Fabrication of PPy Nanosphere/rGO Composites via a Facile Self-Assembly Strategy for Durable Microwave Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.1.1. Synthesis of PPy Nanospheres
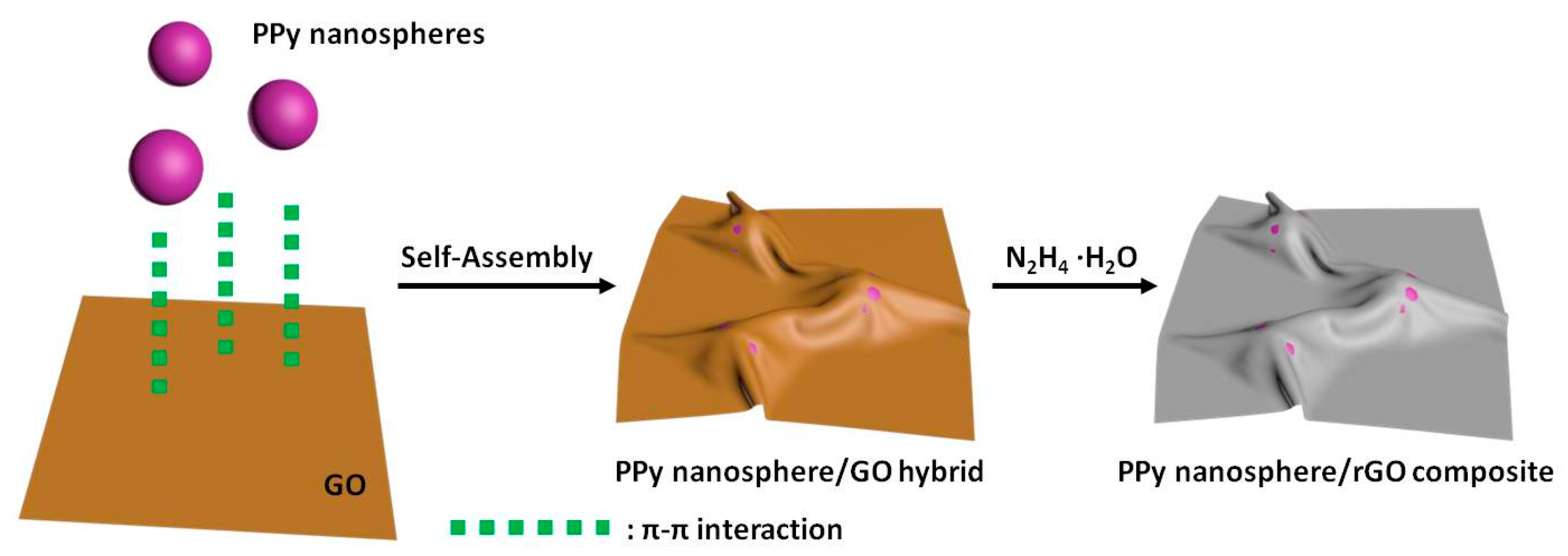
2.1.2. Synthesis of PPy Nanosphere/rGO Composites
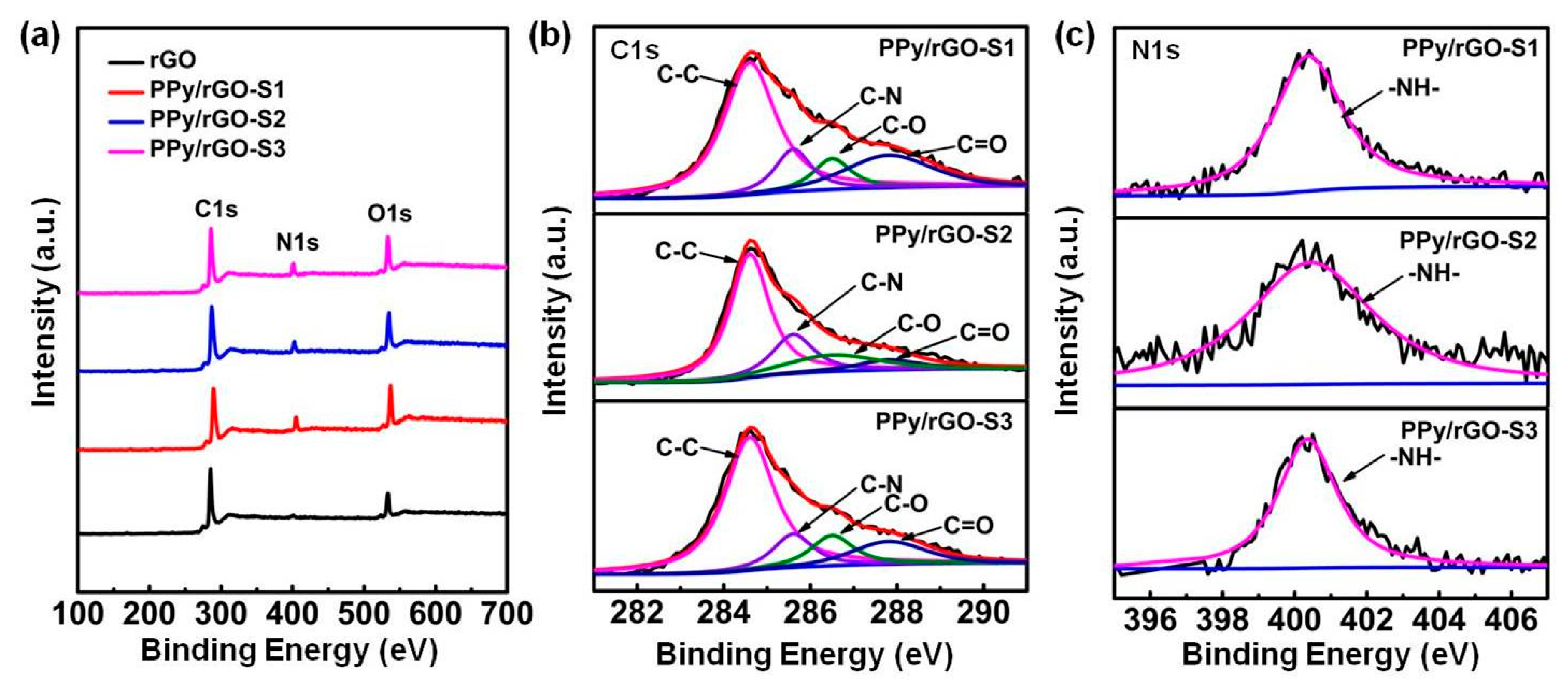
2.2. Physical Characterization
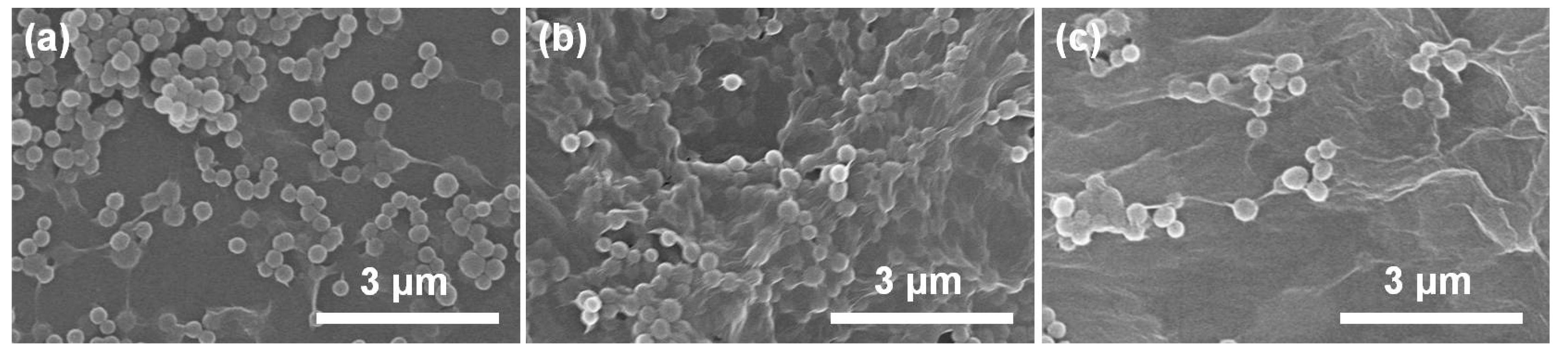
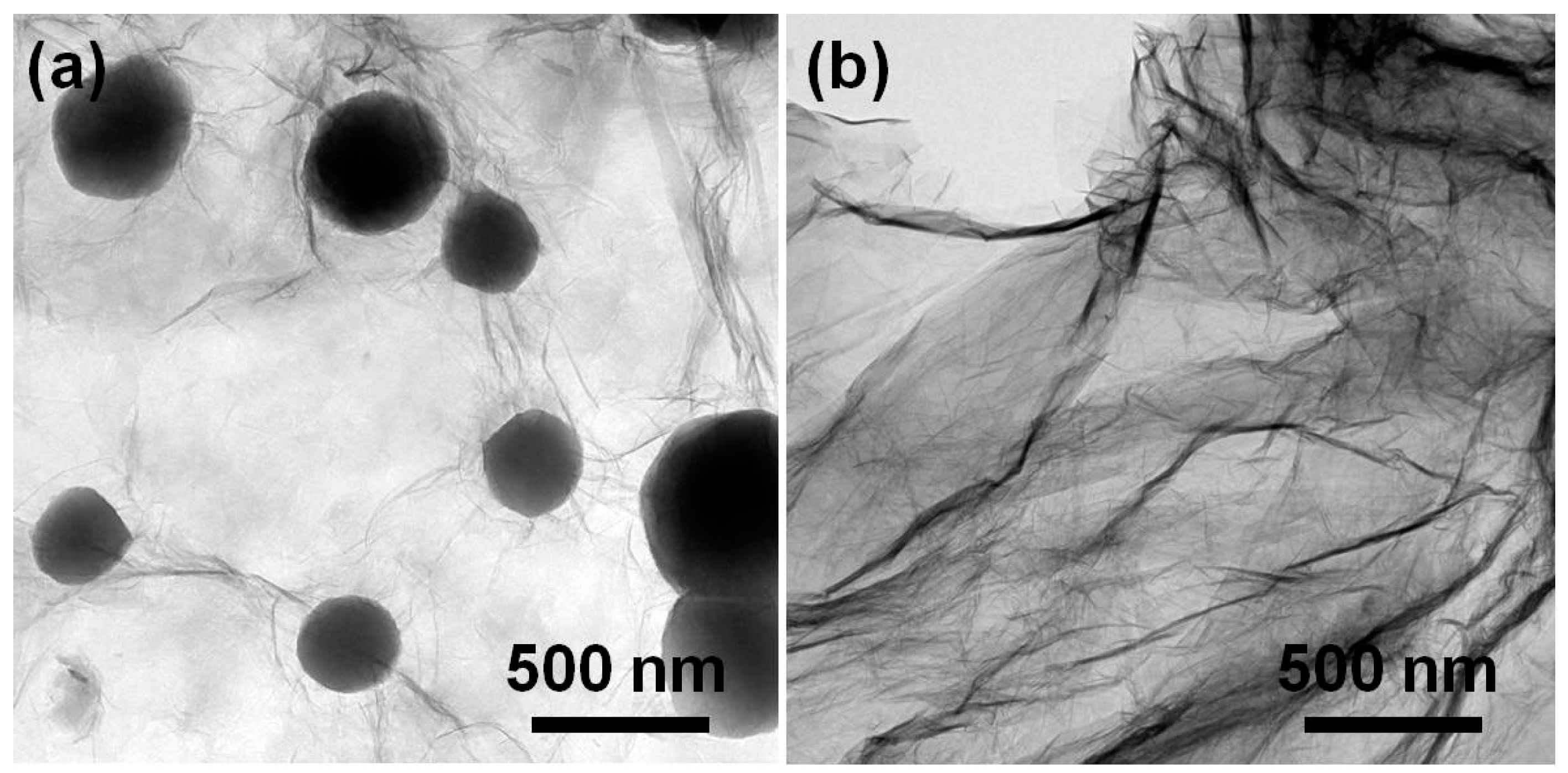
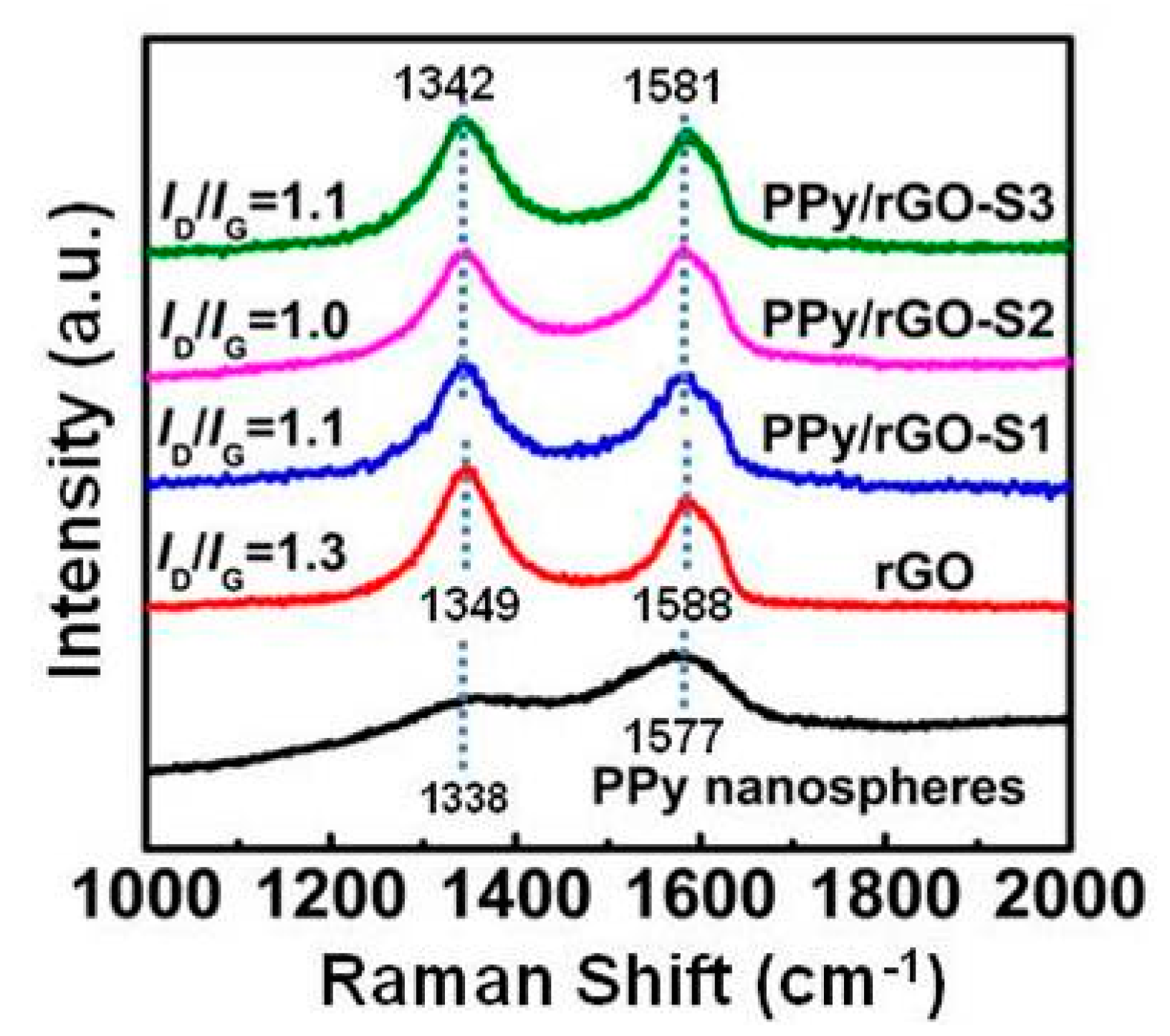
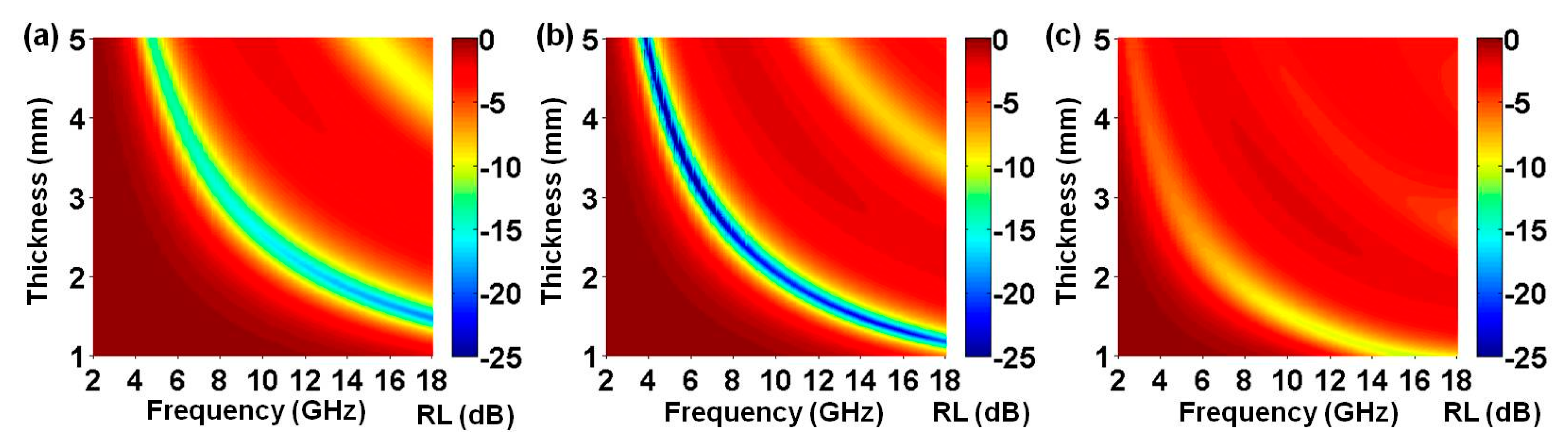
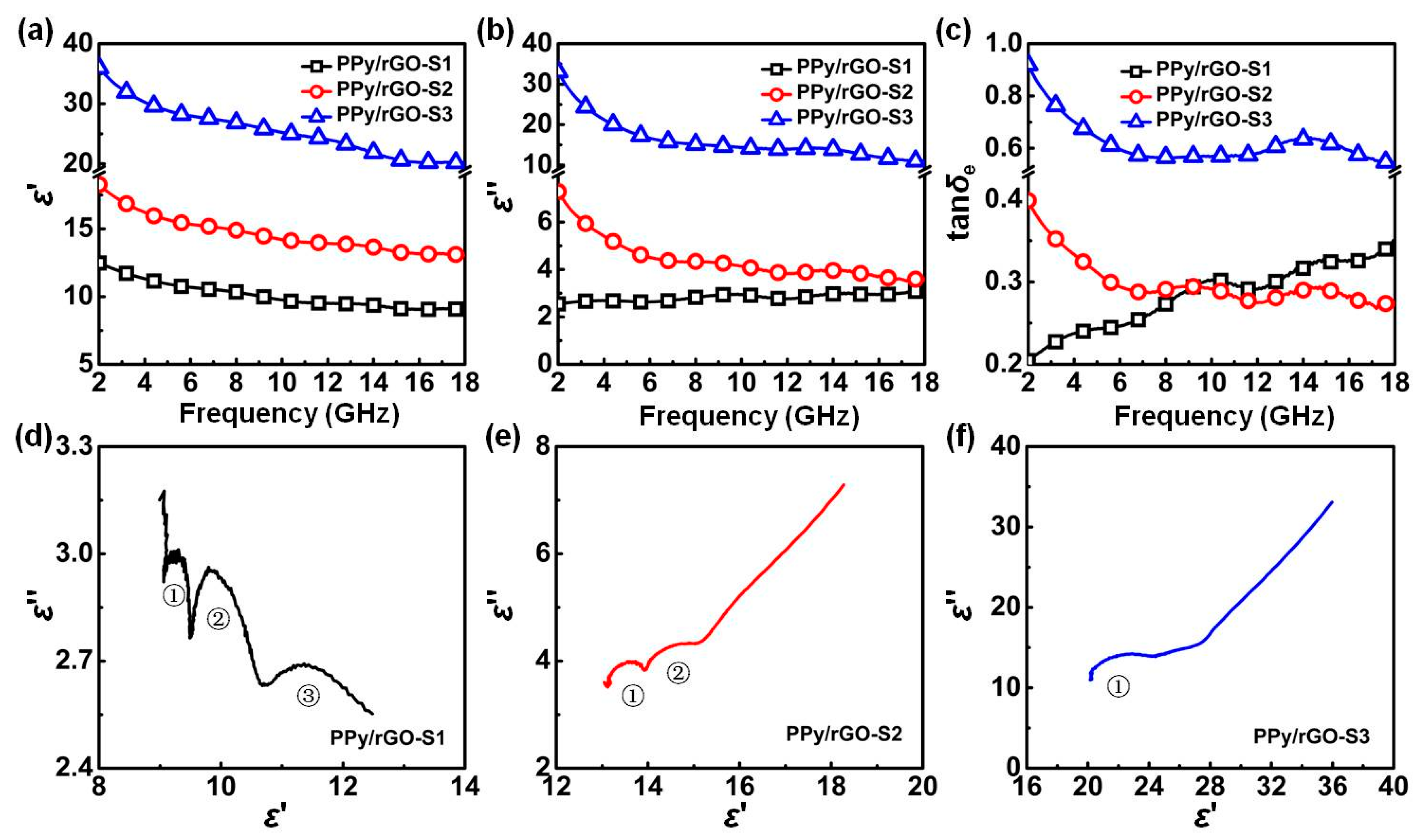
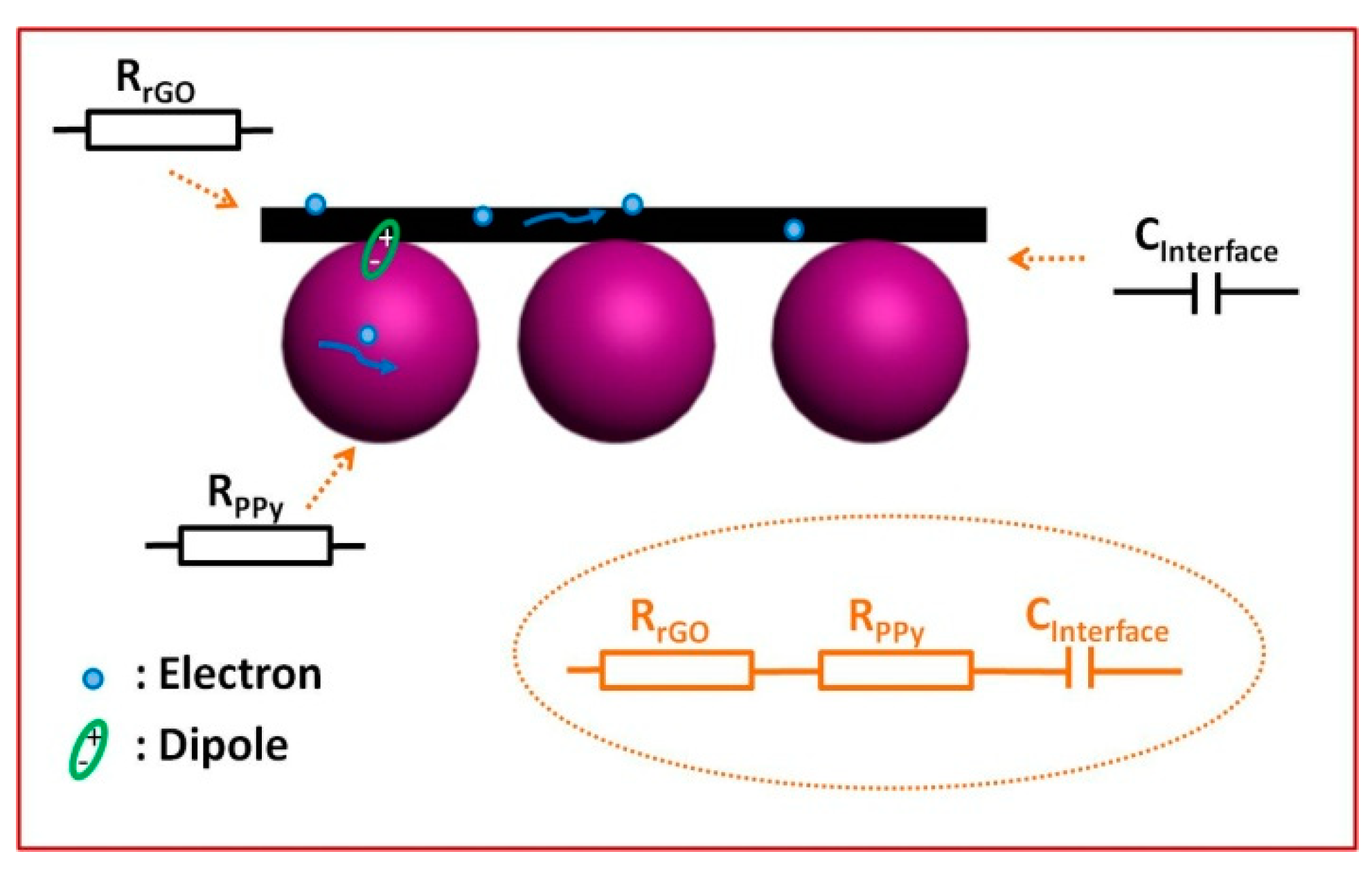
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Xing, H.L.; Gao, S.T.; Ji, X.L.; Shen, Z.Y. Porous flower-like NiO@graphene composites with superior microwave absorption properties. J. Mater. Chem. C 2017, 5, 2005–2014. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, X.X.; Cao, M.S. Confinedly implanted NiFe2O4-rGO: Cluster tailoring and highly tunable electromagnetic properties for selective-frequency microwave absorption. Nano Res. 2018, 11, 1426–1436. [Google Scholar] [CrossRef]
- Xu, H.L.; Yin, X.W.; Li, M.H.; Ye, F.; Han, M.K.; Hou, Z.X.; Li, X.L.; Zhang, L.T.; Cheng, L.F. Mesoporous carbon hollow microspheres with red blood cell like morphology for efficient microwave absorption at elevated temperature. Carbon 2018, 132, 343–351. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.S.; Ma, Y.T.; Zheng, H.F.; Lin, L.; Zhang, Q.F.; Chen, Y.Z.; Qiu, Y.L.; Peng, D.L. Enhanced microwave absorption properties by tuning cation deficiency of perovskite oxides of two-dimensional LaFeO3/C composite in X-band. ACS Appl. Mater. Interfaces 2017, 9, 7601–7610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, Y.C.; Xu, P.; Qiang, R.; Han, X.J. Recent advances in conjugated polymer-based microwave absorbing materials. Polymers 2017, 9, 29. [Google Scholar] [CrossRef]
- Quan, B.; Liang, X.H.; Ji, G.B.; Cheng, Y.; Liu, W.; Ma, J.N.; Zhang, Y.N.; Li, D.R.; Xu, G.Y. Dielectric polarization in electromagnetic wave absorption: Review and perspective. J. Alloys Compd. 2017, 728, 1065–1075. [Google Scholar] [CrossRef]
- Duan, W.J.; Li, X.D.; Wang, Y.; Qiang, R.; Tian, C.H.; Wang, N.; Han, X.J.; Du, Y.C. Surface functionalization of carbonyl iron with aluminum phosphate coating toward enhanced anti-oxidative ability and microwave absorption properties. Appl. Surf. Sci. 2018, 427, 594–602. [Google Scholar] [CrossRef]
