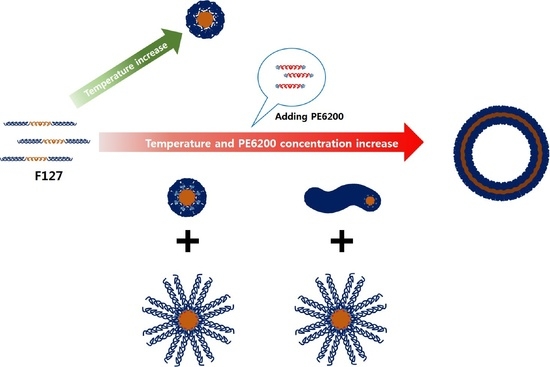
Self-Assembly of Temperature Sensitive Unilamellar Vesicles by a Blend of Block Copolymers in Aqueous Solution
Abstract
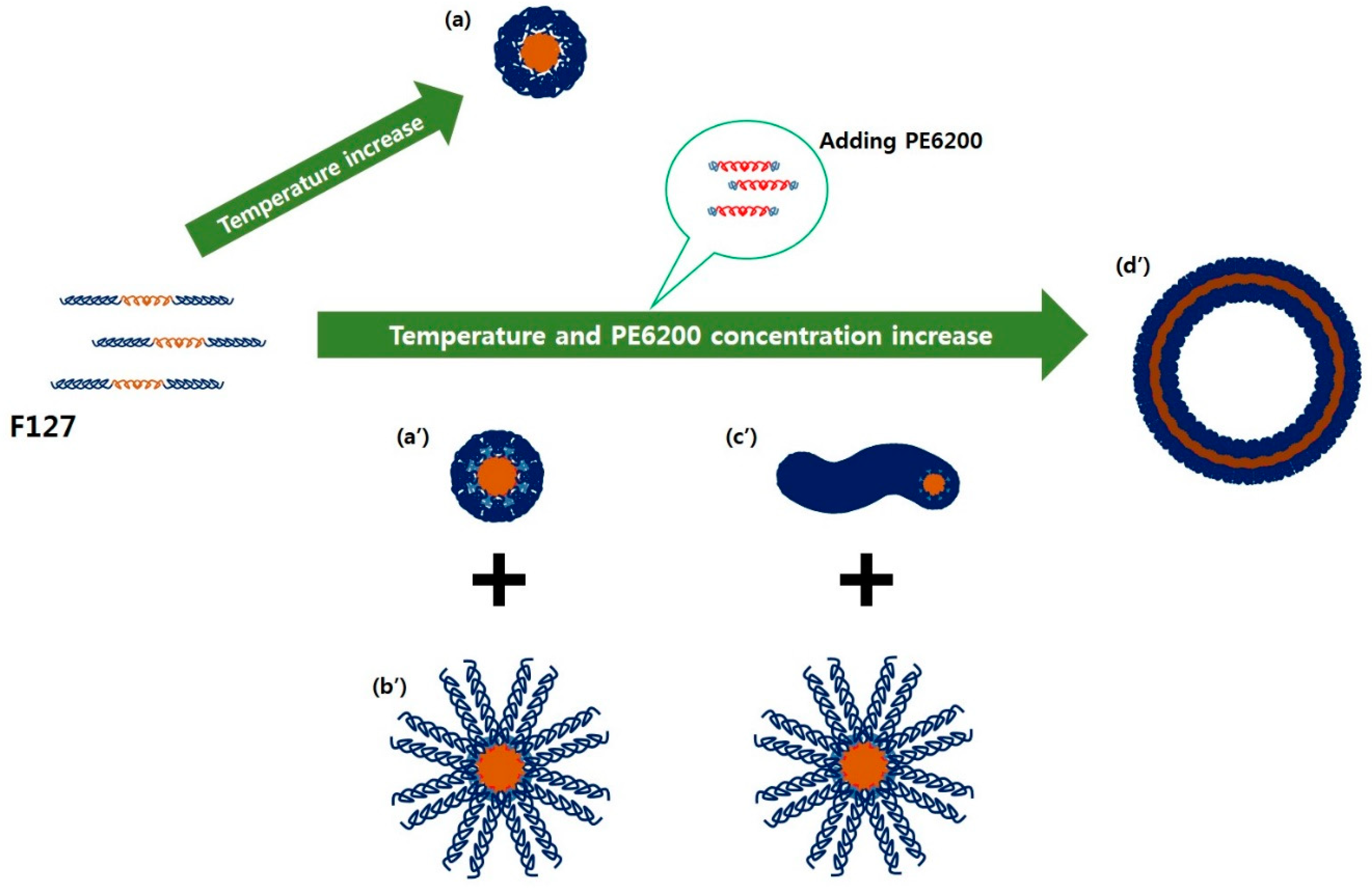
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
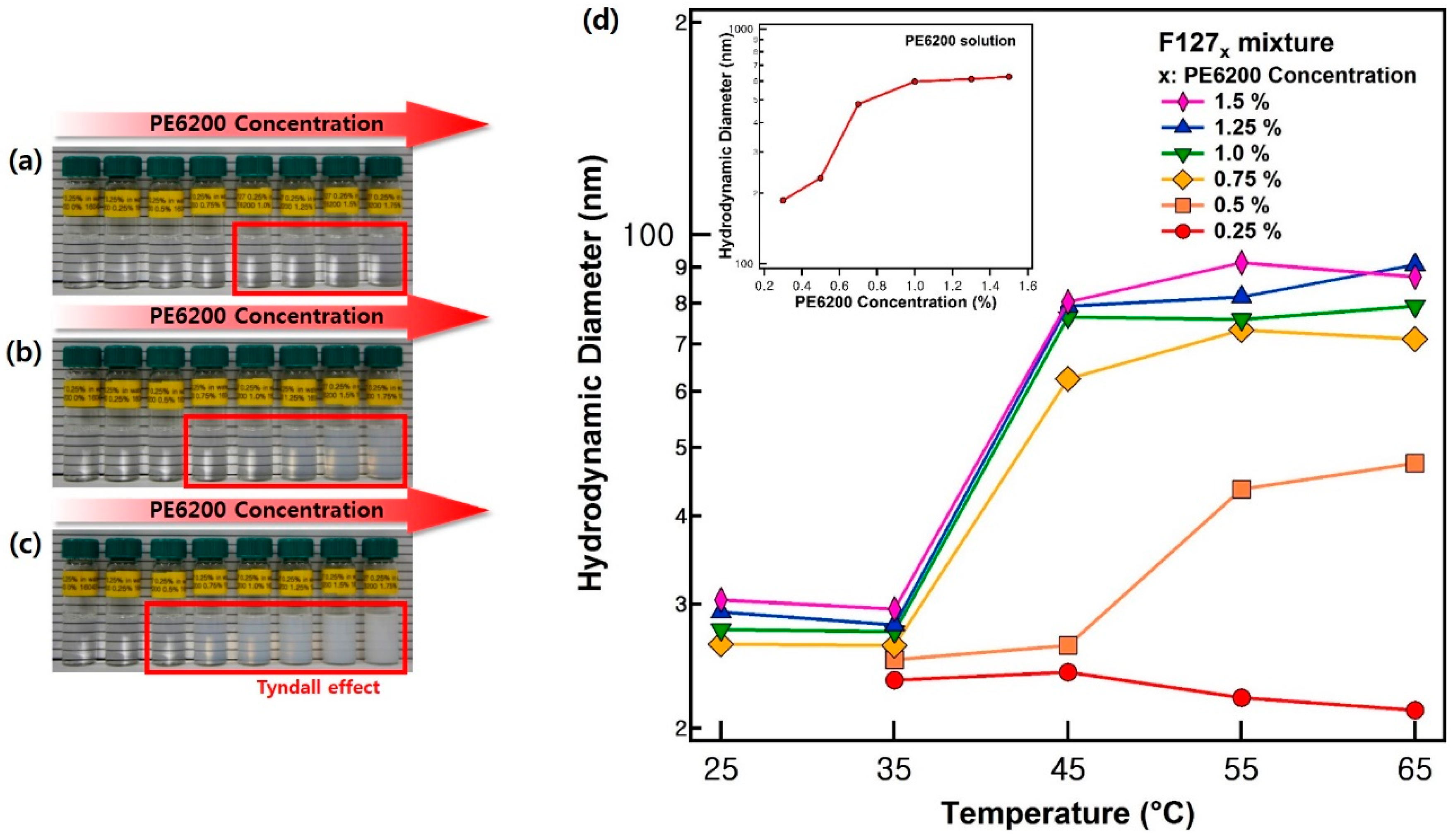
2.3. Dynamic Light Scattering (DLS) Measurements
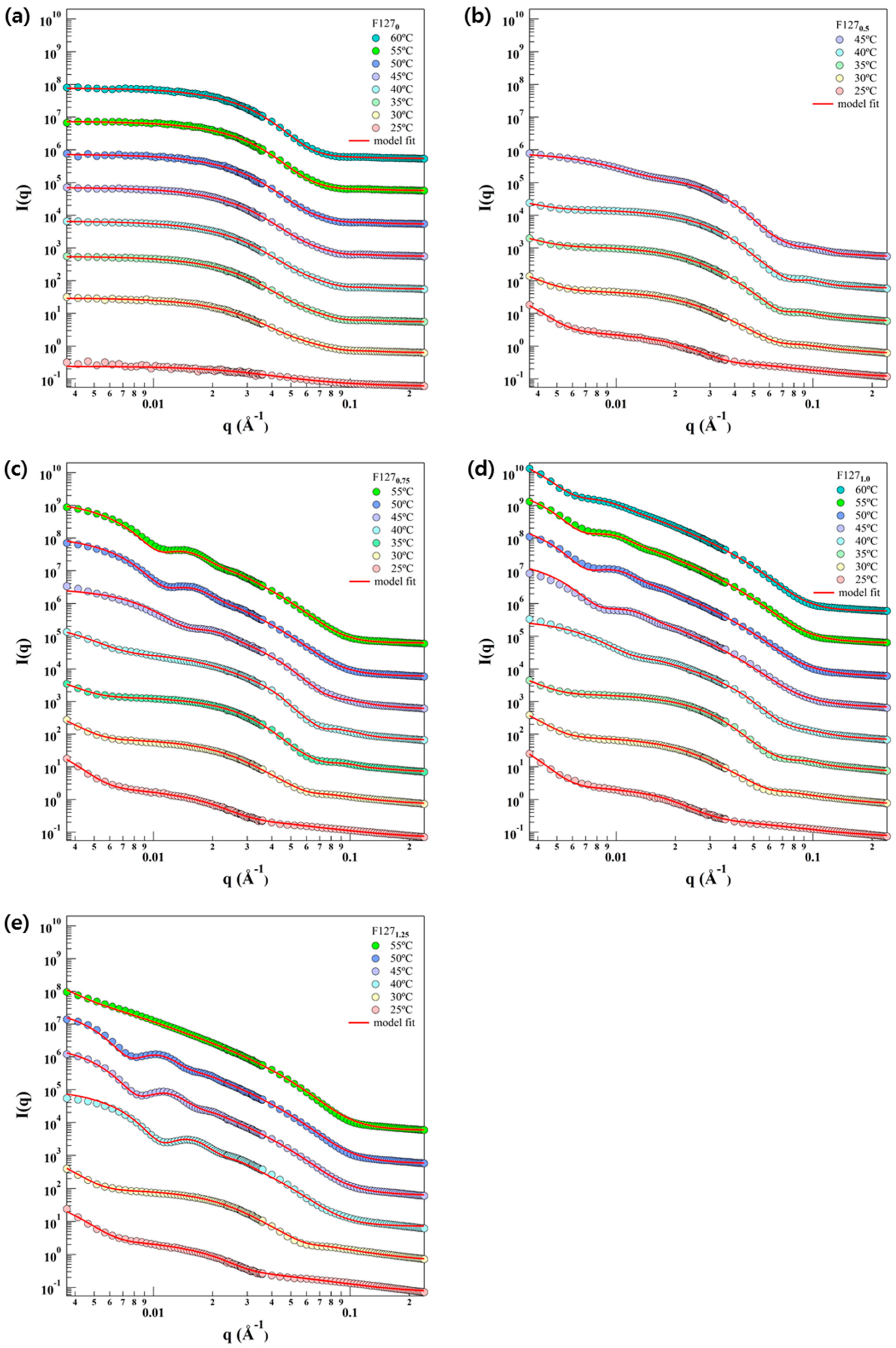
2.4. Small Angle Neutron Scattering (SANS) Measurement
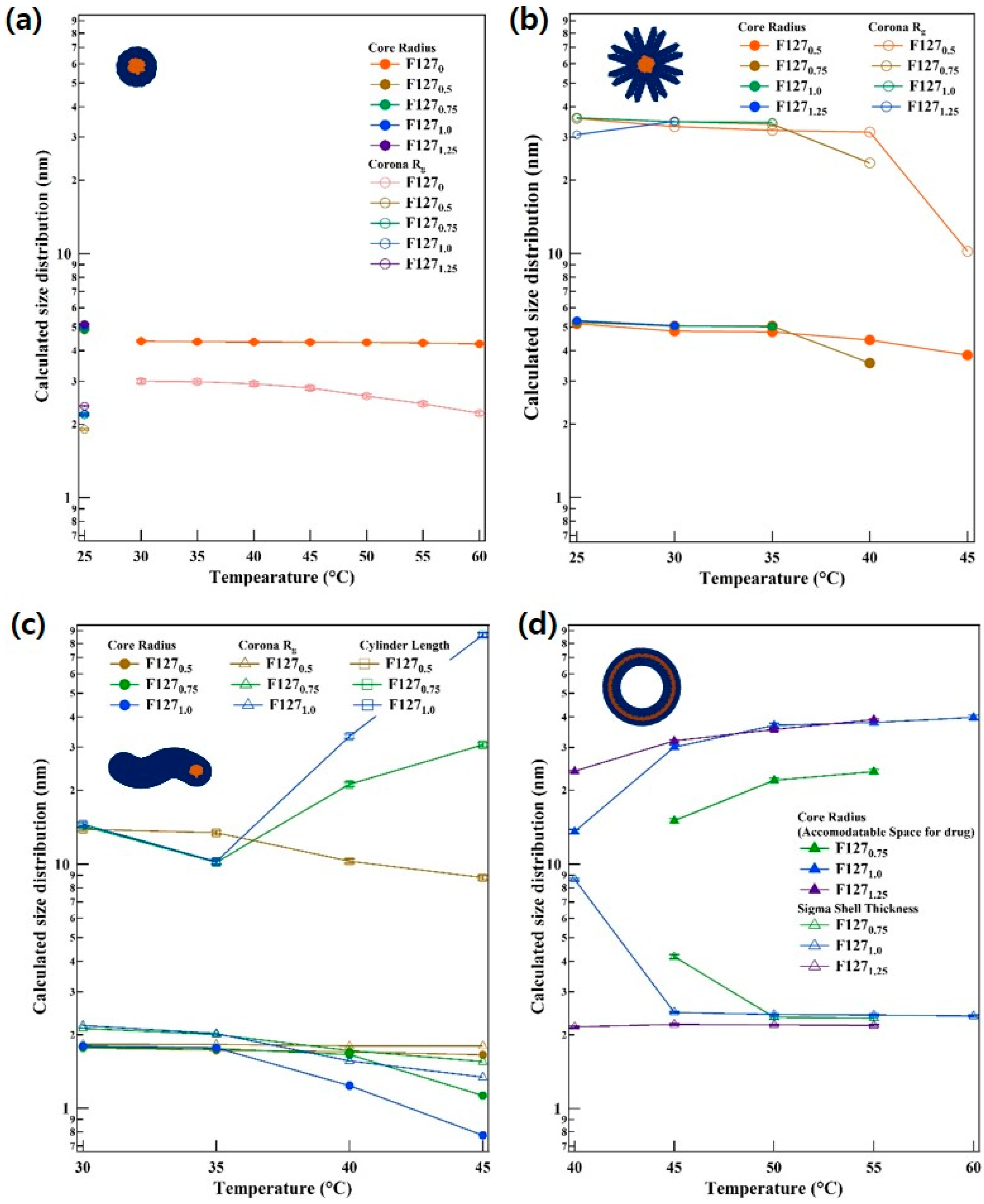
2.5. Small Angle Neutron Scattering (SANS) Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalization and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Liggins, R.T.; Burt, H.M. Polyether-polyester diblock copolymers for the preparation of paclitaxel load polymeric micelle formulations. Adv. Drug Deliv. Rev. 2002, 54, 191–202. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Kingston, D.G.I.; Tamarkin, L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006, 67, 47–54. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of PEGylated liposomal doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, C.; Kostarelos, K.; Prato, M.; Bianco, A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim. Biophys. Acta 2006, 