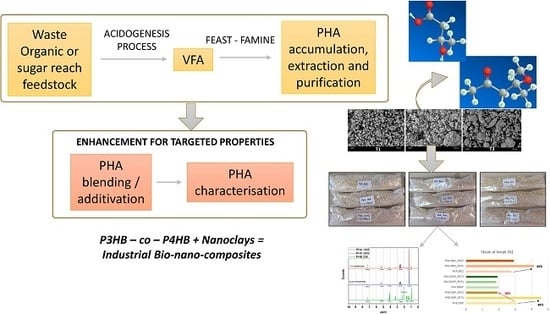
Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite
Abstract
:1. Introduction
- Create an effective after-use plastics economy by improving the economics and uptake of recycling, reuse and controlled biodegradation for targeted applications;
- Drastically reduce leakage of plastics into natural systems (in particular, the ocean);
- Decouple plastics from fossil feedstocks by exploring and adopting renewably sourced feedstocks.
2. Materials and Methods
2.1. Materials
- T1: Modified sepiolite: organically modified through aminosilane groups on the surface;
- T2: Natural sepiolite without modifications on its surface (naturally containing silanol groups, commercially marketed as Pangel 9);
- T3: Natural sodium montmorillonite (Na-MMT) modified with a quaternary ammonium salt; it is an anionic organoclay (highly compatible with nonpolar polymers).
2.2. Nanobiocomposite Preparation
2.3. General Characterisation Methods
3. Results and Discussion
3.1. Mechanical Results (Flexural and Tensile)
3.2. NMR
3.3. DSC—Differential Scanning Calorimetry
3.4. WAX—X-ray Diffraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plastics Europe, Plastics—the Facts 2018. An analysis of European plastics production, demand and waste data. Available online: https://www.plasticseurope.org/es/resources/publications/619-plastics-facts-2018 (accessed on 1 March 2019).
- Plastics Europe, Plastics—The Facts 2015. An analysis of European plastics production, demand and waste data. Available online: https://www.plasticseurope.org/93-plastics-facts-2015 (accessed on 1 March 2019).
- Available online: https://www.cnbc.com/2018/04/16/climate-change-china-bans-import-of-foreign-waste-to-stop-pollution.html (accessed on 1 March 2019).
- Available online: https://www.reuters.com/article/us-eu-environment/eu-targets-recycling-as-china-bans-plastic-waste-imports-idUSKBN1F51SP (accessed on 1 March 2019).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- European Commision. A European Strategy for Plastics in a Circular Economy. 2015. Available online: http://ec.europa.eu/environment/circular-economy/pdf/plastics-strategy-brochure.pdf (accessed on 1 March 2019).
- Kuang, T.; Ju, J.; Yang, Z.; Geng, L.; Peng, X. A facile approach towards fabrication of lightweight biodegradable poly(butylene succinate)/carbon fiber composite foams with high electrical conductivity and strength. Compos. Sci. Technol. 2018, 159, 171–179. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 1–21. [Google Scholar] [CrossRef]
- Kuang, T.; Chang, L.; Chen, F.; Sheng, Y.; Fu, D.; Peng, X. Facile preparation of lightweight high-strength biodegradable polymer/multi-walled carbon nanotubes nanocomposite foams for electromagnetic interference shielding. Carbon 2016, 105, 305–313. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Data Report. 2016. Available online: https://docs.european-bioplastics.org/publications/EUBP_Bioplastics_market_data_report_2016.pdf (accessed on 1 March 2019).
- Dreizen, C. 2020 Bioplastics Market Forecast. Sustainable Packaging Coalition (GREENBLUE). 2017. Available online: https://sustainablepackaging.org/2020-bioplastics-market-forecast/ (accessed on 1 March 2019).