- Lv, H.L.; Liang, X.H.; Cheng, Y.; Ji, G.B.; Tang, D.M.; Zhang, B.S.; Zhang, H.Q.; Du, Y.W. Facile synthesis of porous coin-like iron and its excellent electromagnetic absorption performance. RSC Adv. 2015, 5, 25936–25941. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.C.; Guo, D.; Qiang, R.; Tian, C.H.; Xu, P.; Han, X.J. Precursor-directed synthesis of porous cobalt assemblies with tunable close-packed hexagonal and face-centered cubic phases for the effective enhancement in microwave absorption. J. Mater. Sci. 2017, 52, 4399–4411. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, C.L.; Xia, Y.X.; Wang, T.; Li, F.S. Synthesis and microwave absorption properties of FeCo nanoplates. J. Alloys Compd. 2010, 493, 549–552. [Google Scholar] [CrossRef]
- Liu, Q.H.; Cao, Q.; Zhao, X.B.; Han, B.; Wang, C.; Wu, D.S.; Che, R.C. Insights into size-dominant magnetic microwave absorption properties of CoNi microflowers via off-axis electron holography. ACS Appl. Mater. Interfaces 2015, 7, 4233–4240. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.Y.; Pong, Z.W.; Wu, Y.P.; Ho, P.; Qiu, J.J.; Kong, L.B.; Han, G.C. Electrodeposition of granular FeCoNi films with large permeability for microwave applications. J. Mater. Chem. 2011, 21, 16042–16048. [Google Scholar] [CrossRef]
- Pei, S.F.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Wang, C.; Han, X.J.; Xu, P.; Zhang, X.L.; Du, Y.C.; Hu, S.R.; Wang, J.Y.; Wang, X.H. The electromagnetic property of chemically reduced graphene oxide and its application as microwave absorbing material. Appl. Phys. Lett. 2011, 98, 072906. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhang, T.F.; Chang, H.C.; Xiao, P.S.; Chen, H.H.; Huang, Z.Y.; Chen, Y.S. Broadband and tunable high-performance microwave absorption of an ultralight and highly compressible grapheme foam. Adv. Mater. 2015, 27, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.R.; Wang, X.X.; Cao, W.Q.; Han, C.; Yang, H.J.; Yuan, J.; Cao, M.S. A facile fabrication and highly tunable microwave absorption of 3D flower-like Co3O4-rGO hybrid-architectures. Chem. Eng. J. 2018, 339, 487–498. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, G.S.; Cao, W.Q.; Wei, Y.Z.; Liang, J.F.; Guo, L.; Cao, M.S. Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478. [Google Scholar] [CrossRef] [PubMed]
- Zong, M.; Huang, Y.; Zhao, Y.; Sun, X.; Qu, C.H.; Luo, D.D.; Zheng, J.B. Facile preparation, high microwave absorption and microwave absorbing mechanism of RGO–Fe3O4 composites. RSC Adv. 2013, 3, 23638–23648. [Google Scholar] [CrossRef]