1758, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Shum, H.C.; Zhao, Y.J.; Kim, S.H.; Weitz, D.A. Multicompartment polymersomes from double emulsions. Angew. Chem. Int. Ed. 2011, 50, 1648–1651. [Google Scholar] [CrossRef]
- Mabrouk, E.; Cuvelier, D.; Brochard-Wyart, F.; Nassoy, P.; Li, M.H. Bursting of sensitive polymersomes induced by curling, Spotted vesicles, striped micelles and Janus assemblies induced by ligand binding. Proc. Natl. Acad. Sci. USA 2009, 106, 7294–7298. [Google Scholar] [CrossRef] [PubMed]
- Christian, D.A.; Tian, A.; Ellenbroek, W.G.; Levental, I.; Rajagopal, K.; Janmey, P.A.; Liu, A.J.; Baumgart, T.; Discher, D.E. Spotted vesicles, striped micelles and Janus assemblies induced by ligand binding. Nat. Mater. 2009, 8, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.; Dewhirst, M.W. The development and testing of a temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Deliv. Rev. 2001, 53, 285–305. [Google Scholar] [CrossRef]
- Willis, M.; Forssen, E. Ligand-targeted liposomes. Adv. Drug Deliv. Rev. 1998, 29, 249–271. [Google Scholar] [PubMed]
- Steve, F. Sutureless Method for Joining Blood Vessels Invented; Stanford University Medical Center Press: Stanford, CA, USA, 2011. [Google Scholar]