- La Rosa, A.D. Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials, 1st ed.; Woodhead Publishing: Cambridge, UK, 2016; ISBN 9780081002148. [Google Scholar]
- Ipsita, R.; Visakh, P.M. Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites. R. Soc. Chem. 2015. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Iwata, T. Sustainability of Biobased and Biodegradable Plastics. Clean 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Jirage, A.; Baravkar, V.; Kate, V.; Payghan, S.; Disouza, J. Poly-β-Hydroxybutyrate: Intriguing Biopolymer in Biomedical Applications and Pharma Formulation Trends. Int. J. Pharm. Biol. Arch. 2013, 4, 1107–1118. [Google Scholar]
- Shrivastav, A.; Kim, H.Y.; Kim, Y.R. Advances in the Applications of Polyhydroxyalkanoate Nanoparticles for Novel Drug Delivery System. Biomed Res. Int. 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.; Hasirci, V. Chapter 15: Polyhydroxybutyrate and Its Copolymers: Applications in the Medical Field. In Tissue Engineering and Novel Delivery Systems; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Chaos, A.; Sangroniz, A.; Gonzalez, A.; Iriarte, V.; Sarasua, J.R.; del Río, J.; Etxeberria, A. Tributyl citrate as an effective plasticizer for biodegradable polymers: Effect of the plasticizer on the free volume, transport and mechanical properties. Polym. Int. 2018. [Google Scholar] [CrossRef]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Choi, J.I.; Lee, S.Y. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol. Bioeng. 1999, 62, 546–553. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Chiellini, E.; Braunegg, G. Extraction of short-chain-length poly-[(R)-hydroxyalkanoates] (scl-PHA) by the antisolvent acetone under elevated temperature and pressure. Biotechnol. Lett. 2013, 35, 1023–1028. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Vasheghani-Farahani, E. Application of supercritical fluid extraction in biotechnology. Crit. Rev. Biotechnol. 2005, 25, 1–12. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; Ragaert, K.; Erkoç, M.; Van Brempt, W.; Faelens, F.; Cardon, L. Heat resistance of biobased materials, evaluation and effect of processing techniques and additives. Polym. Eng. Sci. 2017, 58, 513–520. [Google Scholar] [CrossRef]
- Kai, D.; Chong, H.M.; Chow, L.P.; Jiang, L.; Lin, Q.; Zhang, K.; Loh, X.J. Strong and biocompatible lignin/poly(3-hydroxybutyrate) composite nanofibers. Compos. Sci. Technol. 2018, 158, 26–33. [Google Scholar] [CrossRef]