- Feng, J.; Pu, F.Z.; Li, Z.X.; Li, X.H.; Hu, X.Y.; Bai, J.T. Interfacial interactions and synergistic effect of CoNi nanocrystals and nitrogen-doped graphene in a composite microwave absorber. Carbon 2016, 104, 214–225. [Google Scholar] [CrossRef]
- Sun, C.; Jiang, W.; Wang, Y.J.; Sun, D.P.; Liu, J.; Li, P.Y.; Li, F.S. Magnetic and electromagnetic absorption properties of FeNi alloy nanoparticles supported by reduced graphene oxide. Phys. Status Solidi RRL 2014, 8, 141–145. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chen, D.L.; Yin, X.; Xu, P.; Wu, F.; He, M. Hybrid of MoS2 and reduced graphene oxide: A lightweight and broadband electromagnetic wave absorber. ACS Appl. Mater. Interfaces 2015, 7, 26226–26234. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Y.M.; Chen, J.C.; Wang, L.; Guo, L.X.; Ouyang, J.H.; Jia, D.C.; Zhou, Y. Reduced graphene oxide decorated with in-situ growing ZnO nanocrystals: Facile synthesis and enhanced microwave absorption properties. Carbon 2016, 108, 52–60. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.C.; Xiang, J.Y.; Su, C.; Mu, C.P.; Wen, F.S.; Liu, Z.Y. Microwave absorption properties of CoS2 nanocrystals embedded into reduced graphene oxide. ACS Appl. Mater. Interfaces 2017, 9, 28868–28875. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, H.T.; Du, S.F.; Wang, Y.D. A facile hydrothermal synthesis of MnO2 nanorod-reduced grapheme oxide nanocomposites possessing excellent microwave absorption properties. RSC Adv. 2015, 5, 88979–88988. [Google Scholar] [CrossRef]
- Wang, X.K.; Yin, L.Y.; Chen, C.; Yu, J.S.; Zhou, X.G.; Wang, H.L.; Xu, B.S.; Wei, S.C. Synthesis of tremella-like grapheme@SiC nanostructure for electromagnetic wave absorbing material application. J. Alloys Compd. 2018, 741, 205–210. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.J.; Kang, L.L.; Qiang, R.; Liu, W.W.; Du, Y.C. Synthesis and characterization of polyaniline nanoparticles with enhanced microwave absorption. RSC Adv. 2013, 3, 12694–12701. [Google Scholar] [CrossRef]
- Xie, A.; Wu, F.; Sun, M.X.; Dai, X.Q.; Xu, Z.H.; Qiu, Y.Y.; Wang, Y.; Wang, M.Y. Self-assembled ultralight three-dimensional polypyrrole aerogel for effective electromagnetic absorption. Appl. Phys. Lett. 2015, 106, 222902. [Google Scholar] [CrossRef]
