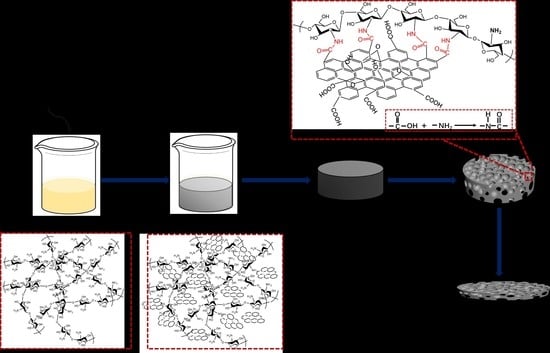
Synthesis and Characterization of Graphene Oxide/Chitosan Composite Aerogels with High Mechanical Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oxidation of Graphite Powders Process
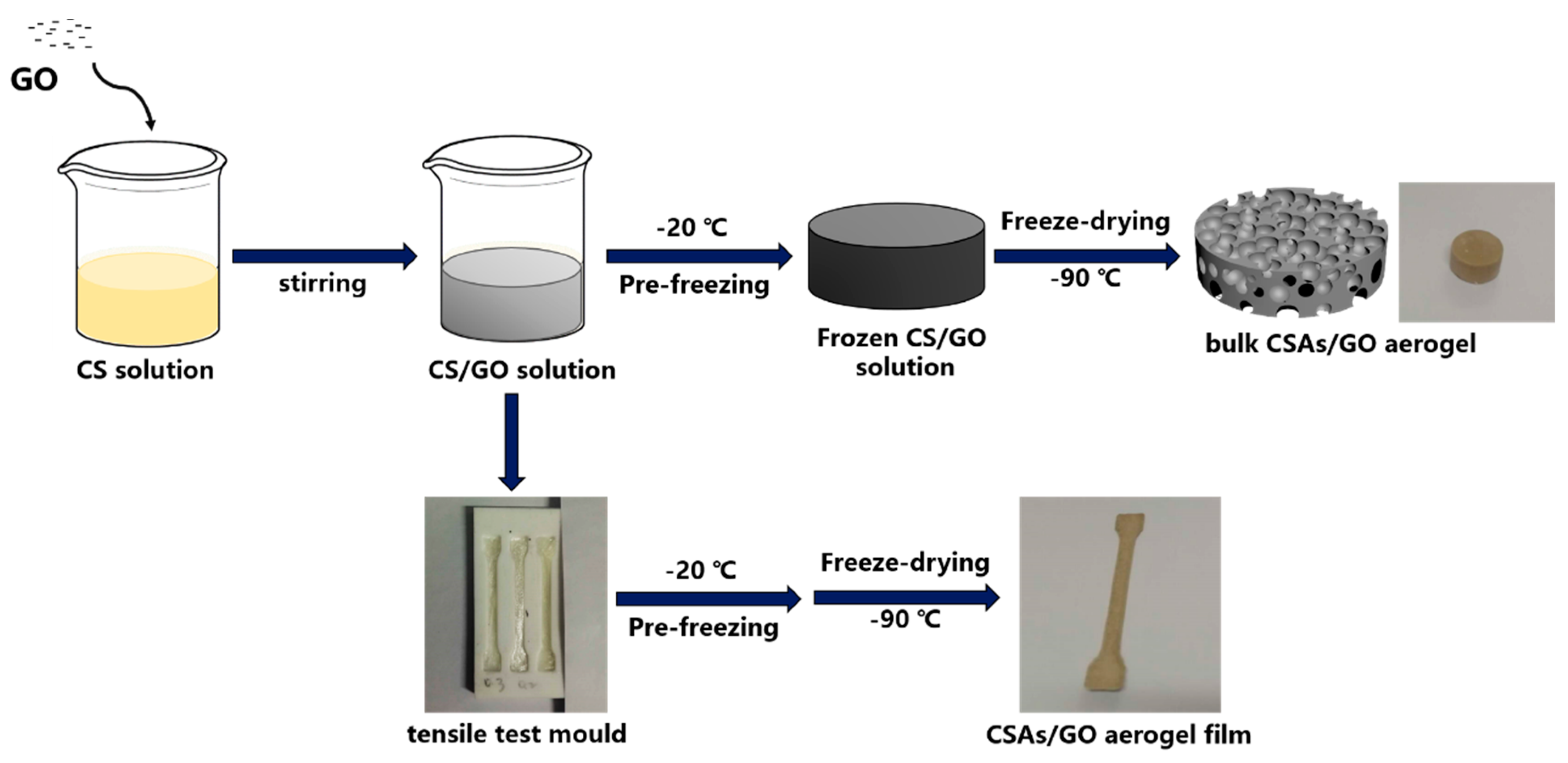
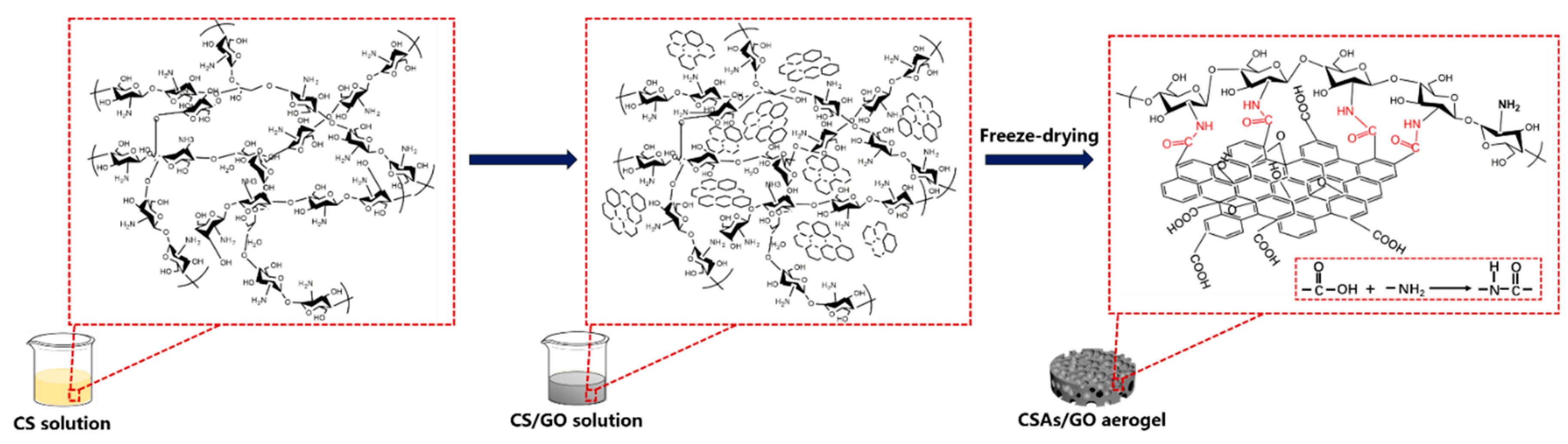
2.3. Preparation of CSAs/GO
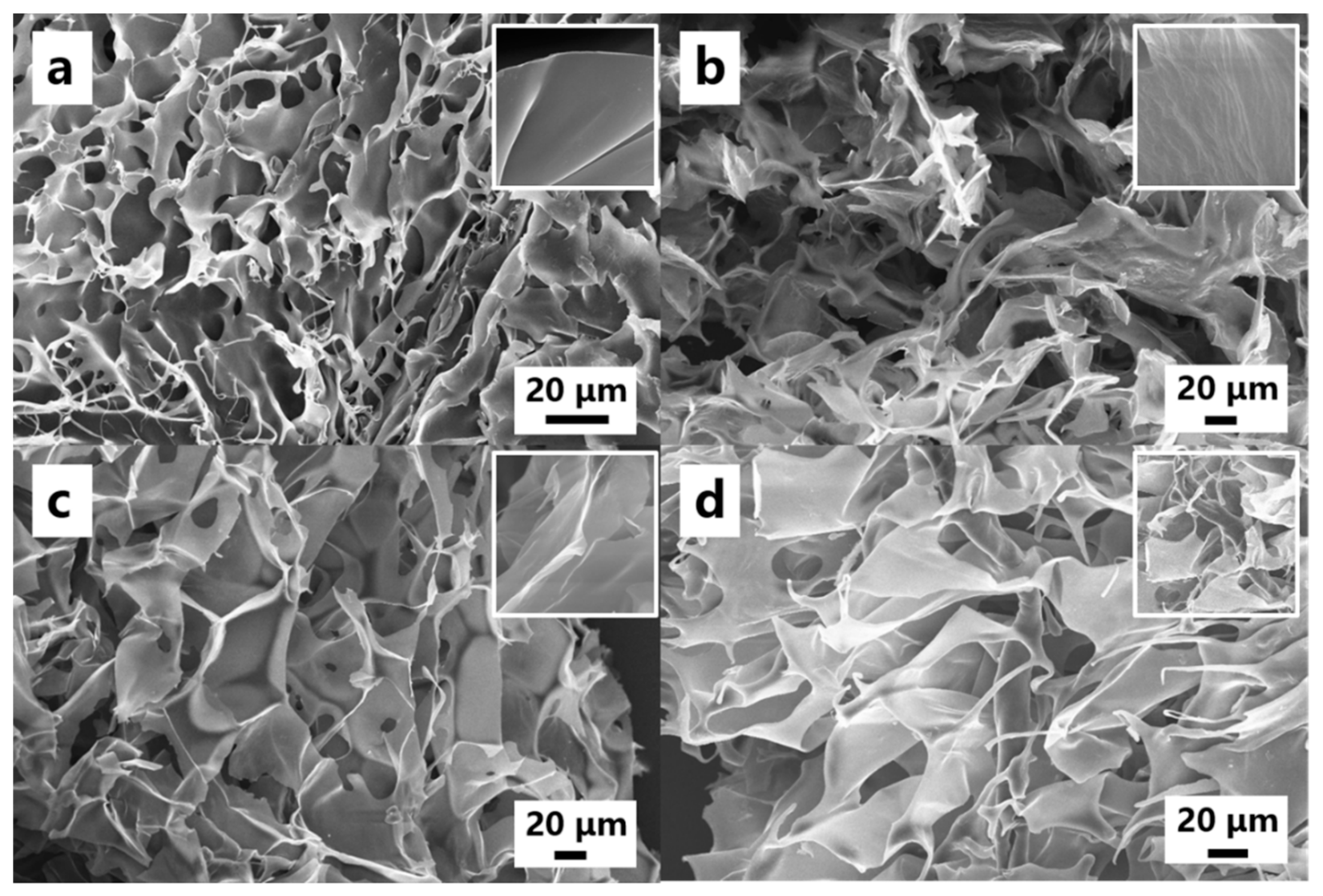
2.4. Characterization
2.5. Mechanical Test
2.6. Testing of Crystallinity
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Cavicchi, K.A.; Li, R.; Yager, K.G.; Fukuto, M.; Vogt, B.D. Thickness Limit for Alignment of Block Copolymer Films Using Solvent Vapor Annealing with Shear. Macromolecules 2018, 51, 4213–4219. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Pandiselvi, K.; Thambidurai, S. Chitosan-ZnO/polyaniline ternary nanocomposite for high-performance supercapacitor. Ionics 2014, 20, 551–561. [Google Scholar] [CrossRef]
