3.1. Background to the Mechanistic Study
The groundwork for the mechanisms proposed for the initiation of epoxy resins using imidazoles and related species was laid in 1968 by Farkas and Ströhm [
4], who studied the curing of PGE with 2-ethyl-4(5)-methylimidazole and suggested that the first stage of the reaction required the attack of the secondary N-3 atom on the terminal carbon atom of the epoxy group to generate a 1:1 adduct. They postulated that both nitrogen atoms on the imidazole ring participated in the curing process in the initial stages and become incorporated into the system [
19]. Barton and Shepherd [
20] revised this mechanism (
Figure 1), having observed that the rate of reaction of the 1:1 adduct (formed from the imidazole and an epoxy) with another epoxide ring was of the same order as the rate of reaction of the original imidazole (2-ethyl-4(5)-methylimidazole) with epoxide.
This led to the conclusion that the N-1 nitrogen, as opposed to the N-3 nitrogen, was the reactive species. It was additionally noted that the rate of adduct formation appeared to be higher than the rate of polymerisation.
Many studies have focused on epoxy/imidazole systems and, more specifically, on the reaction between PGE and various imidazoles [
4,
19,
20,
21]. It has been observed that the formation of epoxy/imidazole adducts appears to be the first stage in the curing process, as both nitrogen atoms are involved in the forming of adducts, with the imidazole becoming permanently incorporated into the network [
4,
20,
22]. The N-1 and N-3 atoms are believed to act in the same manner as pyridine, and form both 1:1 and 2:1 epoxy/imidazole adducts through the ring opening of the epoxide group, with the 2:1 adduct thought to be the catalyst that initiates the polymerisation process [
20,
21,
22]. Further studies on the reaction of the diglycidyl ether of bisphenol A (DGEBA) with low concentrations of 2-ethyl-4-methylimidazole resulted in a reaction mechanism, based on the previous work with PGE [
20,
21], being proposed. The proposed reaction mechanism, as shown in
Figure 2, suggests that four different reactions are possible: two adduct reactions and two etherification reactions [
22].
Heise and Martin [
23] analysed the different reactions that occurred in DGEBA/imidazole systems and observed the effect they had on the thermal properties of the resulting network. The first adduct formation results from an attack of the 3-N atom of the imidazole on the epoxide group, causing it to open and form the 1:1 molar OH adduct through rearrangement of the zwitterion intermediate and a proton transfer. The second adduct is formed through an attack of the 1-N atom of the OH adduct on another epoxide ring to form the 2:1 molar O
−/OH adduct. The latter, as mentioned previously, is believed to catalyse the polymerisation reaction due its highly reactive alkoxide ion. Owing to the fact that both adducts have similar rates of formation, it can be assumed that the N-1 atom is involved in both reactions rather than the hydrogen atom on N-3 reacting with the epoxide ring in a similar manner to an amine-curing agent, as was previously thought [
4,
20,
21,
22]. Previous work by the same authors had already established that adduct formation is necessary before the etherification reaction can proceed, and, hence, this is the rate-limiting step of the reaction [
23]. Etherification can proceed via two different pathways, namely O-etherification or OH-etherification, and it is the chain growth via these two reaction points that allows for the cross-linking of the resin and the final network properties to be determined. It has been established that in order for both adducts to form, it is necessary to use a 1-unsubstituted imidazole in order to have a hydrogen atom present on the secondary nitrogen (N-3) atom that is capable of rearrangement during the zwitterion intermediate state. Therefore, in the case of e.g., 2-ethyl-4-methylimidazole, imidazole, and 1-unsubstituted imidazoles, both the OH (1:1) adduct and the O
−/OH (2:1) adduct are formed, whereas for 1-substituted imidazoles, e.g., 1,2-dimethylimidazole and 1-methylimidazole, only the O
− (1:1) adduct is formed.
FTIR spectroscopy has been used to track epoxide concentration and, hence, to indirectly monitor the formation of the O
−/OH adduct. The presence and subsequent disappearance of the N–H stretching peak in the FTIR spectrum allows for the formation of the OH-adduct to be directly monitored. In the case of 2-ethyl-4-methylimidazole, at a reaction temperature of 80 °C and an imidazole concentration of less than 25 mol %, the adduct formation reaction nears completion before the etherification reaction begins; there is no evidence of the formation of aliphatic ethers in the IR spectrum. Adduct formation for 1-unsubstituted imidazoles is characterised by a slow rate of epoxide conversion and a relatively constant glass transition temperature. The start of the etherification reaction is indicated by a rapid and sudden increase in the glass transition temperature, an increase in the rate of epoxide conversion, and the developing presence of a broad aliphatic ether band occurring in the IR spectrum in the region of 1140–1110 cm
−1 [
22]. FTIR spectroscopy data suggest that the adduct formation reaction approaches completion prior to the start of the etherification reaction. The etherification reaction is characterised by the presence of a second exothermic peak in an isothermal DSC thermogram. Thus, when comparatively higher initiator contents (e.g., 50 mol % imidazole) are used the adduct formation predominates and, consequently, consumes the majority of the epoxide groups, leading to a single exothermic peak. At 50 mol % or higher concentrations, the initiator is sufficiently present to cause a reaction of all of the epoxide groups to form adducts, the etherification reaction may begin earlier, and, in this case, the single exothermic peak will show a broad tail at the end rather than a separate exothermic peak. At intermediate imidazole concentrations (between 5 and 25 mol %), a second exothermic peak is clearly observed, and is attributed O-etherification, which increases as the concentration of imidazole decreases (i.e., fewer epoxide groups are available to form adducts).
