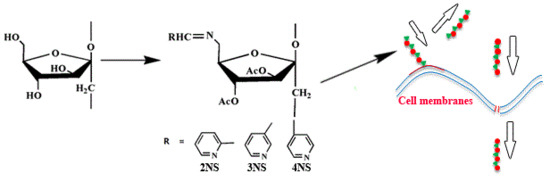
Synthesis, Characterization, and Antifungal Activity of Schiff Bases of Inulin Bearing Pyridine ring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
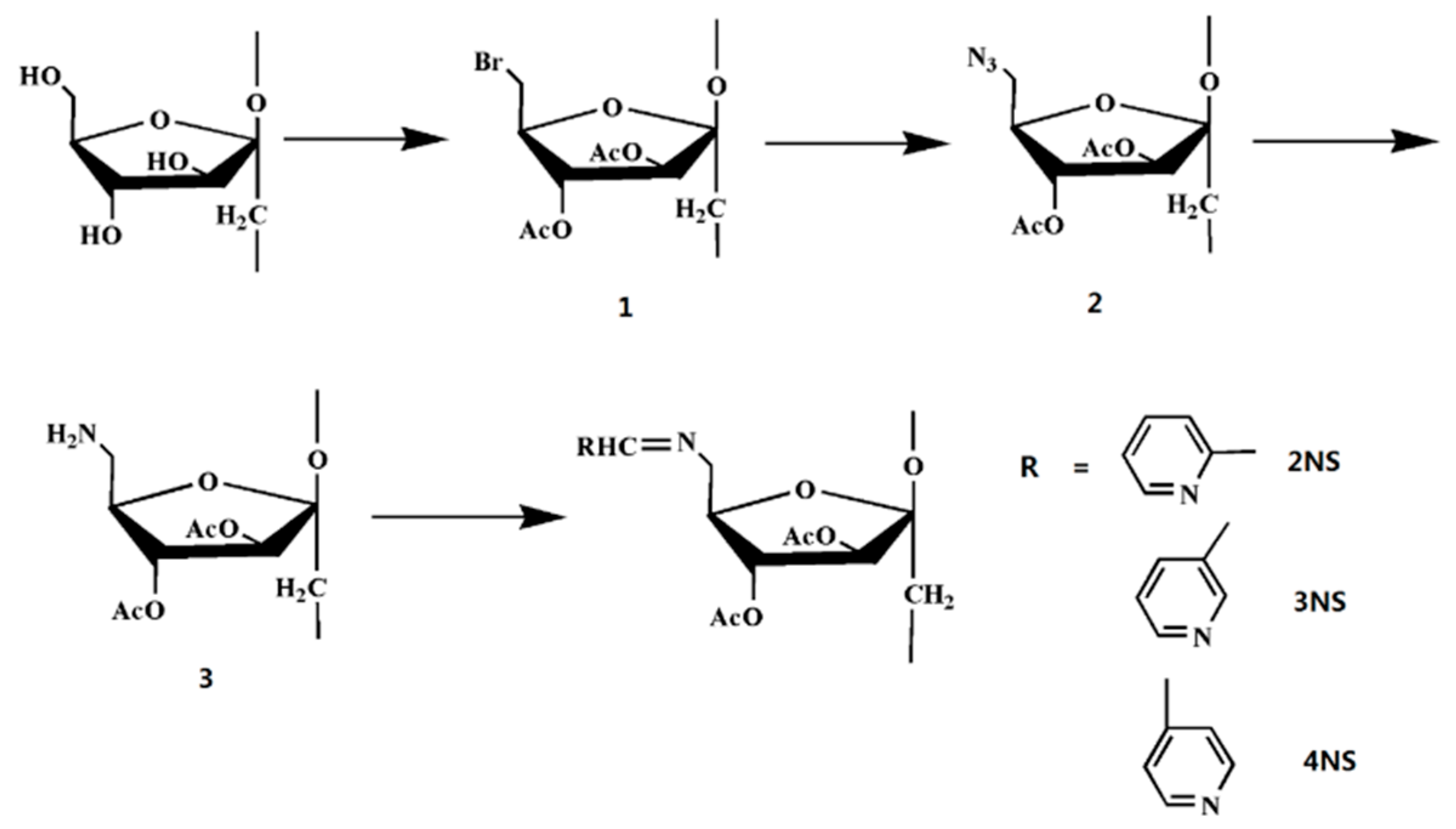
2.3. The Synthesis of the Inulin Derivatives
2.3.1. Synthesis of 6-bromo-6-deoxy-3,4-acetyl Inulin (1)
2.3.2. Synthesis of 6-azido-6-deoxy-3,4-acetyl Inulin (2)
2.3.3. Synthesis of 6-amino-6-deoxy-3,4-acetyl Inulin (3)
2.3.4. Synthesis of Schiff Bases of Inulin Bearing Pyridine Rings (2NS, 3NS, and 4NS)
2.4. Antifungal Assay
3. Results and Discussion
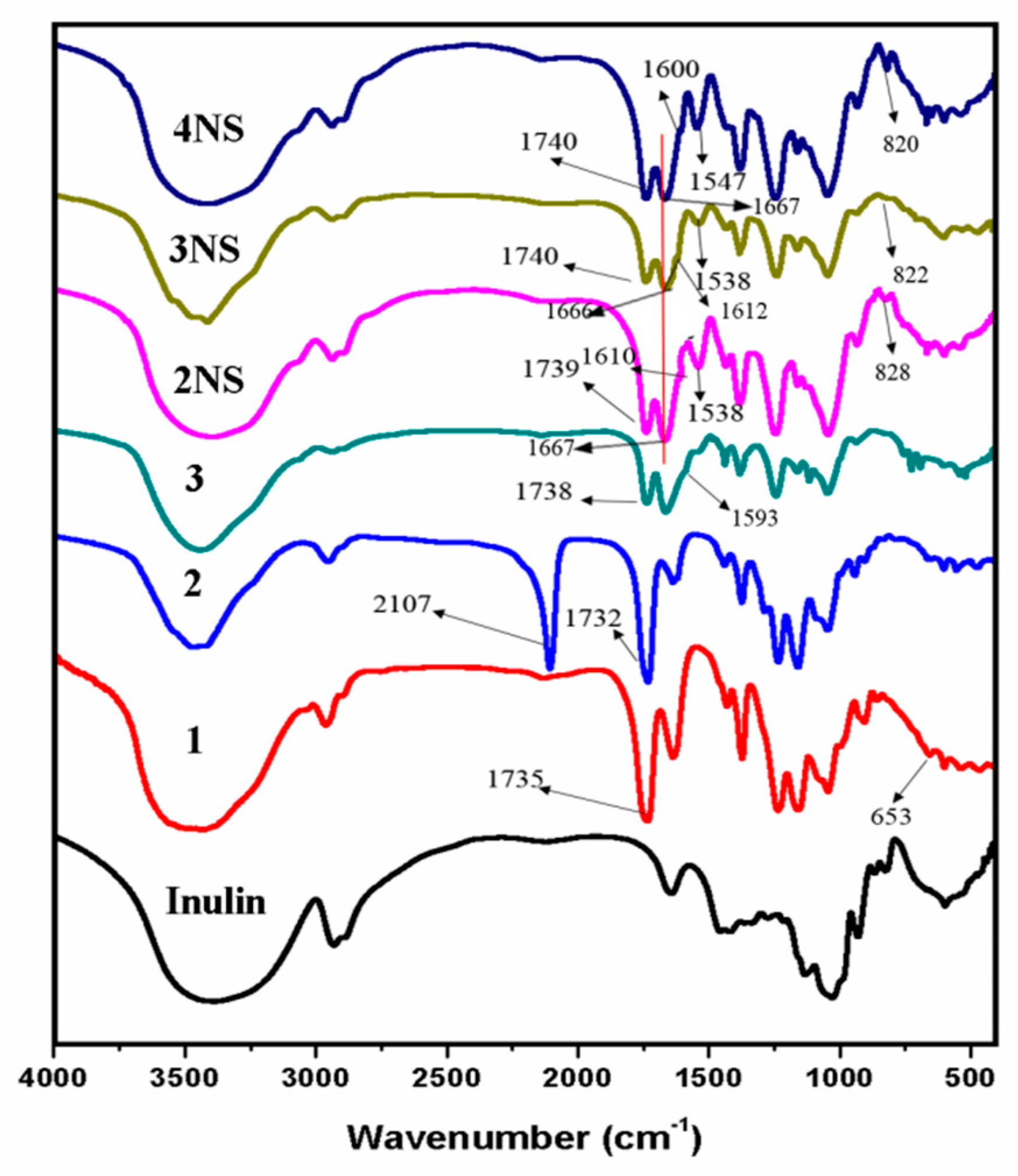
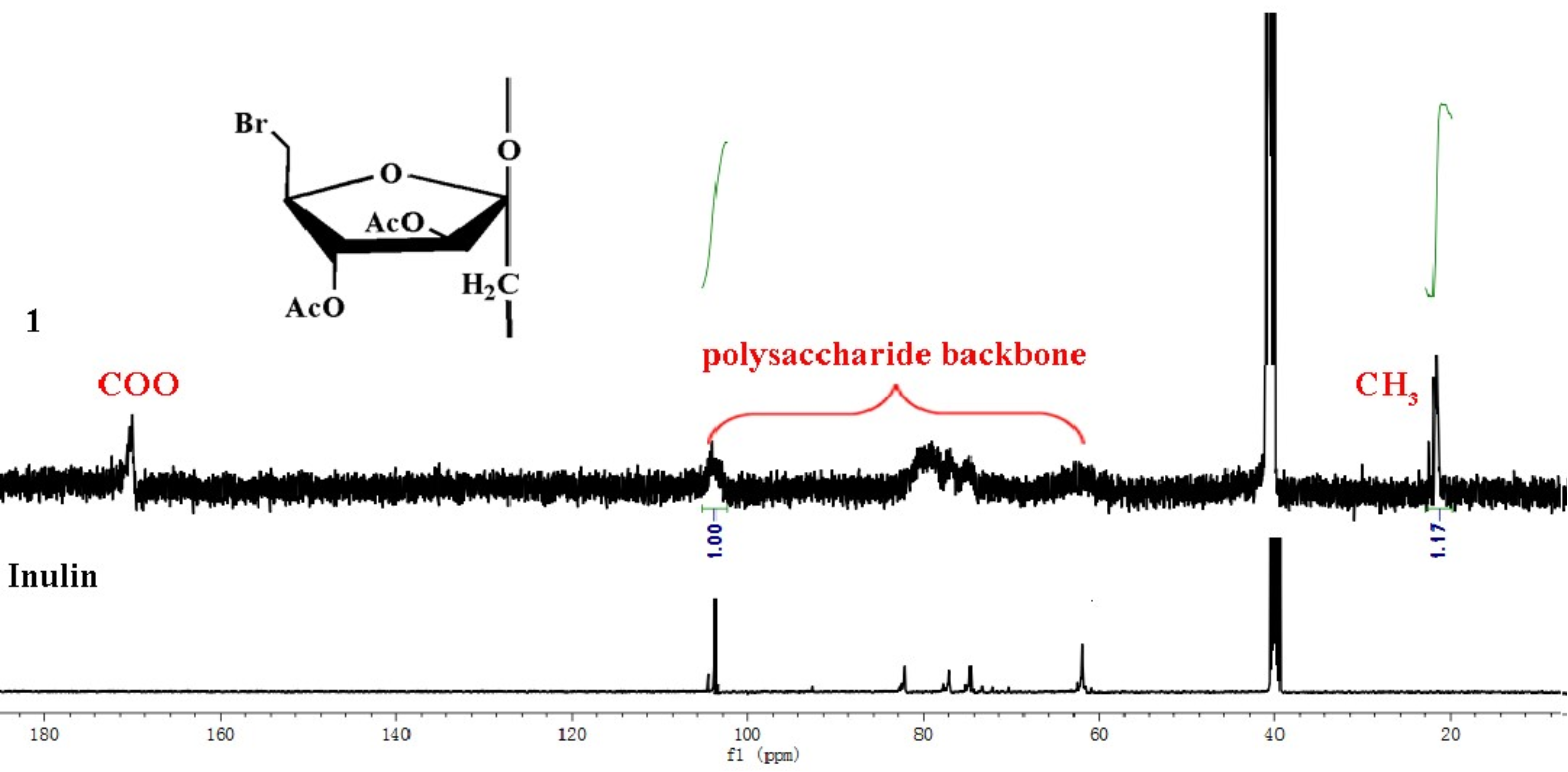
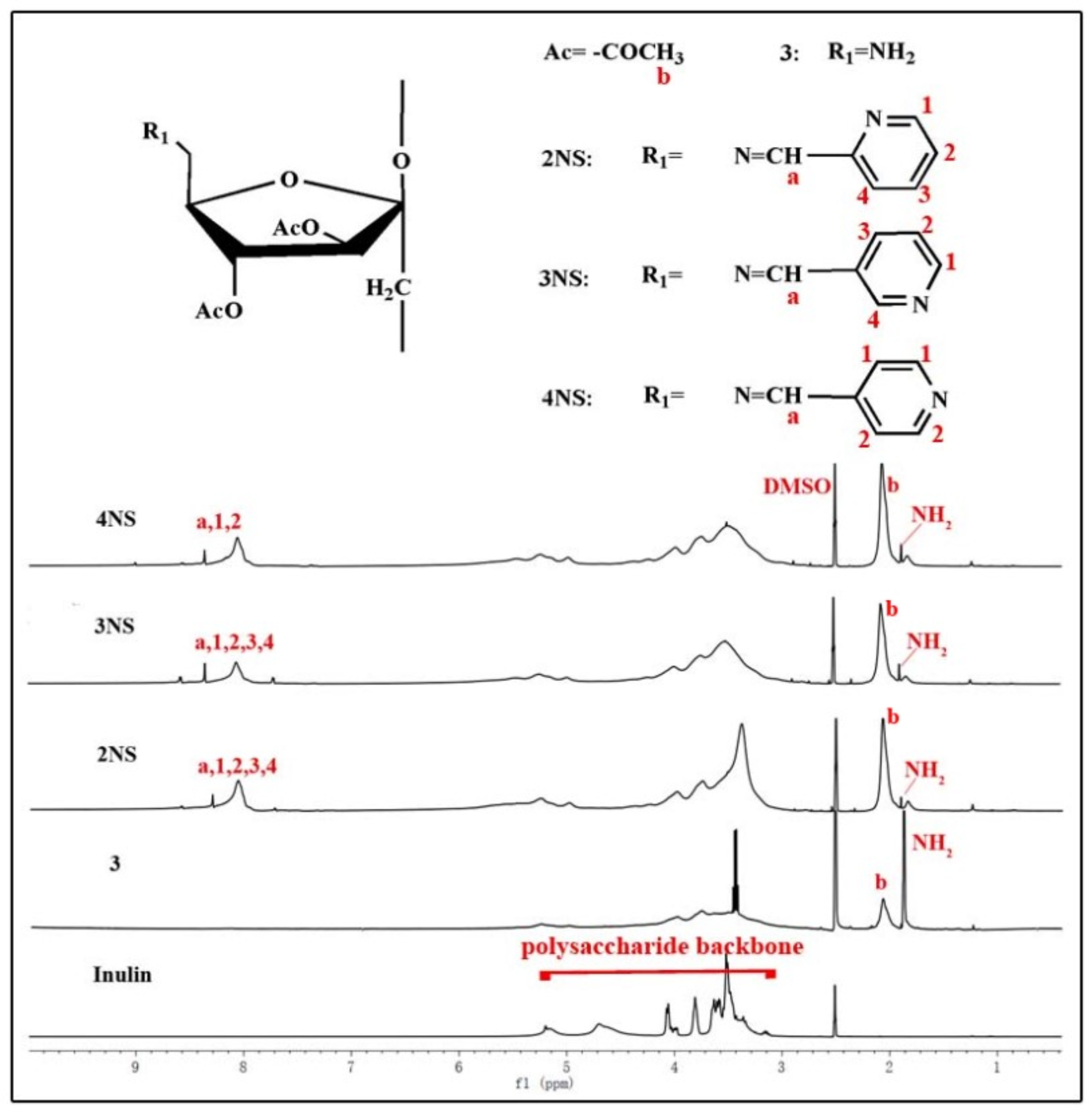
3.1. Structure of Schiff Bases of Inulin
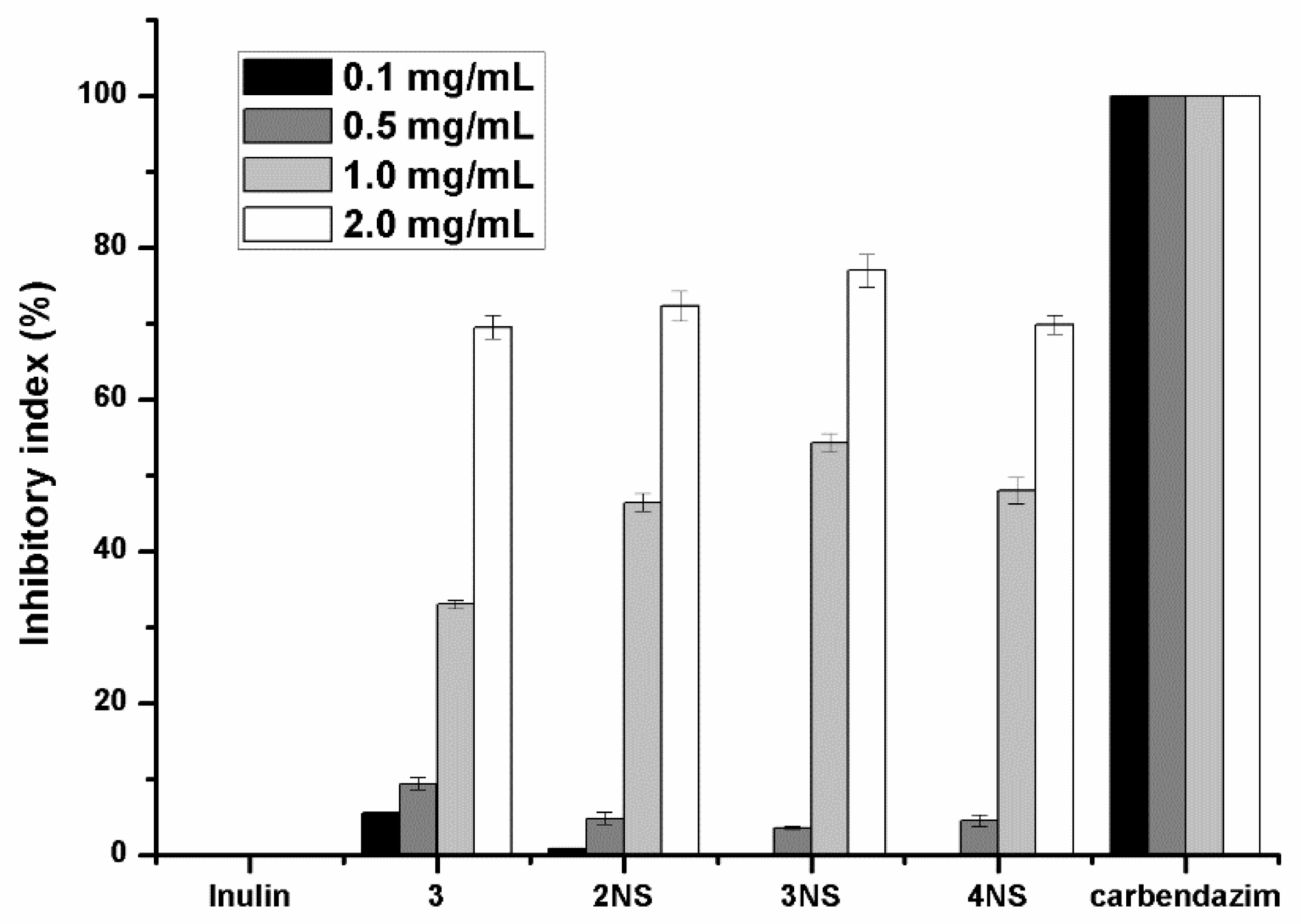
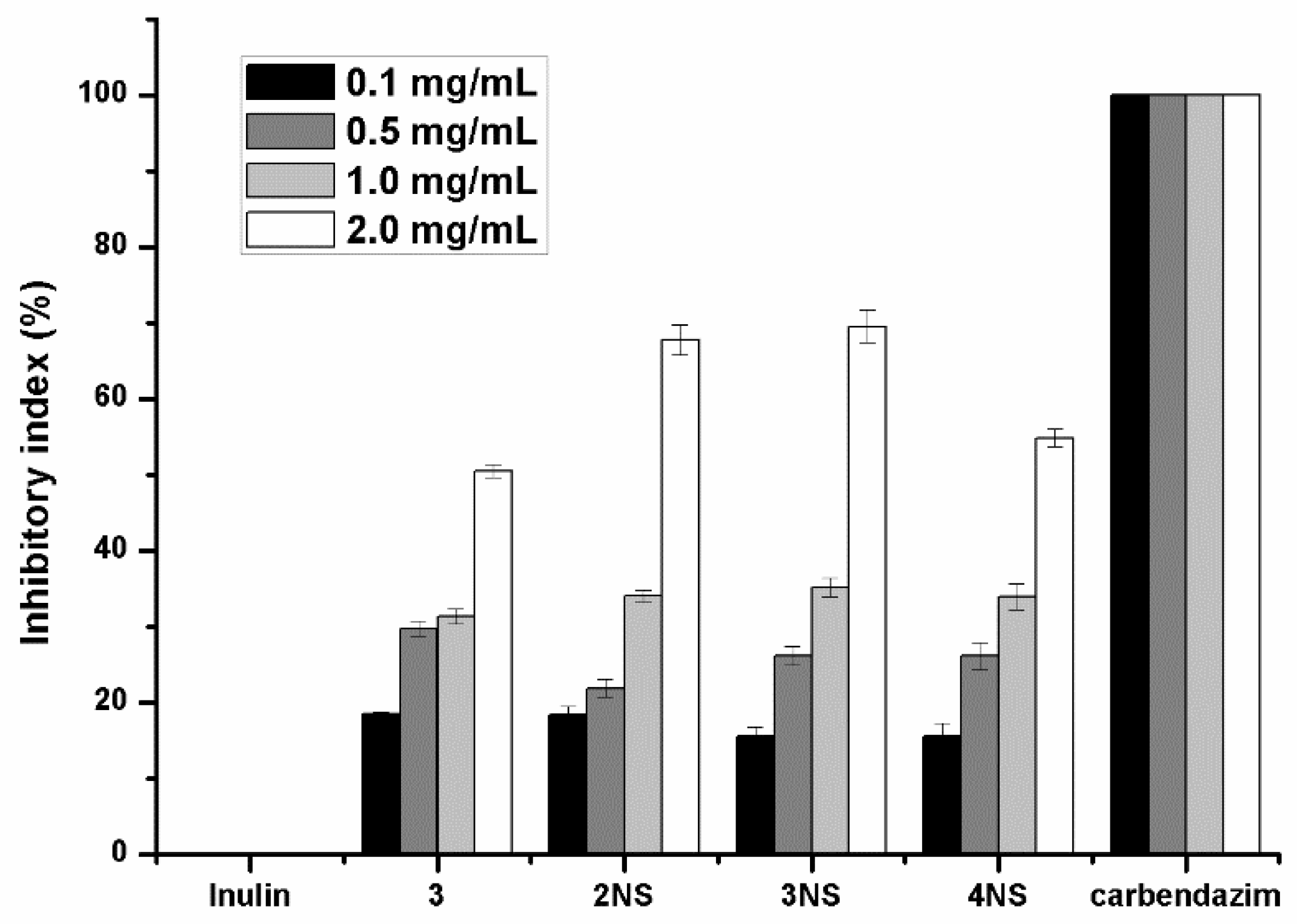
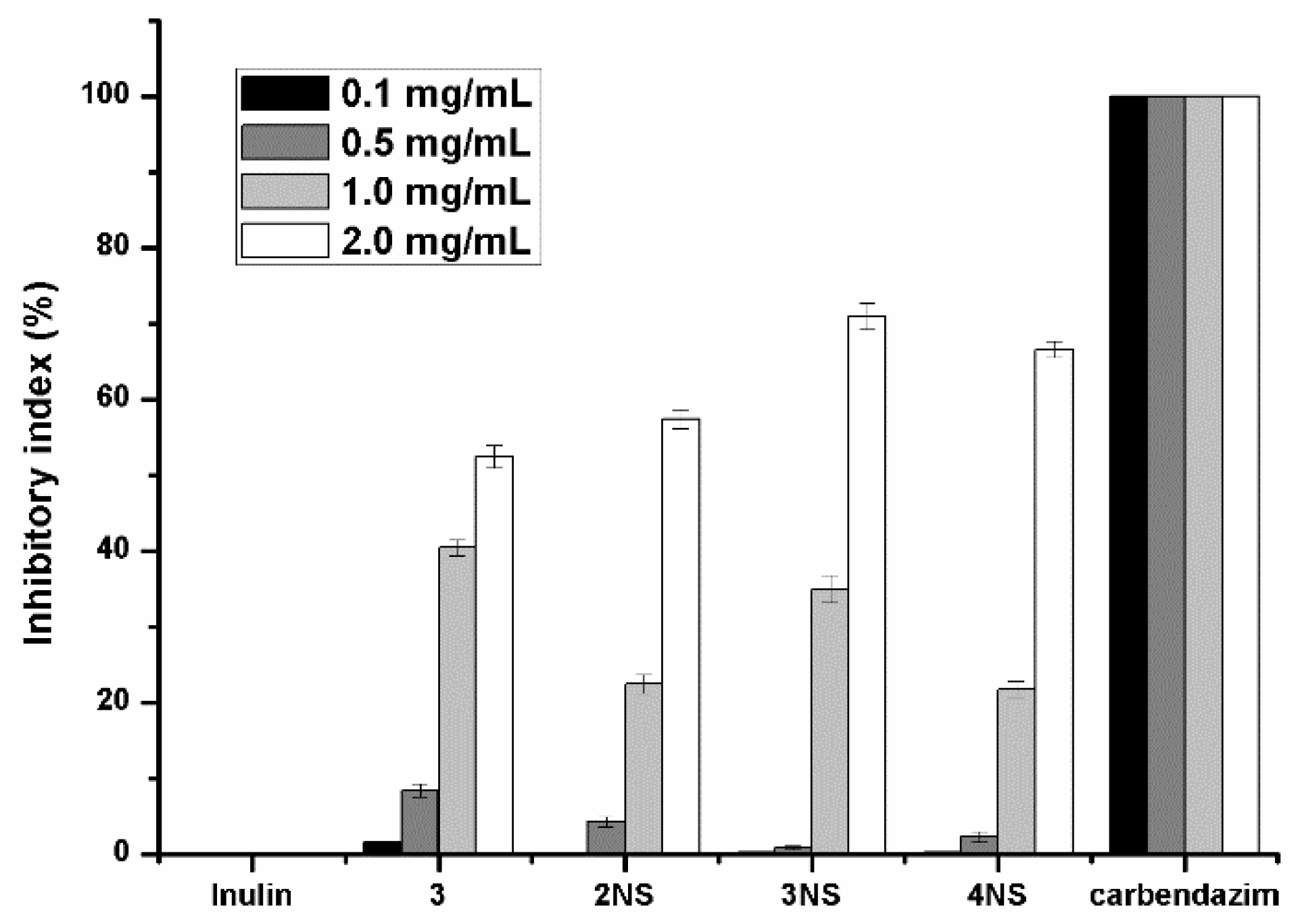
3.2. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Ren, J.; Dong, F.; Feng, Y.; Gu, G.; Guo, Z. Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr. Res. 2013, 373, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everts, K.L.; Egel, D.S.; Langston, D.; Zhou, X.G. Chemical management of Fusarium wilt of watermelon. Crop Prot. 2014, 66, 114–119. [Google Scholar] [CrossRef]
