Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Oligomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
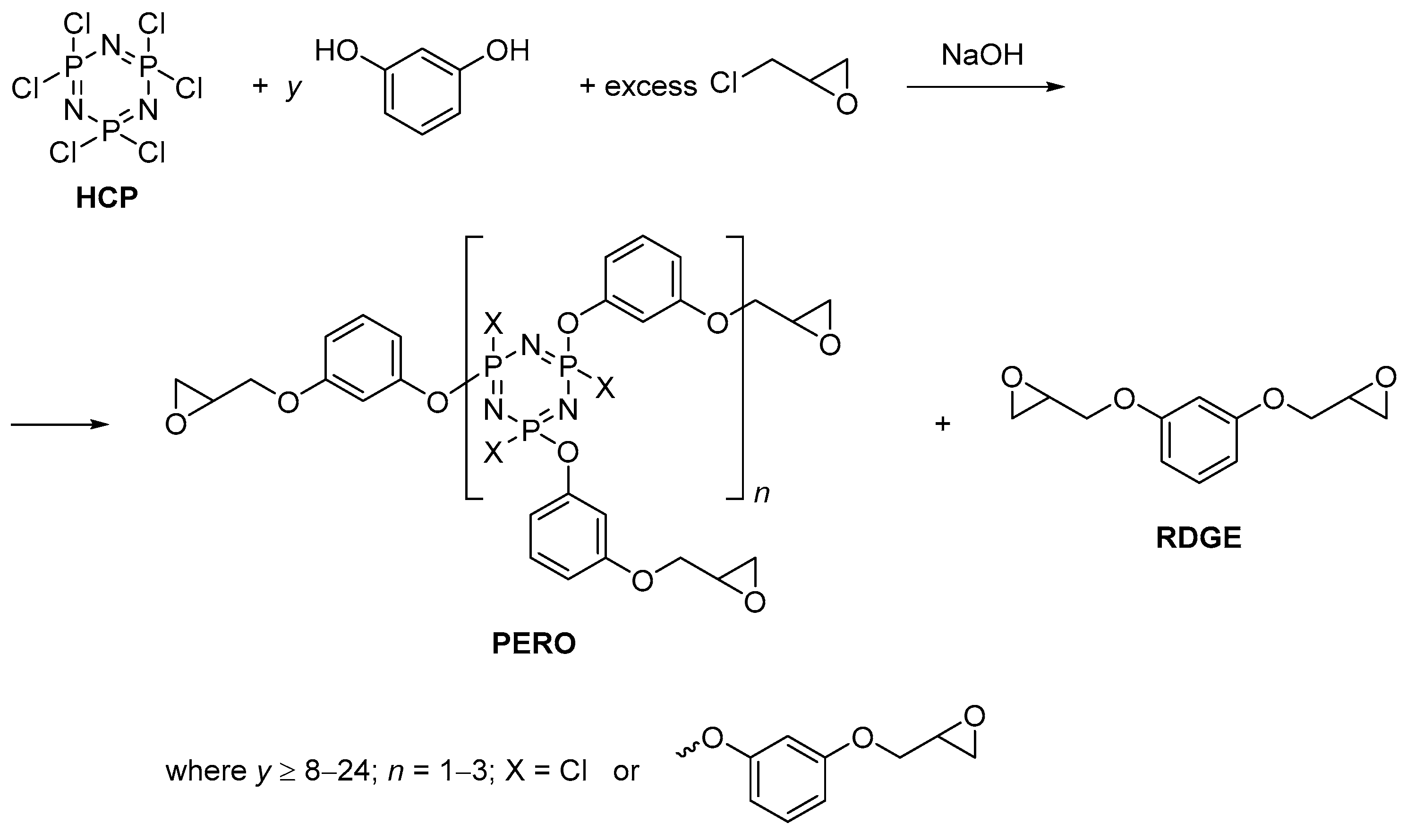
2.2. Synthesis of Epoxyphosphazenes
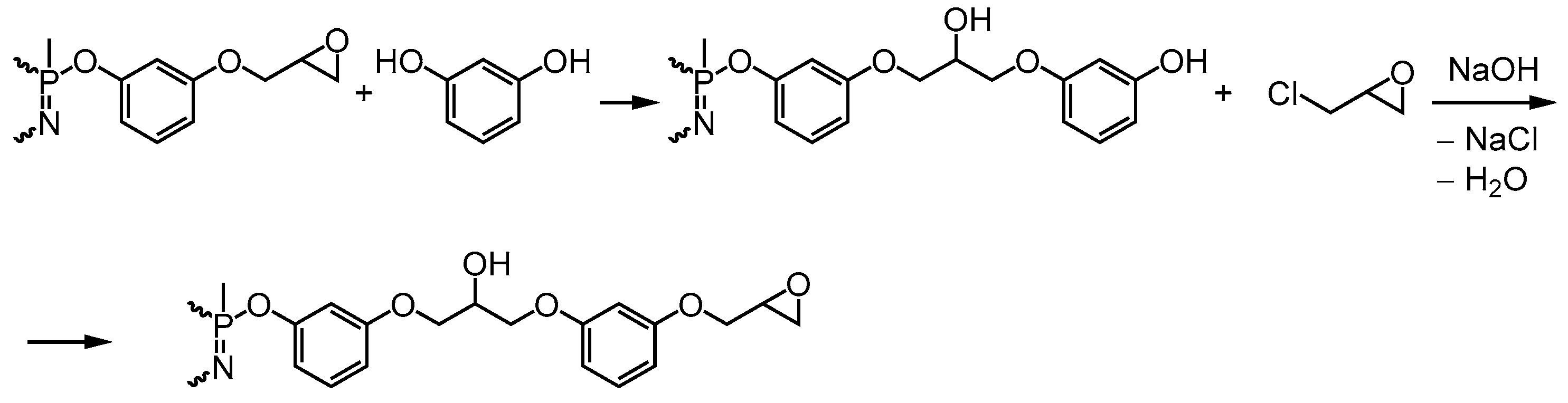
2.2.1. Synthesis of Phosphazene-Containing Resorcin-Based Epoxy Resin with the Introduction of the Entire Amount of Alkali at the Beginning of the Reaction
2.2.2. Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Resin with the Gradual Loading of Alkali
2.3. Methods of Analysis
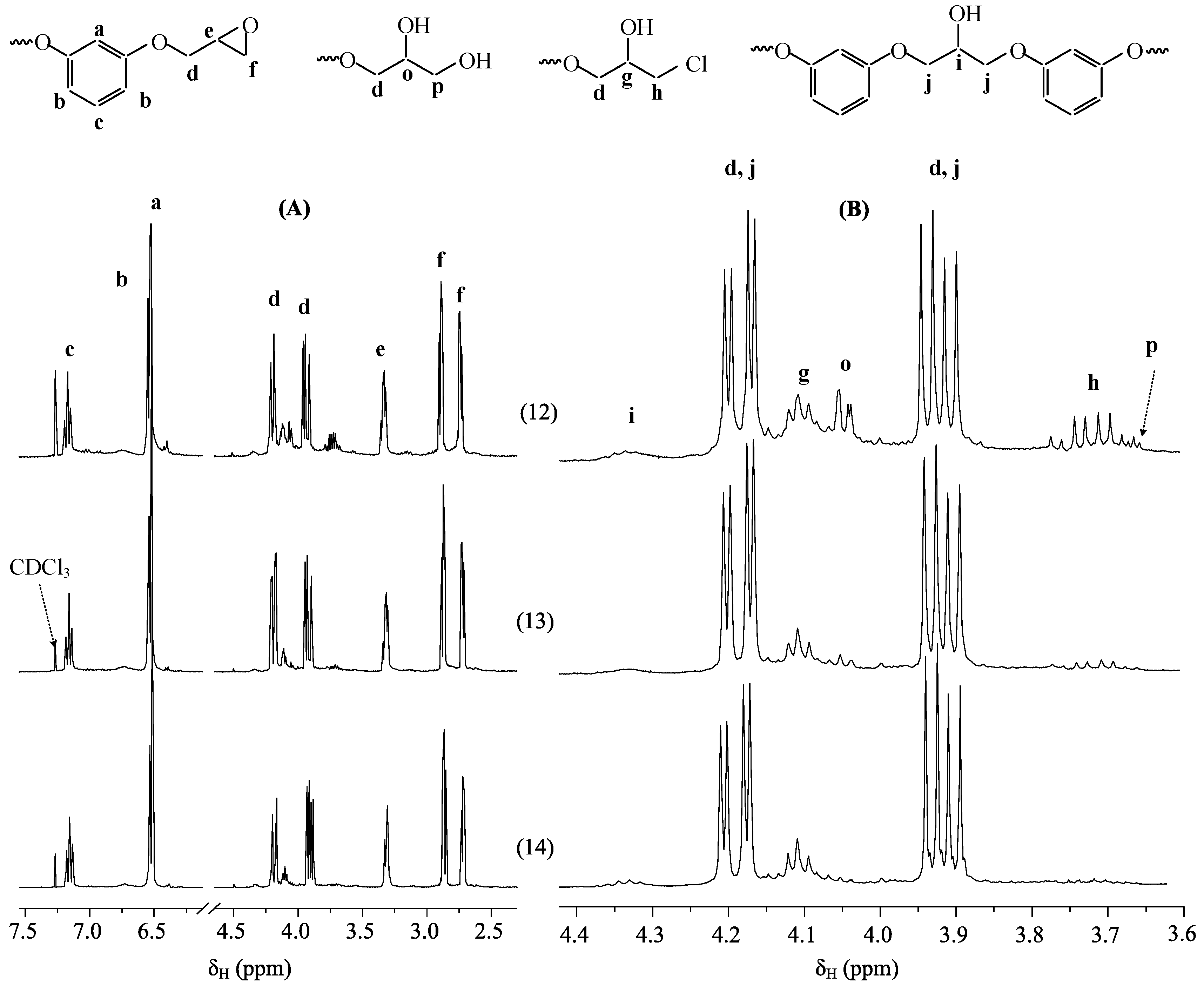
3. Results and Discussion
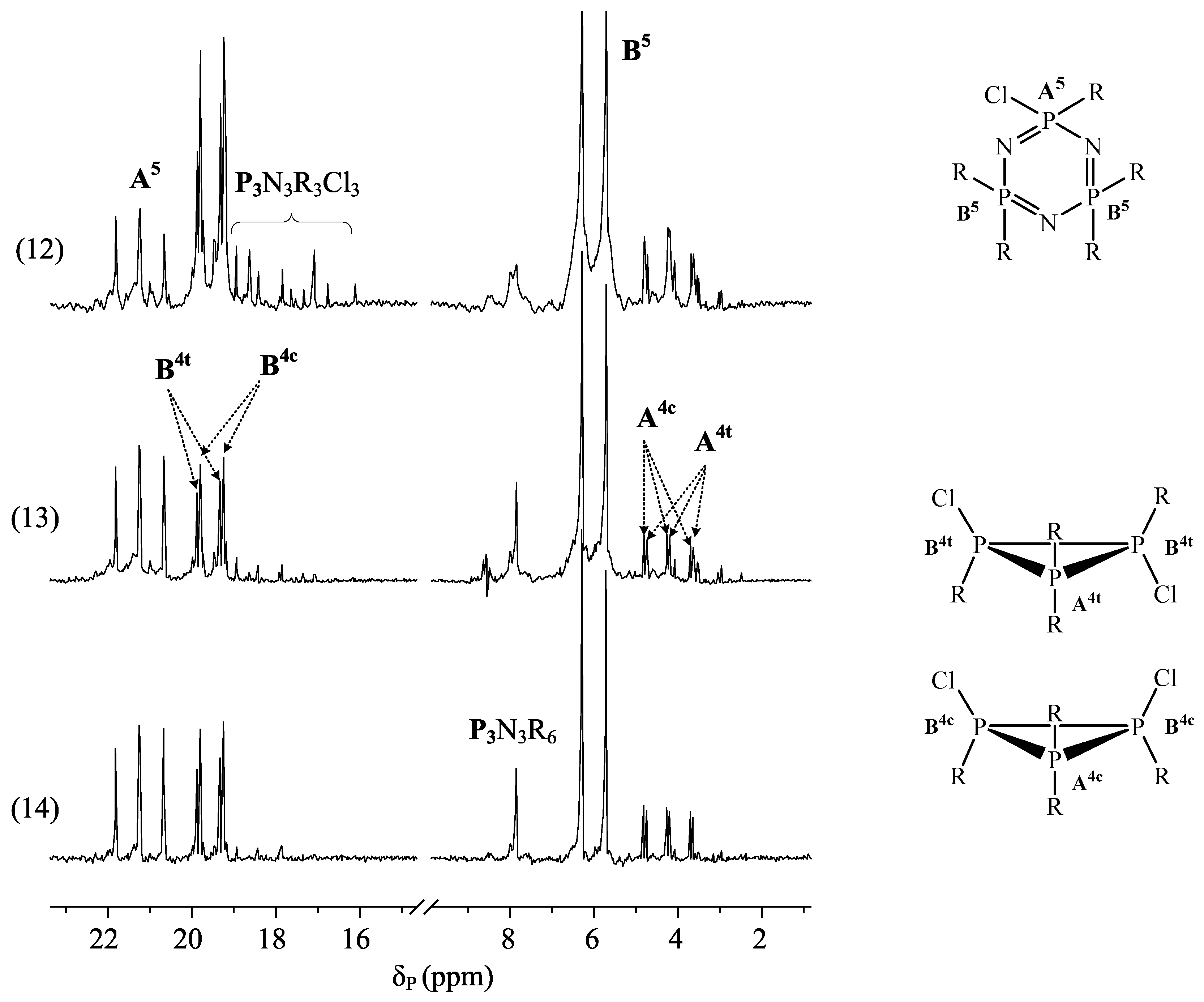
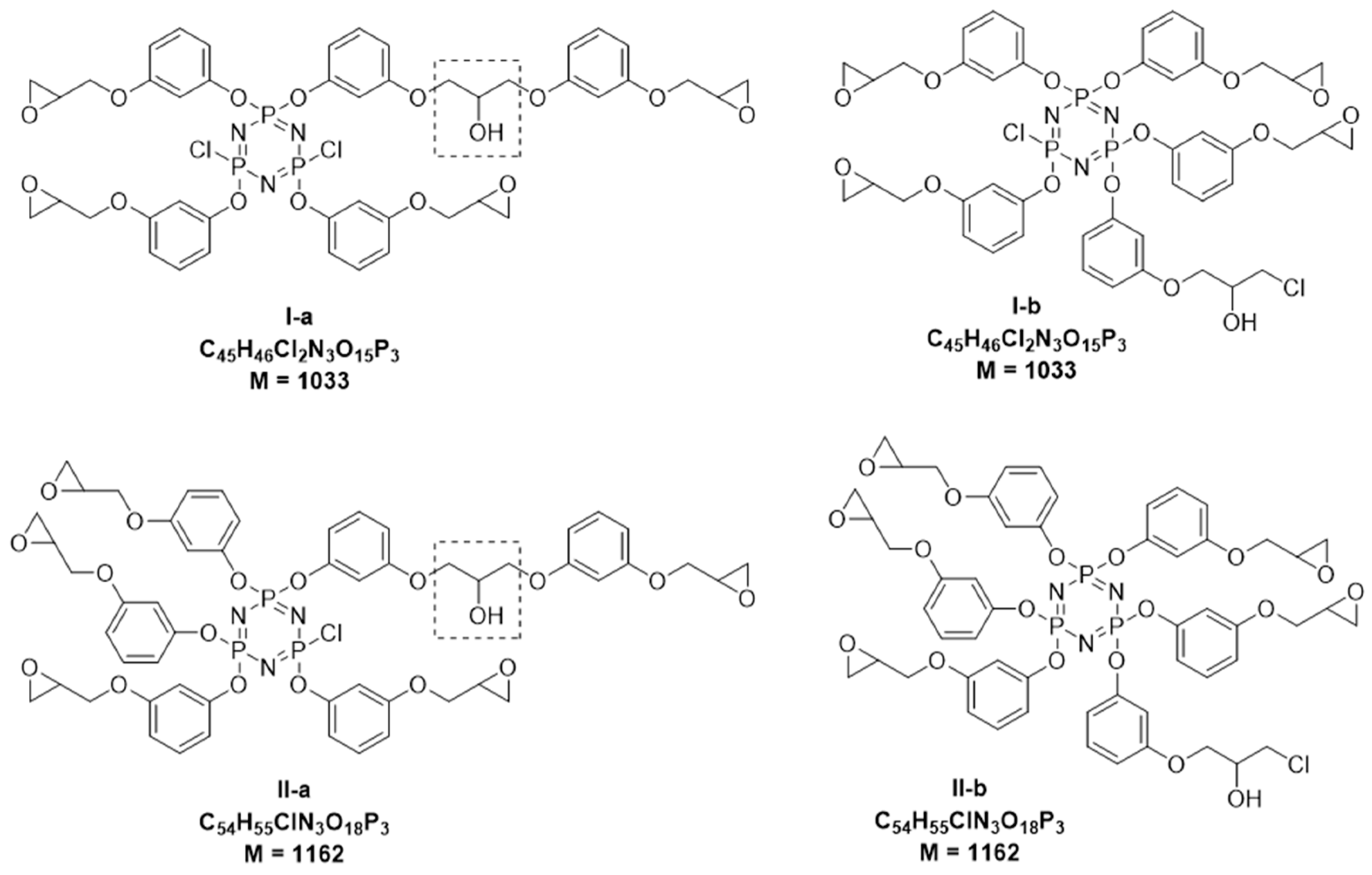
- more complex PERO compositions are formed at the molar ratios of HCP:resorcinol = 1:12, and there is greater presence of compounds whose molecules contain two triphosphazene cycles (m/z > 1200) in their composition;
- in the composition of the phosphazene fractions of oligomers synthesized at the HCP:resorcinol ratio > 1:12, there are mainly tetra- (m/z = 866) and penta-epoxy (m/z = 996) derivatives with content predominantly being of the latter;
- there is an insignificant amount or almost complete absence of hexa-(3-glicidyloxyphenoxy)-cyclotriphosphazene (m/z = 1126) in the composition of PERO;
- the most homogeneous composition of the phosphazene fraction is PERO synthesized at molar ratios of HCP:resorcinol = 1:16 and 1:24, especially in the case of gradual loading of solid alkali (Fig. 2, spectra 13 and 14). At a ratio of 1:24, predominantly tetra- (m/z = 866) and penta-substituted (m/z = 996) compounds are present in the phosphazene fraction.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biron, M. Thermosets and Composites: Material Selection, Applications, Manufacturing and Cost Analysis; Elsevier: Oxford, UK, 2013; ISBN 978-1-4557-3125-1. [Google Scholar]
- Dodiuk, H.; Goodman, S.H. Handbook of Thermoset Plastics; William Andrew: San Diego, CA, USA, 2013; ISBN 978-1-4557-3109-1. [Google Scholar]
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw Hill Professional: New York, NY, USA, 2005; ISBN 978-0-07-158908-6. [Google Scholar]
- Flick, E.W. Epoxy Resins, Curing Agents, Compounds, and Modifiers: An Industrial Guide; Noyes Publications: Saddle River, NJ, USA, 2012; ISBN 978-0-8155-1708-5. [Google Scholar]
- Visakh, P.M.; Arao, Y. (Eds.) Flame Retardants: Polymer Blends, Composites and Nanocomposites; Engineering Materials, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-03466-9. [Google Scholar]
- Lu, S.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Rodriguez, F.; Cohen, C.; Ober, C.K.; Archer, L. Principles of Polymer Systems, 6th ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4822-2378-1. [Google Scholar]
- Amirova, L.M.; Andrianova, K.A. Gradient polymeric materials based on poorly compatible epoxy oligomers. J. Appl. Polym. Sci. 2006, 102, 96–103. [Google Scholar] [CrossRef]