Subsequent work by Ricciardi et al. [
19] focused on the fact that differences in the catalytic ability of various imidazoles were observed, which is not accounted for in terms of the mechanism proposed by Barton and Shepherd [
20]. Regardless of the imidazole, a similar adduct was formed with PGE (see
Supplementary data Figure S3), yet it has been observed that the overall rate of reaction varies greatly depending on the nature of the imidazole [
19]. Additionally, as the rate of adduct formation is faster than the rate of polymerisation, this is not this stage of the reaction that is rate-determining. The alkoxide anion, which was believed to be the reactive species in the etherification reactions, was shown to be less effective as a catalyst than the imidazole wherein the addition of alkoxide anions to the curing reaction mixture had little effect on the rate of the reaction. In contrast, the addition of an equivalent amount of an imidazole significantly increased the rate of the reaction. Consequently, the authors inferred that the imidazole curing agent was active throughout the polymerisation process rather than just at the start, during adduct formation, as was previously thought [19]. This was presumed to be the reactive species, however, when the authors attempted to prepare this adduct, a mixture of several compounds resulted. In most cases the methyl group on the imidazole was removed, and in only a few cases had this group been transferred to the hydroxyl group on the side chain. Additionally, some 2-substituted imidazoles were formed (see
Supplementary data Figure S4).
In order to isolate the effect of the imidazole portion of the system’s reactivity from the PGE chain contribution, a 1,3-disubstituted imidazole (1,3-dimethylimidazolium iodide), displaying an increased electrophilic imidazole ring due to the quaternization process, was prepared and treated with various nucleophiles ranging in character from weak bases, such as the phenyl selenate anion, to strong bases, such as the methoxide ion, to observe the effects of nucleophilicity. Nuclear magnetic resonance (NMR) spectroscopy showed that in all cases the imidazolium ring remained intact, although N-demethylated product was also observed in each case. In the case of 1,3-dimethylimidazolium iodide, the cleavage of the N–C bond—essentially the reverse of the salt-forming reaction—resulted in the regeneration of 1-methylimidazole. It was observed that, upon heating the imidazolium salt to above 200 °C, demethylation occurred, yielding 1-methylimidazole and methyl iodide. Subsequent investigation showed that heating the corresponding hydroxide salt (1,3-dimethylimidazolium hydroxide) to above 100 °C resulted in 1-methylimidazole being regenerated.
The potential of ionic liquids as processible, easily-formulated, and stable initiators has already been mentioned, which makes them increasingly attractive in technological applications. The thermal decomposition of imidazolinium-based ionic liquids has been studied extensively, with authors generally concluding that a dealkylation mechanism occurs. Ohtani et al. [
24] investigated a range of 1,3-dialkylated imidazolium-based ionic liquids and found that the thermal decomposition of the imidazolium-based ionic liquids occurs mainly through the cleavage of the C–N bonds within the chains (although C–C cleavage also occurs in longer alkyl chains). If a halide is present, then the corresponding haloalkanes and 1-alkylimidazoles are generated by nucleophilic attack of the halide on the alkyl residue. The anionic initiation mechanism is complex (
Figure 3) and the thermal decomposition of the ionic liquid yields a highly stabilised
N-heterocyclic carbon structure that may generate imidazole or 1-alkyl derivatives. Several routes are then possible: a “carbene route” (following proton abstraction from the carbon between the nitrogen atoms), an “imidazole route” (involving dealkylation followed by nucleophilic attack of the N-1 atom in the imidazole ring), and a “counter ion route”.
3.2. Examining the Storage Stability of PGE and 1-Ethyl-3-Methylimidazolium Acetate
PGE was selected as the epoxy in the present investigation as there is a significant colour change, from light yellow to dark red (see
Supplementary data Figure S5), which occurs within a short time period (60 min) when it is mixed with 1-ethyl-3-methylimidazolium acetate. Thus, PGE (5 g) and 1-ethyl-3- methylimidazolium acetate (0.25 g) were mixed in a clear glass scintillation vial and analysed every 5 min at a laser power of 150 mW in the region 400–4000 cm
−1 (
Figure 4).
It was apparent that no marked differences were observed between the spectra obtained at different times. During the 60 min analysis period, the sample was observed to turn from a very light yellow to a very dark red/brown, although no obvious increase in viscosity was discerned. In order to ascertain what occurred during this time frame, peaks of interest were studied, such as the epoxy ring stretch and the C–O ether stretch (
Figure 4, bottom). No significant differences were observed between peaks, suggesting that the initial reaction, encompassed by the shoulder reaction, did not involve the epoxy ring to a significant degree.