- Wu, H.-s.; Zhou, X.-d.; Shi, X.; Liu, Y.-d.; Wang, M.-y.; Shang, X.-x.; Gu, D.-l.; Wang, W.-z.; Wu, C.-w. In vitro responses of Fusarium oxysporum f.sp.niveum to phenolic acids in decaying watermelon tissues. Phytochem. Lett. 2014, 8, 171–178. [Google Scholar] [CrossRef]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Meng, X.; Li, R.; Cui, J.; Li, B.; Li, P. Novel thiosemicarbazone chitosan derivatives: Preparation, characterization, and antifungal activity. Carbohydr. Polym. 2012, 87, 2664–2670. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, P.-Y.; Zhang, Y.-J.; Wang, P.-P.; Yu, H.; Jiang, J.-H. Synthesis of biocontrol macromolecules by derivative of chitosan with surfactin and antifungal evaluation. Int. J. Biol. Macromol. 2014, 66, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Q.; Wang, G.; Dong, F.; Zhou, H.; Zhang, J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr. Polym. 2014, 99, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Liu, J.; Dong, F.; Guo, Z. Synthesis and hydroxyl radicals scavenging activity of N-(aminoethyl)inulin. Carbohydr. Polym. 2011, 85, 268–271. [Google Scholar] [CrossRef]
- López-Castejón, M.L.; Bengoechea, C.; Espinosa, S.; Carrera, C. Characterization of prebiotic emulsions stabilized by inulin and β-lactoglobulin. Food Hydrocoll. 2019, 87, 382–393. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, L.; Tan, W.; Gu, G.; Guo, Z. Novel 1,2,3-triazolium-functionalized inulin derivatives: synthesis, free radical-scavenging activity, and antifungal activity. RSC Adv. 2017, 7, 42225–42232. [Google Scholar] [CrossRef] [Green Version]
- Korbelik, M.; Cooper, P.D. Potentiation of photodynamic therapy of cancer by complement: the effect of γ-inulin. Br. J. Cancer 2006, 96, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, S.; Xing, R.; Yu, H.; Qin, Y.; Li, P. Liquid phase adsorption behavior of inulin-type fructan onto activated charcoal. Carbohydr. Polym. 2015, 122, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D.; Carter, M. The anti-melanoma activity of inulin in mice. Mol. Immunol. 1986, 23, 903–908. [Google Scholar] [CrossRef]
- Wang, L.; Barclay, T.; Song, Y.; Joyce, P.; Sakala, I.G.; Petrovsky, N.; Garg, S. Investigation of the biodistribution, breakdown and excretion of delta inulin adjuvant. Vaccine 2017, 35, 4382–4388. [Google Scholar] [CrossRef] [PubMed]
- Rodriguezdel, R.E.; Marradi, M.; Calderongonzalez, R.; Frandecabanes, E.; Petrovsky, N.; Alvarezdominguez, C. A gold glyco-nanoparticle carrying a listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine 2015, 33, 1465–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, C.; Meriggi, A.; Booten, K. Chemical Modification of Inulin, a Valuable Renewable