- Amirova, L.M.; Stroganov, V.F.; Sakhabieva, E.V. Optical Materials the Based on Low Flammable Epoxy Resins. Int. J. Polym. Mater. Polym. Biomater. 2000, 47, 43–60. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Price, D. Advances in Fire Retardant Materials; Woodhead Publishing: Cambridge, UK, 2008; ISBN 978-1-84569-507-1. [Google Scholar]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Allen, C.W. The Use of Phosphazenes as Fire Resistant Materials. J. Fire Sci. 1993, 11, 320–328. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A Review of Recent Progress in Phosphorus-based Flame Retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Allcock, H. Phosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric Systems; Academic Press: New York, NY, USA, 1972; ISBN 978-0-12-050560-9. [Google Scholar]
- Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.A.; Gaan, S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications. Materials 2017, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Phosphazene | Chemical Products | Otsuka Chemical Co., Ltd. Available online: https://www.otsukac.co.jp/en/products/flame-retardant/phosphazene/ (accessed on 23 December 2018).
- Höhne, C.-C.; Wendel, R.; Käbisch, B.; Anders, T.; Henning, F.; Kroke, E. Hexaphenoxycyclotriphosphazene as FR for CFR anionic PA6 via T-RTM: A study of mechanical and thermal properties. Fire Mater. 2017, 41, 291–306. [Google Scholar] [CrossRef]
- Denq, B.-L.; Hu, Y.-S.; Chiu, W.-Y.; Chen, L.-W.; Chiu, Y.-S. Thermal degradation behavior and physical properties for poly (methyl methacrylate) blended with propyl ester phosphazene. Polym. Degrad. Stab. 1997, 57, 269–278. [Google Scholar] [CrossRef]
- Denq, B.; Chiu, W.; Chen, L.; Lee, C. Thermal degradation behavior of polystyrene blended with propyl ester phosphazene. Polym. Degrad. Stab. 1997, 57, 261–268. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Wang, W.; Qian, L. Preparation and characterization of surface-modified ammonium polyphosphate and its effect on the flame retardancy of rigid polyurethane foam. J. Appl. Polym. Sci. 2017, 134, 45369. [Google Scholar] [CrossRef]
- Yang, R.; Wang, B.; Han, X.; Ma, B.; Li, J. Synthesis and characterization of flame retardant rigid polyurethane foam based on a reactive flame retardant containing phosphazene and cyclophosphonate. Polym. Degrad. Stab. 2017, 144, 62–69. [Google Scholar] [CrossRef]
- Nair, P.R.; Nair, C.P.R.; Francis, D.J. Phosphazene-modified polyurethanes: Synthesis, mechanical and thermal characteristics. Eur. Polym. J. 1996, 32, 1415–1420. [Google Scholar] [CrossRef]
- Byczyński, Ł.; Dutkiewicz, M.; Januszewski, R. Thermal behaviour and flame retardancy of polyurethane high-solid coatings modified with hexakis(2,3-epoxypropyl)cyclotriphosphazene. Prog. Org. Coat. 2017, 108, 51–58. [Google Scholar] [CrossRef]