- Han, H.; Wang, X.; Wu, D. Isothermal Crystallization Kinetics, Morphology, and Mechanical Properties of Biocomposites Based on Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and Recycled Carbon Fiber. Ind. Eng. Chem. Res. 2012, 51, 14047–14060. [Google Scholar] [CrossRef]
- Zini, E.; Focarete, M.L.; Noda, I.; Scandola, M. Bio-composite of bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with vegetable fibers. Compos. Sci. Technol. 2007, 67, 2085–2094. [Google Scholar] [CrossRef]
- An, S.; Ma, X. Properties and structure of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/wood fiber biodegradable composites modified with maleic anhydride. Ind. Crop. Prod. 2017, 109, 882–888. [Google Scholar] [CrossRef]
- Al, G.; Aydemir, D.; Kaygin, B.; Ayrilmis, N.; Gunduz, G. Preparation and characterization of biopolymer nanocomposites from cellulose nanofibrils and nanoclays. J. Compos. Mater. 2017, 52, 689–700. [Google Scholar] [CrossRef]
- Larsson, M.; Hetherington, C.J.D.; Wallenberg, R.; Jannasch, P. Effect of hydrophobically modified graphene oxide on the properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polymer 2017, 108, 66–77. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nanoclays in Food and Beverage Packaging—Review article. J. Nanomater. 2019, 1–13. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Pereira, R.N.; Cerqueira, M.A.; Martins, J.R.; Teixeira, J.A.; Malcata, F.X.; Vicente, A.A. Bio-Based Nanocomposites for Food Packaging and Their Effect in Food Quality and Safety. Food Packag. Preserv. 2018, 271–306. [Google Scholar] [CrossRef]
- Franchini, E. Structuration of Nano-Objects in Epoxy-Based Polymer Systems: Nanoparticles & Nanoclusters for Improved Fire Retardant Properties, Institut National des Sciences Appliquées de Lyon, Lyon. Ph.D. Thesis, INSA Lyon, Villeurbanne, France, 2008. [Google Scholar]
- Falco, G.; Giulieri, F.; Volle, N.; Pagnotta, S.; Sbirrazzuoli, N.; Peuvrel Disdier, E.; Mija, A. Self-organization of sepiolite fibbers in a biobased thermoset. Compos. Sci. Technol. 2019, 171, 226–233. [Google Scholar] [CrossRef]
- Zheng, Y.; Zaoui, A. Mechanical behavior in hydrated Na-montmorillonite clay. Phys. A 2018, 505, 582–590. [Google Scholar] [CrossRef]
- Wang, S.; Song, C.; Chen, G.; Guo, T.; Liu, J.; Zhang, B.; Takeuchi, S. Characteristics and biodegradation properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/organophilic montmorillonite (PHBV/OMMT) nanocomposite. Polym. Degrad. Stab. 2005, 87, 69–76. [Google Scholar] [CrossRef]
- Peinado, V.; García, L.; Fernández, A.; Castell, P. Novel lightweight foamed poly(lactic acid) reinforced with different loadings of functionalised sepiolite. Compos. Sci. Technol. 2014, 101, 17–23. [Google Scholar] [CrossRef]