Resource, and Its Industrial Applications. Biomacromolecules 2001, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, F.; Dong, F.; Guo, Z. Highly efficient synthesis and antioxidant activity of O-(aminoethyl)inulin. Carbohydr. Polym. 2011, 83, 1240–1244. [Google Scholar] [CrossRef]
- Ren, J.; Wang, P.; Dong, F.; Feng, Y.; Peng, D.; Guo, Z. Synthesis and antifungal properties of 6-amino-6-deoxyinulin, a kind of precursors for facile chemical modifications of inulin. Carbohydr. Polym. 2012, 87, 1744–1748. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Duan, Y.; Fang, Q.; Wang, X.; Huang, J. Pyridine-grafted chitosan derivative as an antifungal agent. Food Chem. 2016, 196, 381–387. [Google Scholar] [CrossRef]
- Sajomsang, W.; Ruktanonchai, U.; Gonil, P.; Warin, C. Quaternization of N-(3-pyridylmethyl) chitosan derivatives: Effects of the degree of quaternization, molecular weight and ratio of N-methylpyridinium and N,N,N-trimethyl ammonium moieties on bactericidal activity. Carbohydr. Polym. 2010, 82, 1143–1152. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Venkata Subbaiah, M. Removal of Pb(II) ions by using magnetic chitosan-4-((pyridin-2-ylimino)methyl)benzaldehyde Schiff’s base. Int. J. Biol. Macromol. 2016, 93, 408–417. [Google Scholar]
