Thermotropic Liquid-Crystalline and Light-Emitting Properties of Poly(pyridinium) Salts Containing Various Diamine Connectors and Hydrophilic Macrocounterions †
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure for the Synthesis of Polymers I-1–I-5
2.2. General Procedure for the Synthesis of Polymers II-1–II-5
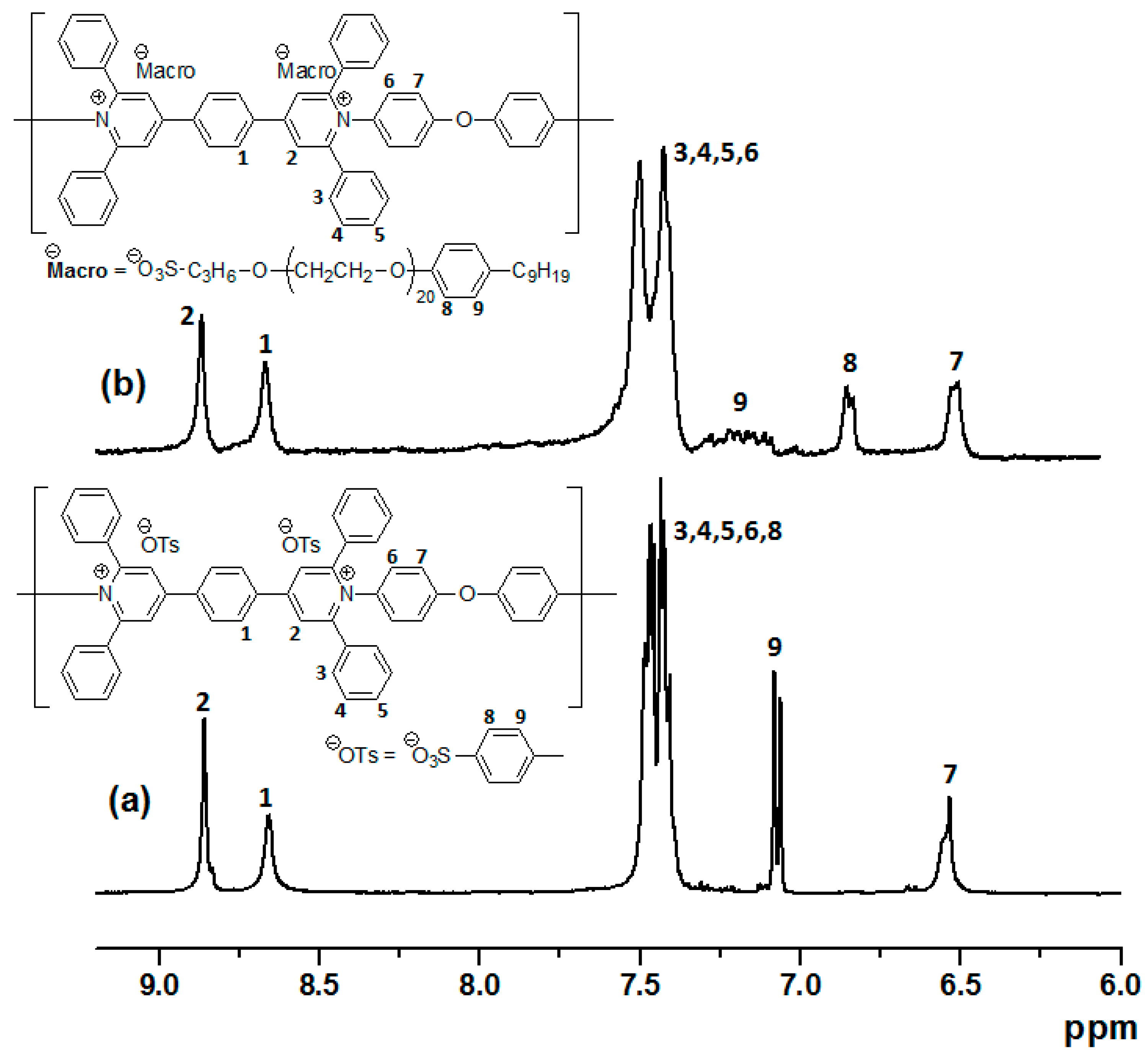