- Khandal, D.; Pollet, E.; Avérous, L. Elaboration and behavior of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)-nano-biocomposites based on montmorillonite or sepiolite nanoclays. Eur. Polym. J. 2016, 81, 64–76. [Google Scholar] [CrossRef]
- Reinsch, V.; Kelley, S. Crystallization of poly(hydroxybutyrate-cohydroxyvalerate) in wood fiber-reinforced composite. J. Appl. Sci. 1997, 64, 1785–1796. [Google Scholar] [CrossRef]
- Koller, M. Poly(hydroxyalkanoates) for Food Packaging: Application and Attempts towards Implementation. Appl. Food Biotechnol. 2014, 1, 3–15. [Google Scholar]
- Cong, C.; Zhang, S.; Xu, R.; Lu, W.; Yu, D. The Influence of 4HB Content on the Properties of Poly(3-hydroxylbutyrate-co-4-hydroxylbutyrate) based on Melt Molded Sheets. J. Appl. Polym. Sci. 2008, 109, 1962–1967. [Google Scholar] [CrossRef]
- Avérous, L. Nano- and Biocomposites. Mater. Today 2010, 13, 57. [Google Scholar] [CrossRef]
- Botana, A.; Mollo, M.; Eisenberg, P.; Torres Sanchez, R.M. Effect of modified montmorillonite on biodegradable PHB nanocomposites. Appl. Clay Sci. 2010, 47, 263–270. [Google Scholar] [CrossRef]
- Czerniecka-Kubicka, A.; Fracz, W.; Jasiorski, M.; Błazejewski, W.; Pilch-Pitera, B.; Pyda, M.; Zarzyka, I. Thermal properties of poly(3-hydroxybutyrate) modified by nanoclay. J. Therm. Anal. Calorim. 2017, 128, 1513–1526. [Google Scholar] [CrossRef]
- Corre, Y.M.; Bruzaud, S.; Audic, J.L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Hu, D.; Chung, A.L.; Wu, L.P.; Zhang, X.; Wu, Q.; Chen, J.C.; Chen, G.Q. Biosynthesis and Characterization of Polyhydroxyalkanoate Block Copolymer P3HB-b-P4HB. Biomacromolecules 2011, 12, 3166–3173. [Google Scholar] [CrossRef]
- Wang, H.-H.; Zhou, X.-R.; Liu, Q.; Chen, G.Q. Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Appl. Microbiol. Biotechnol. 2010, 89, 1497–1507. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Parulekar, Y. Methods of Making Nanocomposites and Compositions of Rubber Toughened Polyhydroxyalkanoates. U.S. Patent US20070015858A1, 2005. [Google Scholar]
- Xiao, N.; Chen, Y.; Shen, X.; Zhang, C.; Yano, S.; Gottschaldt, M.; Schubert, U.S.; Kakuchi, T.; Satoh, T. Synthesis of miktoarm star copolymer Ru(II) complexes by click-to-chelate approach. Polym. J. 2013, 45, 216–225. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Takahashi, I.; Yan, S. Melting and β to α transition behavior of β-PBA and the β-PBA/PVPh blend investigated by synchrotron SAXS and WAXD. RSC Adv. 2014, 4, 39101. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef]