- Pang, R.; Hu, X.J.; Zhou, S.Y.; Sun, C.H.; Yan, J.; Sun, X.M.; Xiao, S.Z.; Chen, P. Preparation of multi-shelled conductive polymer hollow microspheres by using Fe3O4 hollow spheres as sacrificial templates. Chem. Commun. 2014, 50, 12493–12496. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Wang, T.S.; Wen, B.; Lu, M.M.; Xu, Z.; Zhu, C.L.; Chen, Y.J.; Xue, X.Y.; Sun, C.W.; Cao, M.S. Graphene/polyaniline nanorod arrays: Synthesis and excellent electromagnetic absorption properties. J. Mater. Chem. 2012, 22, 21679–21685. [Google Scholar] [CrossRef]
- Wu, F.; Xie, A.M.; Sun, M.X.; Wang, Y.; Wang, M.Y. Reduced graphene oxide (RGO) modified spongelike polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Li, P.B. Enhanced electromagnetic wave absorption properties of poly(3,4-ethylenedioxythiophene) nanofiber-decorated graphene sheets by non-covalent interactions. Nano-Micro Lett. 2016, 8, 131–136. [Google Scholar] [CrossRef]
- Thi, Q.V.; Tung, N.T.; Sohn, D. Synthesis and characterization of graphene-polypyrrole nanocomposites applying for electromagnetic microwave absorber. Mol. Cryst. Liq. Cryst. 2018, 660, 128–134. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.H.; Wang, L.F.; Ren, J.H.; Xu, Y.F. Ultralight graphene aerogel enhanced with transformed micro-structure led by polypyrrole nano-rods and its improved microwave absorption properties. Compos. Part A 2017, 97, 141–150. [Google Scholar] [CrossRef]
- Tian, C.H.; Du, Y.C.; Xu, P.; Qiang, R.; Wang, Y.; Ding, D.; Xue, J.L.; Ma, J.; Zhao, H.T.; Han, X.J. Constructing uniform core–shell PPy@PANI composites with tunable shell thickness toward enhancement in microwave absorption. ACS Appl. Mater. Interfaces 2015, 7, 20090–20099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Poyraz, S.; Zhang, X.Y. Green-nano approach to nanostructured polypyrrole. Chem. Commun. 2011, 47, 4421–4423. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Yu, C.F.; Wu, S.S.; Shen, J. A facilely prepared polypyrrole–reduced graphene oxide composite with a crumpled surface for high performance supercapacitor electrodes. J. Mater. Chem. A 2013, 1, 1–6539. [Google Scholar] [CrossRef]
- Zhang, M.F.; Li, Y.; Su, Z.Q.; Wei, G. Recent advances in the synthesis and applications of graphene-polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., McNeil, S., Eds.; Humana Press: New York, NY, USA, 2011; Volume 697, pp. 63–70. ISBN 978-1-60327-197-4. [Google Scholar]