2.3. Analytic, Spectroscopic, Thermal and Structural Polymers Characterization
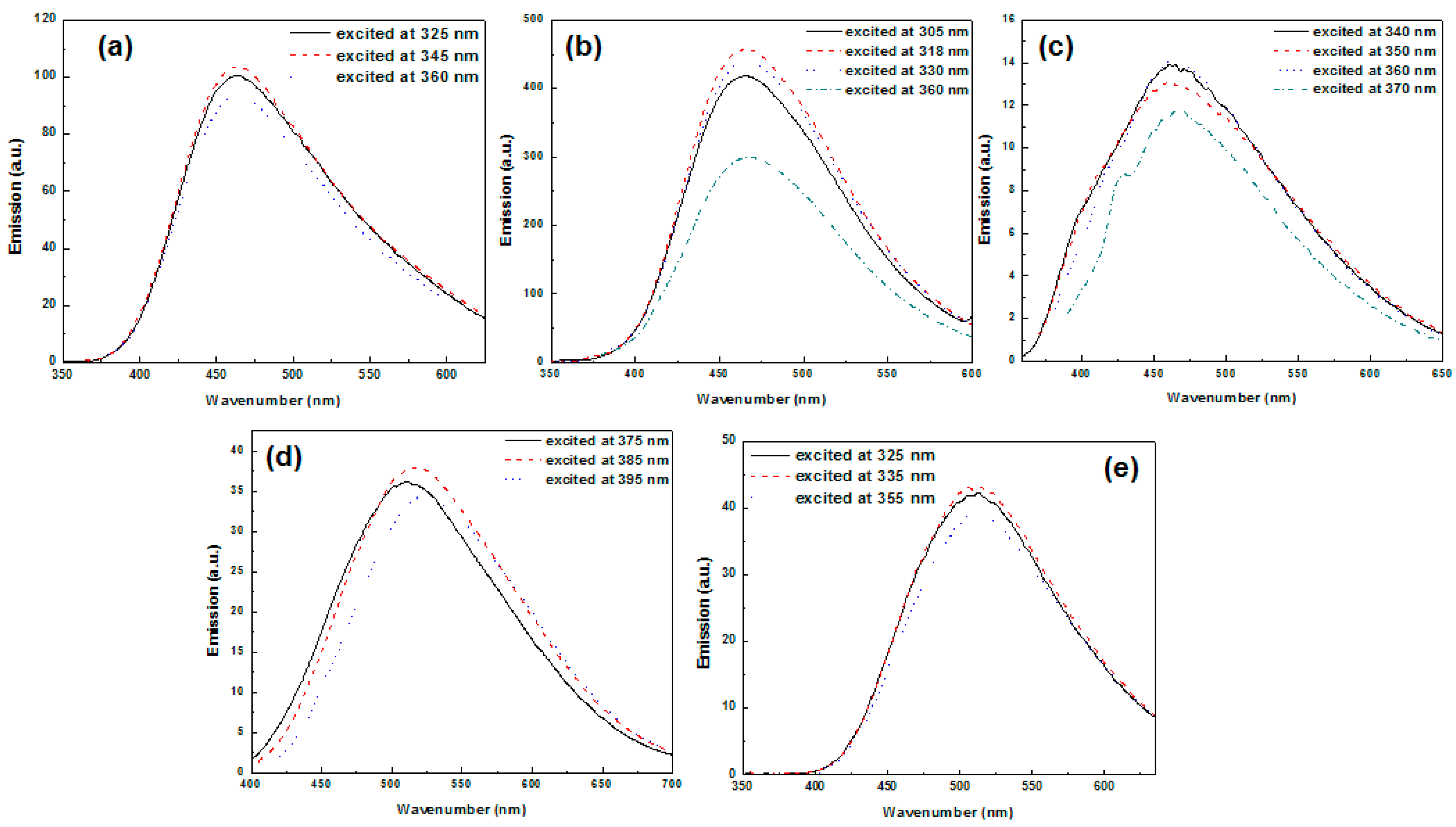
3. Results and Discussion
3.1. Chemical Structures
3.2. Solubility
3.3. Molecular Weight Measurements
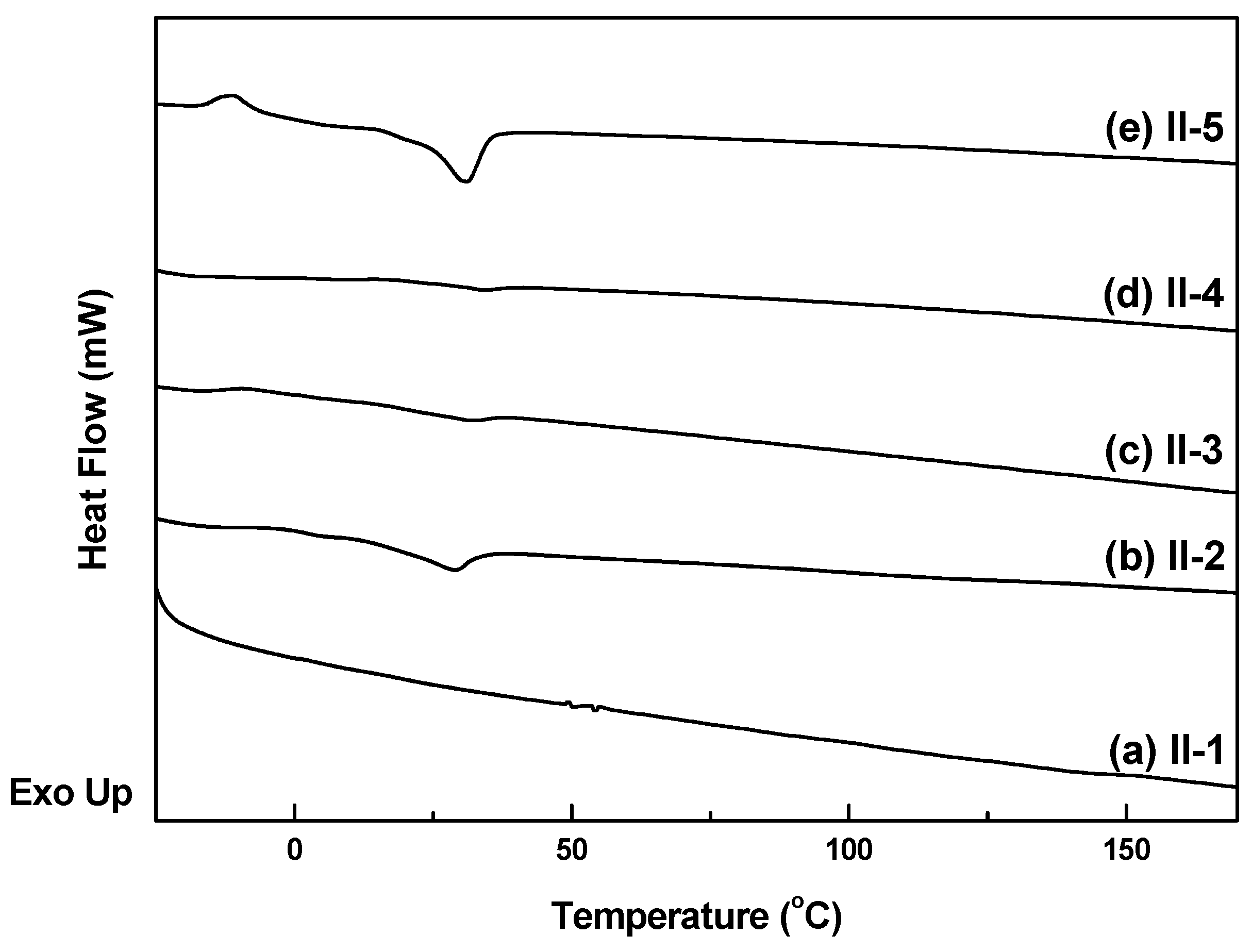
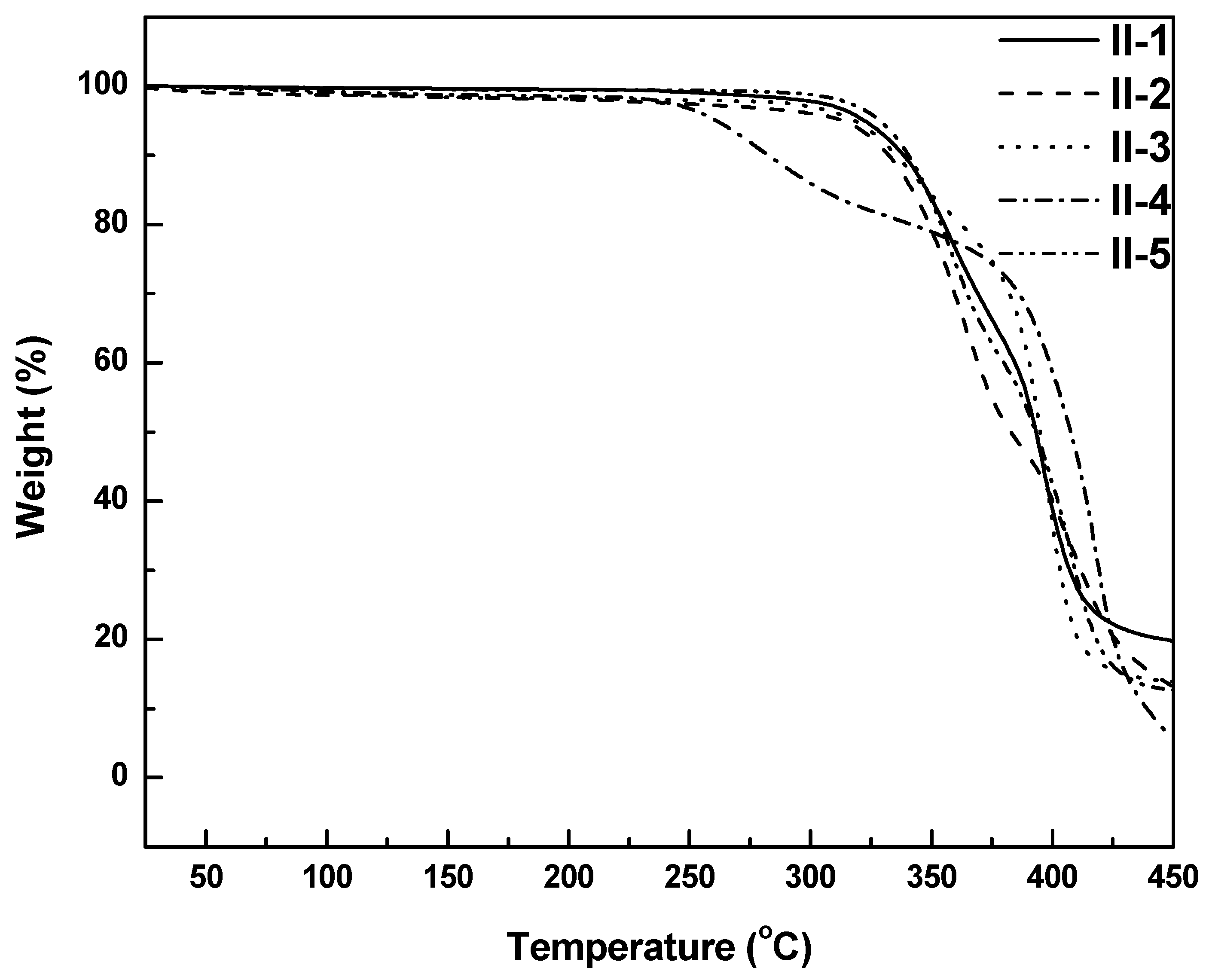
3.4. Thermal Properties
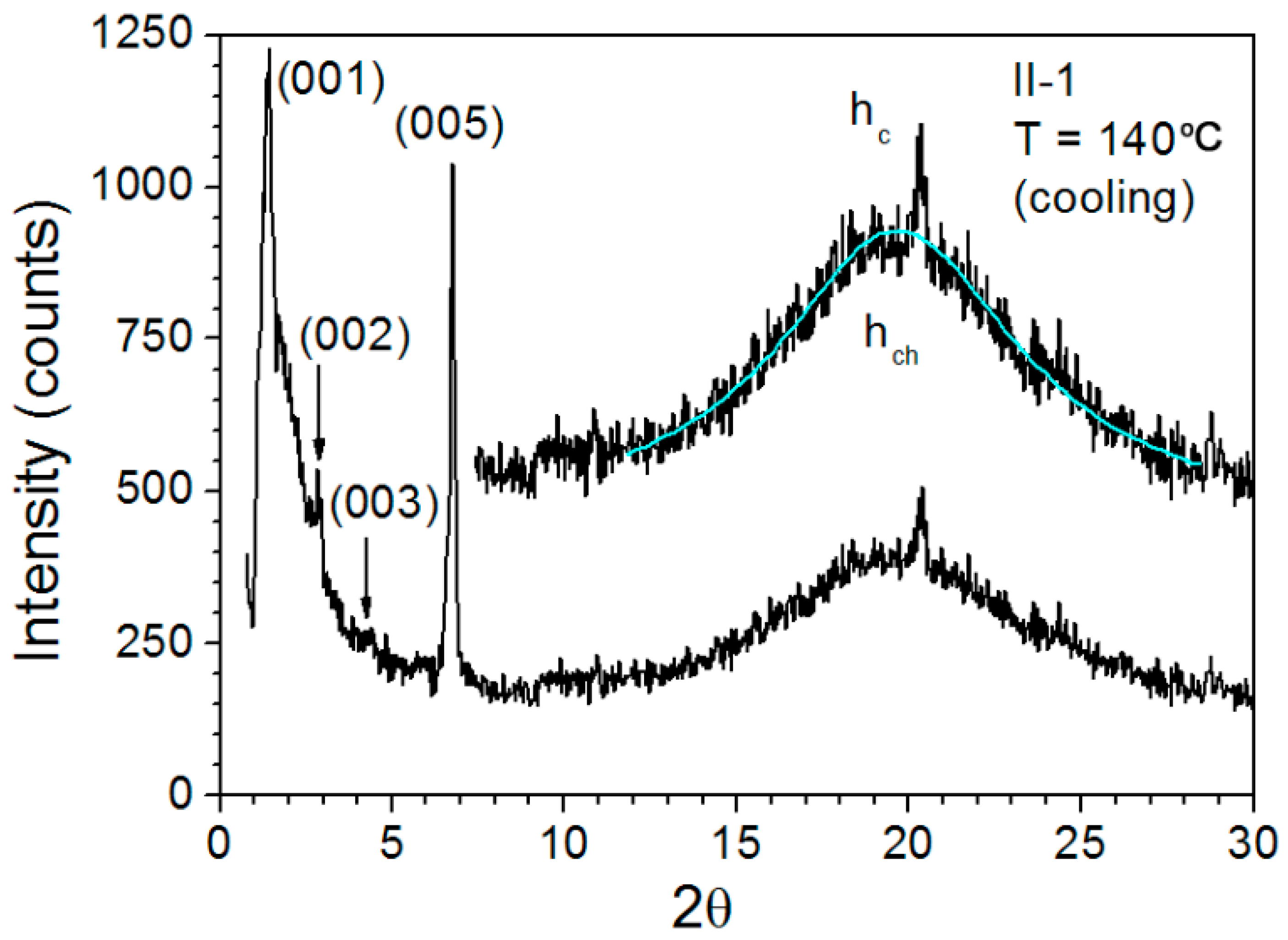