- Madison, L.L.; Huismann, G.W. Metabolic engineering of poly(3-hydroxyalkanoates): From DNA to plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Utracki, A.L.; Jamieson, A.M. Polymer Physics: From Suspensions to Nanocomposites and Beyond; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-19342-6. [Google Scholar]
- Thiré, R.M.; Arruda, L.C.; Barreto, L.S. Morphology and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/attapulgite nanocomposites. Mater. Res. 2011, 14, 340–344. [Google Scholar] [CrossRef]
- Bayarı, S.; Severcan, F. FTIR study of biodegradable biopolymers: P(3HB), P(3HB-co-4HB) and P(3HB-co-3HV). J. Mol. Struct. 2005, 744–747, 529–534. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.J.; Zhu, W.F.; Chen, Z.F.; Pan, J.Y.; Xu, K.T. Effect of nucleation agents on the crystallization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3HB4HB). J. Appl. Polym. Sci. 2009, 2, 1116–1123. [Google Scholar] [CrossRef]
- Inoue, Y.; Yoshie, N. Structure and physical properties of bacterially synthesized polyesters. Prog. Polymer. Sci. 1992, 17, 571–610. [Google Scholar] [CrossRef]
- Kumar, S.; Hideki, A. Practical Guide to Microbial Polyhydroxyalkanoates. Chapter 6: Crystalline and Solid-State Structures of Polyhydroxyalkanoates (PHA); Smithers Rapra: Shrewsbury, UK, 2010; pp. 1–160. ISBN 1847351174. [Google Scholar]
- Di Lorenzo, M.L.; Gazzano, M.; Righetti, M.C. The Role of the Rigid Amorphous Fraction on Cold Crystallization of Poly(3-hydroxybutyrate). Macromolecules 2012, 45, 5684–5691. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, T.L. Property analysis of biodegradable material P(3HB-co-4HB). Adv. Mater. Res. 2012, 380, 168–172. [Google Scholar] [CrossRef]
- Akhtar, S.; Pouton, C.W.; Notarianni, L.J. Crystallization behaviour and drug release from bacterial polyhydroxyalkanoates. Polymer 1992, 33, 117–126. [Google Scholar] [CrossRef]
- Volova, T.G.; Vinogradova, O.N.; Zhila, N.O.; Peterson, I.V.; Kiselev, E.G.; Vasiliev, A.D.; Sukovatiy, A.G.; Shishatskaya, E.I. Properties of a novel quaterpolymer P(3HB/4HB/3HV/3HHx). Polymer 2016, 101, 67–74. [Google Scholar] [CrossRef]
- Kunioka, M.; Tamaki, A.; Doi, Y. Crystalline and Thermal Properties of Bacterial Copolyesters: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1989, 22, 694–697. [Google Scholar] [CrossRef]
- Kabe, T.; Sato, T.; Kasuya, K.; Hikima, T.; Takata, M.; Iwata, T. Transition of spherulite morphology in a crystalline/crystalline binary blend of biodegradable microbial polyesters. Polymer 2014, 55, 271–277. [Google Scholar] [CrossRef]