- Opanasopit, P.; Sajomsang, W.; Ruktanonchai, U.; Mayen, V.; Rojanarata, T.; Ngawhirunpat, T. Methylated N-(4-pyridinylmethyl) chitosan as a novel effective safe gene carrier. Int. J. Pharm. 2008, 364, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Nomura, T. Silver-selective sensor using an electrode-separated piezoelectric quartz crystal modified with a chitosan derivative. Anal. Sci. 2002, 18, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Ruktanonchai, U.R.; Gonil, P.; Nuchuchua, O. Mucoadhesive property and biocompatibility of methylated-aryl chitosan derivatives. Carbohydr. Polym. 2009, 78, 945–952. [Google Scholar] [CrossRef]
- Goncalves, F.J.; Kamal, F.; Gaucher, A.; Gil, R.; Bourdreux, F.; Martineau-Corcos, C.; Gurgel, L.V.A.; Gil, L.F.; Prim, D. Synthesis, characterisation and application of pyridine-modified chitosan derivatives for the first non-racemic Cu-catalysed Henry reaction. Carbohydr. Polym. 2018, 181, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Gao, Z.; Qiu, S.; Dong, F.; Guo, Z. Design, synthesis of novel starch derivative bearing 1,2,3-triazolium and pyridinium and evaluation of its antifungal activity. Carbohydr. Polym. 2017, 157, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Zhang, J.; Yu, C.; Li, Q.; Guo, Z. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. Int. J. Biol. Macromol. 