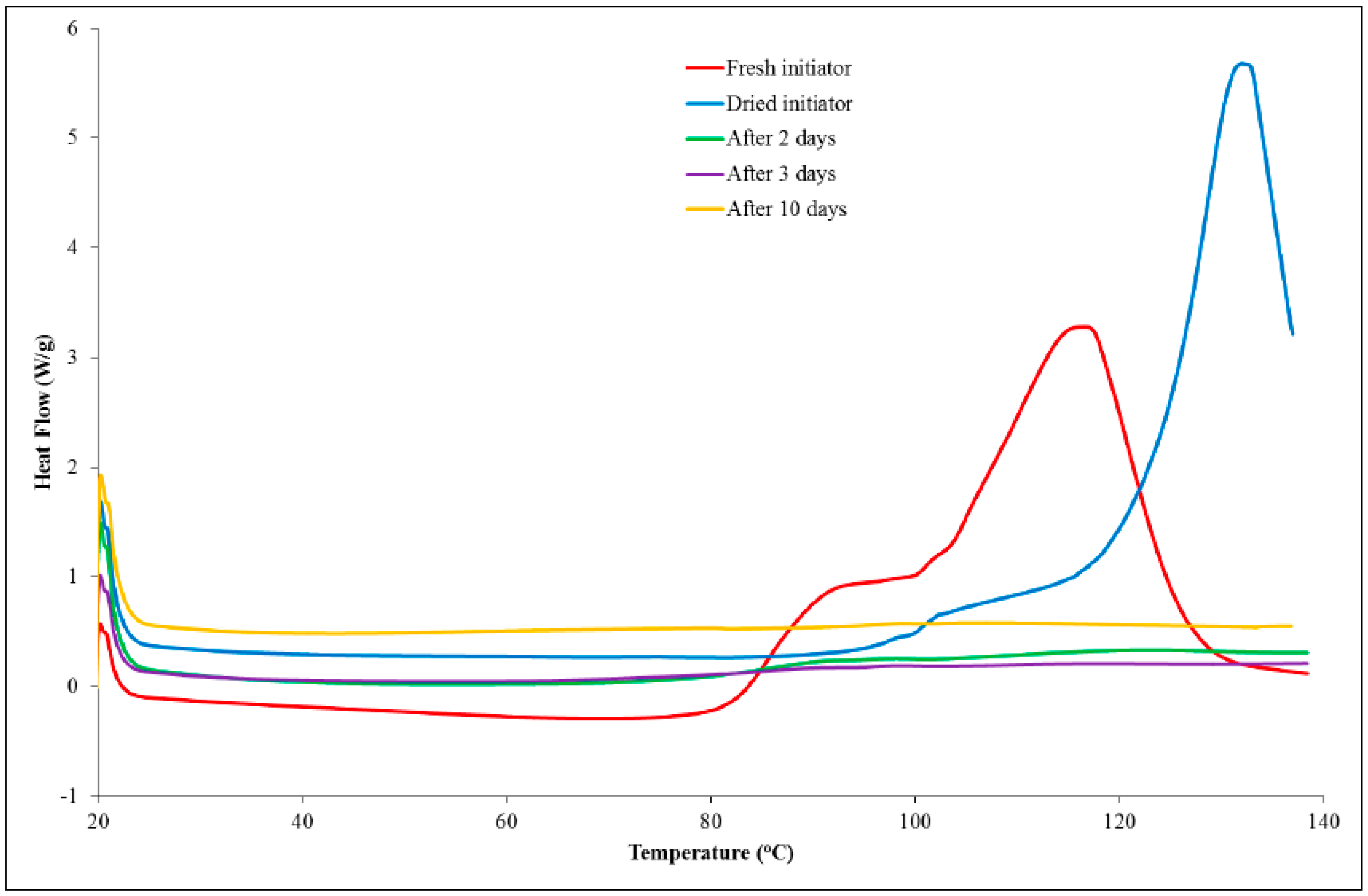
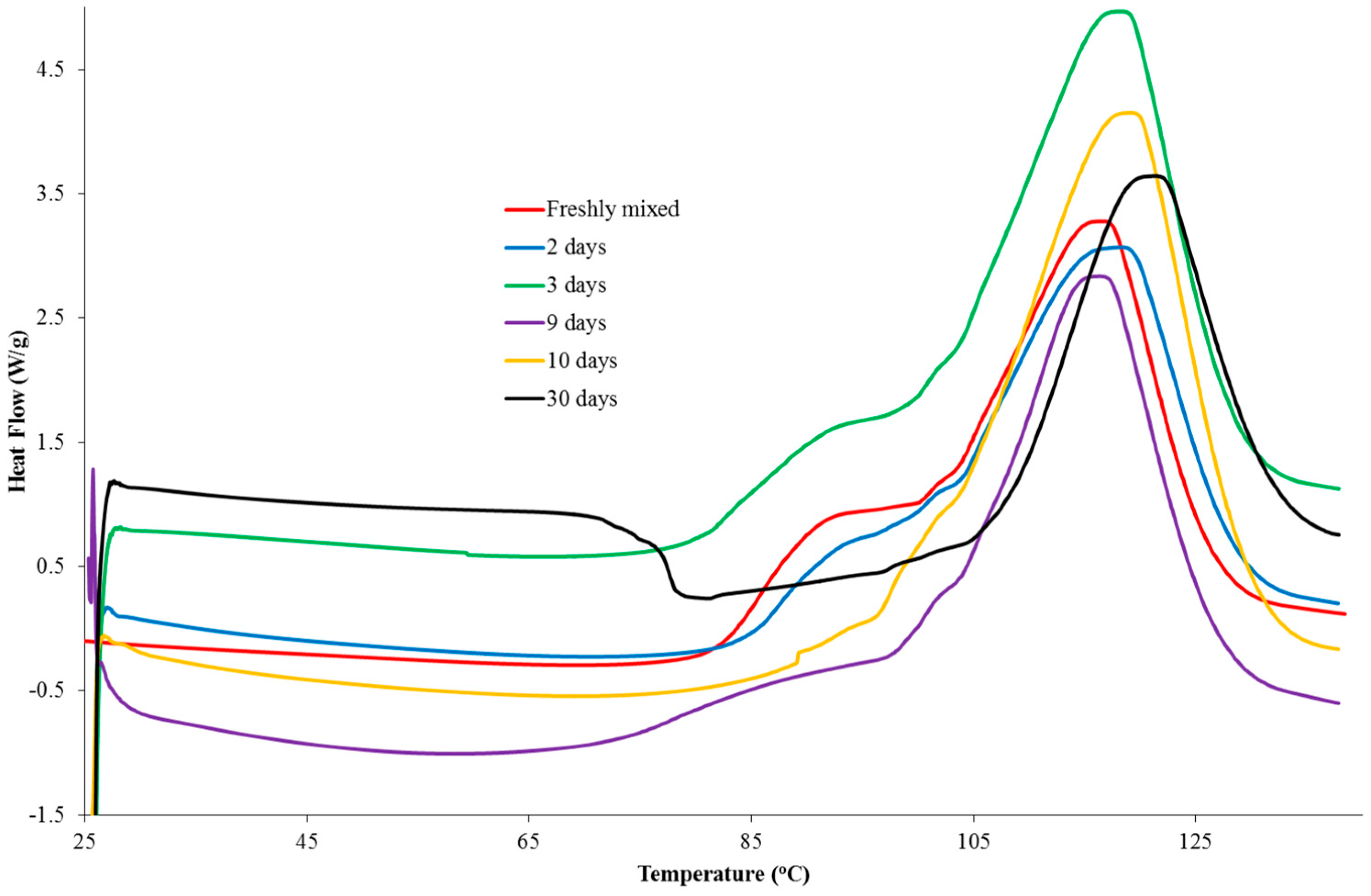
Samples of a formulation, comprising PGE (1 g) and 1-ethyl-3-methylimidazolium acetate (0.05 g), were analysed using DSC at a scan rate of 10 K/min from 20 to 140 °C following different storage times. The dried initiator was also analysed using DSC to confirm that the drying process had not affected the initiating ability of the material. The DSC thermograms are shown in
Figure 5. Upon addition of the ionic liquid to PGE, small globules were formed that remained after mixing, indicating that the two liquids were immiscible. The DSC data for the formulation of 1-ethyl-3-methylimidazolium acetate and PGE, stored at room temperature for a period of 60 min, revealed that a low temperature shoulder peak had indeed been lost or, at least, greatly reduced in size. Subsequent DSC analysis showed that the initiating ability of the ionic liquid had been lost as no exothermic peaks were observed in the thermograms. The dried sample, which had not been exposed to the atmosphere, was shown to delay the onset of the reaction considerably and to shift the T
max value to a higher temperature compared to the sample that was taken freshly from the bottle. The fact that the ionic liquid was shown not to initiate the reaction can be explained by the existence of hydrogen bonding and the proton abstraction effect in the material [
19]. The ability of the dried sample to considerably delay the onset of the reaction may well be a result of the loss of water, which could catalyse the etherification mechanism in the later stages of the reaction. While the main etherification reaction (leading to the major DSC peak) results in changes in the peaks of interest in the Raman spectrum, it is likely that the first reaction does not involve a sufficient number of epoxy groups to cause either a decrease in the peak characteristic of the epoxy group or an increase in the peak, which is attributed to an ether stretch.
