Synthesis and Characterization of Covalently Crosslinked pH-Responsive Hyaluronic Acid Nanogels: Effect of Synthesis Parameters
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials and Chemicals
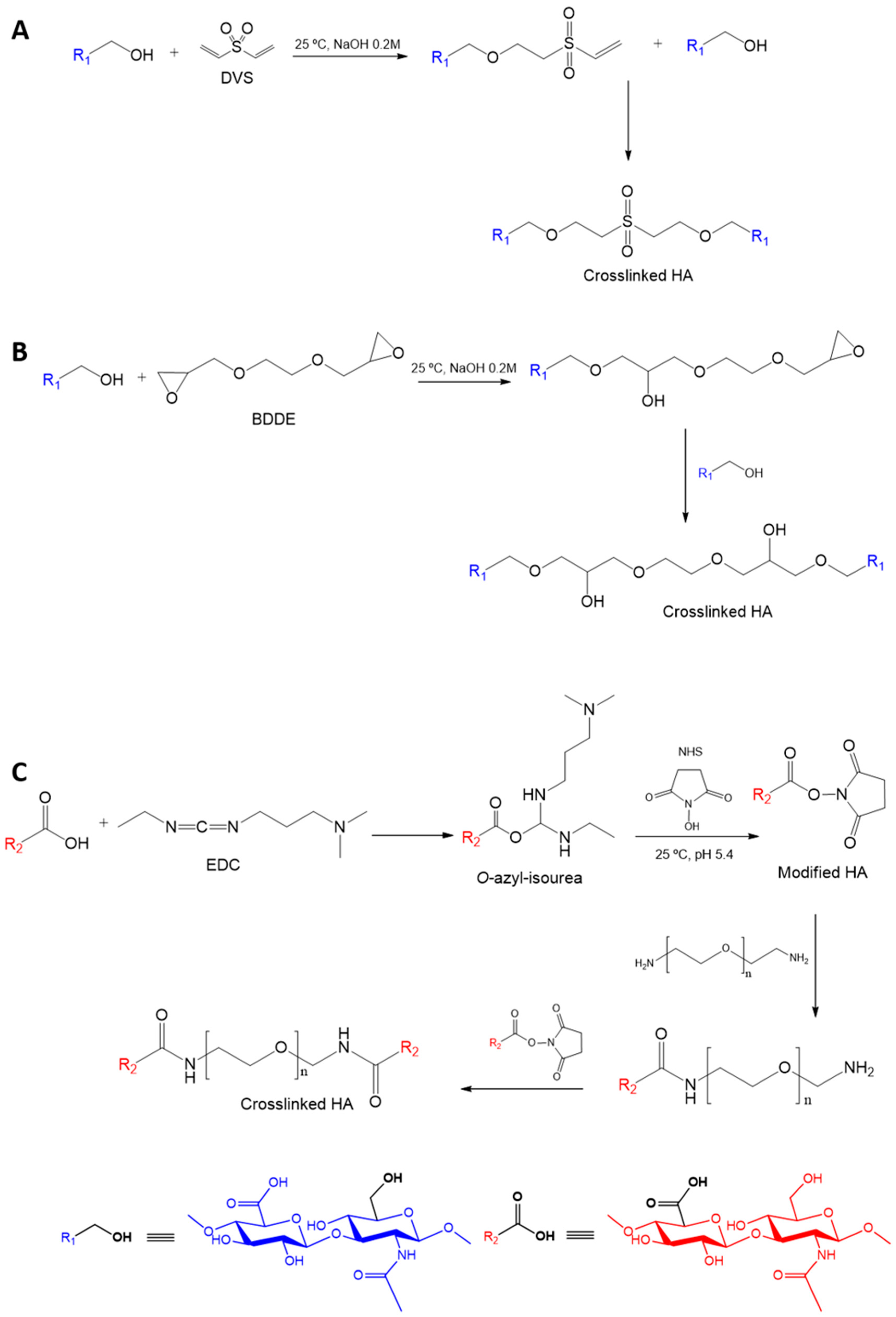
2.2. Synthesis of HA Nanogels
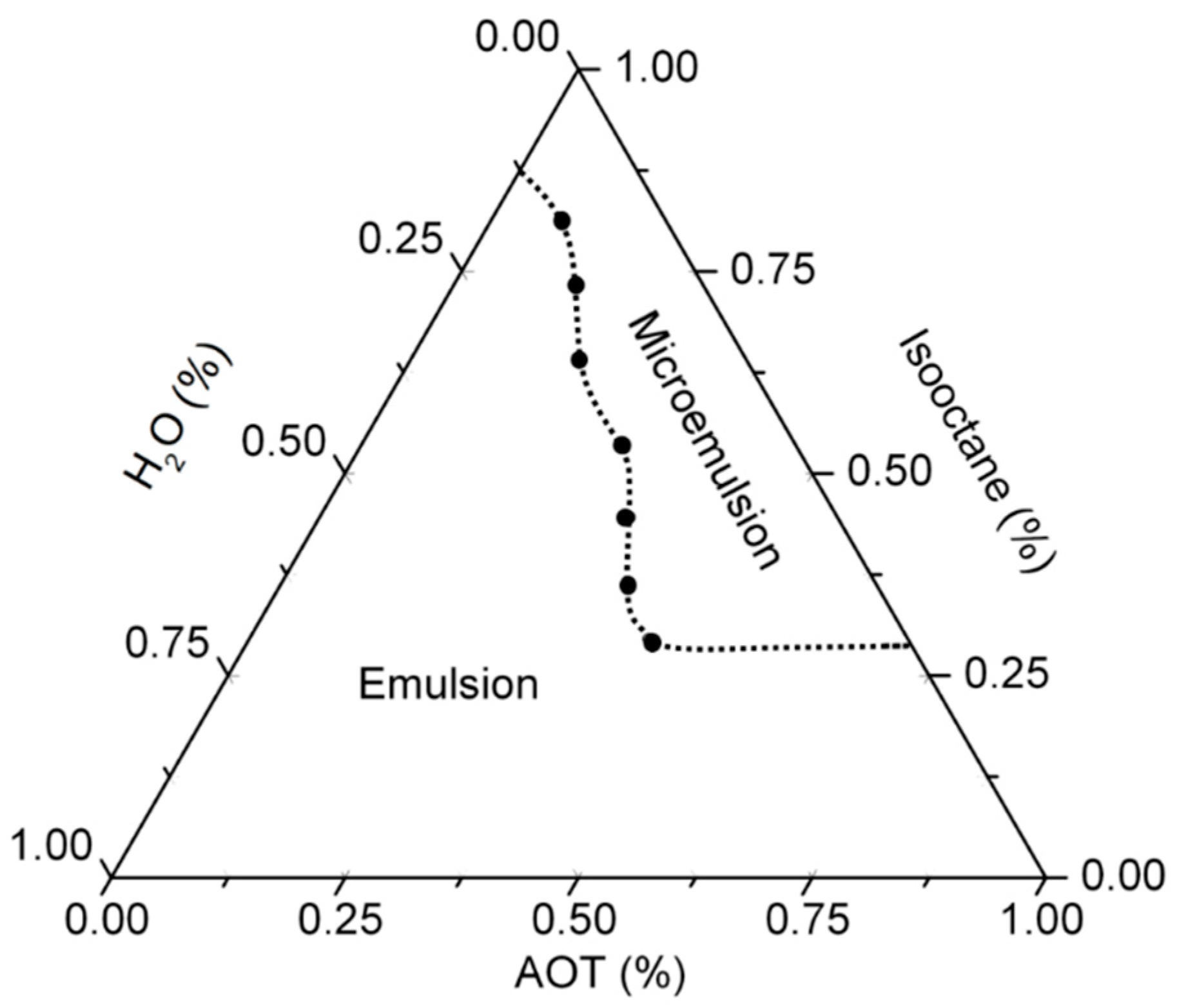
2.3. Determination of the Pseudoternary Phase Diagram
2.4. Characterization Methods
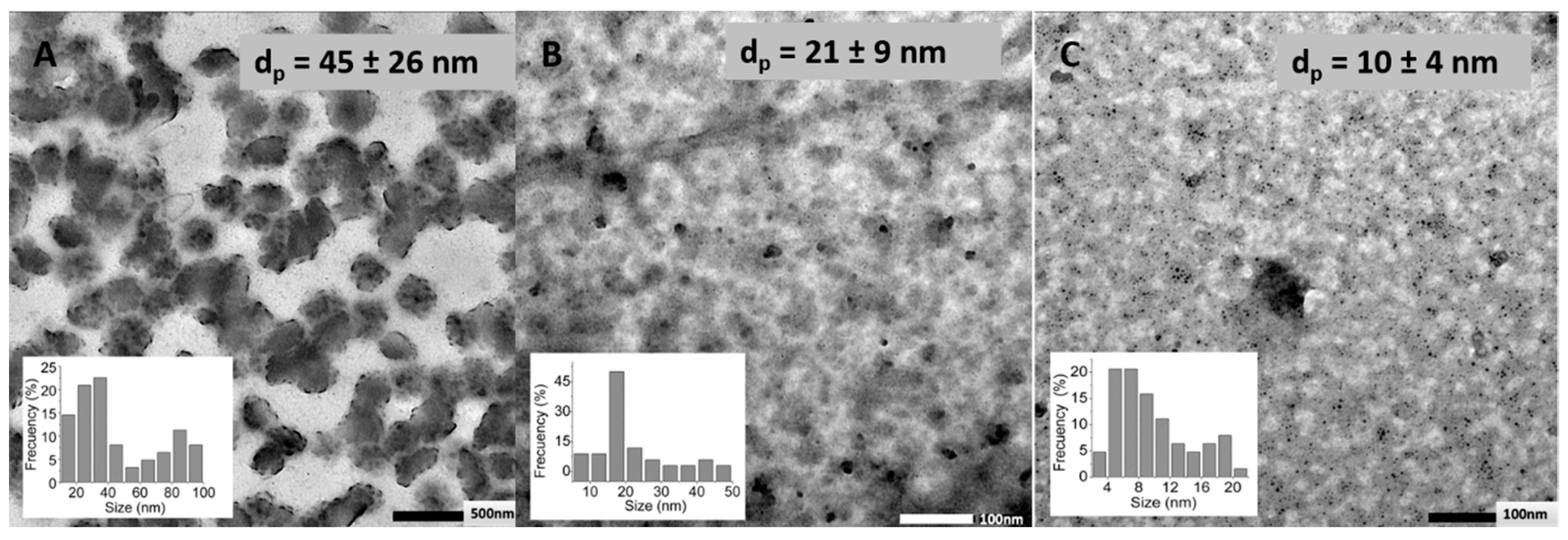
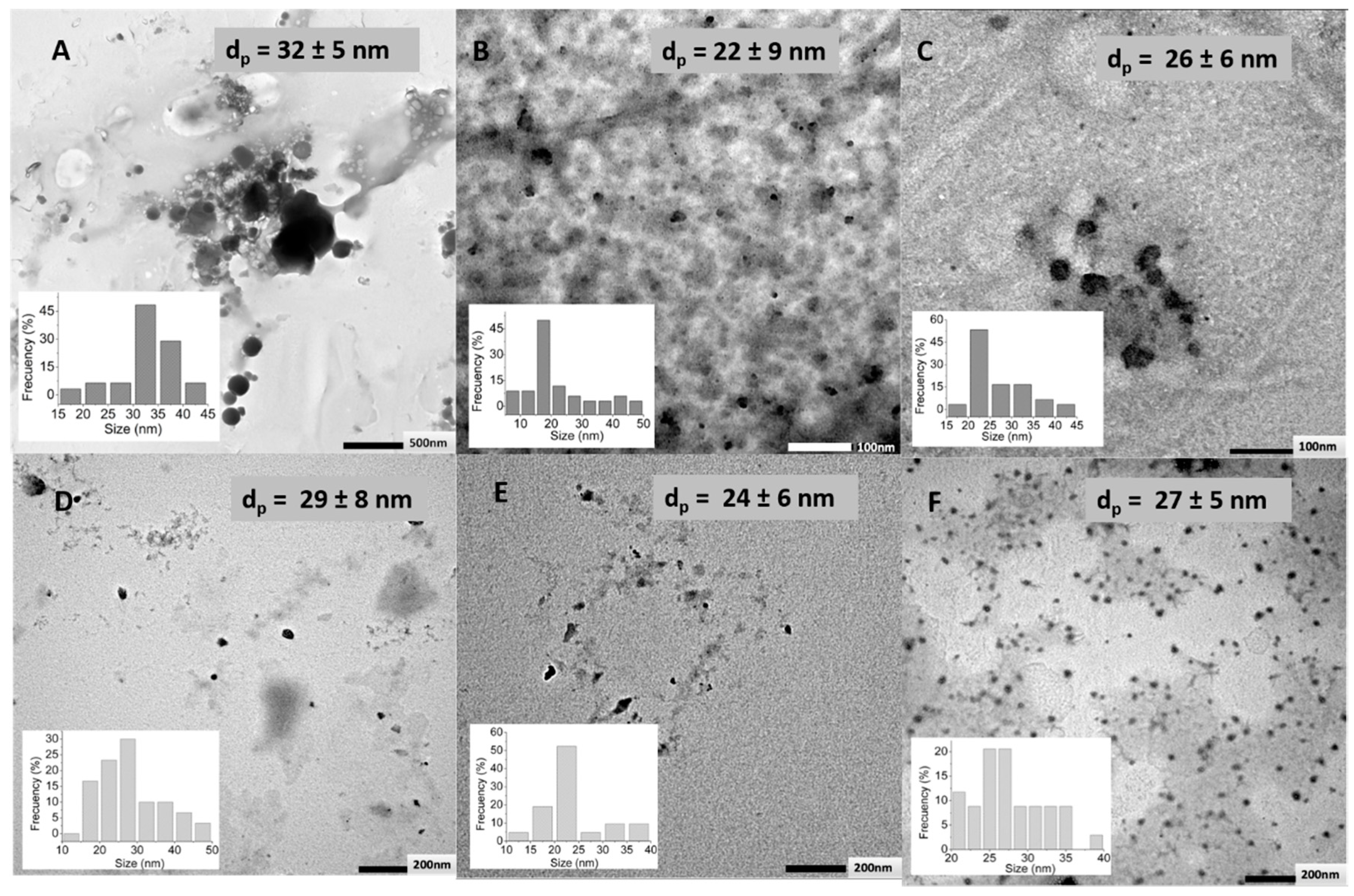
2.4.1. Transmission Electron Microscopy (TEM)
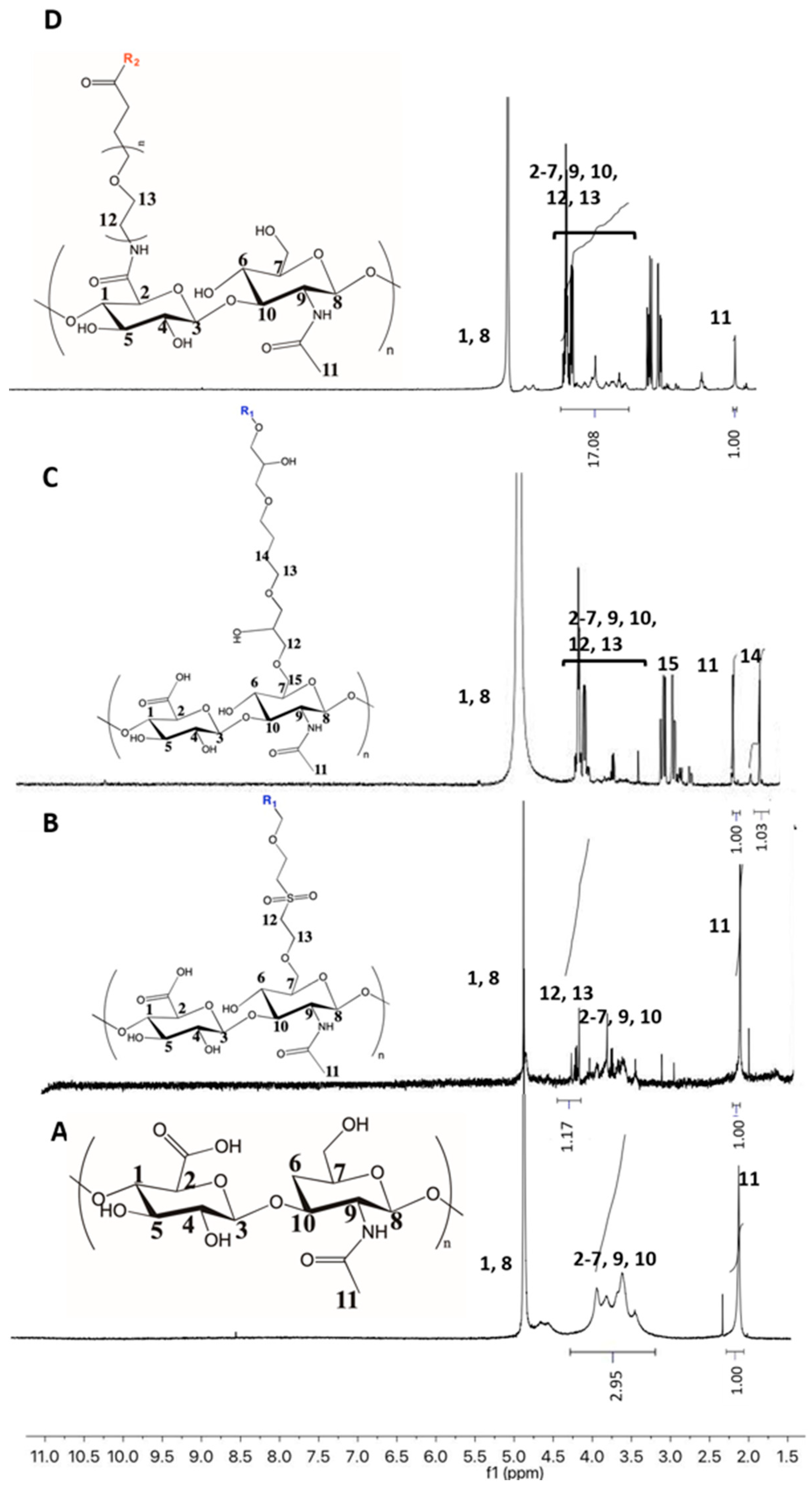
2.4.2. Nuclear Magnetic Resonance (1H-NMR)
2.4.3. Dynamic Light Scattering (DLS)
2.4.4. Zeta Potential
3. Results
3.1. Determination of the Microemulsion Phase Diagram
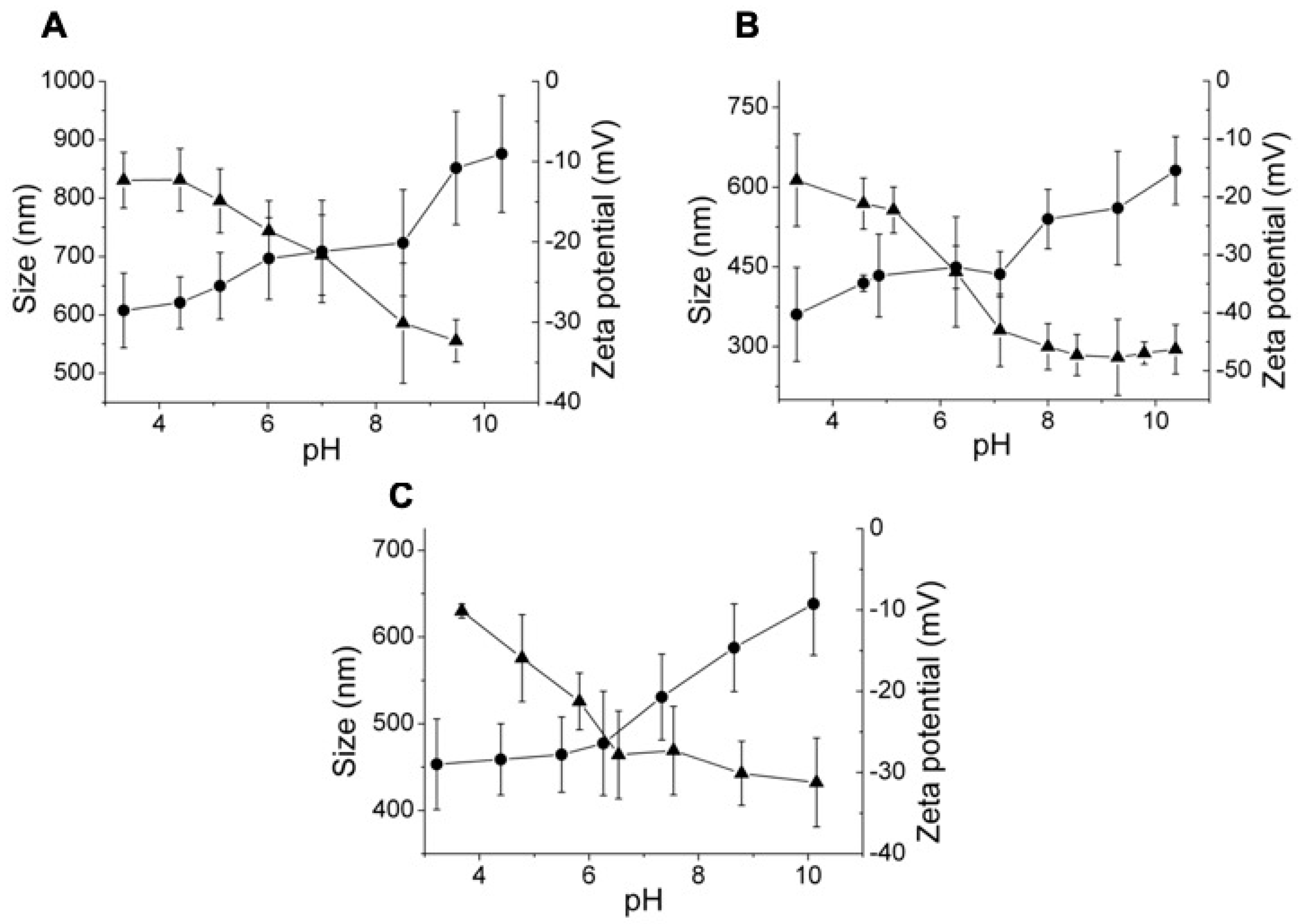
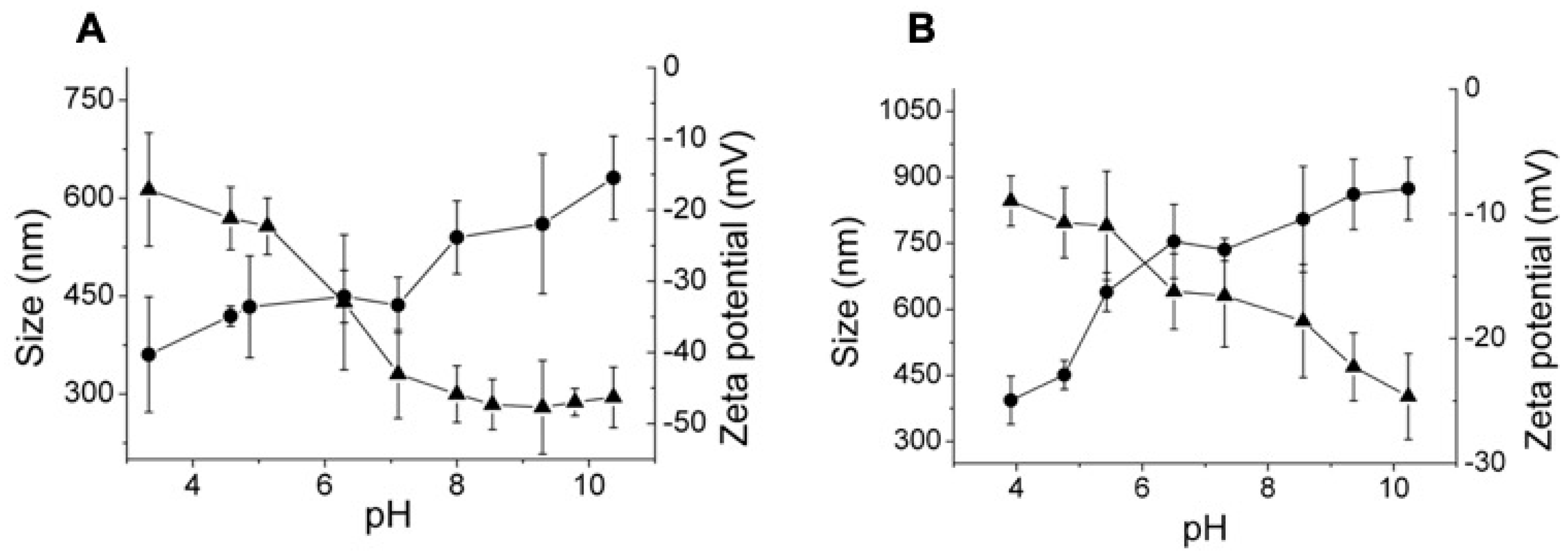
3.2. Effect of the Crosslinking Agent
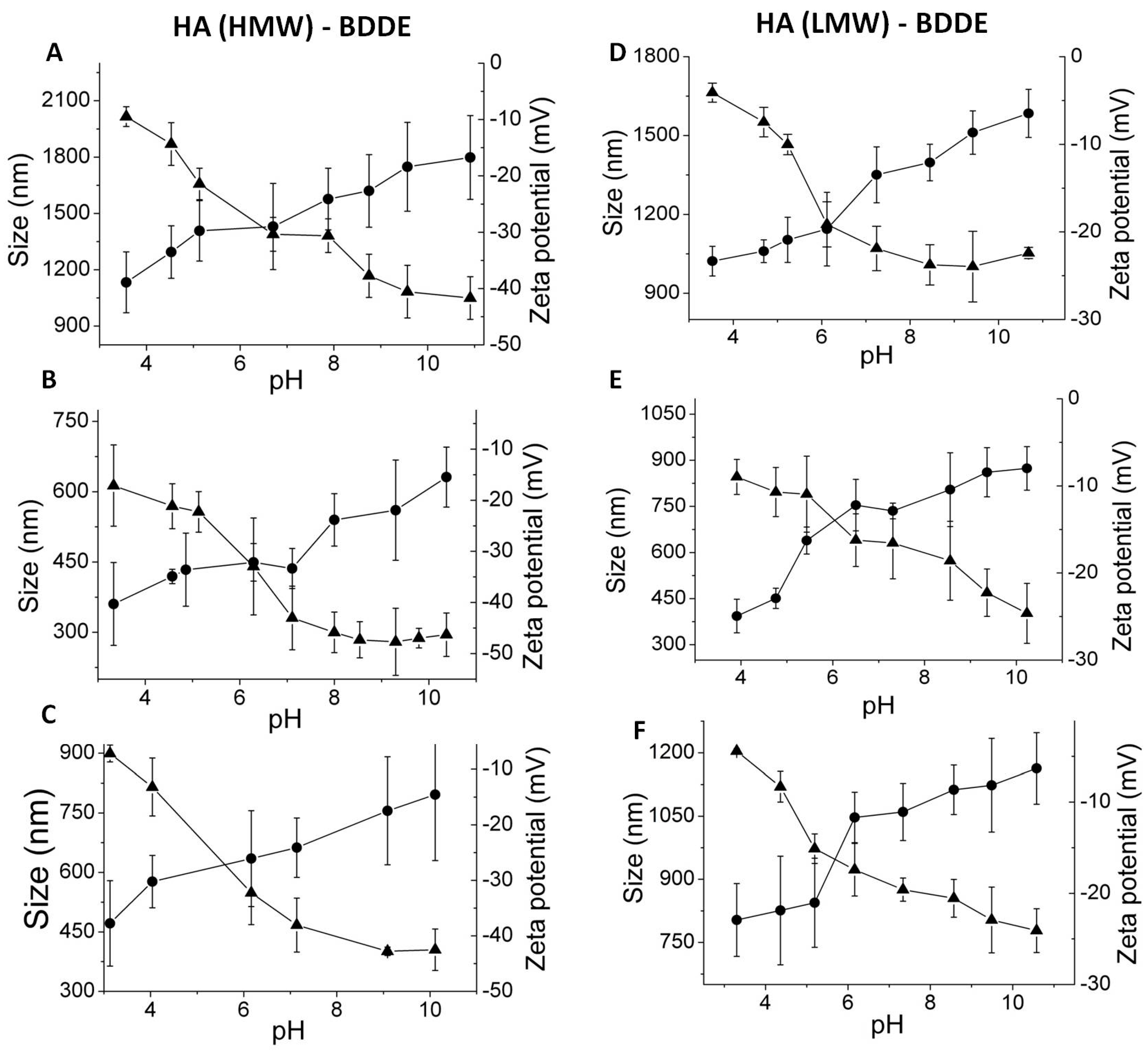
3.3. Effect of HA Molecular Weight and BDDE Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lipinski, C. Poor Aqueous Solubility: An Industry Wide Problem in Drug Delivery Discovery. Am. Pharm. Rev. 2016, 5, 1–16. [Google Scholar]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Tahara, Y.; Akiyoshi, K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv. Drug Deliv. Rev. 2015, 95, 65–76. [Google Scholar] [CrossRef]