- Machotova, J.; Stranska, E.; Skornok, J.; Zarybnicka, L.; Melanova, K.; Rychly, J.; Ruckerova, A. Fluorine containing self-crosslinking acrylic latexes with reduced flammability and their application as polymer binders for heterogeneous cation-exchange membranes. J. Appl. Polym. Sci. 2017, 134, 45467. [Google Scholar] [CrossRef]
- Chen-Yang, Y.W.; Lee, H.F.; Yuan, C.Y. A flame-retardant phosphate and cyclotriphosphazene-containing epoxy resin: Synthesis and properties. J. Polym. Sci. Part Polym. Chem. 2000, 38, 972–981. [Google Scholar] [CrossRef]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Ziraoui, R.; Rafik, M.; El Harfi, A. A phosphazene compound multipurpose application-Composite material precursor and reactive flame retardant for epoxy resin materials. J Mater Env. Sci 2011, 2, 319–334. [Google Scholar]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Thermal degradation of a reactive flame retardant based on cyclotriphosphazene and its blend with DGEBA epoxy resin. Polym. Degrad. Stab. 2009, 94, 2101–2106. [Google Scholar] [CrossRef]
- Krishnadevi, K.; Selvaraj, V. Development of cyclophosphazene and rice husk ash incorporated epoxy composites for high performance applications. Polym. Bull. 2017, 74, 1791–1815. [Google Scholar] [CrossRef]
- Qian, L.-J.; Ye, L.-J.; Xu, G.-Z.; Liu, J.; Guo, J.-Q. The non-halogen flame retardant epoxy resin based on a novel compound with phosphaphenanthrene and cyclotriphosphazene double functional groups. Polym. Degrad. Stab. 2011, 96, 1118–1124. [Google Scholar] [CrossRef]
- Qian, L.; Ye, L.; Qiu, Y.; Qu, S. Thermal degradation behavior of the compound containing phosphaphenanthrene and phosphazene groups and its flame retardant mechanism on epoxy resin. Polymer 2011, 52, 5486–5493. [Google Scholar] [CrossRef]
- Xu, M.-J.; Xu, G.-R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Terekhov, I.V.; Filatov, S.N.; Chistyakov, E.M.; Borisov, R.S.; Kireev, V.V. Synthesis of oligomeric epoxycyclotriphosphazenes and their properties as reactive flame-retardants for epoxy resins. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 544–554. [Google Scholar] [CrossRef]
- Fantin, G.; Medici, A.; Fogagnolo, M.; Pedrini, P.; Gleria, M.; Bertani, R.; Facchin, G. Functionalization of poly(organophosphazenes)—III. Synthesis of phosphazene materials containing carbon-carbon double bonds and epoxide groups. Eur. Polym. J. 2012, 29, 1571–1579. [Google Scholar] [CrossRef]
- Allcock, H.R.; Nelson, C.J.; Coggio, W.D. Photoinitiated graft poly(organophosphazenes): Functionalized immobilization substrates for the binding of amines, proteins, and metals. Chem. Mater. 1994, 6, 516–524. [Google Scholar] [CrossRef]