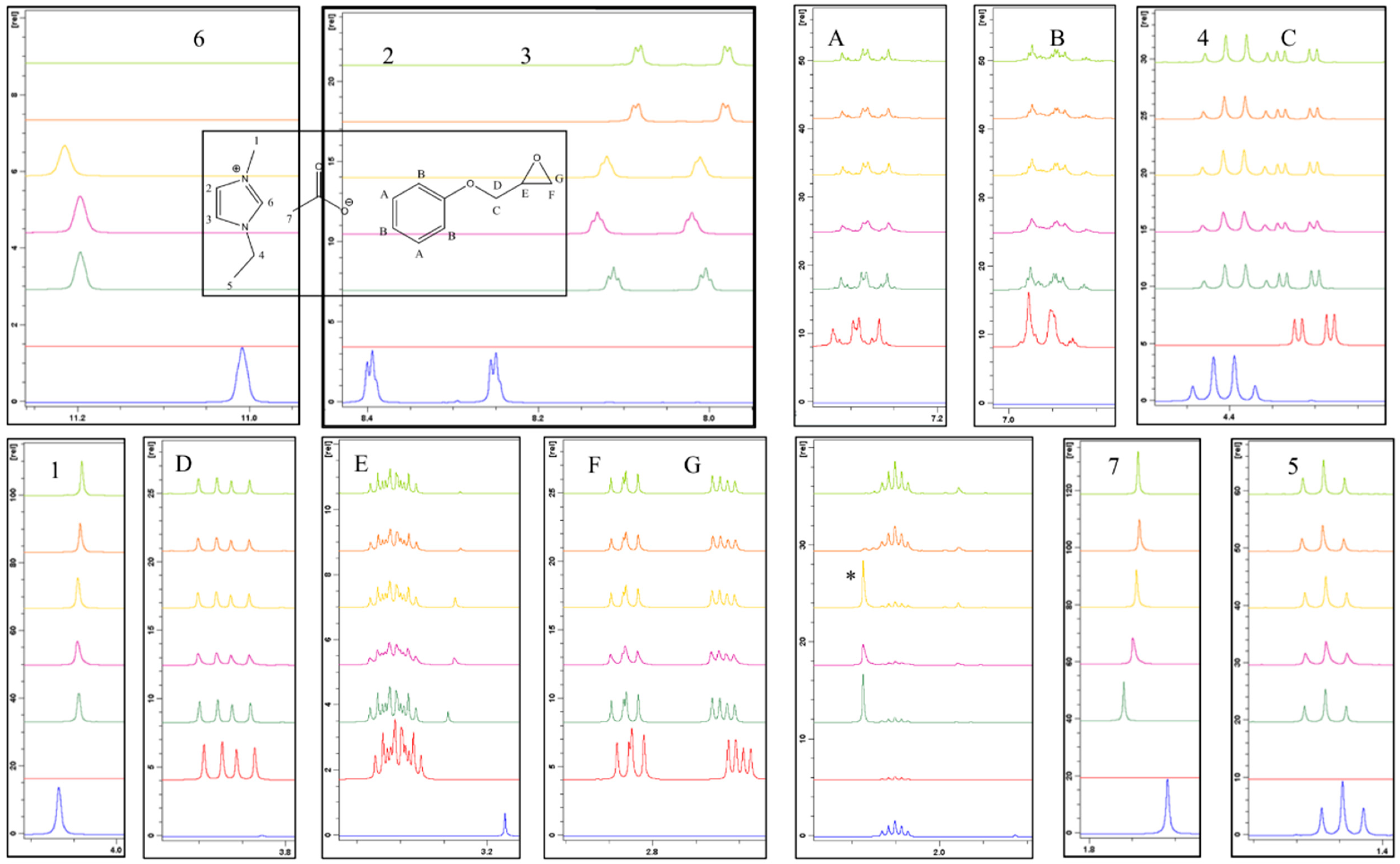
Complementary analysis, via NMR spectroscopy, allowed for more subtle changes in the formulation to be observed. A formulation based on an equimolar 1:1 stoichiometry was employed to highlight chemical changes although, in the absence of heat, this was not expected to impact the reaction mechanism significantly. The expanded overlaid
1H NMR spectra (
Figure 6) included, from bottom to top, 1-ethyl-3-methylimidazolium acetate (dark blue), PGE (red), 0 min (dark green), 30 min (purple), 50 min (yellow), 70 min (orange), and 90 min (light green). Any new peaks, which cannot be assigned to the starting materials, are highlighted with an asterisk (*). In this case, a number of changes occurred over the storage period. The acidic proton (H
6) shifted downfield upon the addition of the epoxy and greatly reduced in size until the point at which it was no longer observable in the spectrum without significant magnification. Integration of the peak area suggested that, in general, the peak decreased relative to the aromatic ring protons (H
A), over the storage time, although the trend was not linear (
Table 1). This suggests that the mechanism does not simply consist of one reaction and is likely to be influenced by other processes, which would account for the area of the peak to fluctuate.
