Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cod Gelatin Extraction
2.3. Chemical Composition of Fish Gelatin
2.4. Isoelectric Point
2.5. Amino Acid Composition
2.6. Molecular Mass
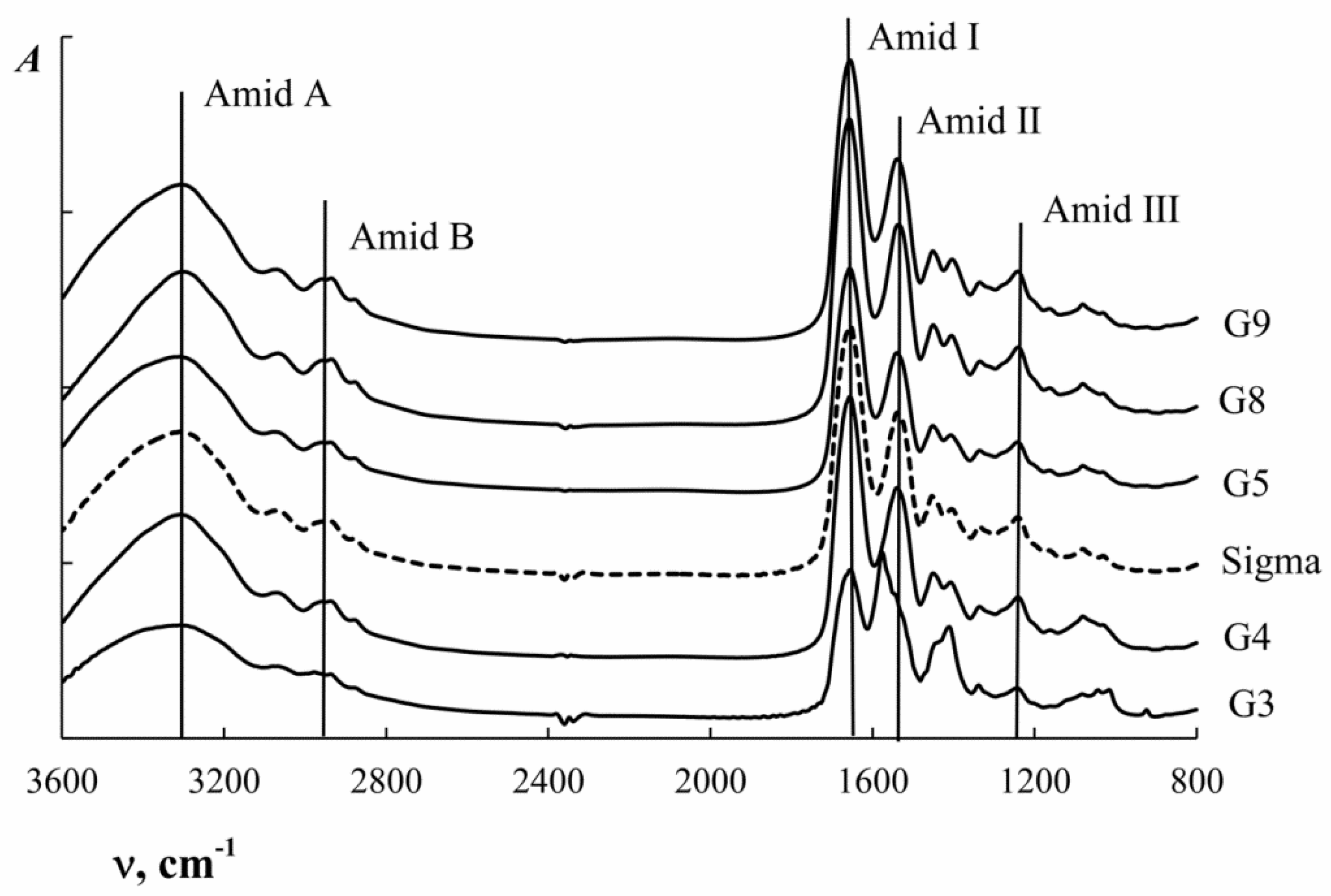
2.7. Secondary Structure of Gelatin
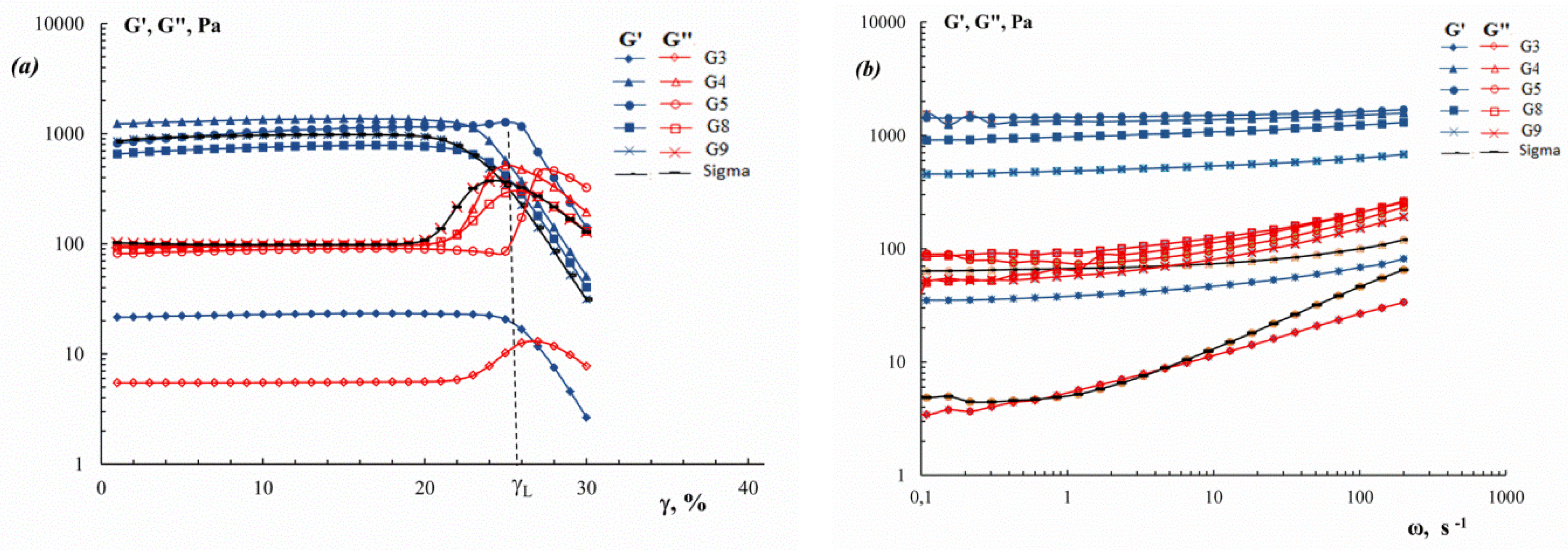
2.8. Thermal Stability and Viscoelastic Properties of Gels
2.9. Differential Scanning Calorimetry (DSC)
2.10. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Isoelectric Point of Gelatin
3.3. Amino Acid Composition
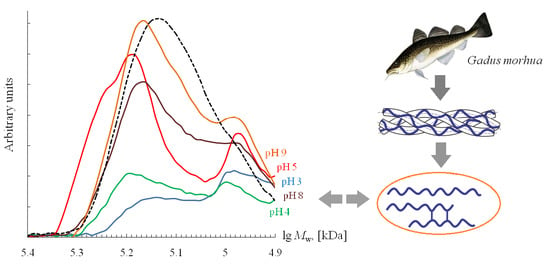
3.4. Molecular Weight Distribution
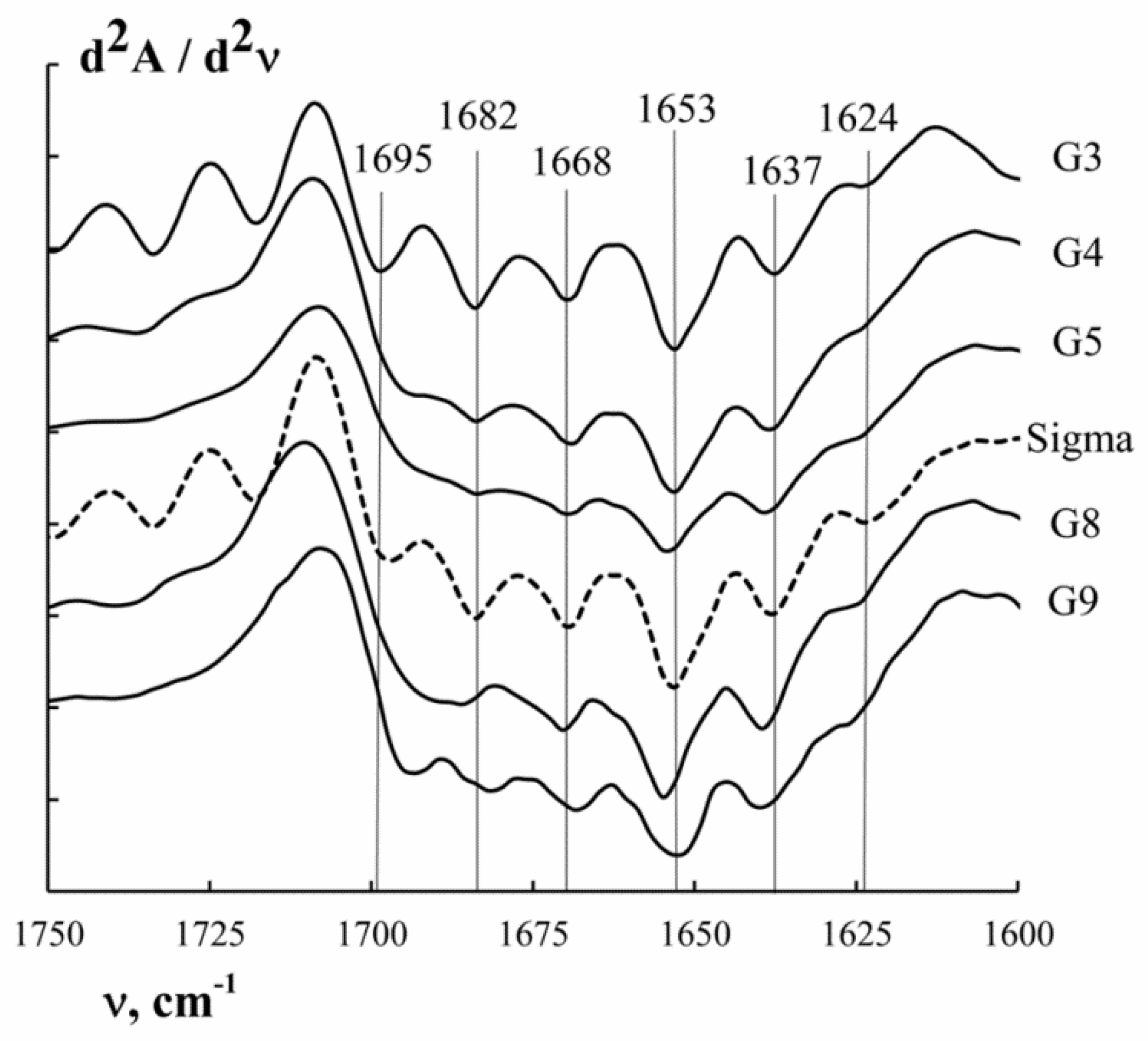
3.5. Secondary Structure of Macromolecules
3.6. Gelation and Melting Temperatures, and Viscoelastic Properties of Gels
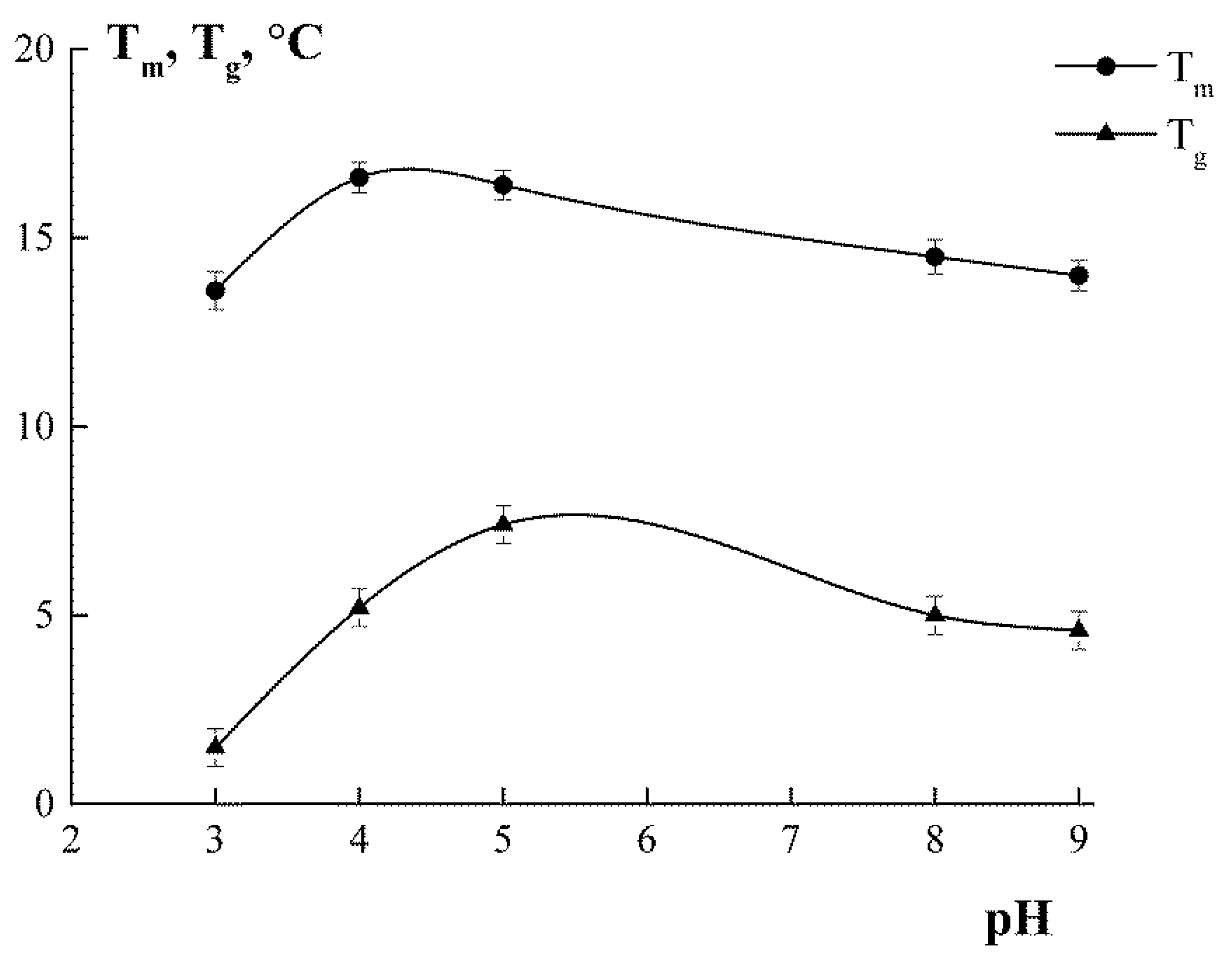