- Alavi, S.; Thomas, S.; Sandeep, K.P.; Kalarikal, N.; Varghese, J.; Yaragalla, S. Polymers for Packaging Applications; Appe Academic Press, CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781926895772. [Google Scholar]
- Ray, D. Biocomposites for High-Performance Applications, Current Barriers and Future Needs Towards Industrial Development; Woodhead Publishing Series in Composites Science and Engineering: Elsevier, Cambridge, UK, 2017; pp. 1–336. ISBN 9780081007945. [Google Scholar]
- Penning, J.P.; St John Manley, R. Miscible Blends of Two Crystalline Polymers. 1. Phase Behavior and Miscibility in Blends of Poly(vinylidene fluoride) and Poly(1,4-butylene adipate). Macromolecules 1996, 29, 77–83. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Alvarez, A.; Santarén, J.; Esteban-Cubillo, A. Developments in Palygorskite-Sepiolite Research. A New Outlook on These Nanomaterials; Galán, E., Singer, A., Eds.; Elsevier, B.V.: Oxford, UK, 2011; pp. 393–452. ISBN 9780444536082. [Google Scholar]
- Ruiz-Hitzky, E.; Van Meerbeek, A. Handbook of Clay Science, Development in Clay Science, ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 583–621. ISBN 9780080457635. [Google Scholar]
- Lvov, Y.; Guo, B.; Fakhrullin, R.F. Functional Polymer Composites with Nanoclays. REC Smart Mater. 2016. [Google Scholar] [CrossRef]
- Karami, S.; Ahmadi, Z.; Nazockdast, H.; Rabolt, J.F.; Noda, I.; Chase, B.D. The effect of well-dispersed nanoclay on isothermal and non-isothermal crystallization kinetics of PHB/LDPE blends. Mater. Res. Express 2018, 5, 015316. [Google Scholar] [CrossRef]
- Bruckner, S.; Meille, S.V.; Malpezzi, L. The Structure of Poly(D-(-)-@-hydroxybutyrate). A Refinement Based on the Rietveld Method. Macromolecules 1988, 21, 967–972. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef]
- Jalali, A.M.; Taromi, F.A.; Atai, M.; Solhi, L. Effect of reaction conditions on silanisation of sepiolite nanoparticles. J. Exp. Nanosci. 2016, 11, 1171–1183. [Google Scholar] [CrossRef]
- Patrício, A.C.L.; da Silva, M.M.; de Sousa, A.K.F.; Mota, M.F.; Freire Rodrigues, M.G. SEM, XRF, XRD, Nitrogen Adsorption, Fosters Swelling and Capacity Adsorption Characterization of Cloisite 30 B. Mater. Sci. Forum 2012, 727–728, 1591–1595. [Google Scholar] [CrossRef]