- Bertani, R.; Boscolo-Boscoletto, A.; Dintcheva, N.; Ghedini, E.; Gleria, M.; La Mantia, F.; Pace, G.; Pannocchia, P.; Sassi, A.; Scaffaro, R.; et al. New phosphazene-based chain extenders containing allyl and epoxide groups. Des. Monomers Polym. 2003, 6, 245–266. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; La Mantia, F.P.; Magagnini, P.; Acierno, D.; Gleria, M.; Bertani, R. Effect of adding new phosphazene compounds to poly(butylene terephthalate)/polyamide blends. I: Preliminary study in a batch mixer. Polym. Degrad. Stab. 2005, 90, 234–243. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X. Synthesis, characterization, thermal properties and flame retardancy of a novel nonflammable phosphazene-based epoxy resin. Polym. Degrad. Stab. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- El Gouri, M.; Cherkaoui, O.; Ziraoui, R.; El Harfi, A. Physico-chemical study of DGEBA epoxy resin flame retarded with an ecological flame retardant based on cyclotriphosphazene. J. Mater. Environ. Sci. 2010, 3, 157–162. [Google Scholar]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Fireproofing amelioration of epoxy resin material by way a reactive flame retardant based on cyclophosphazene. Phys. Chem. News 2010, 56, 128–137. [Google Scholar]
- El Gouri, M.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Synthesis and thermal degradation of phosphazene containing the epoxy group. Ann. Chim. Sci. Mater. 2010, 35, 27–39. [Google Scholar] [CrossRef]
- Liu, F.; Wei, H.; Huang, X.; Zhang, J.; Zhou, Y.; Tang, X. Preparation and Properties of Novel Inherent Flame-Retardant Cyclotriphosphazene-Containing Epoxy Resins. J. Macromol. Sci. Part B 2010, 49, 1002–1011. [Google Scholar] [CrossRef]
- Gu, X.; Huang, X.; Wei, H.; Tang, X. Synthesis of novel epoxy-group modified phosphazene-containing nanotube and its reinforcing effect in epoxy resin. Eur. Polym. J. 2011, 47, 903–910. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Wu, D. Novel cyclolinear cyclotriphosphazene-linked epoxy resin for halogen-free fire resistance: Synthesis, characterization, and flammability characteristics. Ind. Eng. Chem. Res. 2012, 51, 15064–15074. [Google Scholar] [CrossRef]
- El Gouri, M.; El Harfi, A. Chemical modification of hexachlorocyclotriphosphazene—Preparation of flame retardants and ecological flame retardant polymers. J. Mater. Environ. Sci. 2012, 3, 17–33. [Google Scholar]
- Liu, J.; Tang, J.; Wang, X.; Wu, D. Synthesis, characterization and curing properties of a novel cyclolinear phosphazene-based epoxy resin for halogen-free flame retardancy and high performance. RSC Adv. 2012, 2, 5789–5799. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Wu, D. Novel Spirocyclic Phosphazene-Based Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Curing Behaviors, and Flammability Characteristics. Acs Appl. Mater. Interfaces 2012, 4, 4047–4061. [Google Scholar] [CrossRef]
- Feng, H.; Wang, X.; Wu, D. Fabrication of spirocyclic phosphazene epoxy-based nanocomposites with graphene via exfoliation of graphite platelets and thermal curing for enhancement of mechanical and conductive properties. Ind. Eng. Chem. Res. 2013, 52, 10160–10171. [Google Scholar] [CrossRef]