3.7. Melting Temperature and Glass Transition Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cai, L.; Feng, J.; Regenstein, J.; Lv, Y.; Li, J. Confectionery gels: Effects of low calorie sweeteners on the rheological properties and microstructure of fish gelatin. Food Hydrocolloids 2017, 67, 157–165. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food hydrocolloids 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatin Handbook. Theory and Industrial Practice; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Mahmood, K.; Muhammad, L.; Ariffin, F.; Kamilah, H.; Razak, A.; Sulaiman, S. Review of Fish Gelatin Extraction, Properties and Packaging Applications. Food Sci. Qual. Management 2016, 56, 47–59. [Google Scholar]
- Mariod, A.A.; Fadul, H. Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Tech. Aliment. 2013, 12, 135–147. [Google Scholar]
- Veis, A. The Macromolecular Chemistry of Gelatin; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Khiari, Z.; Rico, D.; Martin-Diana, B.A.; Barry-Ryana, C. Comparison between gelatines extracted from mackerel and blue whiting bones after pre-treatments. Food Chem. 2013, 139, 347–354. [Google Scholar] [CrossRef]
- Sultana, S.; Hossain, M.A.M.; Zaidul, I.S.M.; Ali, M.E. Multiplex PCR to discriminate bovine, porcine, and fish DNA in gelatin and confectionery products. LWT Food Sci. Technol. 2018, 92, 169–176. [Google Scholar] [CrossRef]
- Gilsenan, P.M.; Ross-Murphy, S.B. Rheological characterization of gelatins from mammalian and marine sources. Food Hydrocolloids 2000, 14, 191–195. [Google Scholar] [CrossRef]
- Grand View Research. Gelatin market size expected to reach $4.08 billion by 2024. Available online: https://www.grandviewresearch.com/press-release/global-gelatin-market (accessed on 18 March 2019).