2014, 70, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Anush, S.M.; Vishalakshi, B.; Kalluraya, B.; Manju, N. Synthesis of pyrazole-based Schiff bases of Chitosan: Evaluation of antimicrobial activity. Int. J. Biol. Macromol. 2018, 119, 446–452. [Google Scholar] [CrossRef]
- Grivani, G.; Bruno, G.; Rudbari, H.A.; Khalaji, A.D.; Pourteimouri, P. Synthesis, characterization and crystal structure determination of a new oxovanadium(IV) Schiff base complex: The catalytic activity in the epoxidation of cyclooctene. Inorg. Chem. Commun. 2012, 18, 15–20. [Google Scholar] [CrossRef]
- Naeimi, H.; Safari, J.; Heidarnezhad, A. Synthesis of Schiff base ligands derived from condensation of salicylaldehyde derivatives and synthetic diamine. Dyes Pigments 2007, 73, 251–253. [Google Scholar] [CrossRef]
- Güngör, Ö.; Gürkan, P. Synthesis and characterization of higher amino acid Schiff bases, as monosodium salts and neutral forms. Investigation of the intramolecular hydrogen bonding in all Schiff bases, antibacterial and antifungal activities of neutral forms. J. Mol. Struct. 2014, 1074, 62–70. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorg. Med. Chem. Lett. 2005, 15, 4600–4603. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lian, Z.; Wang, H.; Jin, X.; Liu, Y. Synthesis and antimicrobial activity of Schiff base of chitosan and acylated chitosan. J. Appl. Polym. Sci. 2012, 123, 3242–3247. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, J.; Yu, C.; Li, Q.; Ren, J.; Wang, G.; Gu, G.; Guo, Z. Synthesis of amphiphilic aminated inulin via “click chemistry” and evaluation for its antibacterial activity. Bioorg. Med. Chem. Lett. 2014, 24, 4590–4593. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr. Res. 2007, 342, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, W.; Li, Q.; Dong, F.; Gu, G.; Guo, Z. Synthesis of inulin derivatives with quaternary phosphonium salts and their antifungal activity. Int. J. Biol. Macromol. 2018, 113, 1273–1278. [Google Scholar] [CrossRef]
- Ce’rantola, S.; Kervarec, N.; Pichon, R.; Magne’, C.; Bessieres, M.; Deslandes, E. NMR characterisation of inulin-type fructooligosaccharides as the major water-soluble carbohydrates from Matricaria maritima (L.). Carbohydr. Res. 2004, 339, 2445–2449. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Tan, W.; Wang, G.; Dong, F.; Li, Q.; Guo, Z. Antioxidant activity of inulin derivatives with quaternary ammonium. Starch Stärke 2017, 69, 11–12. [Google Scholar] [CrossRef]
- Sajomsang, W.; Tantayanon, S.; Tangpasuthadol, V.; Thatte, M.; Daly, W.H. Synthesis and characterization of N-aryl chitosan derivatives. Int. J. Biol. Macromol. 2008, 43, 79–87. [Google Scholar] [CrossRef]