Protons H
2 and H
3 on the imidazolium ring experienced a slight upfield shift upon the addition of the epoxy, however, they retained a fairly constant chemical shift over the storage period and integrated to the same value relative to the protons labelled as H
A. This upfield shift is consistent with the deprotonation that occurs as the electron density of the ring increases, meaning that the protons on the ring and alkyl groups will be more shielded. Protons H
A, H
B, H
C, H
D, H
E, H
F, and H
G, which are attributable to the PGE component, did not show significant changes in their chemical shifts when combined with the ionic liquid, however, integration of the peaks attributed to protons H
E, H
F, and H
G, which are associated with the epoxy group, revealed a decrease in area over the storage period. The intensity of the aromatic ring protons (H
A) should not change during the reaction, since these protons do not participate in the reaction mechanism. The ratio of the integral change for each proton was expressed with respect to H
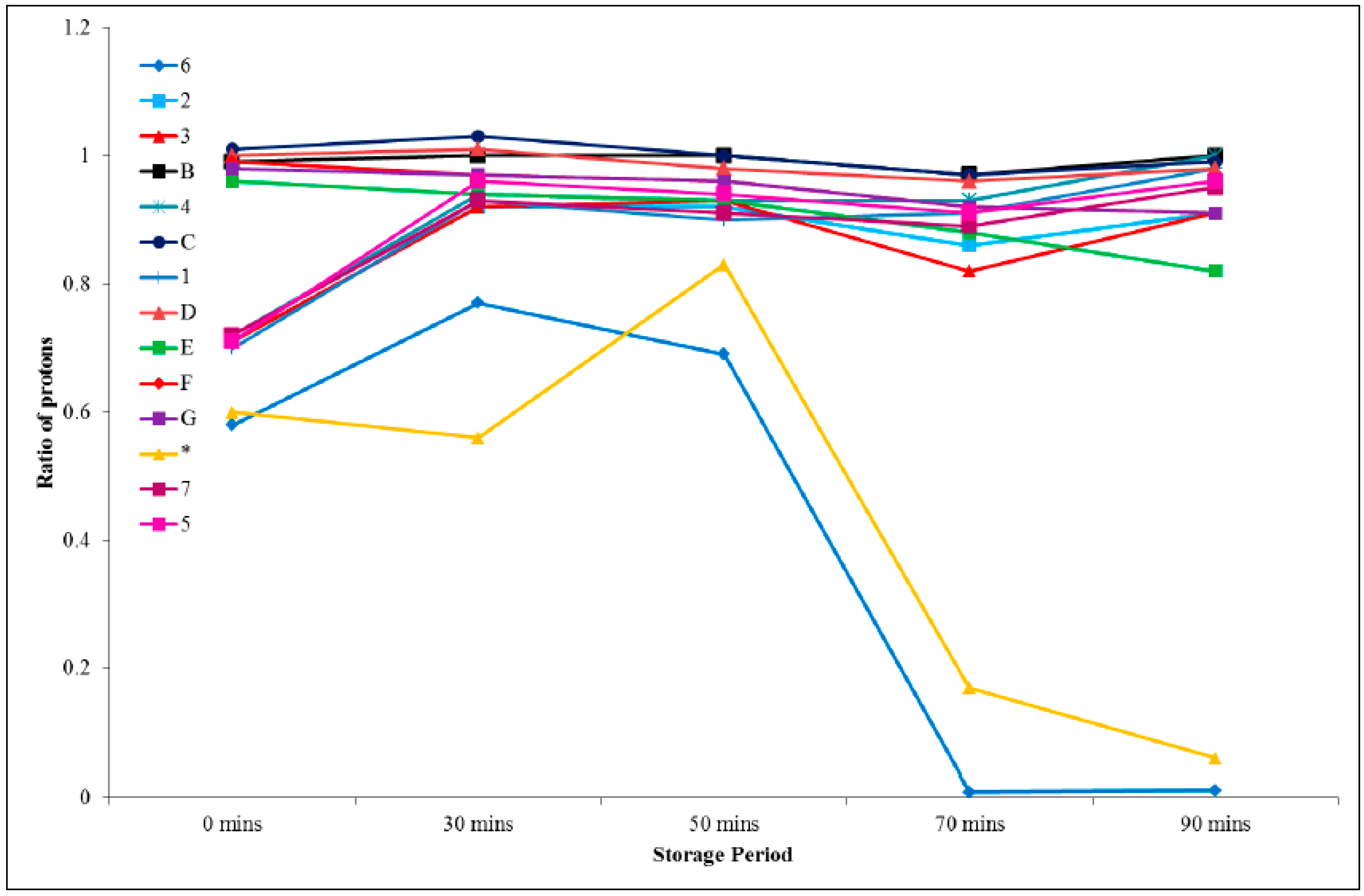
A and the results are represented graphically in
Figure 7. From this treatment, it can be seen that proton H
6 exhibits the greatest change over time.
The 13C NMR spectra (not shown) revealed the presence of no new peaks, but showed that the peak attributed to the NCHN carbon was not visible in the spectrum after a period of 70 min of storage time. This supports the 1H NMR spectral data that suggest the suppression of the NCHN proton after the same storage period.