- Wu, H.Q.; Wang, C.C. Biodegradable smart nanogels: A new platform for targeting drug delivery and biomedical diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef] [PubMed]
- Asadian-Birjand, M.; Sousa-Herves, A.; Steinhilber, D. Functional nanogels for biomedical applications. Curr. Med. Chem. 2012, 19, 5029–5043. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro- and nanogels with labile crosslinks-from synthesis to biomedical applications. Chem. Soc. Rev. 2015, 44, 1948–1973. [Google Scholar] [CrossRef]
- O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Cross-linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006, 35, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.H.; Ho, V.T.; Huang, W.C.; Huang, Y.F.; Chern, C.S.; Chiu, H.C. Dual stimuli-responsive polymeric hollow nanogels designed as carriers for intracellular triggered drug release. Langmuir 2012, 28, 15056–15064. [Google Scholar] [CrossRef]
- Sanson, N.; Rieger, J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 2010, 1, 965–977. [Google Scholar] [CrossRef]
- Elsabahy, M.; Heo, G.S.; Lim, S.M.; Sun, G.; Wooley, K.L. Polymeric Nanostructures for Imaging and Therapy. Chem. Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef] [PubMed]
- Desale, S.S.; Cohen, S.M.; Zhao, Y.; Kabanov, A.V.; Bronich, T.K. Biodegradable hybrid polymer micelles for combination drug therapy in ovarian cancer. J. Control. Release 2013, 171, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Siegwart, D.J.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Washburn, N.R.; Matyjaszewski, K. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 2009, 30, 5270–5278. [Google Scholar] [CrossRef]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Control. Release 2010, 142, 483–489. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Washburn, N.R.; Matyjaszewski, K. Synthesis by AGET ATRP of degradable nanogel precursors for in situ formation of nanostructured hyaluronic acid hydrogel. Biomacromolecules 2009, 10, 2499–2507. [Google Scholar] [CrossRef]
- Garg, H.G.; Hales, C.A. Chemistry and Biology of Hyaluronan, 1st ed.; Elsevier: Boston, MA, USA, 2004. [Google Scholar]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Modification of natural polymers: Hyaluronic acid. In Methods of Tissue Engineering; Academic Press: San Diego, CA, USA, 2001; Volume 1, pp. 539–553. [Google Scholar]
- Aigner, J.; Tegeler, J.; Hutzler, P.; Campoccia, D.; Pavesio, A.; Hammer, C.; Kastenbauer, E.; Naumann, A. Cartilage tissue engineering with novel nonwomen structured biomaterial based on hyaluronic acid benzyl ester. J. Biomed. Mater. Res. Banner 1998, 42, 22–24. [Google Scholar]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef]