- Chang, E.I.; Galvez, M.G.; Glotzbach, J.P.; Hamou, C.D.; El-ftesi, S.; Rappleye, C.T.; Sommer, K.M.; Rajadas, J.; Abilez, O.J.; Fuller, G.G.; et al. Vascular anastomosis using controlled phase transitions in poloxamer gels. Nat. Med. 2011, 17, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Cogger, V.C.; Hilmer, S.N.; Sullivan, D.; Muller, M.; Fraser, R.; Le Couteur, D.G. Hyperlipidemia and surfactants: The liver sieve is a link. Atherosclerosis 2006, 189, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agarwal, P.; Zhao, S.; Xu, R.X.; Yu, J.; Lu, X.; He, X. Hyaluronic acid-decorated dual responsive nanoparticles of Pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorucin and irinotecan to eliminate cancer stem-like cells. Biomaterials 2015, 72, 74–89. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide) poly(propylene oxide) poly(ethylene oxide) block copolymer surfactants in aqueous solution and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Atanase, L.I.; Riess, G. Self-assembly of block and graft copolymer in organic solvents: An overview of recent advances. Polymers 2018, 10, 62. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Han, Y.S.; Jang, J.D.; Seong, B.S. SANS study on self-assembled structures of Pluronic F127 triblock copolymer induced by additives and temperature. J. Appl. Crystallogr. 2014, 47, 53–59. [Google Scholar] [CrossRef]
