An Albumin Biopassive Polyallylamine Film with Improved Blood Compatibility for Metal Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Plasma Polymerization of the Polyallylamine Films
2.2.2. Surface Characterization of the Polyallylamine Films
2.2.3. Film Stability
2.2.4. BSA Immobilization and Characterization
2.2.5. In vitro Hemocompatibility Evaluation
2.3. Statistical Analysis
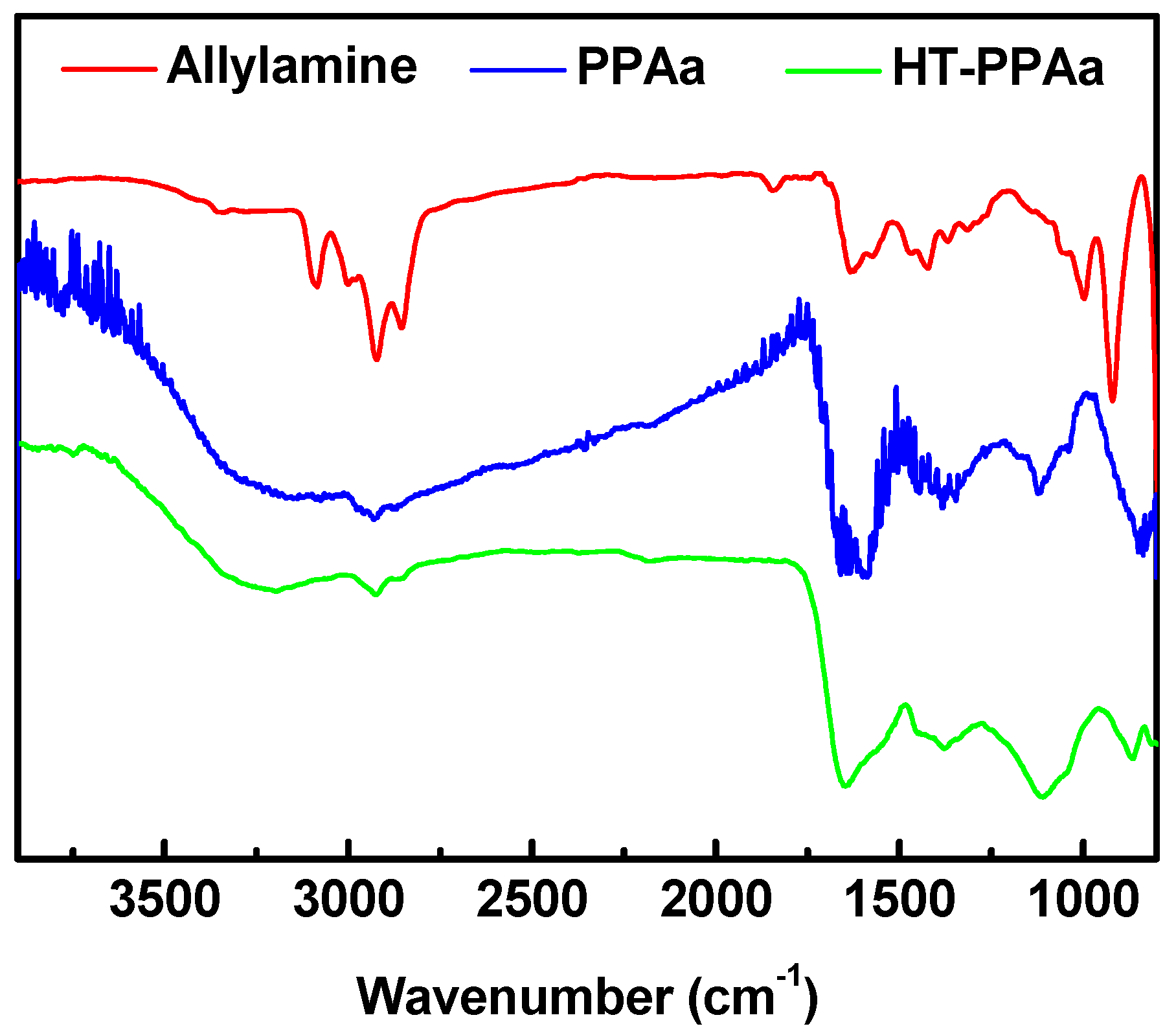
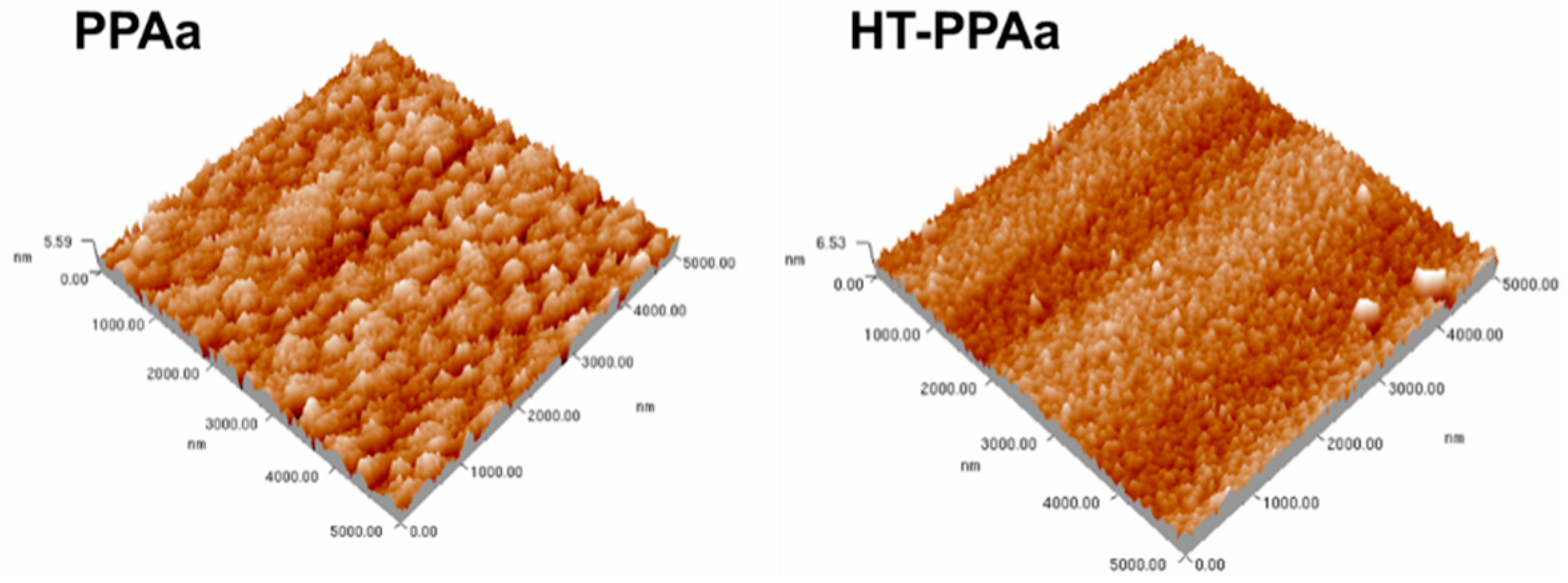
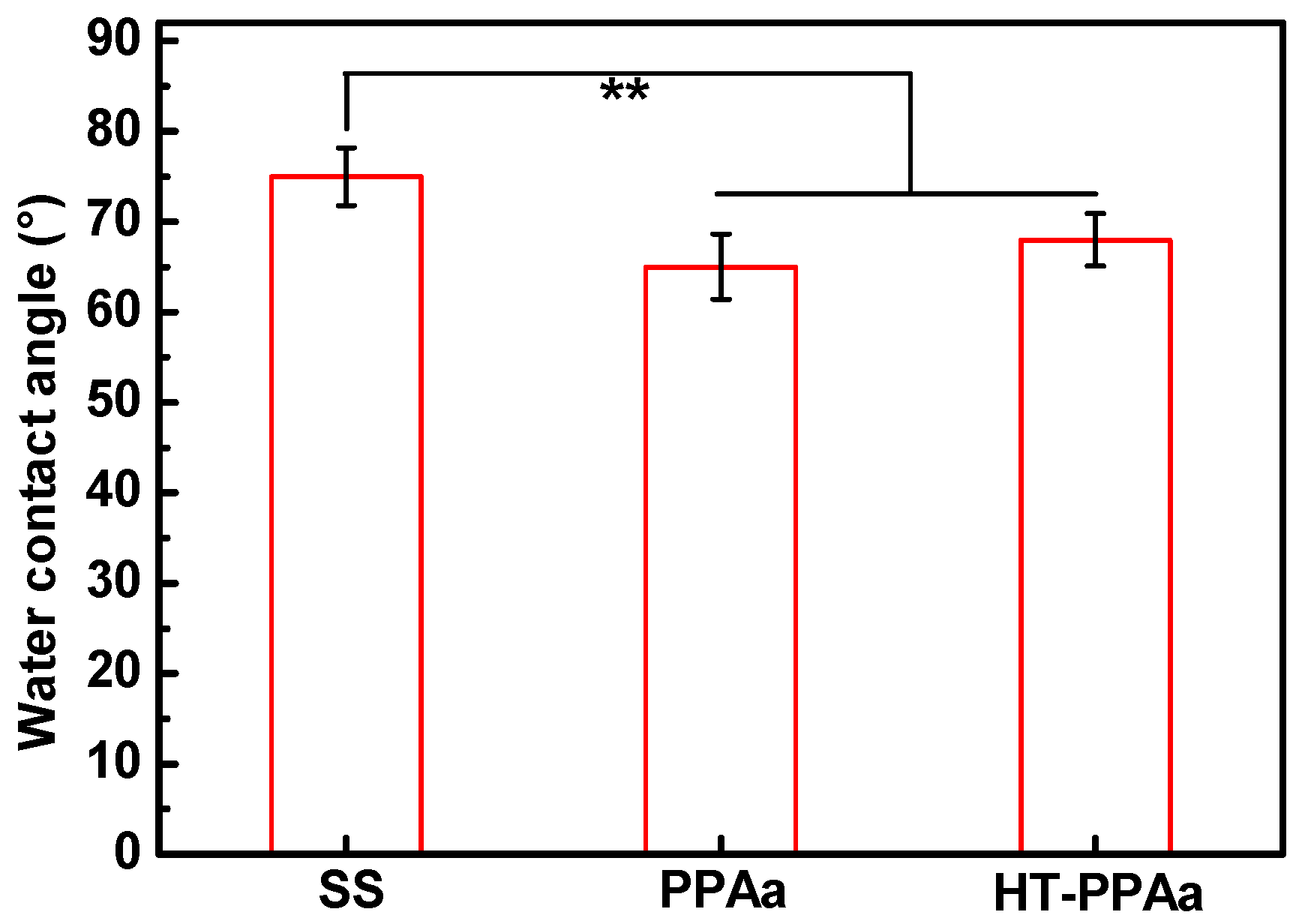
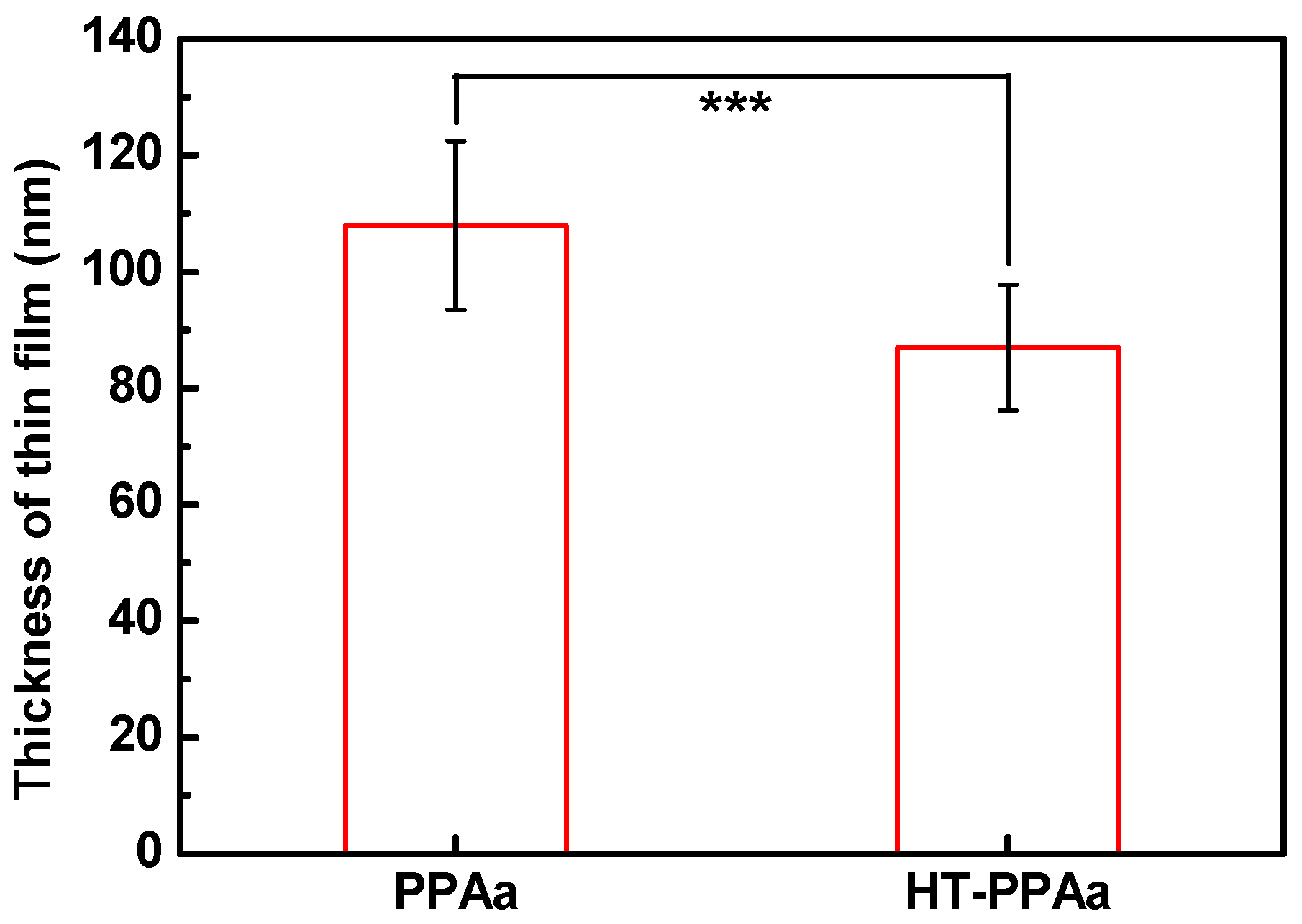
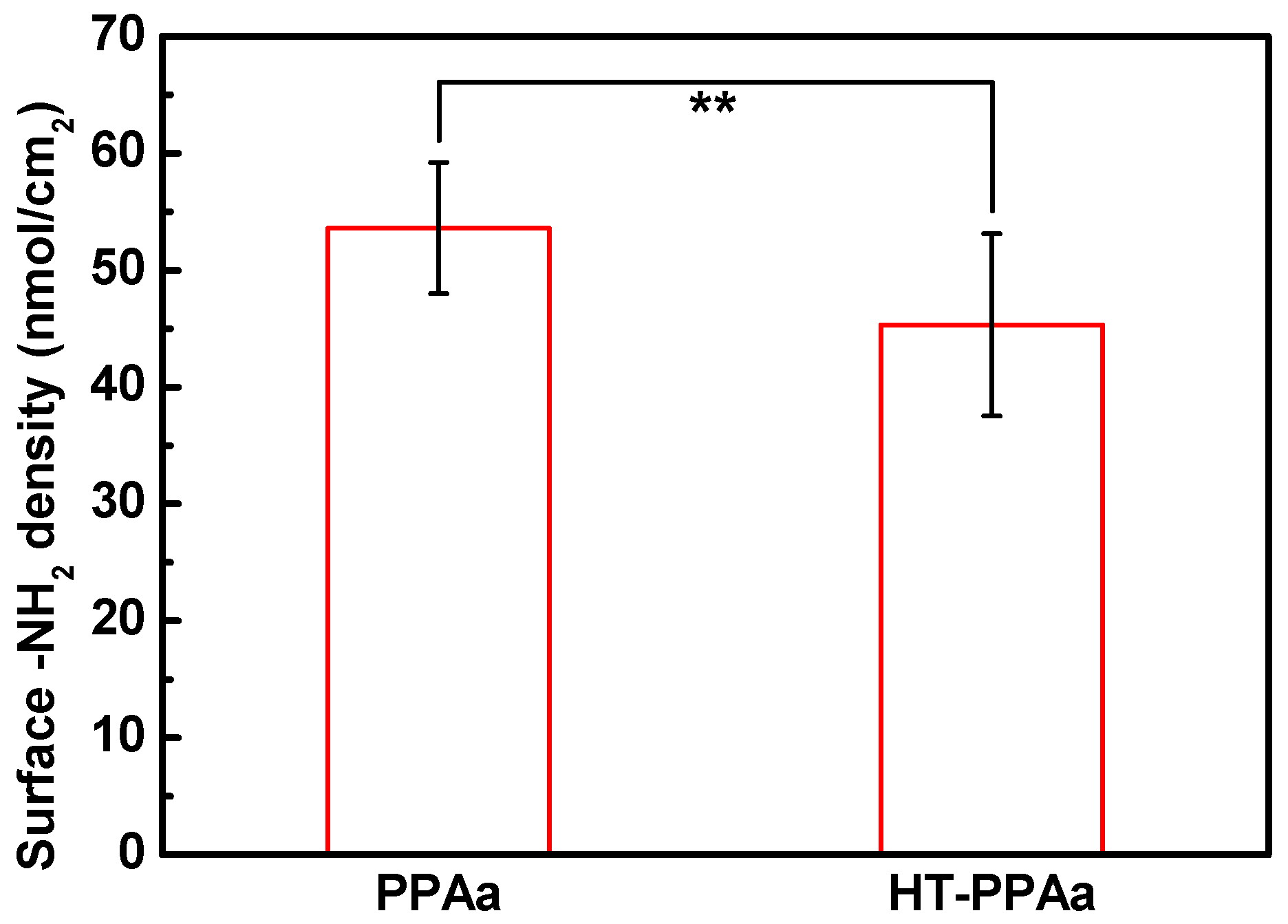
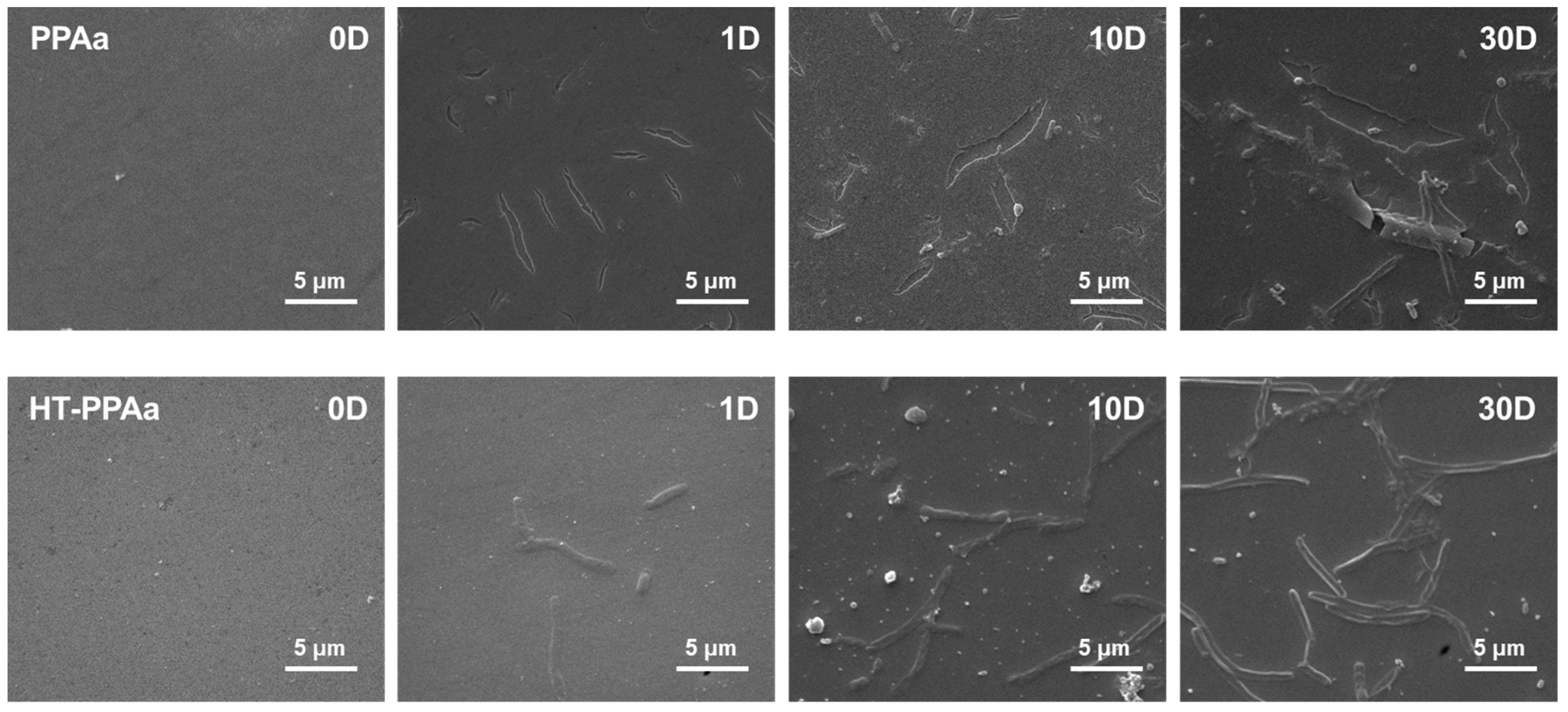
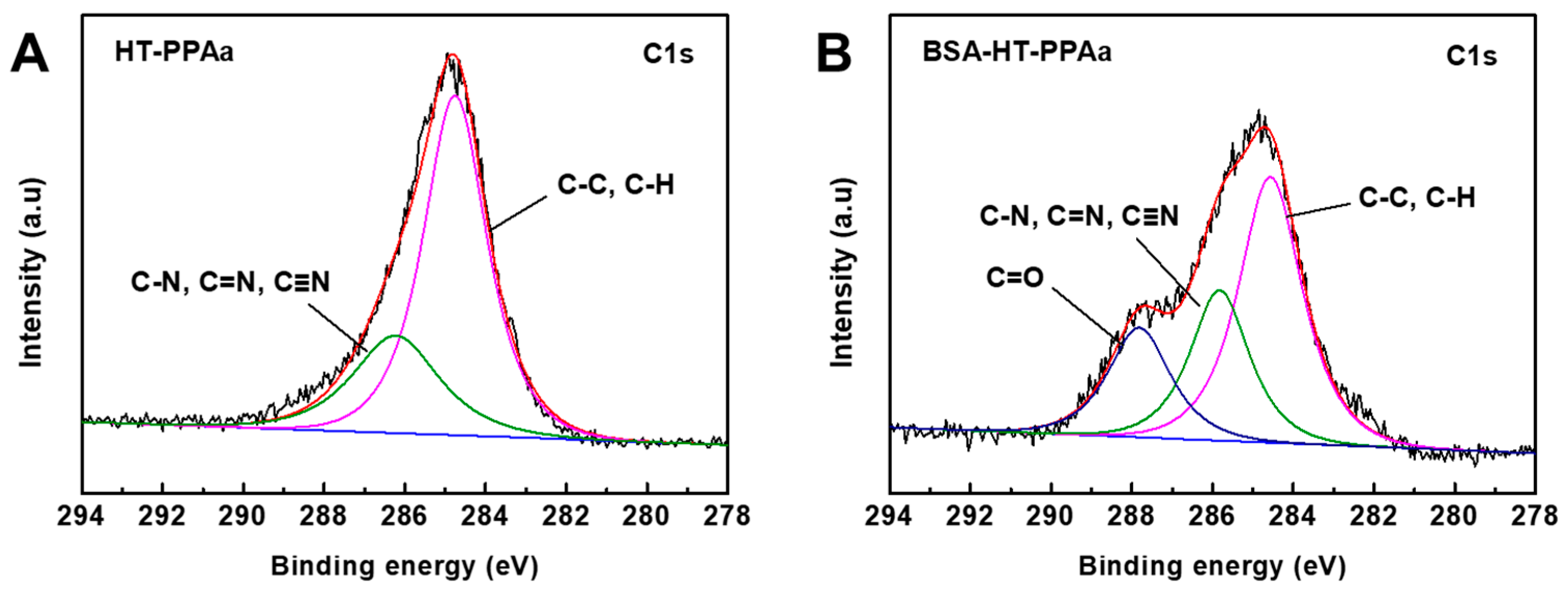
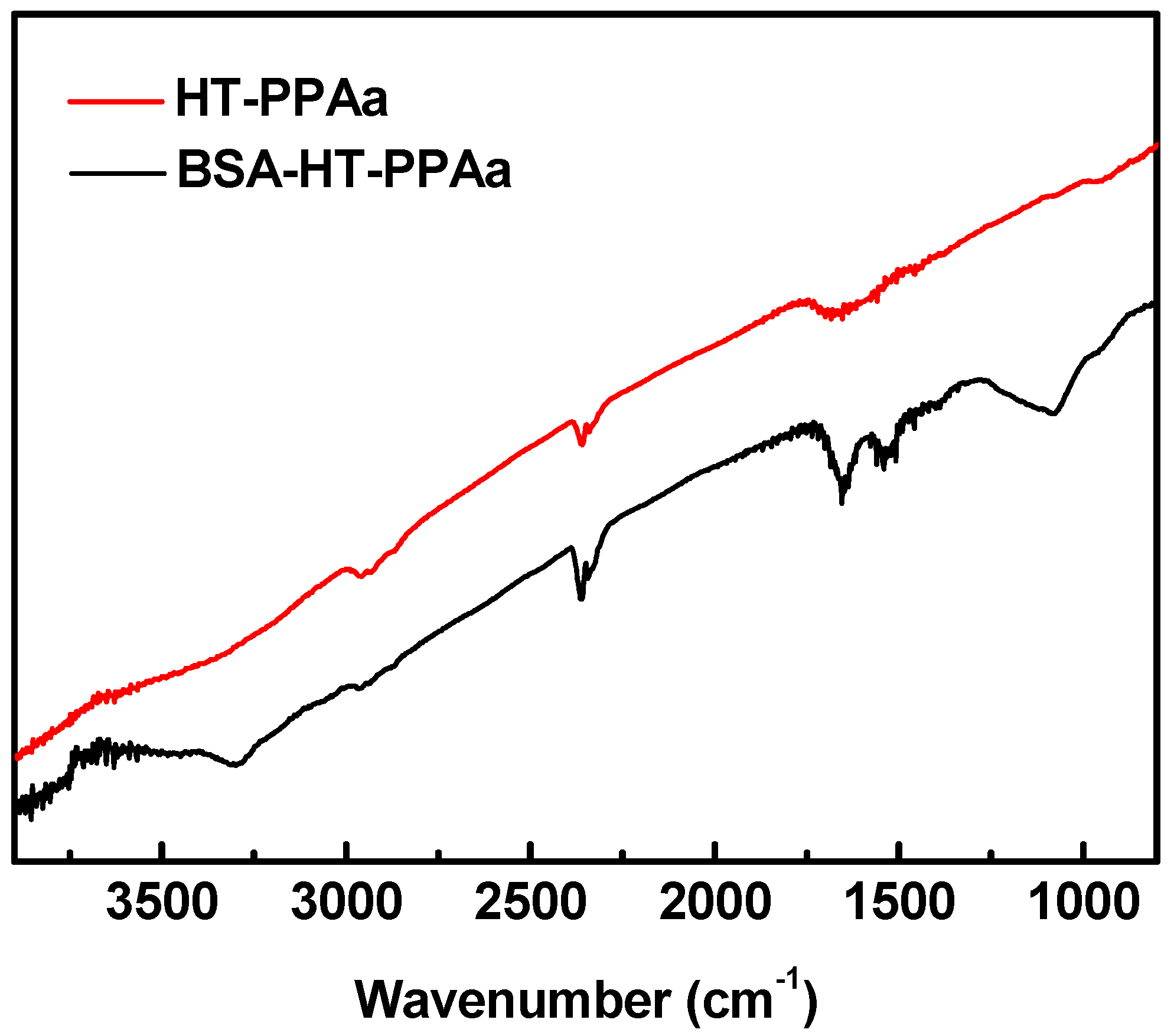
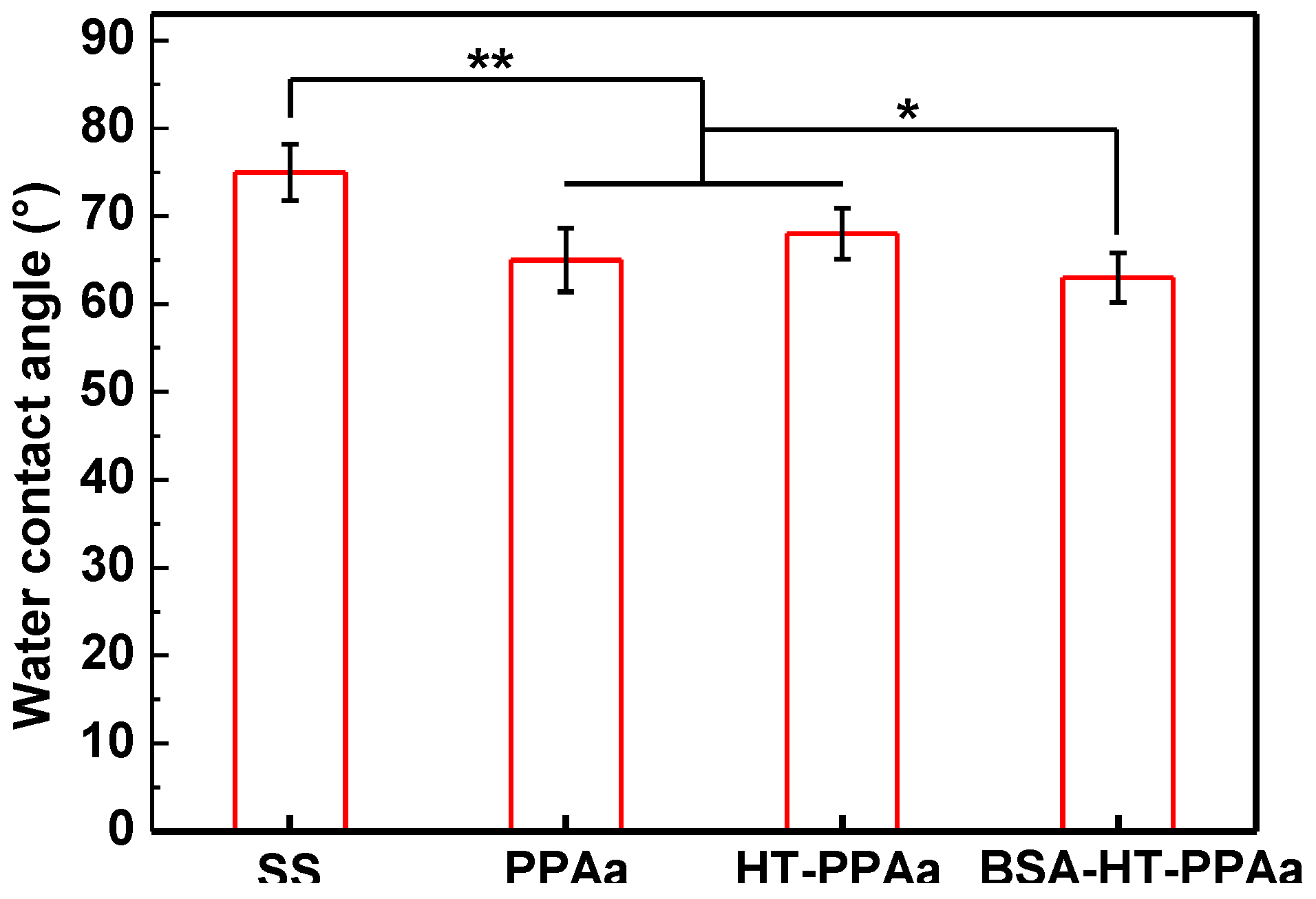
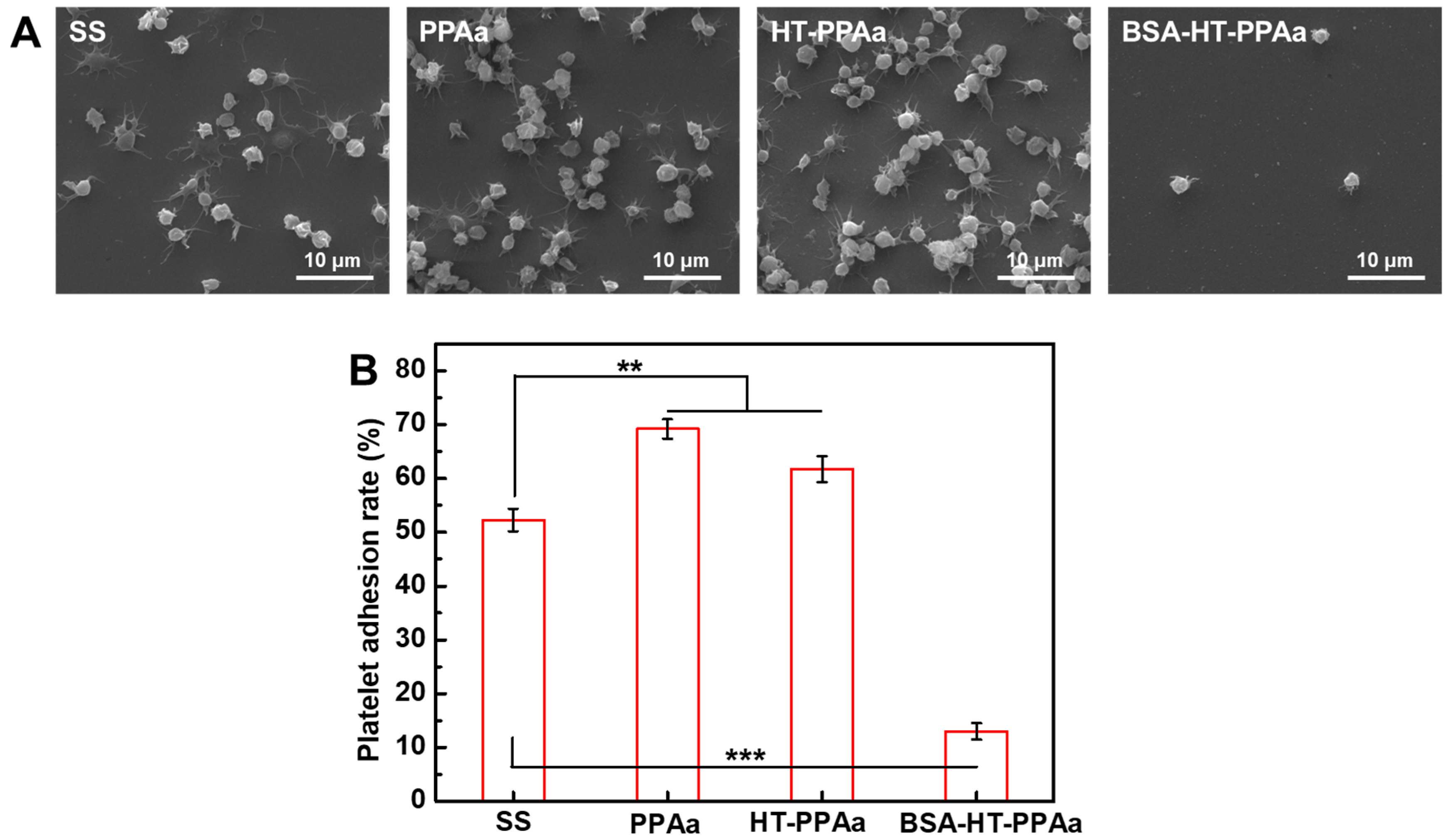
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef]
- Fischer, M.; Maitz, M.; Werner, C. Coatings for biomaterials to improve hemocompatibility. In Hemocompatibility of Biomaterials for Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–190. [Google Scholar]
- Deppisch, R.; Storr, M.; Buck, R.; Gohl, H. Blood material interactions at the surfaces of membranes in medical applications. Sep. Purif. Technol. 1998, 14, 241–254. [Google Scholar] [CrossRef]