- Jridi, M.; Nasri, R.; Lassoued, I.; Souissi, N.; Mbarek, A.; Barkia, A.; Nasri, M. Chemical and biophysical properties of gelatins extracted from alkali-pretreated skin of cuttlefish (Sepia officinalis) using pepsin. Food Res. Int. 2013, 54, 1680–1687. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Zhou, P.; Mulvaney, S.J.; Regenstein, J.M. Properties of Alaska pollock skin gelatin: A comparison with tilapia and pork skin gelatins. J. Food Sci. 2006, 71, 313–321. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Saidi, G.S.; Guizani, M. Thermal characterisation of gelatin extracted from yellowfin tuna skin and commercial mammalian gelatin. Food Chem. 2008, 108, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dutilleul, P.; Li, C.; Simpson, B.K. Alcalase-assisted production of fish skin gelatin rich in high molecular weight (HMW) polypeptide chains and their characterization for film forming capacity. LWT Food Sci. Technol. 2019, 110, 117–125. [Google Scholar] [CrossRef]
- Kamer, D.D.A.; Palabiyik, I.; Işık, N.O.; Akyuz, F.; Demirci, A.S.; Gumus, T. Effect of confectionery solutes on the rheological properties of fish (Oncorhynchus mykiss) gelatin. LWT Food Sci. Technol. 2019, 101, 499–505. [Google Scholar] [CrossRef]
- Alfaro, A.T.; Biluca, F.C.; Marquetti, C.; Tonial, I.B.; Nilson, E.; de Souza, N.E. African catfish (Clarias gariepinus) skin gelatin: Extraction optimization and physical–chemical properties. Food Res. Int. 2014, 65, 416–422. [Google Scholar] [CrossRef]
- Eysturskard, J.; Haug, I.J.; Elharfaoui, N.; Djabourov, M.; Draget, K.I. Structural and mechanical properties of fish gelatin as a function of extraction conditions. Food Hydrocolloids 2009, 23, 1702–1711. [Google Scholar] [CrossRef]
- Silva, R.S.G.; Bandeira, S.F.; Pinto, L.A.A. Characteristics and chemical composition of skins gelatin from cobia (Rachycentron canadum). LWT Food Sci. Technol. 2014, 57, 580–585. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from marine species: a comparative study. Food Hydrocolloids 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Tech. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Lv, S.; Lu, J.; Jiang, S.; Regenstein, J.M.; Lin, L. Comparison of collagen and gelatin extracted from the skins of Nile tilapia (Oreochromis niloticus) and channel cat fish (Ictalurus punctatus). Food Biosci. 2016, 13, 41–48. [Google Scholar] [CrossRef]