- Cho, B.K.; Jain, A.; Gruner, S.M.; Wiesner, U. Mesophase structure-mechanical and ionic transport correlations in extended amphiphilic dendrons. Science 2004, 305, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, J.H.; Chung, S.W.; Mirkin, C.A. Self-assembly of mesoscopic metal-polymer amphiphiles. Science 2004, 303, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Holowka, E.P.; Sun, V.Z.; Kamei, D.T.; Deming, T.J. polyarginine segments in block copolypeptides drive both vesicular assembly and intracellular delivery. Nat. Mater. 2007, 6, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Bates, F.S.; Lodge, T.P. Structure of poly(styrene-b-ethylene-alt-propylene) diblock copolymer micelles in squalene. J. Phys. Chem. B 2009, 113, 13840–13848. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- Guinier, A.; Fournet, G. Small Angle Scattering of X-rays; Wiley Press: New York, NY, USA, 1955. [Google Scholar]
- Kraemer, E.O.; Dexter, S.T. The light-scattering capacity (Tyndall effect) and colloidal behavior of gelatin sols and gels. J. Phys. Chem. 1927, 31, 764–782. [Google Scholar] [CrossRef]
- Gentry, J.W.; Lin, J.C. The Legacy of John Tyndall in aerosol science. J. Aerosol Sci. 1996, 27, 503–504. [Google Scholar] [CrossRef]
- Jung, Y.W.; Lee, H.; Kim, J.Y.; Koo, E.J.; Oh, K.S.; Yuk, S.H. Pluronic-based core/shell nanoparticles for drug delivery and diagnosis. Curr. Med. Chem. 2013, 20, 3488–3499. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Gerstenberg, M.C. Scattering form factor of block copolymer micelles. Macromolecules 1996, 29, 1363–1365. [Google Scholar] [CrossRef]
- Roe, R.J. Method of X-ray and Neutron Scattering in Polymer Science; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Higgins, J.A.; Benoit, H.C. Polymers and Neutron Scattering; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Manet, S.; Lecchi, A.; Imperor-Clerc, M.; Zholobenko, V.; Durand, D.; Oliveira, C.L.; Pedersen, J.S.; Grillo, I.; Meneau, F.; Rochas, C. Structure of micelles of a nonionic block copolymer determined by SANS and SAXS. J. Phys. Chem. 2011, 115, 11318–11329. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Song, C.; Han, Y.S.; Jang, J.D.; Choi, M.C. Spontaneous unilamellar polymer vesicles in aqueous solution. Soft Matter 2014, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Qipeng, G. Polymer Morphology: Principles, Characterization, and Processing; Wiley Press: New York, NY, USA, 2016. [Google Scholar]