- Santos-López, G.; Argüelles-Monal, W.; Carvajal-Millan, E.; López-Franco, Y.L.; Recillas-Mota, M.T.; Lizardi-Mendoza, J. Aerogels from Chitosan Solutions in Ionic Liquids. Polymers 2017, 9, 722. [Google Scholar] [CrossRef]
- Praveen, E.; Murugan, S.; Jayakumar, K. Investigations on the existence of piezoelectric property of a bio-polymer – chitosan and its application in vibration sensors. RSC Adv. 2017, 7, 35490–35495. [Google Scholar] [CrossRef]
- Lalov, I. Treatment of waste water from distilleries with chitosan. Water Res. 2000, 34, 1503–1506. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Keshipour, S.; Mirmasoudi, S.S. Cross-linked chitosan aerogel modified with Au: Synthesis, characterization and catalytic application. Carbohydr. Polym. 2018, 196, 494–500. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Shi, R.; Chen, L.; Fan, M. A robust salt-tolerant superoleophobic chitosan/nanofibrillated cellulose aerogel for highly efficient oil/water separation. Carbohydr. Polym. 2018, 200, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Nazrul, H.; Dutta, P.K.; Santosh, K.; Ranajit, B.; Neeladri, D. Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 2018, 125, 300–306. [Google Scholar]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y.; Li, L. Ultra-low shrinkage chitosan aerogels trussed with polyvinyl alcohol. Mater. Des. 2018, 156, 398–406. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Yu, H.-C.; Lee, J.; Jeon, J.; Im, J.; Jang, J.; Jin, S.-W.; Kim, K.-K.; Cho, S.; Chung, C.-M. Preparation of Polyimide/Graphene Oxide Nanocomposite and Its Application to Nonvolatile Resistive Memory Device. Polymers 2018, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.A. Graphene-based Composite Materials. Nature 2006, 422, 282–286. [Google Scholar]