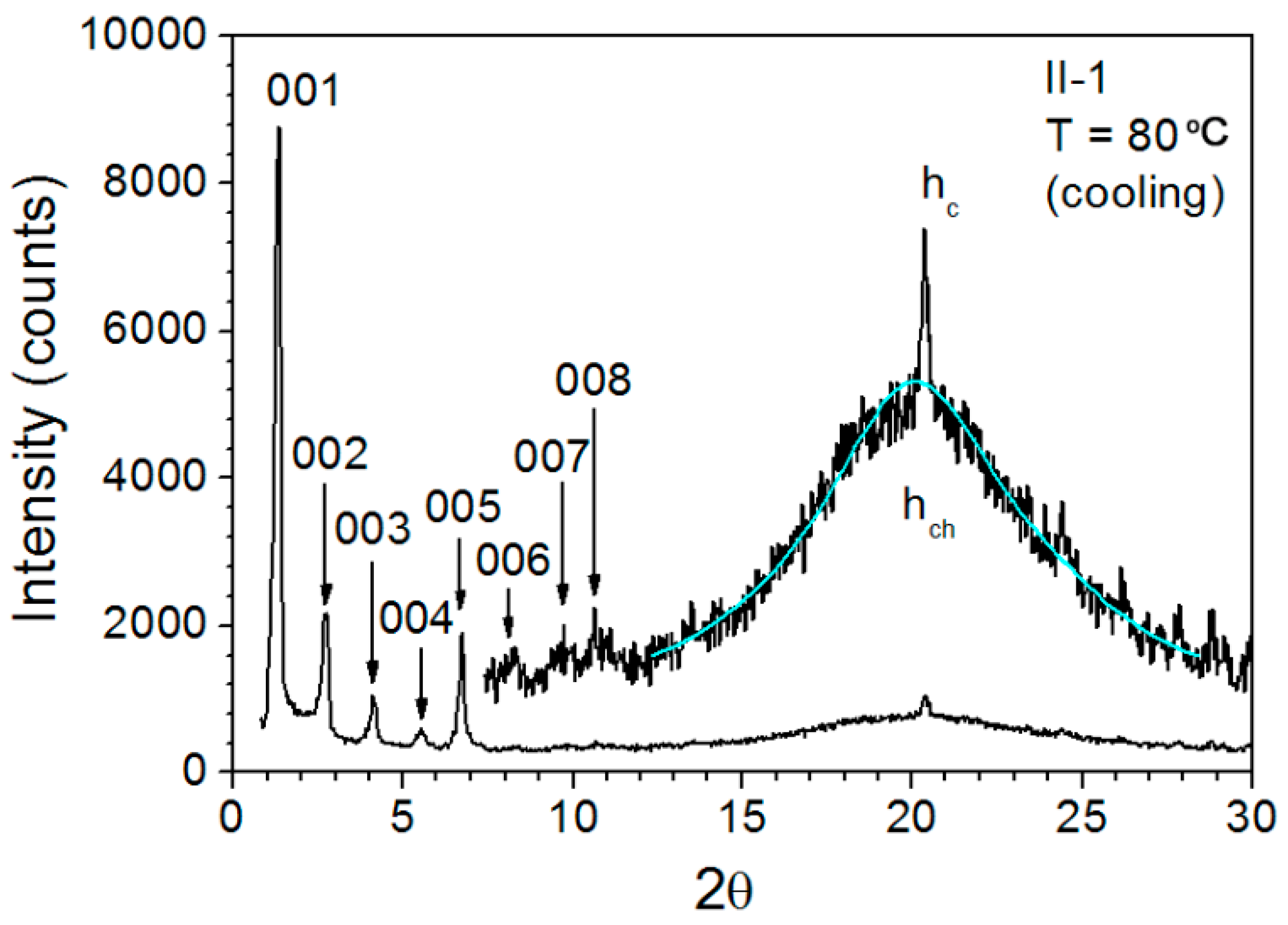
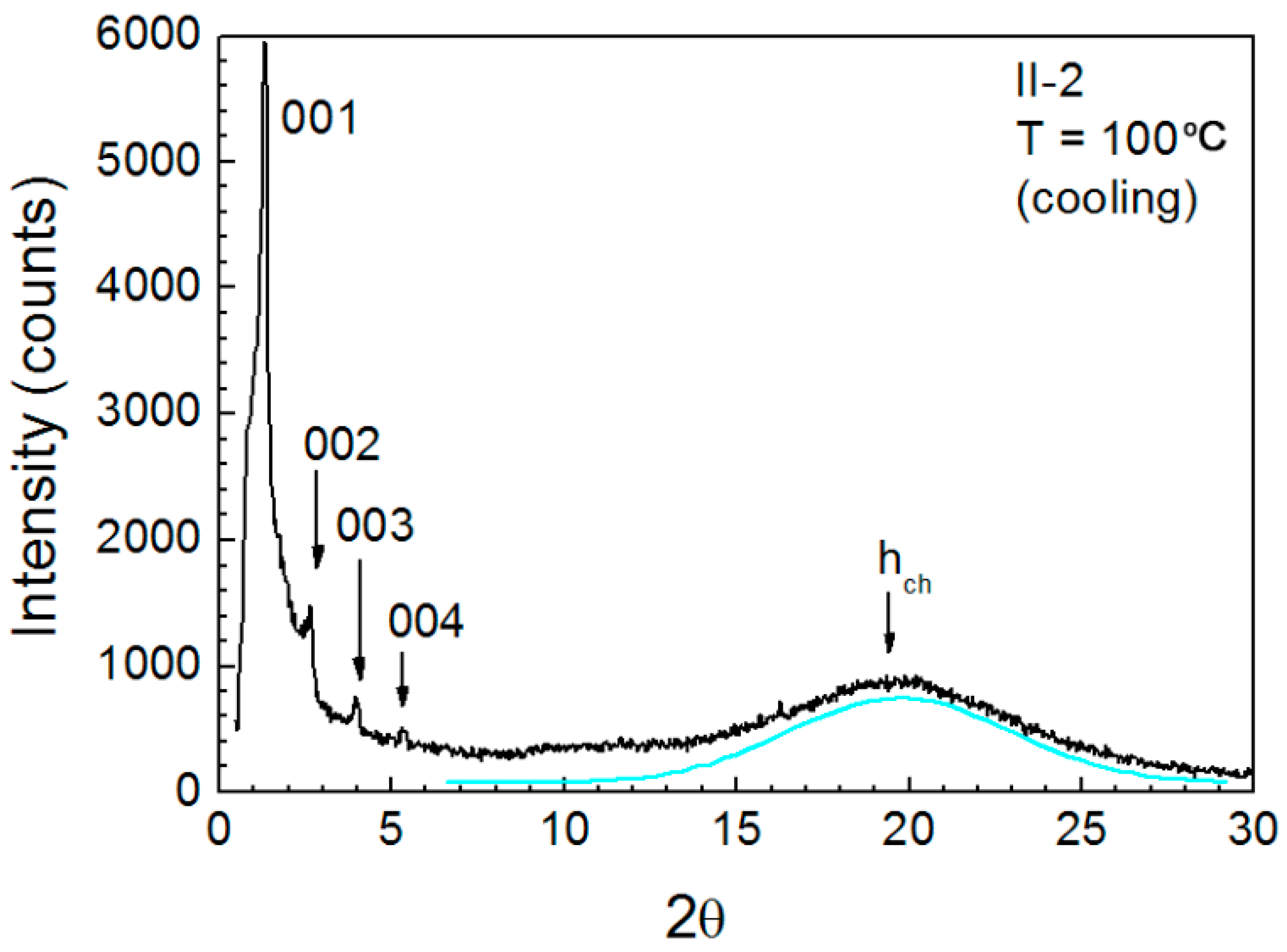
3.5. Variable-Temperature SAXS Measurement
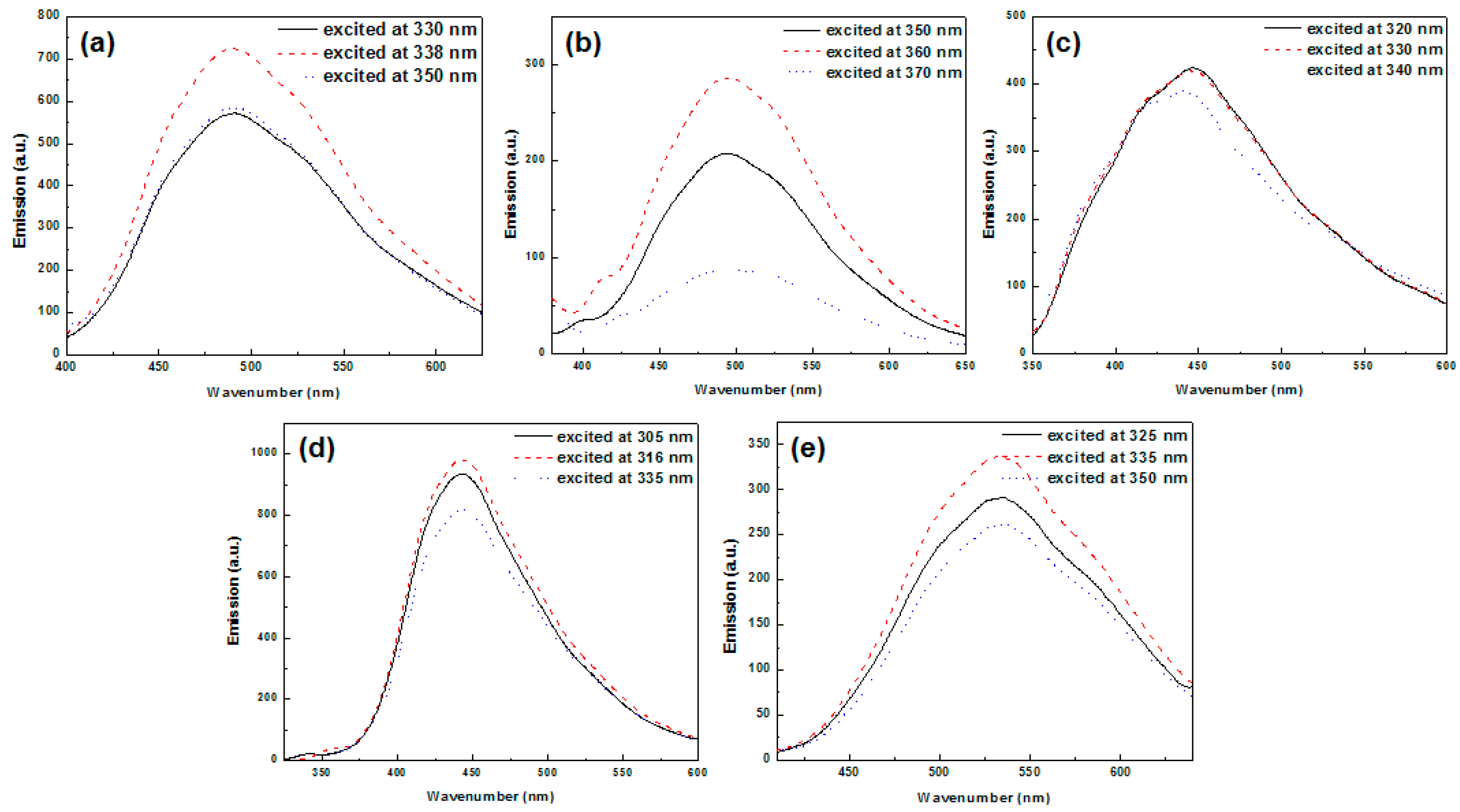
3.6. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The design of polymeric ionic liquids for the preparation of functional materials. J. Macromol. Sci. Part C Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Anderson, E.B.; Long, T.E. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer 2010, 51, 2447–2454. [Google Scholar] [CrossRef]
- Green, M.D.; Long, T.E. Designing imidazole-based ionic liquids and ionic liquid monomers for emerging technologies. J. Macromol. Sci. Part C Polym. Rev. 2009, 49, 291–314. [Google Scholar] [CrossRef]
- Kokorin, A. (Ed.) Advanced Applications of Ionic Liquids in Polymer Science, Ionic Liquids: Applications and Perspectives. ISBN: 978-953-307-248-7. Available online: http://www.intechopen.com/books/ionic-liquids-applications-and-perspectives/advanced-applications-of-ionic liquids-in-polymer-science (accessed on 30 March 2019).
