Ea (kJ/mol) | |
|---|---|---|---|---|---|---|
| Ozawa | Kissinger | |||||
| SBR | 3 | 157.74 | 171.55 | 13.31 | 83.2 | 77.4 |
| 5 | 164.76 | 178.81 | 17.08 | |||
| 10 | 176.01 | 197.65 | 16.17 | |||
| 15 | 184.8 | 203.12 | 13.57 | |||
| SBR/HS10 | 3 | 158.2 | 176.56 | 12.83 | 100.3 | 92.7 |
| 5 | 166.01 | 181.55 | 13.83 | |||
| 10 | 180.22 | 196.72 | 16.65 | |||
| 15 | 186.97 | 203.4 | 16.03 | |||
| SBR/HS30 | 3 | 160.9 | 181.05 | 10.35 | 101.4 | 93.6 |
| 5 | 168.4 | 189.43 | 11.71 | |||
| 10 | 180.39 | 200.74 | 11.81 | |||
| 15 | 187.44 | 210.43 | 10.31 | |||
| SBR/HS-s-M10 | 3 | 161.65 | 174.25 | 15.96 | 96.8 | 89.2 |
| 5 | 170.3 | 185.19 | 13.56 | |||
| 10 | 181.53 | 196.39 | 16.6 | |||
| 15 | 187.84 | 203.82 | 16.1 | |||
| SBR/HS-s-M30 | 3 | 161.94 | 176.66 | 10.86 | 97.7 | 90.1 |
| 5 | 170.44 | 186.79 | 12.76 | |||
| 10 | 180.7 | 198.7 | 13.91 | |||
| 15 | 187.32 | 206.8 | 10.97 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Hu, D.; Luo, Y.; Zhong, B.; Chen, Y.; Jia, Z.; Jia, D. Functionalized Halloysite Nanotubes–Silica Hybrid for Enhanced Curing and Mechanical Properties of Elastomers. Polymers 2019, 11, 883. https://doi.org/10.3390/polym11050883
Lin J, Hu D, Luo Y, Zhong B, Chen Y, Jia Z, Jia D. Functionalized Halloysite Nanotubes–Silica Hybrid for Enhanced Curing and Mechanical Properties of Elastomers. Polymers. 2019; 11(5):883. https://doi.org/10.3390/polym11050883
Chicago/Turabian StyleLin, Jing, Dechao Hu, Yuanfang Luo, Bangchao Zhong, Yongjun Chen, Zhixin Jia, and Demin Jia. 2019. "Functionalized Halloysite Nanotubes–Silica Hybrid for Enhanced Curing and Mechanical Properties of Elastomers" Polymers 11, no. 5: 883. https://doi.org/10.3390/polym11050883
APA StyleLin, J., Hu, D., Luo, Y., Zhong, B., Chen, Y., Jia, Z., & Jia, D. (2019). Functionalized Halloysite Nanotubes–Silica Hybrid for Enhanced Curing and Mechanical Properties of Elastomers. Polymers, 11(5), 883. https://doi.org/10.3390/polym11050883