- Werner, C.; Maitz, M.F.; Sperling, C. Current strategies towards hemocompatible coatings. J. Mater. Chem. 2007, 17, 3376–3384. [Google Scholar] [CrossRef]
- Peckham, S.M.; Turitto, V.T.; Glantz, J.; Puryear, H.; Slack, S.M. Hemocompatibility studies of surface-treated polyurethane-based chronic indwelling catheters. J. Biomater. Sci. Polym. E 1997, 8, 847–858. [Google Scholar] [CrossRef]
- Ye, X.; Shao, Y.L.; Zhou, M.; Li, J.; Cai, L. Research on micro-structure and hemo-compatibility of the artificial heart valve surface. Appl. Surf. Sci. 2009, 255, 6686–6690. [Google Scholar] [CrossRef]
- Athanasoulis, C.A.; Kaufman, J.A.; Halpern, E.F.; Waltman, A.C.; Geller, S.C.; Fan, C.M. Inferior vena caval filters: Review of a 26-year single-center clinical experience. Radiology 2000, 216, 54–66. [Google Scholar] [CrossRef]
- Maitz, M.F.; Pham, M.T.; Wieser, E. Blood compatibility of titanium oxides with various crystal structure and element doping. J. Biomater. Appl. 2003, 17, 303–319. [Google Scholar] [CrossRef]