- Gold, M. The science and art of hyaluronic acid dermal filler use in esthetic applications. J. Cosmet. Dermatol. 2009, 8, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, D. Nanostructured hyaluronic acid-based materials for active delivery to cancer. Expert Opin. Drug Deliv. 2010, 7, 681–703. [Google Scholar] [CrossRef]
- Jha, A.K.; Malik, M.S.; Farach-Carson, M.C.; Duncan, R.L.; Jia, X. Hierarchically structured, hyaluronic acid-based hydrogel matrices via the covalent integration of microgels into macroscopic networks. Soft Matter 2010, 6, 5045–5055. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Kweon, D.K.; Park, H.J. Investigations on skin permeation of hyaluronic acid based nanoemulsion as transdermal carrier. Carbohydr. Polym. 2011, 86, 837–843. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chau, Y. One-Step “Click” Method for Generating Vinyl Sulfone Groups on Hydroxyl-Containing Water-Soluble Polymers. Biomacromolecules 2012, 13, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Kenne, L.; Gohil, S.; Nilsson, E.M.; Karlsson, A.; Ericsson, D.; Helander Kenne, A.; Nord, L.I. Modification and cross-linking parameters in hyaluronic acid hydrogels—Definitions and analytical methods. Carbohydr. Polym. 2013, 91, 410–418. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Fidalgo, J.; Deglesne, P.; Arroyo, R.; Sepúlveda, L.; Deprez, P. Detection of a new reaction by-product in BDDE cross-linked autoclaved hyaluronic acid hydrogels by LC– S analysis. Med. Devices Evid. Res. 2018, 1, 367–376. [Google Scholar] [CrossRef]
- Lai, J. Relationship between structure and cytocompatibility of divinyl sulfone cross-linked hyaluronic acid. Carbohydr. Polym. 2014, 101, 203–212. [Google Scholar] [CrossRef]
- Bulpitt, P.; Aeschlimann, D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. [Google Scholar] [CrossRef]
- Choi, K.Y.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef]
- Bogdan Allemann, I.; Baumann, L. Hyaluronic acid gel preparations in the treatment of facial wrinkled and folds. Clin. Interv. Aging 2008, 3, 629–634. [Google Scholar]
- Boonme, P.; Krauel, K.; Graf, A.; Rades, T.; Junyaprasert, V.B. Characterization of microemulsion structures in the pseudoternary phase diagram of isopropyl palmitate/water. AAPS PharmSciTech 2006, 7, E1–E6. [Google Scholar] [CrossRef]
- Strasser, S.; Slugovc, C. Nucleophile-mediated oxa-Michael addition reactions of divinyl sulfone—A thiol-free option for step-growth polymerisations. Catal. Sci. Technol. 2015, 5, 5091–5094. [Google Scholar] [CrossRef]