- Yu, A.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite Nanoplatelet−Epoxy Composite Thermal Interface Materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.W.; An, J.H.; Ruoff, R. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Wu, Y.; Zhan, K.; Yang, Z.; Sun, W.; Zhao, B.; Yan, Y.; Yang, J. Graphene oxide/Al composites with enhanced mechanical properties fabricated by simple electrostatic interaction and powder metallurgy. J. Alloy Compd. 2019, 775, 233–240. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Mondal, T.; Butler, P.; Ashkar, R.; Bhowmick, A.K.; Krishnamoorti, R. Graphene Nanocomposites with High Molecular Weight Poly(ε-caprolactone) Grafts: Controlled Synthesis and Accelerated Crystallization. ACS Macro Lett. 2016, 5, 278–282. [Google Scholar] [CrossRef]
- Nie, W.Y.; Tsai, H.; Blancon, J.C.; Liu, F.Z.; Stoumpos, C.C.; Traore, B.; Kepenekian, M.; Durand, O.; Katan, C.; Tretiak, S.; et al. Critical Role of Interface and Crystallinity on the Performance and Photostability of Perovskite Solar Cell on Nickel Oxide. Adv. Mater. 2017, 30, 1703879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Niu, J.; Song, H.; Zhu, L.; Zhou, J.; Chen, X.; Liu, J.; Hong, S.; Song, R. Can closed shell graphitic materials be exfoliated? Defect induced porphyra-like graphene from the cooperation of activation and oxidation. J. Mater. Chem. A 2013, 1, 14103–14107. [Google Scholar] [CrossRef]
- Harish, K.V.; Kittur, F.S.; Tharanathan, R.N. Solid state structure of chitosan prepared under different N-deacetylating condition. Carbohydr. Polym. 2002, 50, 27–33. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Fukada, E. Piezoelectricity in polymers and biological materials. Ultrasonics 1968, 6, 229–234. [Google Scholar] [CrossRef]