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.A.; Doré, K.; Boissnoit, M.; Bergeron, M.G.; Tanguay, R.M.; Boudreau, D.; Leclerc, M. Direct molecular detection of nucleic acids by fluorescence signal amplification. J. Am. Chem. Soc. 2005, 127, 12673–12676. [Google Scholar] [CrossRef]
- Chen, Y.; Pu, K.-Y.; Fan, Q.-L.; Qi, X.-Y.; Huang, Y.-Q.; Lu, X.-M.; Huang, W. Water-soluble anionic conjugated polymers for metal Ion sensing: Effect of interchain aggregation. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5057–5067. [Google Scholar] [CrossRef]
- Balamurugan, A.; Reddy, M.L.P.; Jayakannan, M. Carboxylic-unctionalized water-soluble pi-conjugated polymer: Highly selective and efficient chemosensor for mercury(II) ions. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5144–5157. [Google Scholar] [CrossRef]
- An, L.; Wang, S. Conjugated polyelectrolytes as new platforms for drug screening. Chem. Asian J. 2009, 4, 1196–1206. [Google Scholar] [CrossRef]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors. Chem. Soc. Rev. 2010, 39, 2411–2419. [Google Scholar] [CrossRef]
- Liang, J.; Li, K.; Liu, B. Visual sensing with conjugated polyelectrolytes. Chem. Sci. 2013, 4, 1377–1394. [Google Scholar] [CrossRef]
- Cagnoli, R.; Lanzi, M.; Libertini, E.; Mucci, A.; Paganin, L.; Parenti, F.; Preti, L.; Schenetti, L. Organic- and water-soluble aminoalkylsulfanyl polythiophenes. Macromolecules 2008, 41, 3785–3792. [Google Scholar] [CrossRef]
- Zhai, L.; McCullough, R.D. Layer-by-layer assembly of polythiophene. Adv. Mater. 2002, 14, 901–905. [Google Scholar] [CrossRef]
- Richter, T.V.; Bühler, C.; Ludwigs, S. Water- and ionic-liquid-soluble branched polythiophenes bearing anionic and cationic moieties. J. Am. Chem. Soc. 2012, 134, 43–46. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G.; Wuest, J.D. Controlling the morphology and performance of bulk heterojunctions in solar cells. Lessons learned from the benchmark poly(3-hexylthiophene): [6,6]-Phenyl-C61-butyric acid methyl ester system. Chem. Rev. 2013, 113, 3734–3765. [Google Scholar] [CrossRef]
- Qin, C.; Wu, X.; Gao, B.; Tong, H.; Wang, L. Amino acid-functionalized polyfluorene as a water- soluble Hg2+ chemosensor with high solubility and high photoluminescence quantum yield. Macromolecules 2009, 42, 5427–5429. [Google Scholar] [CrossRef]
- Chong, H.; Duan, X.; Yang, Q.; Liu, L.; Wang, S. Synthesis and characterization of degradable water-soluble fluorescent polymers. Macromolecules 2010, 43, 10196–10200. [Google Scholar] [CrossRef]
- Li, L.; He, F.; Wang, X.; Ma, N.; Li, L. Reversible pH-responsive fluorescence of water-Soluble polyfluorenes and their application in metal ion detection. ACS Appl. Mater. Interfaces 2012, 4, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-C.; Wu, H.; Lee, C.-L.; Chen, W.-C.; He, H.-J.; Chen, M.-T. Triphenylamine-based linear conjugated polyfluorenes with various pendant groups: Synthesis, characterization, and ion responsive properties. Polymer 2013, 54, 1080–1090. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Miller, E.K.; Moses, D.; Bazan, G.C.; Heeger, A.J. Photoluminescence of Water-Soluble Conjugated Polymers: Origin of Enhanced Quenching by Charge Transfer. Macromolecules 2000, 33, 5153–5158. [Google Scholar] [CrossRef]
- Harris, F.W.; Chuang, K.C.; Huang, S.A.X.; Janimak, J.J.; Cheng, S.Z.D. Aromatic poly(pyridinium salt)s: Synthesis and structure of organo-soluble, rigid-rod poly(pyridinium tetrafluoroborates)s. Polymer 1994, 35, 4940–4948. [Google Scholar] [CrossRef]
- Huang, S.A.X.; Chuang, K.C.; Cheng, S.Z.D.; Harris, F.W. Aromatic poly(pyridinium salt)s Part 2. Synthesis and properties of organo-soluble, rigid-rod poly(pyridinium triflate)s. Polymer 2000, 41, 5001–5009. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Burchett, R.A.; Han, H.; Cebe, J.J. Synthesis and characterization of poly(pyridinium salt)s with organic counterion exhibiting both lyotropic liquid-crystalline and light-emitting properties. Macromolecules 2001, 34, 7579–7581. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Burchett, R.A.; Han, H.; Cebe, J.J. Synthesis and characterization of poly(pyridinium salt)s with organic counterion exhibiting both lyotropic liquid-crystalline and light-emitting properties. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2710–2715. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Burchett, R.A.; Han, H.; Cebe, J.J. Synthesis and characterization of poly(pyridinium salt)s with organic counterion exhibiting both lyotropic liquid-crystalline and light-emitting properties. Polymer 2002, 43, 1953–1958. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Cebe, J.J.; Nedeltchev, I.K.; Kang, S.-W.; Kumar, S. Synthesis and characterization of poly(pyridinium salt)s with organic counterions exhibiting both thermotropic liquid-crystalline and light-emitting properties. Macromolecules 2004, 37, 2688–2694. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, A.K. Synthesis and characterization of poly(pyridinium salt)s with anthracene moieties exhibiting both lyotropic liquid-crystalline and UV light-emitting properties. Polymer 2006, 47, 8281–8288. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, A.K. Synthesis and characterization of poly(pyridinium salt)s with organic counterions exhibiting both thermotropic liquid-crystalline and light-emitting properties. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1028–1041. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Kamatam, S.; Han, H.; Nedeltchev, A.K. Synthesis and characterization of poly(pyridinium salt)s with oxyalkylene units exhibiting amphotropic liquid-crystalline and photoluminescence properties. Polymer 2008, 49, 1748–1760. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, A.K.; Mandal, H.D.; Jimenez-Hernandez, J.A.; McGannon, P.M. Poly(pyridinium salt)s with organic counterions derived from an aromatic diamine containing oxyethylene unit exhibiting amphotropic liquid-crystalline and photoluminescence properties. Polymer 2009, 50, 3128–3135. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.; Nedeltchev, A.K.; Mandal, H.D.; Jimenez-Hernandez, J.A.; McGannon, P.M. Poly(pyridinium salt)s with organic counterions derived from an aromatic diamine containing tetraoxyethylene units exhibiting amphotropic liquid-crystalline and photoluminescence properties. J. Appl. Polym. Sci. 2010, 116, 1197–1206. [Google Scholar] [CrossRef]
- Nedeltchev, A.K.; Han, H.; Bhowmik, P.K. Solution, thermal and optical properties of new poly(pyridinium salt)s derived from bisquinoline diamines. Polym. Chem. 2010, 1, 908–915. [Google Scholar] [CrossRef]
- Nedeltchev, A.K.; Han, H.; Bhowmik, P.K. Solution, thermal and optical properties of novel poly(pyridinium salt)s derived from conjugated pyridine diamines. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4408–4418. [Google Scholar] [CrossRef]
- Nedeltchev, A.K.; Han, H.; Bhowmik, P.K. Solution, thermal, and optical properties of poly(pyridinium salt)s derived from an ambipolar diamine consisting of diphenylquinoline and triphenyl amine moieties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4611–4620. [Google Scholar] [CrossRef]
- Nedeltchev, A.K.; Han, H.; Bhowmik, P.K.; Ma, L. Solution, thermal and optical properties of new poly(pyridinium salt)s derived from conjugated quinoline diamines. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1907–1918. [Google Scholar] [CrossRef]
- Jo, T.S.; Nedeltchev, A.K.; Biswas, B.; Han, H.; Bhowmik, P.K. Synthesis and characterization of poly(pyridinium salt)s derived from various aromatic diamines. Polymer 2012, 53, 1063–1071. [Google Scholar] [CrossRef]
- Spiliopoulos, I.K.; Mikroyannidis, J.A. Poly(pyridinium salt)s with stilbene or distyrylbenzene chromophores. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2454–2462. [Google Scholar] [CrossRef]
- Al-Hariri, L.A.; Schlenoff, J.B. Macro-counterions in a precursor to poly(phenylene vinylene): Toward defect-free luminescent films. Polymer 2010, 51, 2993–2997. [Google Scholar] [CrossRef]
- Al-Hariri, L.A.; Zheng, L.; Yang, W.; Schelenoff, J.B. Thermal elimination of precursors to poly(phenylenevinylene) with a macrocounterion versus a small counterion: A coordinated experimental and simulation study. Macromolecules 2011, 44, 6663–6668. [Google Scholar] [CrossRef]
- Feld, W.A.; Ramalingam, B.; Harris, F.W. Polyimides containing oxyethylene units. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 319–328. [Google Scholar] [CrossRef]