- Díaz-Calderón, P.; Flores, E.; González-Muñoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocolloids 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Sila, A.; Martinez-Alvarez, O.; Krichen, F.; Gomez-Guillen, M.C.; Bougatef, A. Gelatin prepared from European eel (Anguilla anguilla) skin: physicochemical, textural, viscoelastic and surface properties. Colloid Surf. A 2017, 529, 643–650. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction and characterization of gelatin from Altantic salmon (Salmo salar) skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of κ-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef]
- Brent, L.; Frederick, W. A method for quantitative amino acid analysis using precolumn o-phthalaldehyde derivatization and high performance liquid chromatography. J. Chromatogr. Sci. 1981, 19, 259–265. [Google Scholar] [CrossRef]
- Moore, S.; Stein, I.; Skierka, E.; Sadowska, M.; Kołodziejski, W.; Niecikowska, C. Effect of extracting time and temperature on yield of gelatin from fish offal. Food Chem. 2008, 107, 700–706. [Google Scholar] [CrossRef]
- Ozcan, S.; Senyuva, H.Z. Improved and simplified liquid chromatography/atmospheric pressure chemical ionization mass spectrometry method for the analysis of underivatized free amino acids in various foods. J. Chromatogr. A 2006, 1135, 179–185. [Google Scholar] [CrossRef]
- Westermeier, R. Electrophoresis in Practice; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Rabek, J.F. Experimental methods in polymer chemistry. Physical principles and applications; John Wiley & Sons: New York, NY, USA, 1983. [Google Scholar]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Bioch. Bioph. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Prystupa, D.A.; Donald, A.M. Infrared study of gelatin conformations in the gel and sol states. Polym. Gels Netw. 1996, 4, 87–110. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A.I. Rheology: Concept, Methods, and Applications; ChemTec Publishing: Toronto, Canada, 2017. [Google Scholar]
- GOST 11293–89. Gelatin. Tekhnicheskie Usloviya [State Standard 11293–89. Gelatin. Technical Specification]; Standartinform Publ.: Moscow, Russia, 2008; 24p. (In Russian) [Google Scholar]