- Xue, M.-L.; Yu, Y.-L.; Li, P. Preparation, Dispersion, and Crystallization of the Poly(trimethylene terephthalate)/Organically Modified montmorillonite (PTT/MMT) Nanocomposites. J. Macromol. Sci. Part B 2010, 49, 1105–1116. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E.; Ersoy, S. The modification of Na-montmorillonite by salts of fatty acids: An easy intercalation process. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 40–49. [Google Scholar] [CrossRef]
- Høgsaa, B.; Fini, E.H.; Christiansen, J.D.; Hung, A.; Mousavi, M.; Jensen, E.A.; Pahlavan, F.; Pedersen, T.H.; Sanporean, C.G. A Novel Bioresidue to Compatibilize Sodium montmorillonite and Linear Low Density Polyethylene. Ind. Eng. Chem. Res. 2018, 57, 1213–1224. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental Study of montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
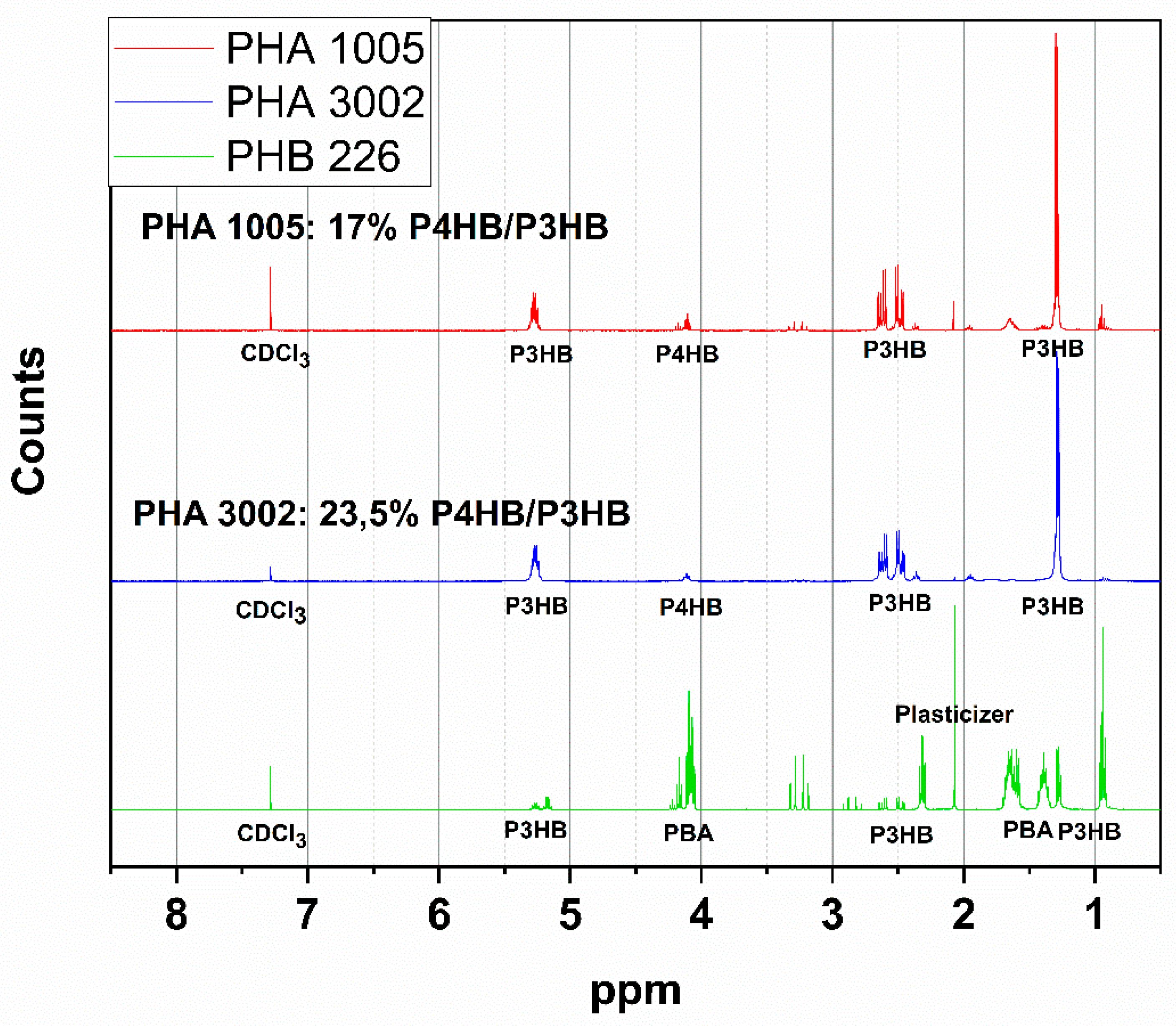
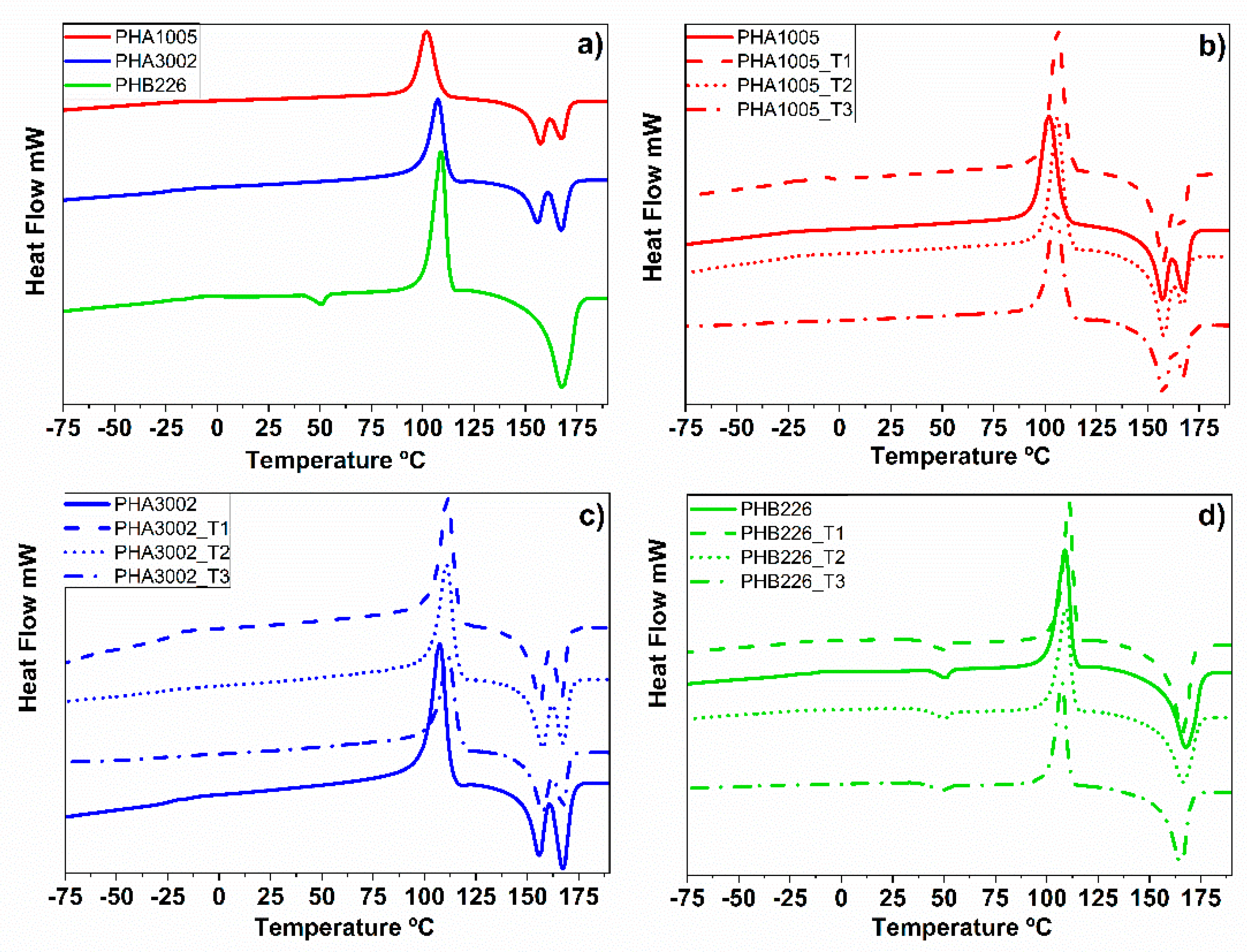
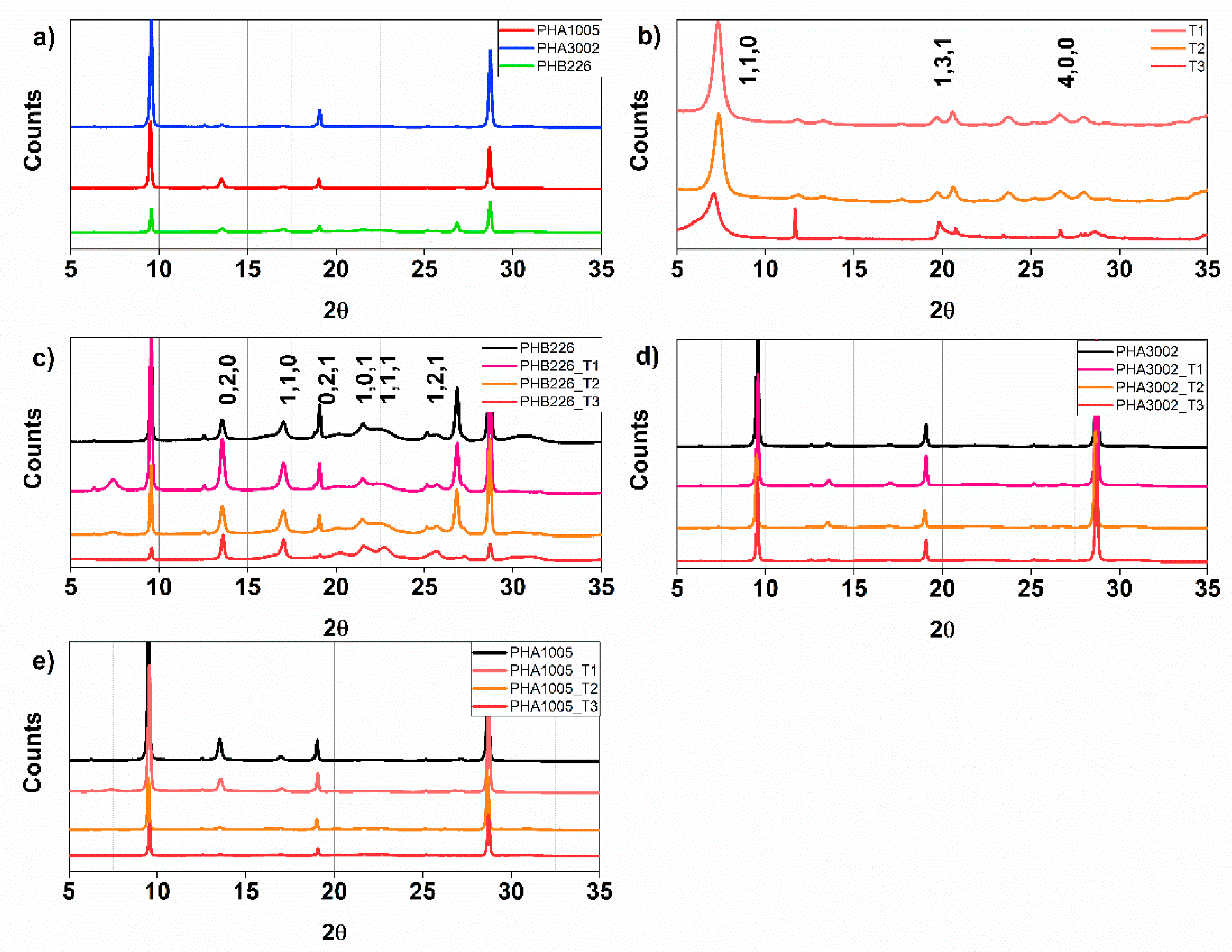
| Material Formulation | Commercial Matrix Used | Nature of the PHA | Type of Reinforcement (3 wt.%) |
|---|---|---|---|
| PHA1005 | PHA1005 | P3HB-co-P4HB | 17% P4HB/P3HB and talc |
| PHA1005_T1 | PHA1005 | P3HB-co-P4HB | T1: Aminosilane sepiolite |
| PHA1005_T2 | PHA1005 | P3HB-co-P4HB | T2: Natural sepiolite |
| PHA1005_T3 | PHA1005 | P3HB-co-P4HB | T3: Sodium montmorillonite: quaternary ammonium salt |
| PHA3002 | PHA3002 | P3HB-co-P4HB | 23.5% P4HB/P3HB and talc |
| PHA3002_T1 | PHA3002 | P3HB-co-P4HB | T1: Aminosilane sepiolite |
| PHA3002_T2 | PHA3002 | P3HB-co-P4HB | T2: Natural sepiolite |
| PHA3002_T3 | PHA3002 | P3HB-co-P4HB | T3: Sodium montmorillonite: quaternary ammonium salt |
| PHB226 | PHB226 | P3HB | Traces of PBA (polybutyladipate), plasticiser, and talc found |
| PHB226_T1 | PHB226 | P3HB | T1: Aminosilane sepiolite |
| PHB226_T2 | PHB226 | P3HB | T2: Natural sepiolite |
| PHB226_T3 | PHB226 | P3HB | T3: Sodium montmorillonite: quaternary ammonium salt |
| Material | E (MPa) | σM (MPa) | εB (%) | UT (MPa) | Ef (MPa) | σfM (MPa) | εfB (%) | UfT (MPa) |
|---|---|---|---|---|---|---|---|---|
| PHB 226 | 2028 ± 68 | 21.8 ± 0.5 | 3.1 ± 0.3 | 53.9 | 1316 ± 28 | 30.2 ± 0.4 | 4.4 ± 0.2 | 98.8 |
| PHB 226_T1 | 2004 ± 66 | 23.7 ± 0.6 | 4.6 ± 0.7 | 83.9 | 1306 ± 17 | 30.1 ± 1.0 | 4.8 ± 0.3 | 115.8 |
| PHB 226_T2 | 2435 ± 88 | 22.1 ± 1.1 | 1.9 ± 0.3 | 33.6 | 1318 ± 68 | 34.1 ± 0.9 | 4.7 ± 0.4 | 111.5 |
| PHB 226_T3 | 2006 ± 65 | 22.5 ± 0.3 | 4.2 ± 0.3 | 69.9 | 1871 ± 9 | 42.3 ± 0.3 | 3.6 ± 0.1 | 99.3 |
| PHA 1005 | 2770 ± 99 | 22.4 ± 0.7 | 2.0 ± 0.1 | 32 | 1801 ± 39 | 31.5 ± 0.4 | 3.9 ± 0.1 | 92.8 |
| PHA 1005_T1 | 3411 ± 98 | 25.2 ± 0.5 | 1.8 ± 0.1 | 33.9 | 2066 ± 71 | 32.2 ± 0.8 | 3.2 ± 0.1 | 71.9 |