- Al-Sibani, M.; Al-Harrasi, A.; Neubert, R.H.H. Study of the effect of mixing approach on cross-linking efficiency of hyaluronic acid-based hydrogel cross-linked with 1,4-butanediol diglycidyl ether. Eur. J. Pharm. Sci. 2016, 91, 131–137. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Pires, A.M.B.; Lichy, R.; Santana, M.H.A. The performance of crosslinking with divinyl sulfone as controlled by the interplay between the chemical modification and conformation of hyaluronic acid. J. Braz. Chem. Soc. 2015, 26, 506–512. [Google Scholar] [CrossRef]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef]
- Zhibing, H.; Xiaohu, X.; Liping, T. Process for Synthesizing Oil and Surfactant-Free Hyaluronic Acid Nanoparticles and Microparticles. U.S. Patent 7,601,704 B2, 13 October 2009. [Google Scholar]
- Jeon, O.; Song, S.J.; Lee, K.J.; Park, M.H.; Lee, S.H.; Hahn, S.K.; Kim, S.; Kim, B.S. Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities. Carbohydr. Polym. 2007, 70, 251–257. [Google Scholar] [CrossRef]
- Prestwich, G.D.; Marecak, D.M.; Marecek, J.F.; Vercruysse, K.P.; Ziebell, M.R. Controlled chemical modification of hyaluronic acid: Synthesis, applications, and biodegradation of hydrazide derivatives. J. Control. Release 1998, 53, 93–103. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.; Zhu, Y.; Wu, X.; Wang, Y.; Liang, F.; Song, S. Hyaluronic-Acid-Based pH-Sensitive Nanogels for Tumor-Targeted Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 2410–2419. [Google Scholar] [CrossRef]
- Labhasetwar, V.; Leslie-Pelecky, D.L. Nanogels: Chemistry to drug delivery. In Biomedical Applications of Nanotechnology; Wiley: Weinheim, Germany, 2007; pp. 131–170. [Google Scholar]
- Kobayashi, Y.; Okamoto, A.; Nishinari, K. Vicoelasticity of hyaluronic acid with different molecular weights. Biorheology 1994, 31, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sáez Martínez, V.; Pérez-Álvarez, L.; Hernáez, E.; Herrero, T.; Katime, I. Synthesis, Characterization, and Influence of Synthesis Parameters on Particle Sizes of a New Microgel Family. Polym. Chem. 2007, 45, 3833–3842. [Google Scholar] [CrossRef]
| HA Molecular Weight | BDDE Equivalents | Swelling Degree (%) (Dried-swollen pH = 4) | Swelling Degree (%) (∆pH) | Crosslinking (%) 1 |
|---|---|---|---|---|
| 2.1 MDa | 1–0.2 | 28 | 59 | 15 |
| 1–1 | 17 | 75 | 78 | |
| 1–10 | 21 | 38 | - | |
| 751 KDa | 1–0.2 | 35 | 53 | 22 |
| 1–1 | 18 | 94 | 73 | |