- Huang, X.; Wei, W.; Wei, H.; Li, Y.; Gu, X.; Tang, X. Preparation of heat-moisture resistant epoxy resin based on phosphazene. J. Appl. Polym. Sci. 2013, 130, 248–255. [Google Scholar] [CrossRef]
- El Gouri, M.; El Mansouri, A.; El Gouri, R.; Hadik, N.; Cherkaoui, O.; Outzourhit, A.; El Harfi, A. Physical behaviour of epoxy resin material flame retarded with a reactive flame retardant based on cyclophosphazene. J. Mater. Environ. Sci. 2014, 5, 400–4007. [Google Scholar]
- Lu, L.; Chen, Y.; Wang, S.; Yang, S.; Dong, X. Preparation and flame retardancy of MMT pattern synergy intumescent flame-retardant epoxy resin. Gaofenzi Cailiao Kexue Yu Gongchengpolymeric Mater. Sci. Eng. 2014, 30, 139–144. [Google Scholar]
- Xu, G.R.; Xu, M.J.; Li, B. Synthesis and characterization of a novel epoxy resin based on cyclotriphosphazene and its thermal degradation and flammability performance. Polym. Degrad. Stab. 2014, 109, 240–248. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Novel cyclotriphosphazene-based epoxy compound and its application in halogen-free epoxy thermosetting systems: Synthesis, curing behaviors, and flame retardancy. Polym. Degrad. Stab. 2014, 103, 96–112. [Google Scholar] [CrossRef]
- Lakshmikandhan, T.; Sethuraman, K.; Chandramohan, A.; Alagar, M. Development of phosphazene imine-modified epoxy composites for low dielectric, antibacterial activity, and UV shielding applications. Polym. Compos. 2017, 38, E24–E33. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Synthesis of a novel linear polyphosphazene-based epoxy resin and its application in halogen-free flame-resistant thermosetting systems. Polym. Degrad. Stab. 2005, 118, 45–58. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Jiang, L.; Mao, Y.; Zhao, D. Kinetics of Thermal Decomposition and Flame Retardance of Phosphazene-Containing Epoxy Resin; Combustion Institute: Beijing, China, 2015. [Google Scholar]
- Takahashi, K.; Yamamoto, T.; Itoh, S.; Harakawa, K.; Kajiwara, M. Mechanical Properties of Epoxy Resins Cured by Various Amines. Zair. Soc. Mater. Sci. Jpn. 1988, 37, 454–459. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, K.; Kon, Y.; Harakawa, K. Curing of epoxy resin with phosphazene derivatives. Kobunshi Ronbunshu 1988, 45, 851–856. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, K.; Kon, Y.; Kobayashi, K. Tensile Behavior and Heat Resistance of Epoxy Resin Cured with Phosphazene Derivatives. Kobunshi Ronbunshu 1989, 46, 177–181. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Komori, T.; Yoon, H.-S. Curing of an epoxy resin with P3N3(NH2)2 (OCH2CF3)4 and its mechanical properties. Kobunshi Ronbunshu 1990, 47, 727–734. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Yoon, H.-S. Mechanical properties of epoxy resins cured with P3N3(NH2)2 (OC6H4Cl)4. Kobunshi Ronbunshu 1990, 47, 757–762. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Yoon, H.-S. Resistance of epoxy resins cured with trichloro-tridimethylamino-cyclotriphosphazene against chemical substances. Zair. Soc. Mater. Sci. Jpn. 1990, 39, 1001–1006. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Kohno, T.; Yoon, H.-S. Water resistance of an epoxy resin cured with P3N3Cl3(N(CH3)2)3 and the effect of glass flake reinforcement. Zair. Soc. Mater. Sci. Jpn. 1991, 40, 458–463. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakashima, J.; Ishiguro, S. Mechanical properties of trifunctional epoxy resin with phosphazene derivatives. Kobunshi Ronbunshu 1994, 51, 717–723. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liao, Y.L.; Lin, J.J. Synergistic effect of silicate clay and phosphazene-oxyalkyleneamines on thermal stability of cured epoxies. J. Colloid Interface Sci. 2010, 343, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kireev, V.V.; Bilichenko, Y.V.; Borisov, R.S.; Sirotin, I.S.; Filatov, S.N. Laser Mass Spectrometry Analysis of the Formation of Phosphazene-Containing Epoxy Oligomers. Polym. Sci. Ser. B 2018, 60, 243–262. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Brigadnov, K.A.; Kireev, V.V.; Suraeva, O.V.; Borisov, R.S. Oligomeric hydroxy-aryloxy phosphazene based on cyclic chlorophosphazenes. Russ. J. Appl. Chem. 2013, 86, 1903–1912. [Google Scholar] [CrossRef]
- Kireev, V.V.; Chistyakov, E.M.; Filatov, S.N.; Borisov, R.S.; Prudskov, B.M. Synthesis and modification of oligo(aryloxycyclotriphosphazenes) based on 4,4′-dihydroxydiphenyl-2,2-propane. Polym. Sci. Ser. B 2011, 53, 412. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Onuchin, D.V.; Sarychev, I.A.; Orlov, A.V.; Filatov, S.N.; Kireev, V.V.; Mu, J. Phosphazene-Containing Epoxy Resin and Method for Its Production. RU Patent 2,639,708, 22 December 2017. [Google Scholar]
- Brigadnov, K.A.; Bilichenko, Y.V.; Polyakov, V.A.; Borisov, R.S.; Gusev, K.I.; Rudakova, T.A.; Filatov, S.N.; Kireev, V.V. Epoxy oligomers modified with epoxyphosphazenes. Polym. Sci. Ser. B 2016, 58, 549–555. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Brigadnov, K.A.; Kireev, V.V.; Prudskov, B.M.; Borisov, R.S. Single-stage synthesis of phosphazene-containing epoxy oligomers. Polym. Sci. Ser. B 2014, 56, 471–476. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Suraeva, O.V.; Solodukhin, A.N.; Kireev, V.V. Synthesis of oligomeric chlorophosphazenes in the presence of ZnCl2. Polym. Sci. Ser. B 2013, 55, 63–68. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification; Wiley: Hoboken, NJ, USA, 1986; ISBN 978-0-471-08467-9. [Google Scholar]
- Li, S.; Guan, W.; Wu, Z.; Lu, J.; Guo, J. An Improved Method to Determine Epoxy Index of Epoxy Resins. Polym.-Plast. Technol. Eng. 2007, 46, 901–903. [Google Scholar] [CrossRef]
- Evtushenko, J.M.; Zaytsev, B.E.; Ivanov, V.M.; Evtushenko, G.J.; Berlin, A.A.; Khalturinskiy, N.A. Method of Determining Hydroxyl Group Concentration in and 4,4′-Isopropylidenediphenol-Epoxide Resins. RU Patent 2,155,334, 27 August 2000. [Google Scholar]