- Kittiphattanabawon, P.; Benjakul, S.; Sinthusamran, S.; Kishimura, H. Gelatin from clown featherback skin: Extraction conditions. LWT Food Sci. Technol. 2016, 66, 186–192. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kumar, Y.; Adeyemi, K.D.; Sazili, A.Q. Recent advances on the role of process variables affecting gelatin yield and characteristics with special reference to enzymatic extraction: A review. Food Hydrocolloids 2017, 63, 85–96. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Molecular characteristics and properties of gelatin from skin of seabass with sizes. Int. J. Biol. Macromol. 2015, 73, 146–153. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Vebster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Application; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Dong, A.; Huang, P.; Caughey, W.S. Protein secondary structures in water from second-derivative Amide I infrared spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.J.; Veis, A. Fourier transform IR spectroscopy of collagen and gelatin solution: deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Izmailova, V.N.; Derkach, S.R.; Sakvarelidze, M.A.; Levachev, S.M.; Voron′ko, N.G.; Yampol′skaya, G.P. Gelation in gelatin and gelatin-containing multicomponent blends. Polymer Sci. C 2004, 46, 73–92. [Google Scholar]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocolloids 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Gómez-Estaca, J.; Sila, A.; Martinez-Alvarez, O.; Gómez-Guillén, M.C.; Chaabouni-Ellouz, S.; Ayadi, M.A.; Bougatef, A. Characteristics and functional properties of gelatin extracted from squid (Loligo vulgaris) skin. LWT Food Sci. Technol. 2015, 65, 924–931. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Thitipramote, N.; Benjakul, S. Preparation and functional characterization of fish skin gelatin and comparison with commercial gelatin. Int. J. Food Sci. Tech. 2013, 48, 1093–1102. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocolloids 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
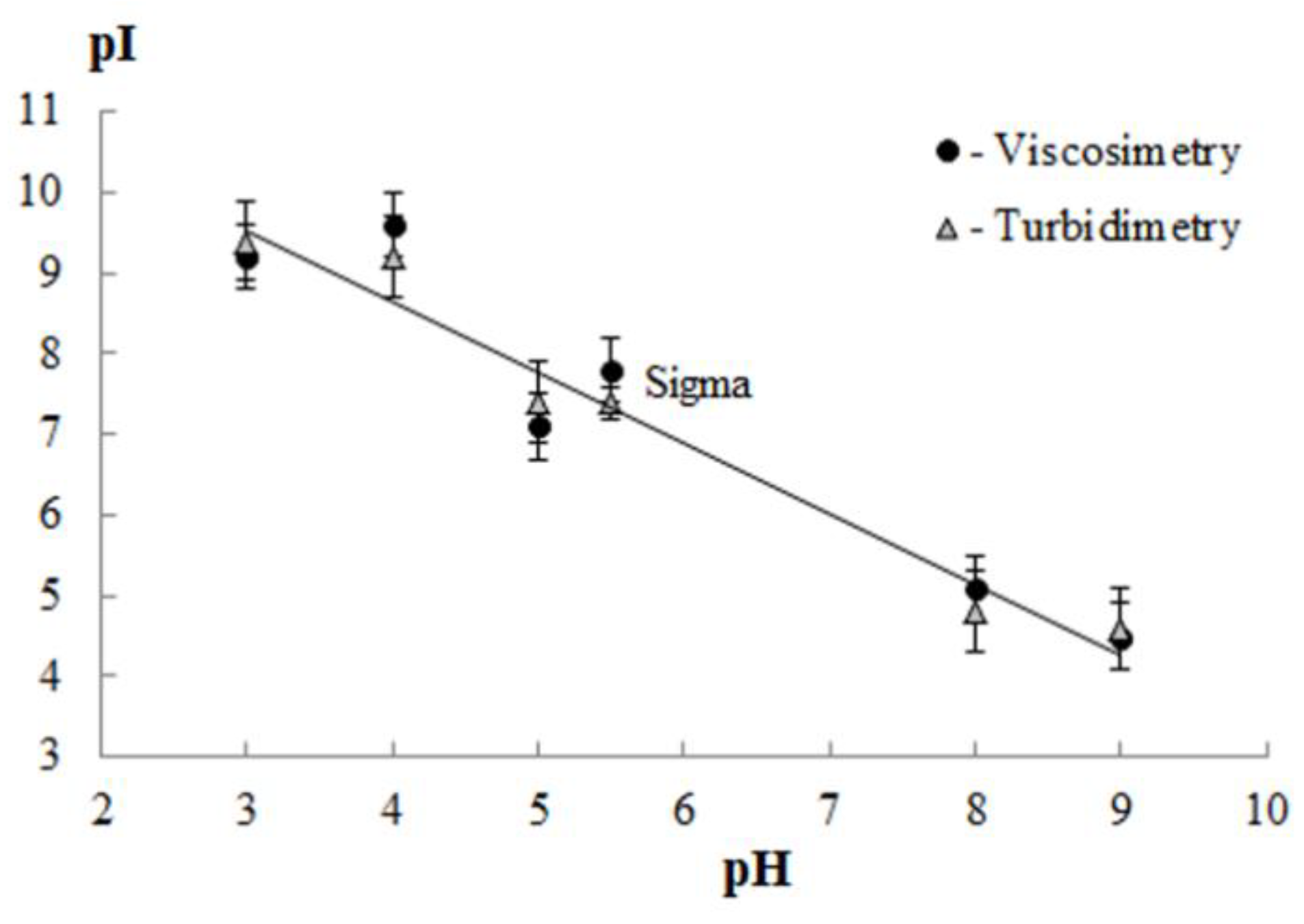
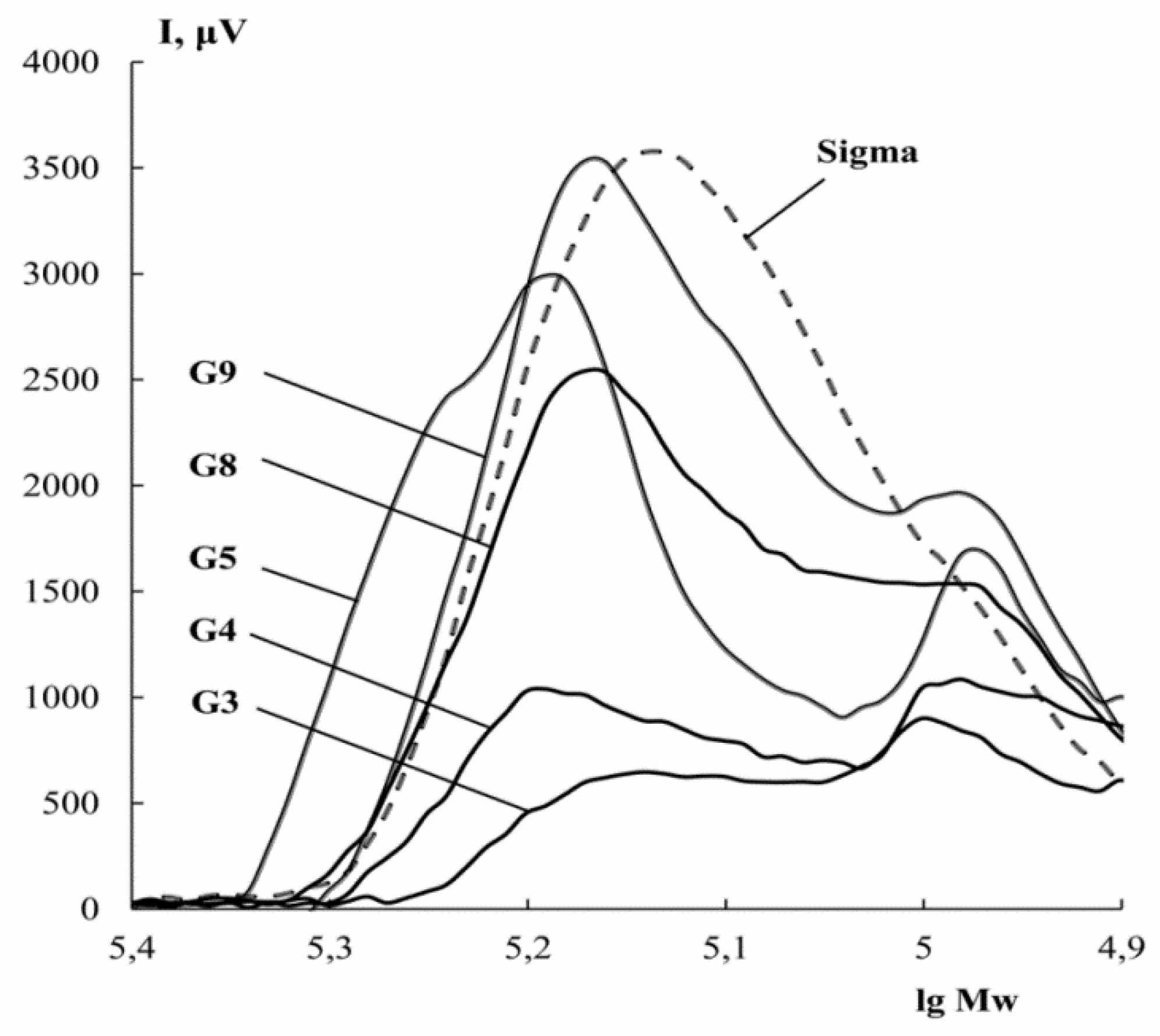
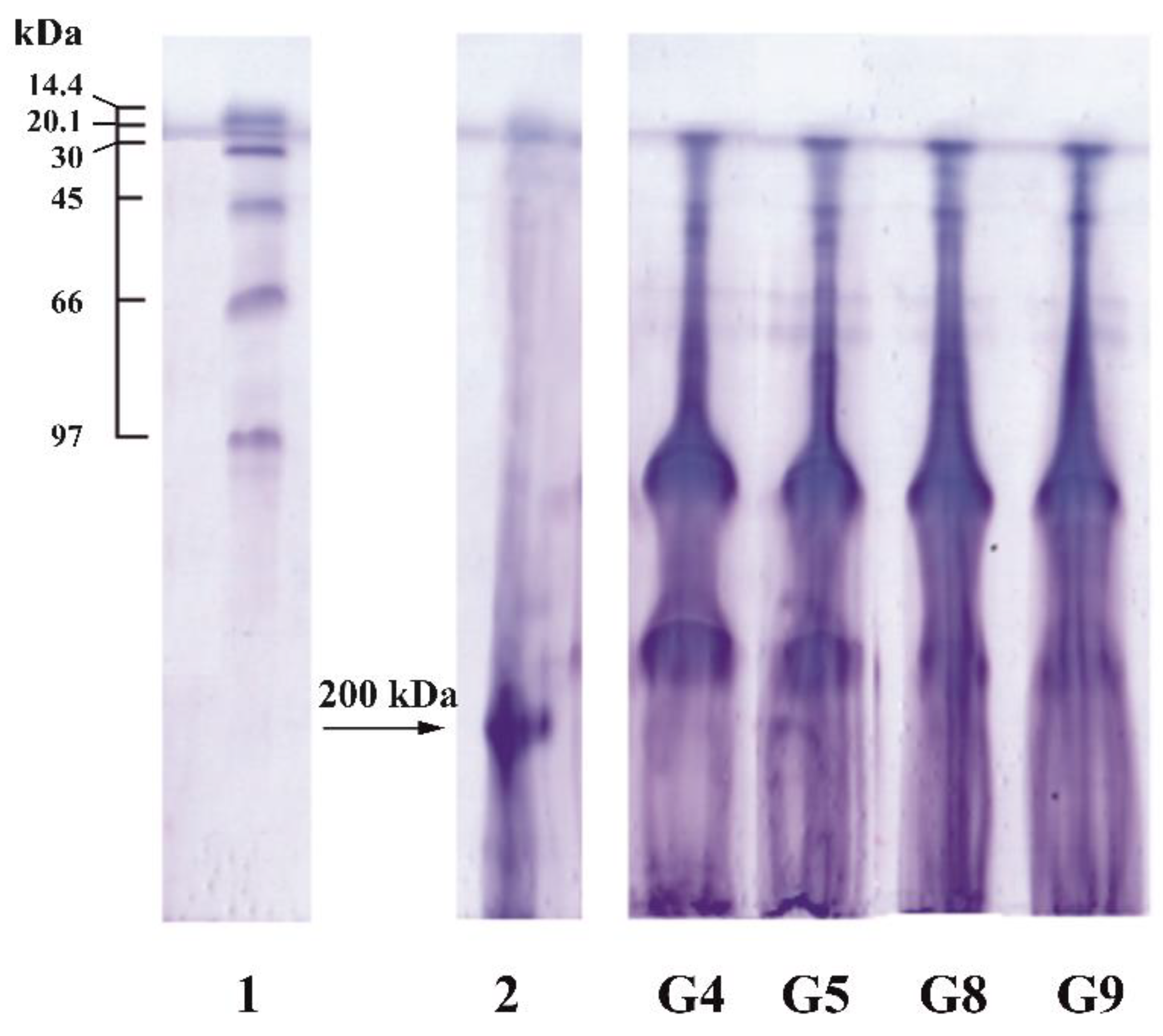