| 1–10 | 29 | 36 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiz-Fernández, S.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Pérez González, R.; Sáez-Martínez, V.; Ruiz Pérez, J.; Vilas-Vilela, J.L. Synthesis and Characterization of Covalently Crosslinked pH-Responsive Hyaluronic Acid Nanogels: Effect of Synthesis Parameters. Polymers 2019, 11, 742. https://doi.org/10.3390/polym11040742
Maiz-Fernández S, Pérez-Álvarez L, Ruiz-Rubio L, Pérez González R, Sáez-Martínez V, Ruiz Pérez J, Vilas-Vilela JL. Synthesis and Characterization of Covalently Crosslinked pH-Responsive Hyaluronic Acid Nanogels: Effect of Synthesis Parameters. Polymers. 2019; 11(4):742. https://doi.org/10.3390/polym11040742
Chicago/Turabian StyleMaiz-Fernández, Sheila, Leyre Pérez-Álvarez, Leire Ruiz-Rubio, Raúl Pérez González, Virginia Sáez-Martínez, Jesica Ruiz Pérez, and José Luis Vilas-Vilela. 2019. "Synthesis and Characterization of Covalently Crosslinked pH-Responsive Hyaluronic Acid Nanogels: Effect of Synthesis Parameters" Polymers 11, no. 4: 742. https://doi.org/10.3390/polym11040742
APA StyleMaiz-Fernández, S., Pérez-Álvarez, L., Ruiz-Rubio, L., Pérez González, R., Sáez-Martínez, V., Ruiz Pérez, J., & Vilas-Vilela, J. L. (2019). Synthesis and Characterization of Covalently Crosslinked pH-Responsive Hyaluronic Acid Nanogels: Effect of Synthesis Parameters. Polymers, 11(4), 742. https://doi.org/10.3390/polym11040742