- Zhou, H.H.; Han, G.Y.; Xiao, Y.M.; Chang, Y.Z.; Zhai, H.J. Facile preparation of polypyrrole/graphene oxide nanocomposites with large areal capacitance using electrochemical codeposition for supercapacitors. J. Power Sources 2014, 263, 259–267. [Google Scholar] [CrossRef]
- Chen, X.N.; Chen, J.J.; Meng, F.B.; Shan, L.M.; Jiang, M.; Xu, X.L.; Lu, J.; Wang, Y.; Zhou, Z.W. Hierarchical composites of polypyrrole/graphene oxide synthesized by in situ intercalation polymerization for high efficiency and broadband responses of electromagnetic absorption. Compos. Sci. Technol. 2016, 127, 71–78. [Google Scholar] [CrossRef]
- Perreault, F.; Faria, A.F.D.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Sov. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, Y.M.; Chen, J.C.; Guo, L.X.; Ouyang, J.H.; Jia, D.C.; Zhou, Y. Microwave absorbing property optimization of starlike ZnO/reduced graphene oxide doped by ZnO nanocrystal composites. Phys. Chem. Chem. Phys. 2017, 19, 14596–14605. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.G.; Jung, Y.C.; So, H.H.; Cho, J.W. Polypyrrole coated carbon nanotubes: Synthesis, characterization, and enhanced electrical properties. Synth. Met. 2007, 157, 374–379. [Google Scholar] [CrossRef]
- Tran, D.N.H.; Kabiri, S.; Losic, D. A green approach for the reduction of graphene oxide nanosheets using non-aromatic amino acids. Carbon 2014, 76, 193–202. [Google Scholar] [CrossRef]
- Liu, C.Y.; Xu, Y.J.; Wu, L.N.; Jiang, Z.H.; Shen, B.Z.; Wang, Z.J. Fabrication of core–multishell MWCNT/Fe3O4/PANI/Au hybrid nanotubes with high-performance electromagnetic absorption. J. Mater. Chem. A 2015, 3, 10566–10572. [Google Scholar] [CrossRef]
- Phan, D.T.; Chung, G.S. Effects of oxygen-functional groups on humidity sensor based graphene oxide thin films. IEEE Sens. 2012, 1–4. [Google Scholar] [CrossRef]
- Hao, J.N.; Liao, Y.Q.; Zhong, Y.Y.; Shu, D.; He, C.; Guo, S.T.; Huang, Y.L.; Zhong, J.; Hu, L.L. Three-dimensional graphene layers prepared by a gas-foaming method for supercapacitor applications. Carbon 2015, 94, 879–887. [Google Scholar] [CrossRef]
- Zhang, J.T.; Chen, P.; Oh, B.H.L.; Chan-Park, M.B. High capacitive performance of flexible and binder-free graphene–polypyrrole composite membrane based on in situ reduction of graphene oxide and self-assembly. Nanoscale 2013, 5, 9860–9866. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.S.; Yang, J.; Song, W.L.; Zhang, D.Q.; Wen, B.; Jin, H.B.; Hou, Z.L.; Yuan, J. Ferroferric oxide/multiwalled carbon nanotube vs polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl. Mater. Interfaces 2012, 4, 6949–6956. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.G.; Yu, M.; Fu, J.; Wang, L.R. Synthesis and microwave absorption properties of hierarchical Fe micro-sphere assembly by nano-plates. J. Alloys Compd. 2017, 721, 449–455. [Google Scholar] [CrossRef]