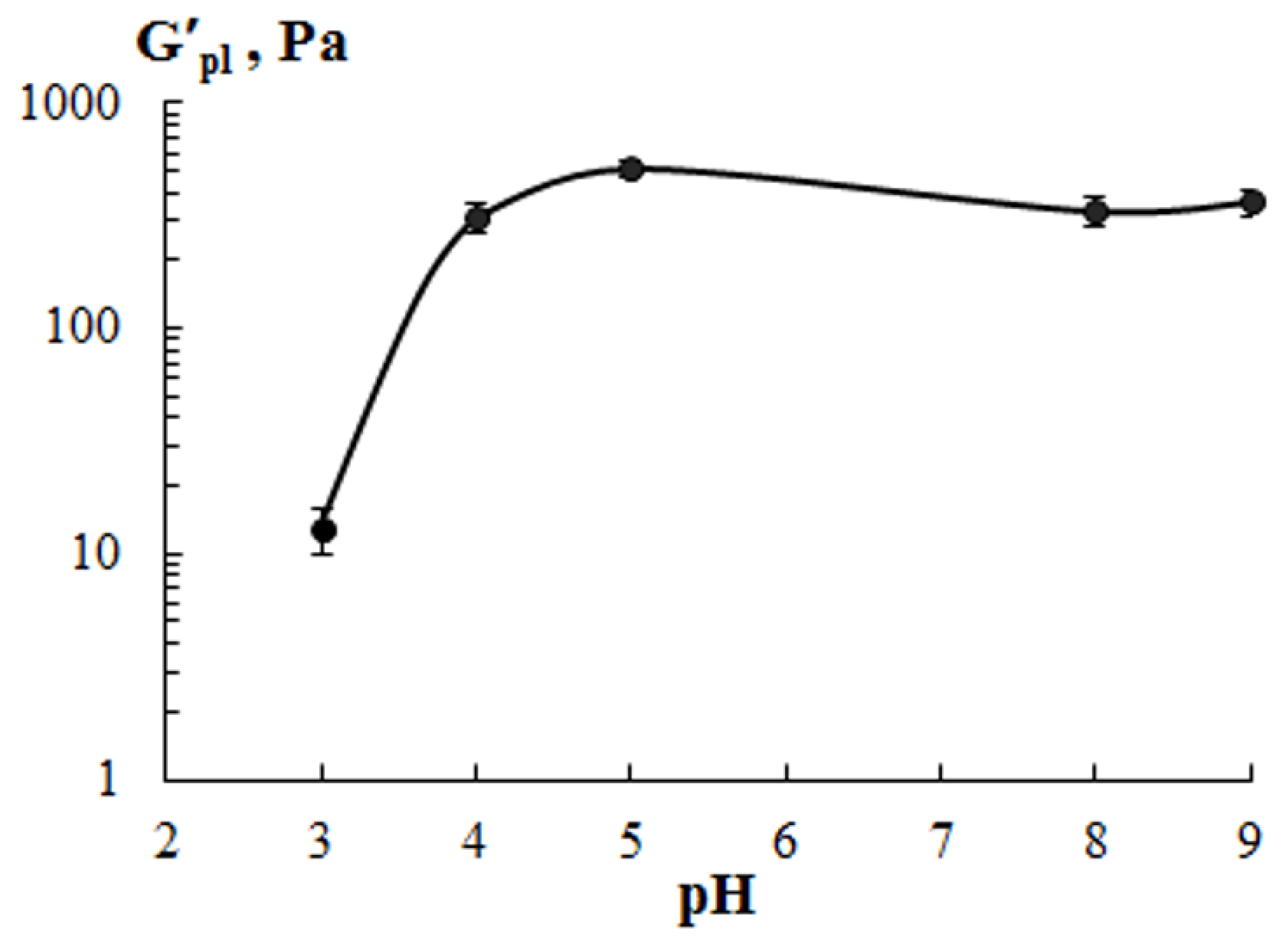
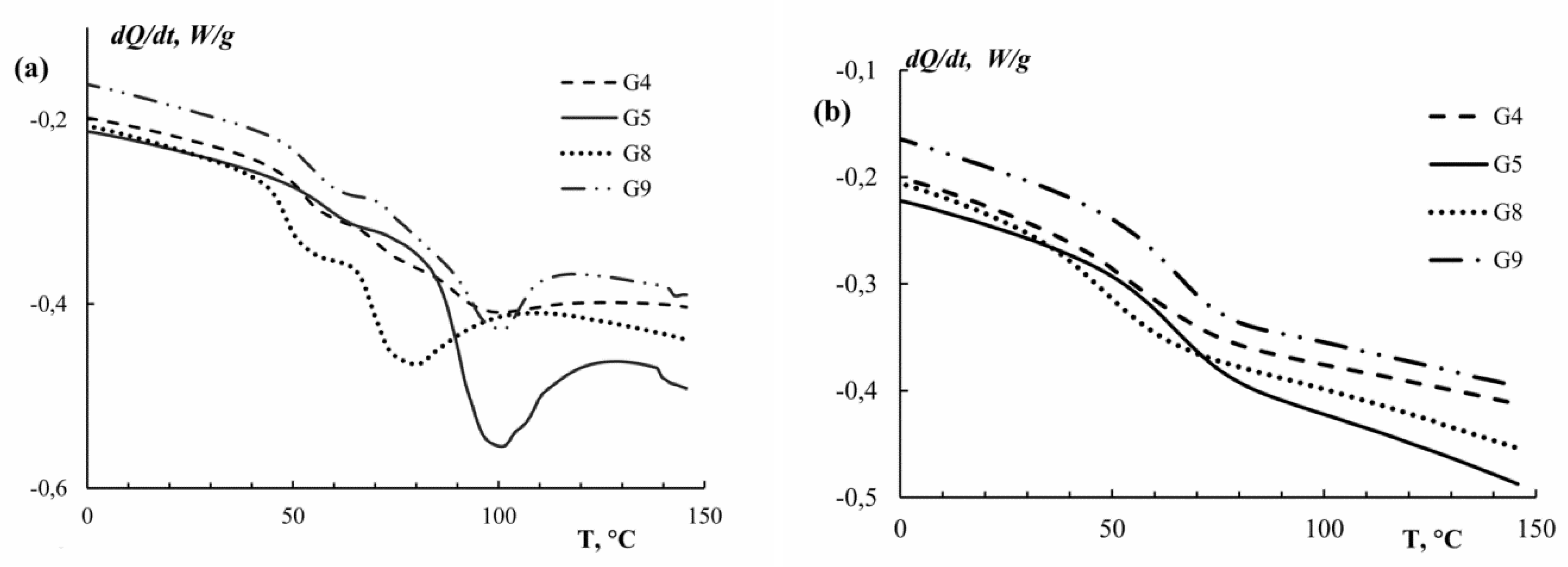
| Sample | Extraction pH | Moisture Content X, % | Amine Nitrogen NA, % | Total Nitrogen NT, % | Protein * P, % | Ash, % | Yield B, % |
|---|---|---|---|---|---|---|---|
| G3 | 3.0 | 8.5 ± 0.5 d | 0.96 ± 0.03 d | 15.6 ± 0.1 a | 86.5 ± 0.6 a | 4.8 ± 0.2 d | 51.1 ± 0.6 a |
| G4 | 4.0 | 9.0 ± 0.7 c | 1.04 ± 0.04 c | 15.8 ± 0.2 b | 87.7 ± 1.0 b | 2.6 ± 0.2 c | 51.2 ± 0.5 a |
| G5 | 5.0 | 6.0 ± 0.5 a | 0.76 ± 0.05 b | 16.8 ± 0.1 c | 92.8 ± 0.6 c | 0.6 ± 0.1 b | 55.4 ± 0.3 b |
| G8 | 8.0 | 7.0 ± 0.5 b | 0.70 ± 0.07 a | 16.5 ± 0.1 d | 90.8 ± 0.6 d | 2.3 ± 0.1 a | 49.3 ± 0.4 c |
| G9 | 9.0 | 7.5 ± 0.4 b | 0.69 ± 0.08 a | 16.2 ± 0.1 e | 89.8 ± 0.6 e | 2.4 ± 0.1 a | 49.1 ± 0.4 c |
| Sigma ** | - | 5.5 ± 0.5 a | 0.53 ± 0.03 e | 17.0 ± 0.1 c | 94.4 ± 0.6 c | - |
| Amino Acids | G3 | G4 | G5 | G8 | G9 | ‘Sigma’ |
|---|---|---|---|---|---|---|
| Glycine | 20.5 | 20.2 | 18.5 | 15.2 | 14.9 | 18.6 |
| Proline | 11.8 | 13.0 | 12.2 | 13.8 | 13.9 | 12.9 |
| Hydroxyproline | 6.9 | 7.2 | 7.5 | 14.6 | 11.5 | 9.6 |
| Aspartic acid | 6.2 | 6.1 | 5.6 | 6.2 | 6.2 | 5.6 |
| Glutamic acid | 9.5 | 9.4 | 9.1 | 9.4 | 9.6 | 9.3 |
| Serine | 6.7 | 6.7 | 6.6 | 6.3 | 6.6 | 6.3 |
| Histidine | 1.6 | 1.6 | 1.9 | 1.3 | 1.5 | 1.7 |
| Threonine | 2.6 | 2.6 | 2.7 | 2.6 | 2.7 | 2.6 |
| Arginine | 7.7 | 7.9 | 7.7 | 7.6 | 7.9 | 7.6 |
| Alanine | 9.5 | 9.1 | 9.3 | 8.5 | 9.0 | 9.4 |
| Taurine | 3.4 | 3.1 | 3.7 | 1.8 | 2.5 | 2.9 |
| Tyrosine | 0.9 | 0.7 | 1.0 | 0.8 | 0.9 | 0.8 |
| Valine | 2.0 | 2.0 | 2.1 | 2.1 | 2.1 | 2.1 |
| Methionine | 1.4 | 1.5 | 1.8 | 1.3 | 1.4 | 1.6 |
| Isoleucine | 1.4 | 1.3 | 1.6 | 1.4 | 1.6 | 1.5 |
| Leucine | 2.8 | 2.7 | 2.9 | 2.6 | 2.8 | 2.8 |
| Lysine | 2.8 | 2.9 | 3.5 | 2.4 | 2.6 | 2.3 |
| Phenylalanine | 2.3 | 2.0 | 2.3 | 2.1 | 2.3 | 2.4 |
| Samples | Mwf, kDa | ω, % * | MW, kDa ** |
|---|---|---|---|
| G3 | ≤ 80 | 13.3 ± 2.8 | 115.2 ± 6.8 c |
| 96.2 ± 5.5 a | 38.2 ± 3.5 a | ||
| 139.3 ± 6.2 b | 48.5 ± 3.1 d | ||
| G4 | 103.5 ± 4.8 a | 42.1 ± 2.4 a | 131.6 ± 7.2 a |
| 152.1 ± 6.1 b | 57.9 ± 3.9 b | ||
| G5 | 112.0 ± 6.9 a | 22.4 ±2.2 c | 153.0 ± 9.0 b |
| 151.0 ± 6.8 b | 39.4 ± 3.1 a | ||
| 179.1 ± 7.0 c | 38.2 ± 3.6 a | ||
| G8 | 105.0 ± 6.0 a | 41.7 ± 2.8 a | 130.2 ± 8.3 a |