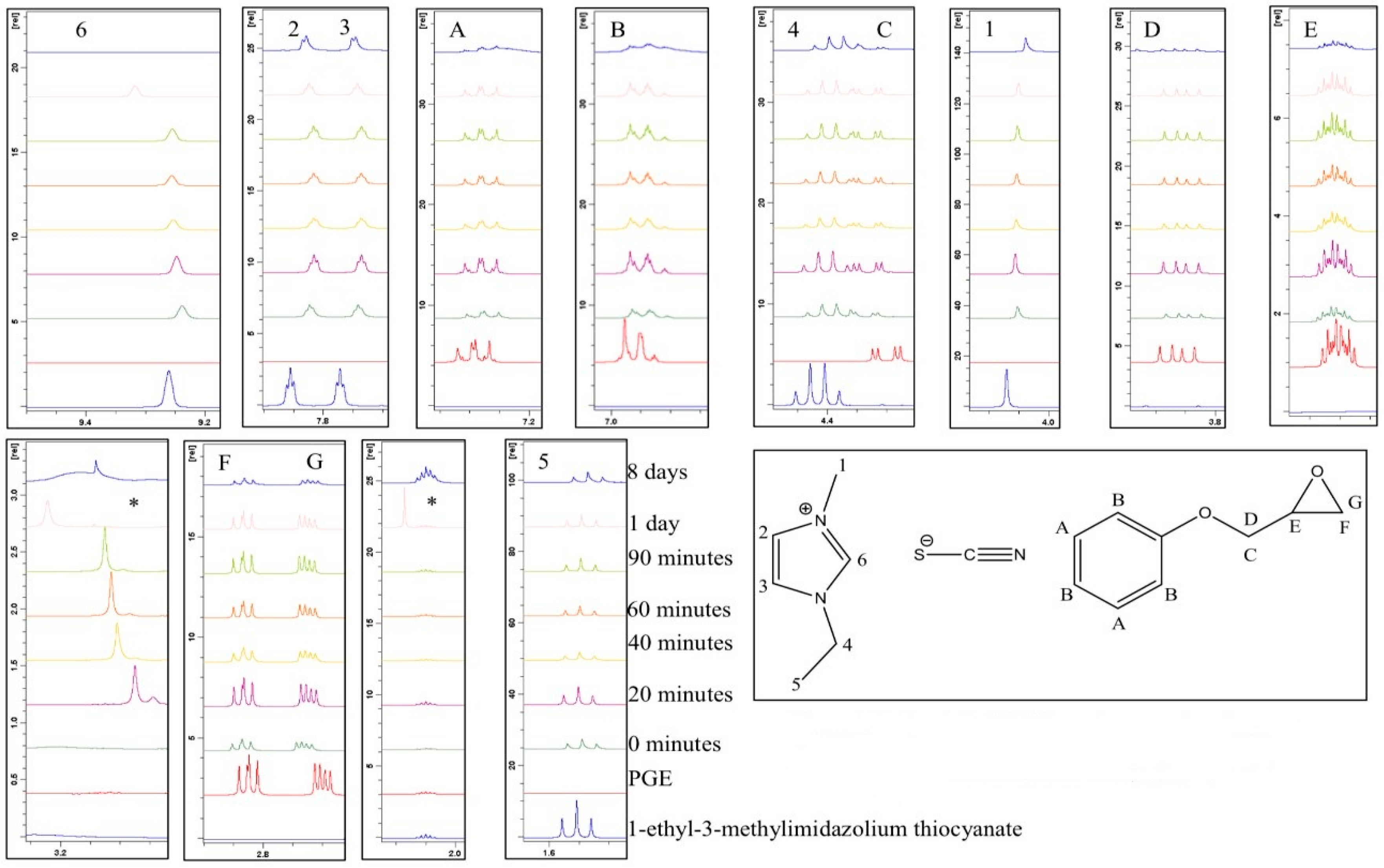
3.3. Examining the Storage Stability of PGE and 1-Ethyl-3-Methylimidazolium Thiocyanate
A similar analysis was performed on a formulation comprising PGE and 1-ethyl-3-methylimidazolium thiocyanate. Maka et al. had previously studied a similar ionic liquid in the context of epoxy cure (with DGEBA) [
17]. They reported that the initiation of the epoxy polymerisation with 1-butyl-3-methylimidazolium thiocyanate proceeded via the aforementioned thermal decomposition and yielded products that included imidazole and alkyl derivatives. In our work, the formulation changed colour markedly from light yellow to dark red over approximately 60 min at room temperature. The emergence of the new peak (*) occurred in the early stages and was attributed to the formation of an intermediate species as a result of the reaction between the thiocyanate anion and the epoxy ring. It was apparent that the chemical shifts remained very similar over the analysis period (
Figure 8).