- Sirotin, I.S.; Bilichenko, Y.V.; Grigandov, K.A.; Kireev, V.V. Method of Obtaining Epoxy Resin, Modified with Epoxyphosphazenes. RU Patent 2,537,403, 10 January 2015. [Google Scholar]
- Simonov-Emel’yanov, I.D.; Apeksimov, N.V.; Kochergina, L.M.; Bilichenko, Y.V.; Kireev, V.V.; Brigadnov, K.A.; Sirotin, I.S.; Filatov, S.N. Rheological and rheokinetic properties of phosphazene-containing epoxy oligomers. Polym. Sci. Ser. B 2016, 58, 168–172. [Google Scholar] [CrossRef]

| The Molar Ratio of HCP:Resorcinol | Reagent Amount, g | Calculated Yield 1 (g) | Calculated Epoxy group Content 1 (%) | ||
|---|---|---|---|---|---|
| HCP | Resorcinol | NaOH | |||
| 1:12 | 1.00 | 3.80 | 2.90 | 7.30 | 32.0 |
| 1:16 | 1.00 | 5.06 | 3.86 | 10.20 | 32.9 |
| 1:24 | 1.00 | 7.59 | 5.79 | 14.96 | 35.2 |
| Experiment No. | Temperature (°C) | Reaction Time (min) | Product Yield (%) | Epoxy Group Content (wt.%) |
|---|---|---|---|---|
| 1 1 | 118 | 30 | 46 | 14.9 |
| 2 | 65 3 | 90 | 58 | 19.9 |
| 3 | 90 | 30 | 71 | 15.8 |
| 4 | 100 | 30 | 55 | 20.2 |
| 5 | 118 | 15 | 75 | 21.6 |
| 6 | 118 | 30 | 69 | 22.2 |
| 7 | 118 | 45 | 75 | 21.4 |
| 8 | 118 | 60 | 68 | 21.9 |
| 9 2 | 118 | 30 | 70 | 27.3 |
| Experiment No. | The Molar Ratio of HCP:Resorcinol | Product Yield (%) | Content (wt.%) 1 | ||||
|---|---|---|---|---|---|---|---|
| Epoxy Groups | –OH Groups | P | Cl | Phosphazene Component | |||
| 10 | 1:8 | 61 | 5.5/28.5 | 5.2 | 6.8/5.6 | 10.8/2.6 | 73.1/60.0 |
| 11 | 1:10 | 71 | 14.5/30.6 | 4.1 | 4.8/4.4 | 8.3/2.3 | 51.3/47.3 |
| 12 | 1:12 | 77 | 21.0/32.0 | 2.2 | 4.0/3.7 | 4.4/2.1 | 42.5/39.8 |
| 13 | 1:16 | 89 | 28.6/32.9 | 2.0 | 3.0/2.7 | 2.4/1.9 | 31.6/29.0 |
| 14 | 1:24 | 90 | 29.6/35.2 | 0.6 | 2.0/1.8 | 1.9/1.7 | 21.2/19.1 |
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| The Number of Radicals X in the Gross Formula | Calculated Molecular Weight | The m/z Values of the Peaks on the MALDI-TOF Spectra of the PERO, in Parentheses—Peak Relative Intensity (%) | |||||||||
| Portioned Addition of NaOH in Experiments No. from Table 3 | Single-Moment Addition of All NaOH in Experiments No. from Table 2 | ||||||||||
| Gly | Gly’ | CH | 14 | 13 | 12 | 11 | 9 | 6 | 1 | 2 | |
| Tetra-Substituted Cyclotriphosphazenes (n = 4) | |||||||||||
| 4 | 867 | 867 (26.4) | 866 (14.0) | 866 (21.2) | 866 (17.5) | 866 (8.2) | 866 (14.2) | ||||
| 3 | 1 | 1033 | 1033 (3.0) | ||||||||
| 3 | 1 | 903 | 904 (9.5) | 904 (3.7) | 904 (5.1) | 904 (8.5) | |||||
| 2 | 2 | 939 | 940 (7.2) | 940 (5.5) | 940 (3.5) | 942 (6.0) | 940 (5.0) | ||||
| 1 | 1 | 2 | 1106 | 1103 (4.9) | 1103 (9.4) | 1106 (6.1) | |||||
| 1 | 3 | 976 | 976 (5.2) | ||||||||
| 1 | 3 | 1142 | 1141 (6.8) | ||||||||
| Penta-Substituted Cyclotriphosphazenes (n = 5) | |||||||||||
| 5 | 996 | 997 (65.0) | 996 (75.1) | 997 (12.5) | 996 (40.9) | 996 (45.1) | 996 (10.0) | 996 (29.4) | |||
| 4 | 1 | 1162 | 1163 (3.9) | 1163 (3.2) | 1163 (6.7) | 1163 (7.5) | 1162 (3.5) | ||||