| PHA 1005_T2 | 3420 ± 218 | 25.9 ± 0.6 | 1.9 ± 0.2 | 38.2 | 2240 ± 88 | 33.9 ± 1.7 | 2.7 ± 0.1 | 60.3 |
| PHA 1005_T3 | 3755 ± 41 | 23.8 ± 0.6 | 1.4 ± 0.1 | 24.9 | 2980 ± 90 | 47.6 ± 2.3 | 2.6 ± 0.2 | 81.6 |
| PHA 3002 | 2263 ± 37 | 24.7 ± 0.5 | 2.8 ± 0.1 | 50 | 1342 ± 71 | 33.9 ± 1.5 | 5.0 ± 0.2 | 121.9 |
| PHA 3002_T1 | 1997 ± 48 | 26.2 ± 0.3 | 4.1 ± 0.2 | 84.6 | 1485 ± 35 | 34.3 ± 1.1 | 4.2 ± 0.1 | 97.6 |
| PHA 3002_T2 | 2961 ± 87 | 28.7 ± 0.3 | 2.9 ± 0.2 | 65.7 | 1807 ± 85 | 43.1 ± 1.1 | 4.4 ± 0.8 | 128.9 |
| PHA 3002_T3 | 2463 ± 115 | 27.4 ± 0.2 | 3.3 ± 0.3 | 68.9 | 2358 ± 7 | 57.0 ± 0.7 | 4.3 ± 0.3 | 134.1 |
| Material | D-spacing (Å) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (0,2,0) | (1,1,0) | (0,2,1) | (1,0,1) | (1,1,1) | (1,2,1) | (1,3,1) | (1,9,1) | (4,0,0) | |
| PHB226 | 6.517 | 5.204 | 4.453 | 4.131 | 3.991 | 3.466 | - | - | - |
| PHA1005 | 6.546 | 5.216 | 4.470 * | - | 3.995 | 3.468 | - | - | - |
| PHA3002 | 6.536 | 5.204 | 4.424 * | - | 3.484 | 3.285 | - | - | - |
| T1 | - | 12.120 | - | - | - | - | 4.311 | 2.565 | 3.344 |
| T2 | - | 12.012 | - | - | - | - | 4.307 | 2.561 | 3.339 |
| T3 | - | 12.444 | - | - | - | - | 4.282 | 2.565 | 3.342 |
| PHB226_T1 | 6.514 | 5.203 | 4.439 | 4.060 | 3.953 | 3.463 | - | - | 3.317 |
| PHB226_T2 | 6.524 | 5.203 | 4.389 | 4.137 | 3.976 | 3.465 | - | - | 3.320 |
| PHB226_T3 | 6.505 | 5.197 | 4.368 | 4.118 | 3.912 | 3.470 | - | - | 3.268 |
| PHA1005_T1 | 6.524 | 5.197 | - | - | - | 3.536 | - | - | - |
| PHA1005_T2 | 6.542 | 5.227 | - | 4.118 | 3.929 | 3.545 | - | - | - |
| PHA1005_T3 | 6.514 | 5.215 | - | 4.096 | 3.893 | 3.534 | - | - | - |
| PHA3002_T1 | 6.514 | 5.197 | - | - | - | 3.534 | - | - | 3.322 |
| PHA3002_T2 | 6.552 | 5.215 | - | - | - | 3.545 | - | - | - |
| PHA3002_T3 | 6.524 | * disappeared | - | 4.104 | - | 3.534 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Quiles, L.; Fernández Cuello, Á.; Castell, P. Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite. Polymers 2019, 11, 696. https://doi.org/10.3390/polym11040696
García-Quiles L, Fernández Cuello Á, Castell P. Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite. Polymers. 2019; 11(4):696. https://doi.org/10.3390/polym11040696
Chicago/Turabian StyleGarcía-Quiles, Lidia, Ángel Fernández Cuello, and Pere Castell. 2019. "Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite" Polymers 11, no. 4: 696. https://doi.org/10.3390/polym11040696
APA StyleGarcía-Quiles, L., Fernández Cuello, Á., & Castell, P. (2019). Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite. Polymers, 11(4), 696. https://doi.org/10.3390/polym11040696