| 148.3 ± 7.9 b | 58.3 ± 4.0 b | ||
| G9 | 100.5 ±6.1 a | 35.6 ± 3.1 a | 129.2 ± 8.5 a |
| 143.9 ± 7.8 b | 64.4 ± 4.2 b |
| Group | Wave Number ν, cm−1 | Type of Vibration |
|---|---|---|
| Amide A | 3400–3300 | N–H stretching vibrations |
| Amide B | 3000–2900 | N–H stretching vibrations |
| Amide I | 1700–1600 | C=O stretching vibrations—80% and C–N stretching vibrations |
| Amide II | 1575–1480 | N–H deformation vibrations—80% and C–N stretching vibrations |
| Amide III | 1300–1230 | C–N stretching vibrations |
| Secondary Structure Elements | Wave Number ν, cm−1 | G3 | G4 | G5 | G8 | G9 | “Sigma” |
|---|---|---|---|---|---|---|---|
| β-Turn/β-sheet | 1624–1626 | 12.8 | 10.5 | 13.6 | 11.9 | 12.4 | 13.5 |
| 1637–1639 | 14.9 | 18.5 | 16.1 | 16.3 | 16.3 | 14.0 | |
| Random coil | 1653–1655 | 23.8 | 20.3 | 20.1 | 18.6 | 23.1 | 24.9 |
| Triple helix | 1668–1670 | 14.7 | 19.7 | 24.8 | 26.9 | 19.9 | 13.8 |
| β-Turn/β-sheet | 1682–1684 | 24.4 | 28.4 | 20.5 | 18.9 | 24.1 | 29.0 |
| 1692–1697 | 9.4 | 2.6 | 4.9 | 7.4 | 4.2 | 4.8 |
| Sample | TGm1, °C | TGm2, °C | ΔHm, J/g | TGglass, °C |
|---|---|---|---|---|
| G3 | 56.4 ± 0.8 a | 118.6 ± 1.2 e | 63.5 ± 1.0 a | 45.4 ± 0.7 f |
| G4 | 56.7 ± 0.8 a | 97.3 ± 0.7 d | 12.9 ± 0.8 b | 56.8 ± 0.6 e |
| G5 | 63.7 ± 0.7 b | 98.7 ± 0.9 c | 24.1 ± 0.7 c | 64.8 ± 0.9 d |
| G8 | 51.7 ± 0.5 c | 78.7 ± 0.6 b | 24.0 ± 0.8 c | 46.8 ± 0.5 c |
| G9 | 59.4 ± 0.6 d | 82.7 ± 0.7 a | 15.8 ± 0.6 d | 65.5 ± 0.7 b |
| Sigma * | 74.3 ± 0.5 | 13.3 ± 0.4 e | 61.7 ± 0.4 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11, 1724. https://doi.org/10.3390/polym11101724
Derkach SR, Kuchina YA, Baryshnikov AV, Kolotova DS, Voron’ko NG. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers. 2019; 11(10):1724. https://doi.org/10.3390/polym11101724
Chicago/Turabian StyleDerkach, Svetlana R., Yuliya A. Kuchina, Andrey V. Baryshnikov, Daria S. Kolotova, and Nikolay G. Voron’ko. 2019. "Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction" Polymers 11, no. 10: 1724. https://doi.org/10.3390/polym11101724
APA StyleDerkach, S. R., Kuchina, Y. A., Baryshnikov, A. V., Kolotova, D. S., & Voron’ko, N. G. (2019). Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers, 11(10), 1724. https://doi.org/10.3390/polym11101724