| Compounds | Yields(%) | Elemental Analysis(%) | Degrees of Substitution | ||
|---|---|---|---|---|---|
| C | N | C/N | |||
| 2 | 85.3 | 40.331 | 14.282 | 2.82 | 1.08 |
| 3 | 83.9 | 45.711 | 5.175 | 8.83 | 1.10 |
| 2NS | 83.5 | 43.719 | 5.874 | 7.95 | 0.32 |
| 3NS | 87.0 | 44.945 | 5.758 | 7.80 | 0.39 |
| 4NS | 84.8 | 40.256 | 5.893 | 7.51 | 0.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Synthesis, Characterization, and Antifungal Activity of Schiff Bases of Inulin Bearing Pyridine ring. Polymers 2019, 11, 371. https://doi.org/10.3390/polym11020371
Wei L, Tan W, Zhang J, Mi Y, Dong F, Li Q, Guo Z. Synthesis, Characterization, and Antifungal Activity of Schiff Bases of Inulin Bearing Pyridine ring. Polymers. 2019; 11(2):371. https://doi.org/10.3390/polym11020371
Chicago/Turabian StyleWei, Lijie, Wenqiang Tan, Jingjing Zhang, Yingqi Mi, Fang Dong, Qing Li, and Zhanyong Guo. 2019. "Synthesis, Characterization, and Antifungal Activity of Schiff Bases of Inulin Bearing Pyridine ring" Polymers 11, no. 2: 371. https://doi.org/10.3390/polym11020371
APA StyleWei, L., Tan, W., Zhang, J., Mi, Y., Dong, F., Li, Q., & Guo, Z. (2019). Synthesis, Characterization, and Antifungal Activity of Schiff Bases of Inulin Bearing Pyridine ring. Polymers, 11(2), 371. https://doi.org/10.3390/polym11020371