- Yang, P.P.; Zhao, X.C.; Liu, Y.; Lai, X.H. Preparation and electromagnetic wave absorption properties of hollow Co, Fe@air@Co and Fe@Co nanoparticles. Adv. Powder Technol. 2018, 29, 289–295. [Google Scholar] [CrossRef]
- Li, X.H.; Guo, X.H.; Liu, T.C.; Zheng, X.L.; Bai, J.T. Shape-controlled synthesis of Fe nanostructures and their enhanced microwave absorption properties at L-band. Mater. Res. Bull. 2014, 59, 137–141. [Google Scholar] [CrossRef]
- Guo, H.; Pu, B.X.; Chen, H.Y.; Yang, J.; Zhou, Y.J.; Yang, J.; Bismark, B.; Li, H.D.; Niu, X.B. Surfactant-assisted solvothermal synthesis of pure nickel submicron spheres with microwave-absorbing properties. Nanoscale Res. Lett. 2016, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Zou, J.P.; Ding, Z.H.; Wu, J.F.; Wang, P.H.; Jin, S.W.; Bi, H. Magnetic and microwave absorption properties of Ni microcrystals with hierarchical branch-like and flowers-like shapes. Mater. Chem. Phys. 2013, 142, 119–123. [Google Scholar] [CrossRef]
- Zhu, Z.T.; Sun, X.; Li, G.X.; Xue, H.R.; Guo, H.; Fan, X.L.; Pan, X.C.; He, J.P. Microwave-assisted synthesis of graphene–Ni composites with enhanced microwave absorption properties in Ku-band. J. Magn. Magn. Mater. 2015, 377, 95–103. [Google Scholar] [CrossRef]
- Pan, G.H.; Zhu, J.; Ma, S.L.; Sun, G.B.; Yang, X.J. Enhancing the electromagnetic performance of Co through the phase-controlled synthesis of hexagonal and cubic Co nanocrystals grown on graphene. ACS Appl. Mater. Interfaces 2013, 5, 12716–12724. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Feng, J.; Du, Y.P.; Bai, J.T.; Fan, H.M.; Zhang, H.L.; Peng, Y.; Li, F.S. One-pot synthesis of CoFe2 O4/graphene oxide hybrids and their conversion into FeCo/graphene hybrids for lightweight and highly efficient microwave absorber. J. Mater. Chem. A 2015, 3, 5535–5546. [Google Scholar] [CrossRef]
- Dong, X.L.; Zhang, X.F.; Huang, H.; Zou, F. Enhanced microwave absorption in Ni/polyaniline nanocomposites by dual dielectric relaxations. Appl. Phys. Lett. 2008, 92, 013127. [Google Scholar] [CrossRef]