- Windecker, S.; Mayer, I.; De Pasquale, G.; Maier, W.; Dirsch, O.; De Groot, P.; Wu, Y.P.; Noll, G.; Leskosek, B.; Meier, B.; et al. Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation 2001, 104, 928–933. [Google Scholar] [CrossRef]
- Manivasagam, G.; Suwas, S. Biodegradable mg and mg based alloys for biomedical implants. Mater. Sci. Technol. Lond. 2014, 30, 515–520. [Google Scholar] [CrossRef]
- Yang, Z.L.; Wang, J.; Luo, R.F.; Maitz, M.F.; Jing, F.J.; Sun, H.; Huang, N. The covalent immobilization of heparin to pulsed-plasma polymeric allylamine films on 316l stainless steel and the resulting effects on hemocompatibility. Biomaterials 2010, 31, 2072–2083. [Google Scholar] [CrossRef]
- Mohan, C.C.; Chennazhi, K.P.; Menon, D. In vitro hemocompatibility and vascular endothelial cell functionality on titania nanostructures under static and dynamic conditions for improved coronary stenting applications. Acta Biomater. 2013, 9, 9568–9577. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Zhang, D.Y.; Shen, F.; Zhang, G.; Song, S.H. Hemocompatibility and anti-endothelialization of copper-titanium coating for vena cava filters. Surf. Coat. Technol. 2012, 206, 3501–3507. [Google Scholar] [CrossRef]