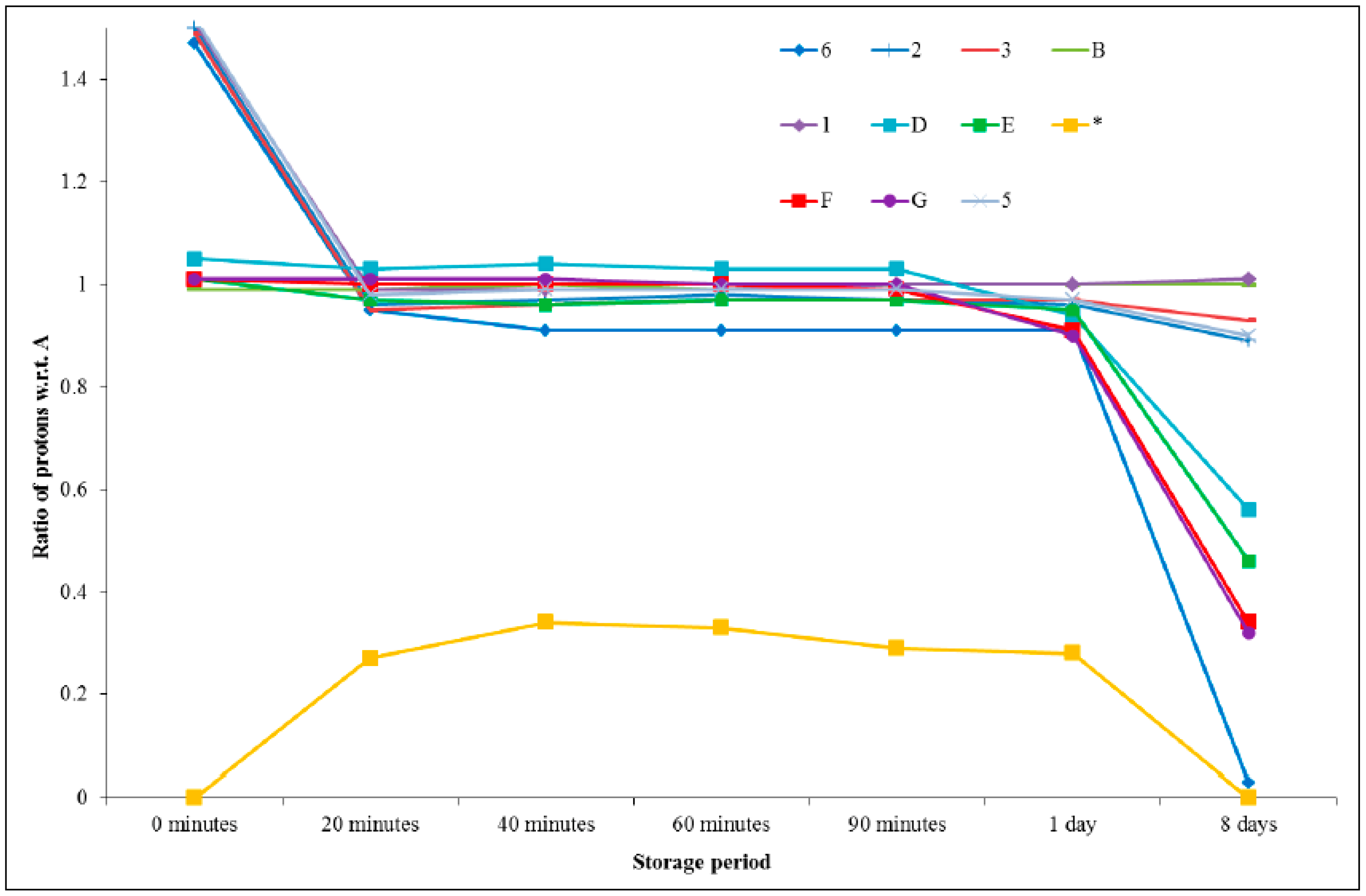
Table 2 provides a summary of all the integrals and ratios for each peak with respect to the aromatic ring protons (H
A), which were thought to remain constant throughout the analysis. These ratio data are presented graphically in
Figure 9, from which it can be seen that the acidic proton (H
6) remained fairly constant in its integral value after an initial decrease in the first 20 min until a period of one day was reached, and then diminished dramatically after 8 days. The protons on the epoxy ring, and in close proximity (H
D, H
E, H
F, and H
G), remained fairly constant for a period of 90 min and were then seen to begin decreasing (in terms of the integration value). The new peak which emerged after 20 min had a relatively constant value, with respect to the aromatic protons, but disappeared after 8 days. The remaining peaks did not vary significantly in intensity.
The fact that the chemical shift for proton H
6 remained visible, and there was not such a marked downfield shift in the imidazolium ring protons, suggests that, at room temperature, the reaction between 1-ethyl-3-methylimidazolium thiocyanate and PGE does not proceed via a carbene initiation pathway. The appearance of a chemical shift at approximately 2.0 ppm (marked with an asterisk) in the spectrum acquired after one day is unidentified, although it is hypothesised that it may be due to residual acetone in the NMR tube after a cleaning process was employed and, as such, will be discounted from any discussion.
Figure 8 shows the expanded overlaid
1H NMR spectra for a sample analysed over a period of 8 days. Any new peaks, which cannot be assigned to the starting materials, are highlighted with an asterisk (*). It can be seen from
Figure 10 that the chemical shifts remained very similar over the analysis period.
Table 2 provides a summary of all the integrals and ratios of each peak with respect to the aromatic ring (H
A).