- Liu, P.B.; Huang, Y. Decoration of reduced grapheme oxide with polyaniline film and their enhanced microwave absorption properties. J. Polym. Res. 2014, 21, 430. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Y.; Wang, M.Y. Using organic solvent absorption as a self-assembly method to synthesize three-dimensional (3D) reduced grapheme oxide (RGO)/poly(3,4-ethylenedioxythiophene) (PEDOT) architecture and its electromagnetic absorption properties. RSC Adv. 2014, 4, 49780–49782. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.C.; Qiang, R.; Tian, C.H.; Xu, P.; Han, X.J. Interfacially engineered sandwich-like rGO/carbon microspheres/rGO composite as an efficient and durable microwave absorber. Adv. Mater. Interfaces 2016, 3, 1500684. [Google Scholar] [CrossRef]
- Jiang, J.J.; Li, D.; Geng, D.Y.; An, J.; He, J.; Liu, W.; Zhang, Z.D. Microwave absorption properties of core double-shell FeCo/C/BaTiO3 nanocomposites. Nanoscale 2014, 6, 3967–3971. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Ohlan, A.; Pham, V.H.; Balasubramaniyan, R.; Varshney, S.; Jang, J.; Hur, S.H.; Choi, W.M.; Kumar, M.; Dhawan, S.K.; et al. Nanostructured graphene/Fe3O4 incorporated polyaniline as a high performance shield against electromagnetic pollution. Nanoscale 2013, 5, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Dong, X.L.; Huang, H.; Liu, Y.Y.; Wang, W.N.; Zhu, X.G.; Lv, B.; Lei, J.P. Microwave absorption properties of the carbon-coated nickel nanocapsules. Appl. Phys. Lett. 2006, 89, 053115. [Google Scholar] [CrossRef]
- Guo, J.; Song, H.X.; Liu, H.; Luo, C.J.; Ren, Y.R.; Ding, T.; Khan, M.A.; Young, D.P.; Liu, X.Y.; Zhang, X.; et al. Polypyrrole-interface-functionalized nano-magnetite epoxy nanocomposites as electromagnetic wave absorbers with enhanced flame retardancy. J. Mater. Chem. C 2017, 5, 5334–5344. [Google Scholar] [CrossRef]
- Chu, W.L.; Wang, Y.; Du, Y.C.; Qiang, R.; Tian, C.H.; Han, X.J. FeCo alloy nanoparticles supported on ordered mesoporous carbon for enhanced microwave absorption. J. Mater. Sci. 2017, 52, 13636–13649. [Google Scholar] [CrossRef]
- Chu, W.L.; Tian, C.H.; Wang, Y.; Chu, J.Y.; Li, Z.G.; Du, Y.C.; Han, X.J. Performance vs convenience of magnetic carbon-metal nanocomposites: A low-cost and facile citrate-derived strategy for FeCo alloy/carbon composites with high-performance microwave absorption. Comments Inorg. Chem. 2017, 37, 301–326. [Google Scholar] [CrossRef]
- Adachi, A.; Yamauchi, J. Effect of UV irradiation on polypyrrole as studied by electron spin resonance. Synth. Met. 1995, 73, 101–105. [Google Scholar] [CrossRef]