- Zimmermann, A.K.; Weber, N.; Aebert, H.; Ziemer, G.; Wendel, H.P. Effect of biopassive and bioactive surface-coatings on the hemocompatibility of membrane oxygenators. J. Biomed. Mater. Res. B 2007, 80, 433–439. [Google Scholar] [CrossRef]
- Tanzi, M.C. Bioactive technologies for hemocompatibility. Expert Rev. Med. Devices 2005, 2, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Winnik, F.M.; Merhi, Y.; Griesser, H.J.; Tabrizian, M. Biomimetic hemocompatible coatings through immobilization of hyaluronan derivatives on metal surfaces. Langmuir 2008, 24, 11834–11841. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Hu, L.; Li, C.; Gan, L.; He, M.; He, X.H.; Tian, W.Q.; Li, M.M.; Xu, L.; Li, Y.P.; et al. Improvement in physical and biological properties of chitosan/soy protein films by surface grafted heparin. Int. J. Biol. Macromol. 2016, 83, 19–29. [Google Scholar] [CrossRef]
- Kakavand, M.; Yazdanpanah, G.; Ahmadiani, A.; Niknejad, H. Blood compatibility of human amniotic membrane compared with heparin-coated eptfe for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Zhang, X.; Zhao, S.; Maitz, M.F.; Zhang, W.T.; Yang, S.; Mao, J.L.; Huang, N.; Wan, G.J. In situ incorporation of heparin/bivalirudin into a phytic acid coating on biodegradable magnesium with improved anticorrosion and biocompatible properties. J. Mater. Chem. B 2017, 5, 4162–4176. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, F.; Qin, Y.; He, J.D.; Xiong, Z.; Deng, G.; Li, Q. A novel natural hirudin facilitated anti-clotting polylactide membrane via hydrogen bonding interaction. J. Membr. Sci. 2017, 523, 505–514. [Google Scholar] [CrossRef]
- Xue, T.; Peng, B.; Xue, M.; Zhong, X.; Chiu, C.Y.; Yang, S.; Qu, Y.Q.; Ruan, L.Y.; Jiang, S.; Dubin, S.; et al. Integration of molecular and enzymatic catalysts on graphene for biomimetic generation of antithrombotic species. Nat Commun 2014, 5, 3200. [Google Scholar] [CrossRef]
- Hansson, K.M.; Tosatti, S.; Isaksson, J.; Wettero, J.; Textor, M.; Lindahl, T.L.; Tengvall, P. Whole blood coagulation on protein adsorption-resistant peg and peptide functionalised peg-coated titanium surfaces. Biomaterials 2005, 26, 861–872. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, Y.M.; Kim, D.M. Platelet adhesion onto segmented polyurethane film surfaces modified by addition and crosslinking of peo-containing block copolymers. Biomaterials 2000, 21, 683–691. [Google Scholar] [CrossRef]
- Abraham, S.; Brahim, S.; Ishihara, K.; Guiseppi-Elie, A. Molecularly engineered p(hema)-based hydrogels for implant biochip biocompatibility. Biomaterials 2005, 26, 4767–4778. [Google Scholar] [CrossRef]
- Ferez, L.; Thami, T.; Akpalo, E.; Flaud, V.; Tauk, L.; Janot, J.M.; Dejardin, P. Interface of covalently bonded phospholipids with a phosphorylicholine head: Characterization, protein nonadsorption, and further functionalization. Langmuir 2011, 27, 11536–11544. [Google Scholar] [CrossRef]
- Sin, M.C.; Chen, S.H.; Chang, Y. Hemocompatibility of zwitterionic interfaces and membranes. Polym. J. 2014, 46, 436–443. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sasakawa, S.; Takase, M.; Osada, Y. Effect of albumin immobilization by plasma polymerization on platelet reactivity. Thromb. Res. 1984, 35, 193–202. [Google Scholar] [CrossRef]
- Noh, H.; Vogler, E.A. Volumetric interpretation of protein adsorption: Mass and energy balance for albumin adsorption to particulate adsorbents with incrementally increasing hydrophilicity. Biomaterials 2006, 27, 5801–5812. [Google Scholar] [CrossRef]