- Valentin, R.; Bonelli, B.; Garrone, E.; Di Renzo, F.; Quignard, F. Accessibility of the Functional Groups of Chitosan Aerogel Probed by FT-IR-Monitored Deuteration. Biomacromolecules 2007, 8, 3646–3650. [Google Scholar] [CrossRef]
- Lambert, J.B. Introduction to Organic Spectroscopy; Macmillan: New York, NY, USA, 1987. [Google Scholar]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.-M. Well-Dispersed Chitosan/Graphene Oxide Nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef]
- Pozzo, L.D.Y.; Da Conceição, T.F.; Spinelli, A.; Scharnagl, N.; Pires, A.T. Chitosan coatings crosslinked with genipin for corrosion protection of AZ31 magnesium alloy sheets. Carbohydr. Polym. 2018, 181, 71–77. [Google Scholar] [CrossRef] [PubMed]
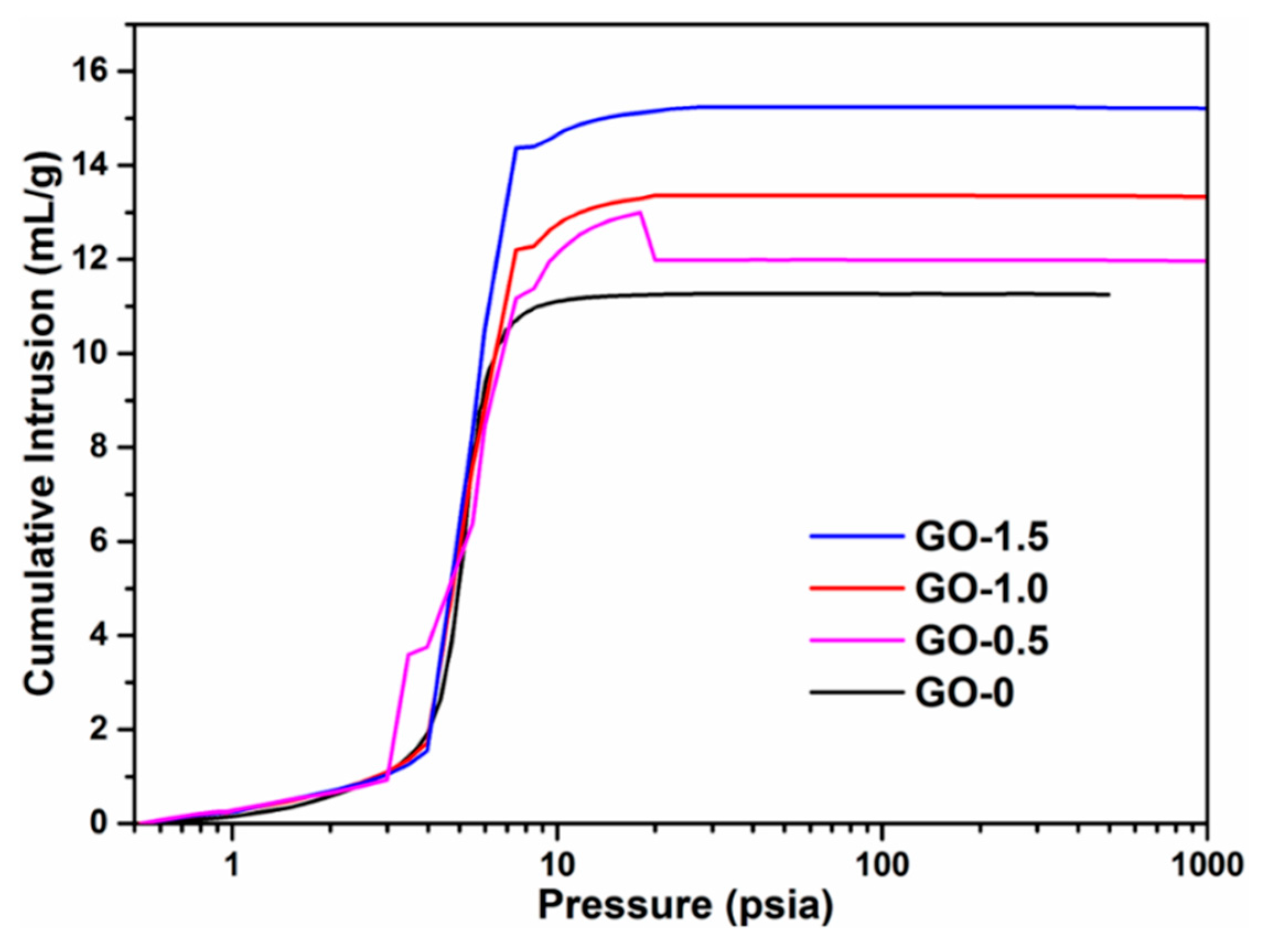
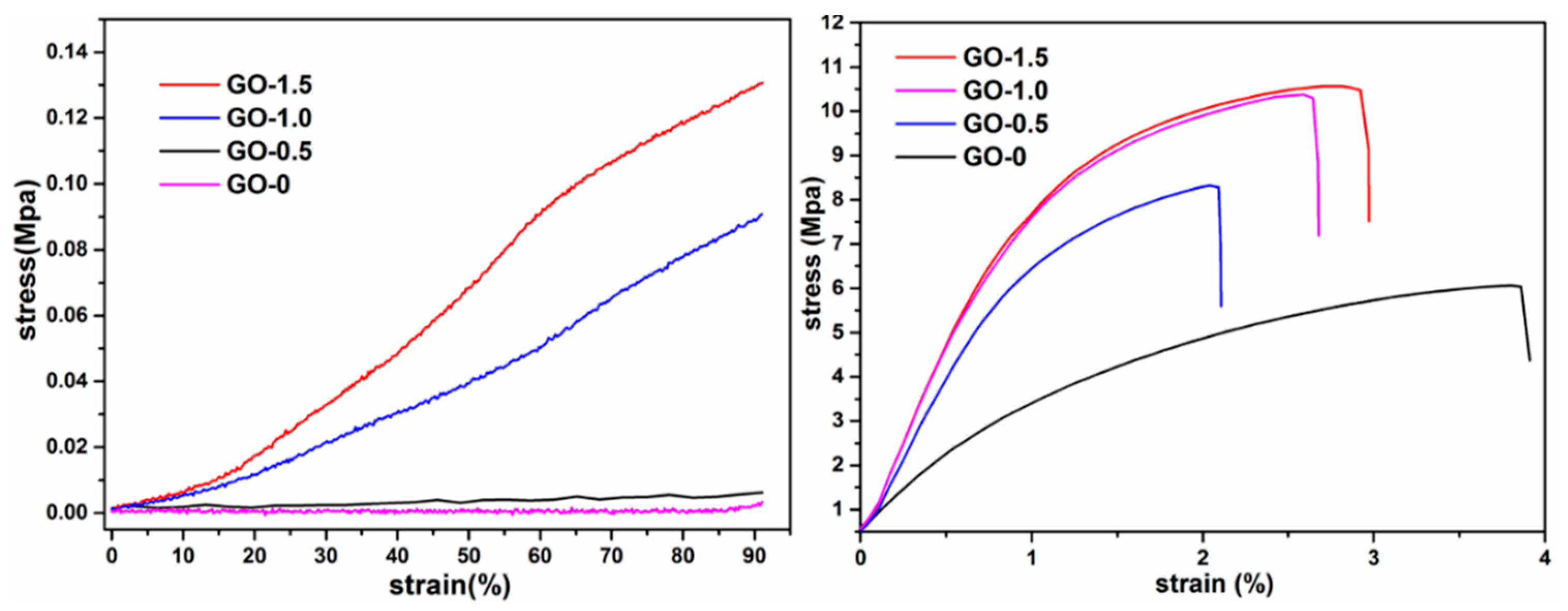
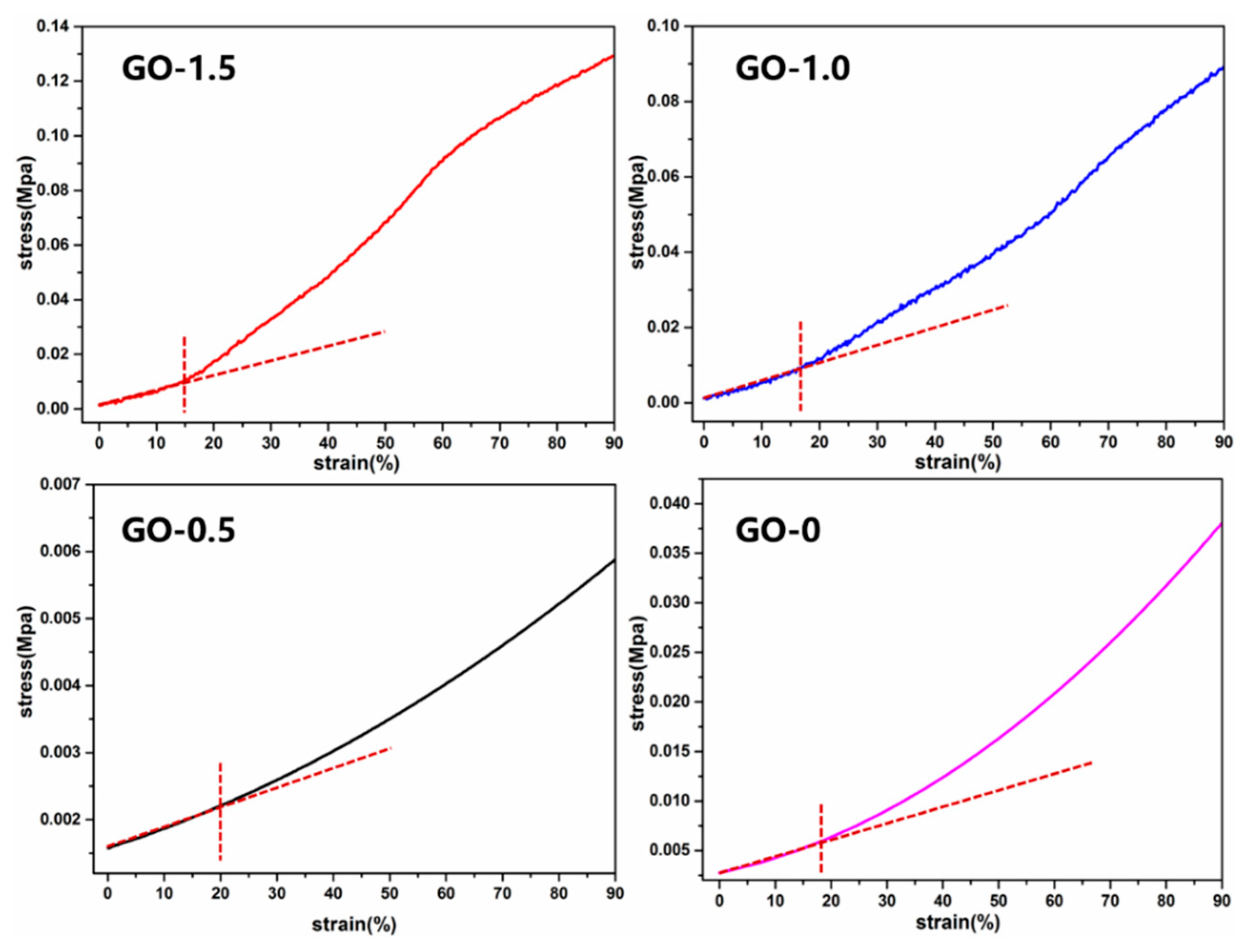

| Samples | Total Pore Volume (mL/g) | Total Pore Area (m2/g) | Porosity (%) |
|---|---|---|---|
| GO-0 | 11.271 | 1.264 | 83.7 |
| GO-0.5 | 12.995 | 1.591 | 85.2 |