- Fisicaro, E.; Compari, C.; Duce, E.; Biemmi, M.; Peroni, M.; Braibanti, A. Thermodynamics of micelle formation in water, hydrophobic processes and surfactant self-assemblies. Phys. Chem. Chem. Phys. 2008, 10, 3903–3914. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.D.; Do, C.; Bang, J.; Han, Y.S.; Kim, T.-H. Self-Assembly of Temperature Sensitive Unilamellar Vesicles by a Blend of Block Copolymers in Aqueous Solution. Polymers 2019, 11, 63. https://doi.org/10.3390/polym11010063
Jang JD, Do C, Bang J, Han YS, Kim T-H. Self-Assembly of Temperature Sensitive Unilamellar Vesicles by a Blend of Block Copolymers in Aqueous Solution. Polymers. 2019; 11(1):63. https://doi.org/10.3390/polym11010063
Chicago/Turabian StyleJang, Jong Dae, Changwoo Do, Joona Bang, Young Soo Han, and Tae-Hwan Kim. 2019. "Self-Assembly of Temperature Sensitive Unilamellar Vesicles by a Blend of Block Copolymers in Aqueous Solution" Polymers 11, no. 1: 63. https://doi.org/10.3390/polym11010063
APA StyleJang, J. D., Do, C., Bang, J., Han, Y. S., & Kim, T.-H. (2019). Self-Assembly of Temperature Sensitive Unilamellar Vesicles by a Blend of Block Copolymers in Aqueous Solution. Polymers, 11(1), 63. https://doi.org/10.3390/polym11010063