- Amiji, M.; Park, H.; Park, K. Study on the prevention of surface-induced platelet activation by albumin coating. J. Biomater. Sci. Polym. E 1992, 3, 375–388. [Google Scholar] [CrossRef]
- Liu, X.L.; Yuan, L.; Li, D.; Tang, Z.C.; Wang, Y.W.; Chen, G.J.; Chen, H.; Brash, J.L. Blood compatible materials: State of the art. J. Mater. Chem. B 2014, 2, 5718–5738. [Google Scholar] [CrossRef]
- Sefton, M.V.; Gemmel, C.H. Nonthrombogenic treatments and strategies. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Zhao, A.S.; Yang, P.; Leng, Y.X.; Sun, H.; Wang, J.; Wan, G.J.; Huang, N. Research on platelet adsorption behavior using enzyme immunoassays and ldh testing. Key Eng. Mater. 2005, 288–289, 515–518. [Google Scholar] [CrossRef]
- Yang, Z.L.; Wang, X.N.; Wang, J.; Yao, Y.; Sun, H.; Huang, N. Pulsed-plasma polymeric allylamine thin films. Plasma Process. Polym. 2009, 6, 498–505. [Google Scholar] [CrossRef]
- Krishnamurthy, V.; Kamel, I.L.; Wei, Y. Analysis of plasma polymerization of allylamine by ftir. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 1211–1224. [Google Scholar] [CrossRef]