| GO-1.0 | 13.243 | 1.625 | 87.2 |
| GO-1.5 | 15.246 | 1.858 | 87.6 |
| Samples | GO-0 | GO-0.5 | GO-1.0 | GO-1.5 |
|---|---|---|---|---|
| maximum load (MPa) | 6.06 | 8.32 | 10.34 | 10.56 |
| Samples | GO-0 | GO-0.5 | GO-1.0 | GO-1.5 |
|---|---|---|---|---|
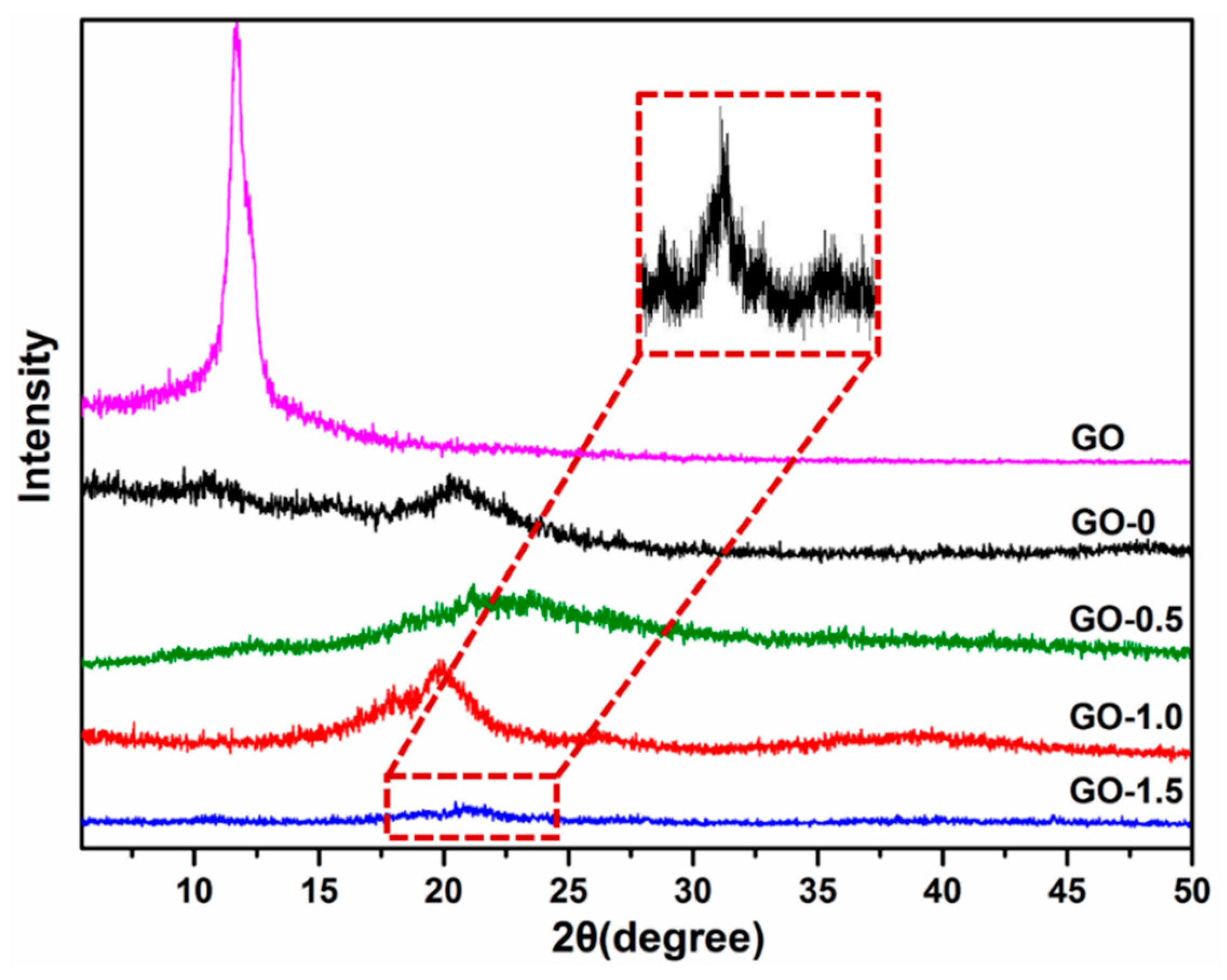
| CrI (%) | 23.7 | 59.5 | 72.5 | 81.1 |
| Samples | GO-0 | GO-0.5 | GO-1.0 | GO-1.5 |
|---|---|---|---|---|
| A1643 | 71.711 | 52.088 | 78.543 | 71.691 |
| A1546 | 70.812 | 51.047 | 76.478 | 66.696 |
| Samples | GO-0 | GO-0.5 | GO-1.0 | GO-1.5 |
|---|---|---|---|---|
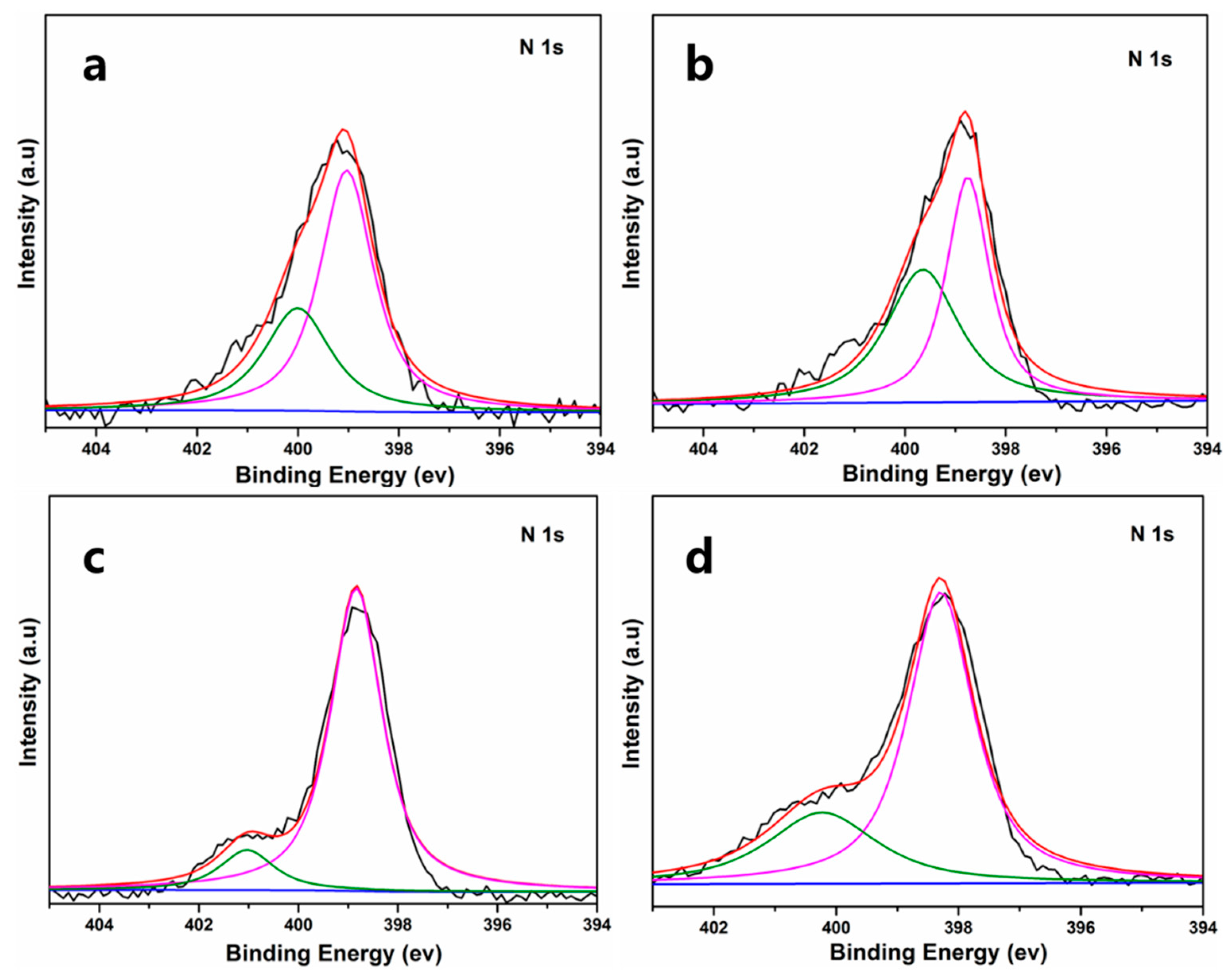
| R (%) | 1.27 | 2.04 | 2.70 | 7.98 |
| Samples | GO-0 | GO-0.5 | GO-1.0 | GO-1.5 |
|---|---|---|---|---|
| R | 0.98 | 1.91 | 2.48 | 7.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Yu, Y.; Kang, H.; Chen, X.; Liu, H.; Zhang, Y.; Sun, Y.; Song, H. Synthesis and Characterization of Graphene Oxide/Chitosan Composite Aerogels with High Mechanical Performance. Polymers 2019, 11, 777. https://doi.org/10.3390/polym11050777
Gong Y, Yu Y, Kang H, Chen X, Liu H, Zhang Y, Sun Y, Song H. Synthesis and Characterization of Graphene Oxide/Chitosan Composite Aerogels with High Mechanical Performance. Polymers. 2019; 11(5):777. https://doi.org/10.3390/polym11050777
Chicago/Turabian StyleGong, Yang, Yingchun Yu, Huixuan Kang, Xiaohong Chen, Hao Liu, Yue Zhang, Yimeng Sun, and Huaihe Song. 2019. "Synthesis and Characterization of Graphene Oxide/Chitosan Composite Aerogels with High Mechanical Performance" Polymers 11, no. 5: 777. https://doi.org/10.3390/polym11050777
APA StyleGong, Y., Yu, Y., Kang, H., Chen, X., Liu, H., Zhang, Y., Sun, Y., & Song, H. (2019). Synthesis and Characterization of Graphene Oxide/Chitosan Composite Aerogels with High Mechanical Performance. Polymers, 11(5), 777. https://doi.org/10.3390/polym11050777