| 3 | 2 | 1329 | 1328 (0.6) | 1325 (2.3) | 1326 (1.5) | 1326 (0.7) | 1325 (2.5) | ||||
| 4 | 1 | 1033 | 1033 (10.6) | 1032 (3.6) | 1034 (21.5) | 1034 (19.1) | 1034 (27.3) | 1032 (17.6) | |||
| 3 | 1 | 1 | 1199 | 1197 (1.9) | 1054 (2.9) | 1199 (2.5) | 1197 (3.7) | ||||
| 2 | 2 | 1 | 1365 | 1363 (3.2) | 1363 (2.5) | 1362 (0.9) | 1364 (2.1) | 1361 (1.0) | |||
| 3 | 2 | 1069 | 1068 (0.9) | 1067 (9.1) | 1068 (7.5) | 1070 (3.3) | 1068 (28.5) | 1067 (5.2) | |||
| 2 | 1 | 2 | 1235 | 1233 (3.5) | 1233 (6.3) | 1233 (2.6) | |||||
| Hexa-Substituted Cyclotriphosphazenes (n = 6) | |||||||||||
| 6 | 1126 | 1127 (1.7) | 1127 (2.8) | 1127 (5.0) | |||||||
| 3 | 2 | 1 | 1495 | 1493 (0.8) | 1491 (3.5) | 1492 (2.2) | 1491 (1.0) | ||||
| 4 | 2 | 1199 | 1197 (4.1) | 1197 (3.4) | |||||||
| Total relative content of compounds with one triphosphazene cycle 1 (%) | 98.3 | 98.0 | 91.4 | 57.5 | 91.0 | 96.2 | 92.5 | 91.7 | |||
| n | m | The Value and the Number of X Radicals in the Gross Formula 1 | Calculated Molecular Weight | Peaks on MALDI-TOF Spectra | ||||
|---|---|---|---|---|---|---|---|---|
| Gly | Gly’ | CH | DH | m/z | Relative Intensity (%) | |||
| 2 | 2 | 3 | 1 | 1269 | 1269 | 6.0 | ||
| 3 | 2 | 4 | 1 | 1399 | 1399 | 4.9 | ||
| 3 | 2 | 3 | 1 | 1 | 1436 | 1435 | 3.8 | |
| 3 | 3 | 5 | 1 | 1529 | 1529 | 3.8 | ||
| 3 | 3 | 4 | 1 | 1 | 1565 | 1566 | 1.7 | |
| 3 | 3 | 3 | 3 | 1620 | 1621 | 1.5 | ||
| 4 | 3 | 6 | 1 | 1659 | 1658 | 3.3 | ||
| 4 | 3 | 4 | 2 | 1991 | 1988 | 2.4 | ||
| 4 | 3 | 5 | 1 | 1 | 1695 | 1693 | 2.8 | |
| 4 | 3 | 4 | 1 | 1 | 1 | 1861 | 1860 | 1.6 |
| 4 | 3 | 2 | 2 | 3 | 2082 | 2081 | 1.6 | |
| 4 | 3 | 4 | 2 | 1 | 1731 | 1731 | 1.5 | |
| 4 | 4 | 7 | 1 | 1788 | 1788 | 0.9 | ||
| 4 | 4 | 6 | 1 | 1 | 1954 | 1952 | 2.5 | |
| 4 | 4 | 6 | 1 | 1 | 1825 | 1824 | 1.7 | |
| - | - | 4 | 1 | 1 | 2116 2 | 2117 | 1.3 | |
| - | - | 5 | 1 | 1 | 2246 2 | 2246 | 1.2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarychev, I.A.; Sirotin, I.S.; Borisov, R.S.; Mu, J.; Sokolskaya, I.B.; Bilichenko, J.V.; Filatov, S.N.; Kireev, V.V. Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Oligomers. Polymers 2019, 11, 614. https://doi.org/10.3390/polym11040614
Sarychev IA, Sirotin IS, Borisov RS, Mu J, Sokolskaya IB, Bilichenko JV, Filatov SN, Kireev VV. Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Oligomers. Polymers. 2019; 11(4):614. https://doi.org/10.3390/polym11040614
Chicago/Turabian StyleSarychev, Igor A., Igor S. Sirotin, Roman S. Borisov, Jianxin Mu, Irina B. Sokolskaya, Julya V. Bilichenko, Sergey N. Filatov, and Vyacheslav V. Kireev. 2019. "Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Oligomers" Polymers 11, no. 4: 614. https://doi.org/10.3390/polym11040614
APA StyleSarychev, I. A., Sirotin, I. S., Borisov, R. S., Mu, J., Sokolskaya, I. B., Bilichenko, J. V., Filatov, S. N., & Kireev, V. V. (2019). Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Oligomers. Polymers, 11(4), 614. https://doi.org/10.3390/polym11040614