| Absorber | Loading (%) | Integrated Thickness (mm) | RLmin (dB) (Frequency, Thickness) | Bandwidth over −10 dB (GHz) | Effective Bandwidth (RL < −10 dB) (GHz) | Ref. |
|---|---|---|---|---|---|---|
| PANI nanorods/graphene | 50 | 2.0–5.0 | −51.1 dB (6.4 GHz, 3.0 mm) | 5.8–18.0 | 12.2 | [29] |
| Flower-like α-Fe particles | 40 | 2.0–5.0 | −33.1 dB (17.5 GHz, 5.5 mm) | 6.0–18.0 | 12.0 | [50] |
| Hollow Co nanoparticles | 70 | 1.1–3.0 | −45.06 dB (8.0 GHz, 1.7 mm) | 5.2–16.5 | 11.3 | [51] |
| Fe nanoparticles | 50 | 1.5–5.0 | −33 dB (1.3 GHz, 5.0 mm) | 0.9–3.7 | 2.8 | [52] |
| Ni-10000 | 75 | 1.0–5.0 | −17.9 dB (17.8 GHz, 1.2 mm) | 4.2–18.0 | 13.8 | [53] |
| Flower-like Ni | 33 | 2.0–5.0 | −17.0 dB (13.0 GHz, 3.0 mm) | 11.5–14.0 | 2.5 | [54] |
| Graphene–Ni composite | 30 | 2.0–4.0 | −42.0 dB (17.6 GHz, 2 mm) | 8.0–18.0 | 10 | [55] |
| α-Co/graphene | 60 | 1.0–5.0 | −47.5 dB (11.9 GHz, 2 mm) | 3.0–15.0 | 12.0 | [56] |
| FeCo/graphene | 50 | 1.5–6.0 | −40.2 dB (8.9 GHz, 2.5 mm) | 3.4–18.0 | 14.6 | [57] |
| Ni/PANI | 50 | 2.0–6.0 | −35.0 dB (17.2 GHz, 5.0 mm) | 4.0–18.0 | 14.0 | [58] |
| PANI/rGO | 50 | 1.5–3.5 | −41.4 dB (13.8 GHz, 2.0 mm) | 6.0–18.0 | 12.0 | [59] |
| PEDOT/rGO | 10 | 2.0–4.0 | −35.5 dB (13.3 GHz, 2.0 mm) | 4.8–16.2 | 11.4 | [60] |
| PPy/rGO-S2 | 30 | 1.0–5.0 | −59.2 dB (5.0 GHz, 3.8 mm) | 3.3–18.0 | 14.7 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Du, Y.; Wu, B.; Han, B.; Dong, S.; Han, X.; Xu, P. Fabrication of PPy Nanosphere/rGO Composites via a Facile Self-Assembly Strategy for Durable Microwave Absorption. Polymers 2018, 10, 998. https://doi.org/10.3390/polym10090998
Wang Y, Du Y, Wu B, Han B, Dong S, Han X, Xu P. Fabrication of PPy Nanosphere/rGO Composites via a Facile Self-Assembly Strategy for Durable Microwave Absorption. Polymers. 2018; 10(9):998. https://doi.org/10.3390/polym10090998
Chicago/Turabian StyleWang, Ying, Yunchen Du, Bo Wu, Binhua Han, Shaoming Dong, Xijiang Han, and Ping Xu. 2018. "Fabrication of PPy Nanosphere/rGO Composites via a Facile Self-Assembly Strategy for Durable Microwave Absorption" Polymers 10, no. 9: 998. https://doi.org/10.3390/polym10090998
APA StyleWang, Y., Du, Y., Wu, B., Han, B., Dong, S., Han, X., & Xu, P. (2018). Fabrication of PPy Nanosphere/rGO Composites via a Facile Self-Assembly Strategy for Durable Microwave Absorption. Polymers, 10(9), 998. https://doi.org/10.3390/polym10090998