- Huang, B.X.; Kim, H.Y.; Dass, C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Courtney, J.M.; Lamba, N.M.K.; Sundaram, S.; Forbes, C.D. Biomaterials for blood-contacting applications. Biomaterials 1994, 15, 737–744. [Google Scholar] [CrossRef]
- Xu, L.C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Li, X.; Wang, K.; Shang, T.; Zhou, L.; Zhang, L.; Wang, J.; Huang, N. An Albumin Biopassive Polyallylamine Film with Improved Blood Compatibility for Metal Devices. Polymers 2019, 11, 734. https://doi.org/10.3390/polym11040734
Lin S, Li X, Wang K, Shang T, Zhou L, Zhang L, Wang J, Huang N. An Albumin Biopassive Polyallylamine Film with Improved Blood Compatibility for Metal Devices. Polymers. 2019; 11(4):734. https://doi.org/10.3390/polym11040734
Chicago/Turabian StyleLin, Shuang, Xin Li, Kebing Wang, Tengda Shang, Lei Zhou, Lu Zhang, Jin Wang, and Nan Huang. 2019. "An Albumin Biopassive Polyallylamine Film with Improved Blood Compatibility for Metal Devices" Polymers 11, no. 4: 734. https://doi.org/10.3390/polym11040734
APA StyleLin, S., Li, X., Wang, K., Shang, T., Zhou, L., Zhang, L., Wang, J., & Huang, N. (2019). An Albumin Biopassive Polyallylamine Film with Improved Blood Compatibility for Metal Devices. Polymers, 11(4), 734. https://doi.org/10.3390/polym11040734