- Genova-Dimitrova, P.; Baradie, B.; Foscallo, D.; Poinsignon, C.; Sanchez, J.Y. Ionomeric membranes for proton exchange membrane fuel cell (PEMFC): Sulfonated polysulfone associated with phosphatoantimonic acid. J. Membr. Sci. 2001, 185, 59–71. [Google Scholar] [CrossRef]
- Žagar, E.; Žigon, M. Solution properties of carboxylated polyurethanes and related ionomers in polar solvents (DMF and LiBr/DMF). Polymer 2000, 41, 3513–3521. [Google Scholar] [CrossRef]
- Moliton, A.; Hiorns, R.C. Review of electronic and optical properties of semiconducting π-conjugated polymers: Applications in optoelectronics. Polym. Int. 2004, 53, 1397–1412. [Google Scholar] [CrossRef]
- Mitschke, U.; Bäuerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles in Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; Chapter 6; pp. 205–235. [Google Scholar]
- Liao, L.; Pang, Y.; Ding, L.; Karasz, F.E. Blue-emitting soluble poly(m-phenylenevinylene) derivatives. Macromolecules 2001, 34, 7300–7305. [Google Scholar] [CrossRef]
- Sarker, A.M.; Strehmel, B.; Neckers, D.C. Synthesis, characterization, and optical properties of copolymers containing fluorine-substituted distyrylbenzene and nonconjugated spacers. Macromolecules 1999, 32, 7409–7413. [Google Scholar] [CrossRef]
- Gettinger, C.L.; Heeger, A.J.; Drake, J.M.; Pine, D.J. A photoluminescence study of poly(phenylene vinylene) derivatives: The effect of intrinsic persistence length. J. Chem. Phys. 1994, 101, 1673–1678. [Google Scholar] [CrossRef][Green Version]
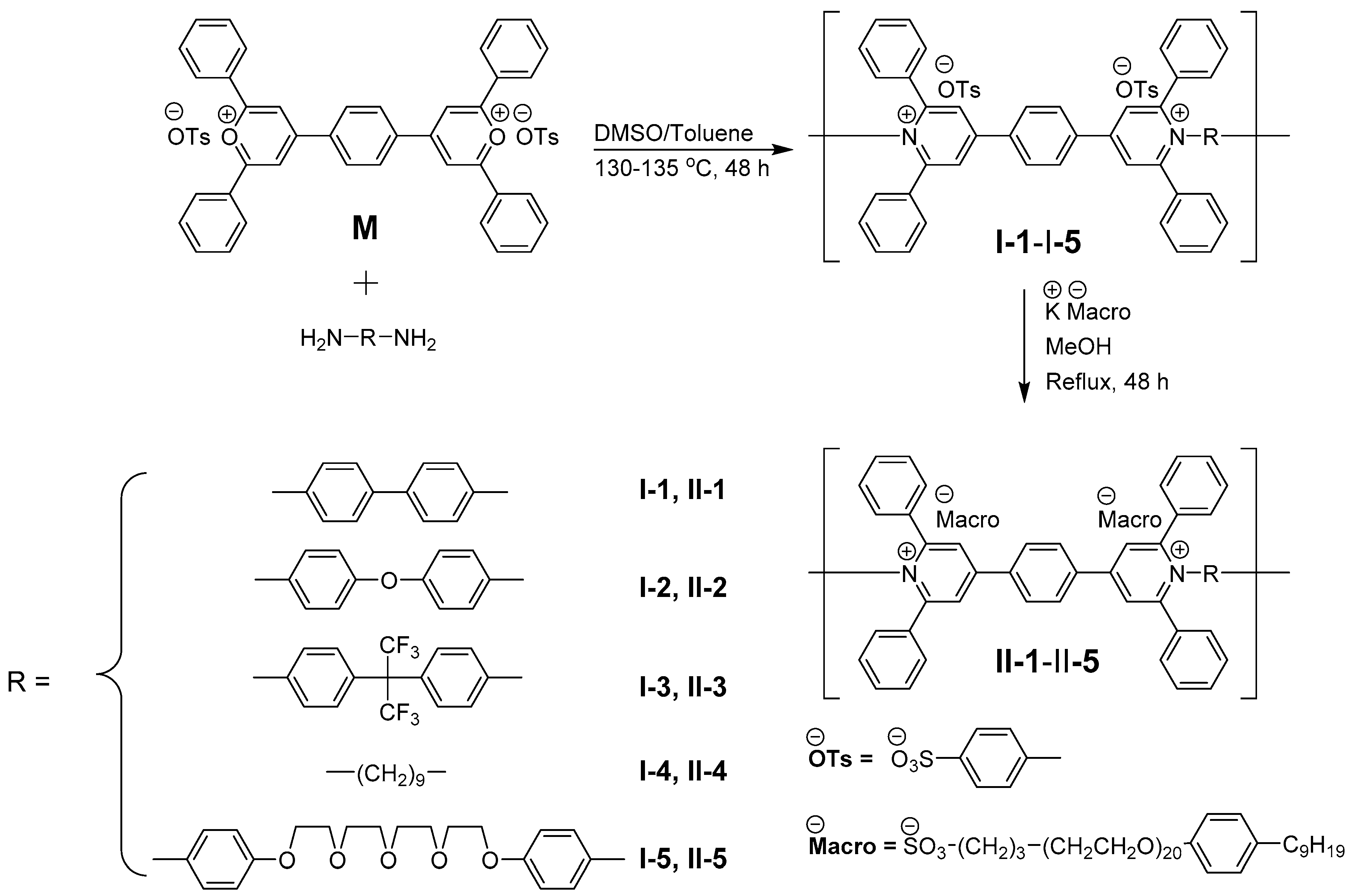

| Polymer | II-1 | II-2 | II-3 | II-4 | II-5 |
|---|---|---|---|---|---|
| Tm/g (°C) | 40 a | 29 c | 32 c | 34 c | 31 d |
| Ti (°C) | 200 b | 160 b | − | − | − |
| Tde (°C) | 322 | 313 | 318 | 261 | 329 |
| Polymer | Temperature | dmeas. (Å) a | I b | Indexation c | dtheor. (Å) a | Aru (Å2) d |
|---|---|---|---|---|---|---|
| hru (Å) | ||||||
| II-1 | 80 °C | 63.9 | VS, sh | 1 | 64.07 | 73.5 |
| 31.96 | S, sh | 2 | 32.04 | 3.44 | ||
| 21.16 | M, sh | 3 | 21.36 | |||
| 15.85 | M, sh | 4 | 16.02 | |||
| 13.1 | S, sh | 5 | 12.81 | |||
| 10.7 | W, sh | 6 | 10.68 | |||
| 9.15 | W, sh | 7 | 9.15 | |||
| 8.02 | W, sh | 8 | 8.01 | |||
| 4.5 | VS, br | − | hch | |||
| 4.35 | M, sh | − | hc | |||
| 140 °C | 64 | VS sh | 1 | 64.15 | 76.2 | |
| 32 | W, sh | 2 | 32.08 | 3.57 | ||
| 21.15 | S, sh | 3 | 21.38 | |||
| 13.03 | VS, br | 5 | 12.83 | |||
| 4.5 | VS, br | − | hch | |||
| 4.36 | M, sh | − | hc | |||
| II-2 | 100 °C | 67 | VS, sh | 1 | 66.95 | 71.5 |
| 33.62 | M, sh | 2 | 33.47 | 3.57 | ||
| 22.2 | M, sh | 3 | 22.31 | |||
| 16.74 | W, sh | 4 | 16.73 | |||
| 4.5 | VS, br | − | hch |
| Polymer | II-1 | II-2 | II-3 | II-4 | II-5 |
|---|---|---|---|---|---|
| UV abs (nm) a | 345 | 337 | 345 | 330 | 337 |
| Band gap (eV) a | 3.04 | 3.00 | 3.04 | 3.21 | 3.00 |
| PL λem H2O (nm) | 492 | 496 | 447 | 445 | 533 |
| PL λem CH3OH (nm) | 497 | 522 | 451 | 443 | 542 |
| PL λem CH3CN (nm) | 500 | 527 | 446 | 442 | 542 |
| PL λem Acetone (nm) | 487 | − | 446 | 442 | 534 |
| PL λem THF (nm) | 492 | 482 | 413 | 439 | 527 |
| PL λem CHCl3 (nm) | 463 | − | 421, 443 | 440 | 500, 520 |
| PL λem film (nm) b | 463 | 466 | 461 | 518 | 512 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, T.S.; Han, H.; Bhowmik, P.K.; Heinrich, B.; Donnio, B. Thermotropic Liquid-Crystalline and Light-Emitting Properties of Poly(pyridinium) Salts Containing Various Diamine Connectors and Hydrophilic Macrocounterions. Polymers 2019, 11, 851. https://doi.org/10.3390/polym11050851
Jo TS, Han H, Bhowmik PK, Heinrich B, Donnio B. Thermotropic Liquid-Crystalline and Light-Emitting Properties of Poly(pyridinium) Salts Containing Various Diamine Connectors and Hydrophilic Macrocounterions. Polymers. 2019; 11(5):851. https://doi.org/10.3390/polym11050851
Chicago/Turabian StyleJo, Tae Soo, Haesook Han, Pradip K. Bhowmik, Benoît Heinrich, and Bertrand Donnio. 2019. "Thermotropic Liquid-Crystalline and Light-Emitting Properties of Poly(pyridinium) Salts Containing Various Diamine Connectors and Hydrophilic Macrocounterions" Polymers 11, no. 5: 851. https://doi.org/10.3390/polym11050851
APA StyleJo, T. S., Han, H., Bhowmik, P. K., Heinrich, B., & Donnio, B. (2019). Thermotropic Liquid-Crystalline and Light-Emitting Properties of Poly(pyridinium) Salts Containing Various Diamine Connectors and Hydrophilic Macrocounterions. Polymers, 11(5), 851. https://doi.org/10.3390/polym11050851







