1. Introduction
Among the diverse polymer-based hydrogels, protein hydrogels have attracted considerable interest due to their advantageous features such as biocompatibility, biodegradability, and low immunogenicity [
1,
2,
3,
4]. These hydrogels are usually fabricated via physical and chemical cross-linking of soluble protein polymers to form water-insoluble three-dimensional networks. Owing to the genetic basis of sequence, molecular weight, folded structure, and stereochemistry [
5,
6], protein polymers thus offer substantial opportunities for the design of protein hydrogels.
Recent trends in protein hydrogel design have evolved from static to dynamic controlling systems, to yield hydrogels with desirable stimuli responsiveness [
7]. Responsive protein hydrogels are playing an increasingly important role in a diverse range of applications in biotechnology and medicine [
8,
9,
10]. They commonly undergo volume and other property changes in response to external stimuli such as temperature [
11], pH [
12], light [
13], and mechanical frequency [
14]. These unique properties of responsive protein hydrogels will not only open up new avenues for a range of experiments in biological drug delivery systems [
7] and tissue engineering studies [
15], but also lead to a new generation of smart biomaterials that may find applications as biosensors [
16] or actuators [
17].
Previously, the carboxyl-terminal (CT) domain of major ampullate spidroin 1 (MaSp1) of the spider
Nephila clavipes (NcCT) was found to form hydrogels when a moderately concentrated solution (15%) of recombinant CT was either cooled to approximately 2 °C or heated to 65 °C [
18]. Intriguingly, the gelation at low temperature was reversible, a process mainly driven by hydrogen bonding and hydrophobic interactions between the globular structures of the recombinant polypeptide. Nevertheless, the gelation at high temperature was irreversible and was speculated to be a process mainly driven by hydrophobic interactions between the partially unfolded biomacromolecules. Such a dual thermosensitive peptide domain was peculiar, because thermally induced sequential gel-sol-gel transition had scarcely been reported for either synthetic or natural biopolymers. Moreover, the CTs of spidroins of different spider species are evolutionarily conserved with respect to amino acid sequences, secondary structures, and formation of dual thermosensitive hydrogels [
18]. Therefore, the CT appears to be a unique building block for
de novo design of a family of new protein polymers and hydrogel materials with tunable physiochemical and mechanical properties. However, in-depth studies on CT-based responsive protein hydrogels are needed to fully explore its potential.
Resilin is another unique protein material that has been well recognized for its remarkable properties, such as high extensibility and exceptional resilience [
19,
20]. This extraordinary material is normally found in specialized regions of the cuticle of most insects [
20,
21]. Elvin et al. reported the first recombinant resilin-like protein, rec1-resilin, which encodes the N-terminal domain (exon 1) in native resilin (fruit fly
Drosophila melanogaster resilin gene CG15920) comprising 18 pentadecapeptide repeats (GGRPSDSYGAPGGGN) [
21]. Further physiochemical analysis revealed that rec1-resilin is an intrinsically disordered polypeptide and dominated by random coils, which exhibits dual-phase transition behavior with an upper critical solution temperature (UCST) and a lower critical solution temperature (LCST) of ~6 and 70 °C, respectively. In addition, the recombinant protein was found to display multiple-stimuli responsiveness to other triggers such as pH, ion, and light [
21,
22]. These unique features and advantages indicate tremendous potential of resilin consensus motifs for the development of dynamic biomaterials [
23]. Indeed, resilin-like homopolymers have been biosynthesized and fabricated into fascinating biomaterials for biomedical applications due to their biomimetically high elasticity and resilience, and attractive biochemical and mechanical properties [
24,
25,
26].
Recently, modular biopolymer designs that combine the unique properties of resilin and other structural or biologically active domains have emerged as an intriguing approach for programming biomaterials to sense and aptly respond to biomechanical demands or changes in the environment [
26,
27,
28]. For instance, Lv et al. combined random-coil-like resilin and folded GB1 domains from the streptococcal B1 immunoglobulin-binding domain of protein G to mimic the complex molecular springs found in the muscle protein titin [
26]. In another example, a polypeptide containing resilin and biologically active domains such as heparin-binding and cell-binding ligands was designed for the fabrication of cross-linked hydrogel scaffold with the capacity for mouse fibroblast cell attachment and proliferation [
27]. More recently, our group designed and recombinantly synthesized a resilin-silk copolymer that was thermosensitive due to the resilin blocks and prone to self-assemble into nano- to microscale fibrils due to the silk blocks (GAGAGS), leading to the formation of cytobiocompatible protein hydrogels [
28]. These examples highlight the exciting opportunities for genetically engineered protein polymers in the design and fabrication of novel dynamic hydrogel materials.
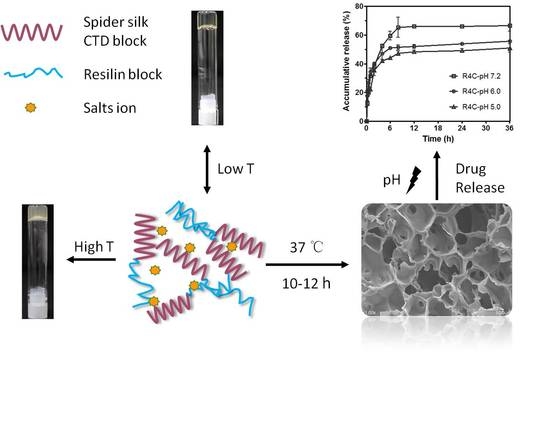
In this study, we hypothesized that the integration of resilin and spider silk CT would result in copolymers that recapitulate the dual thermoresponsive gelation behavior of CT and the multiresponsiveness and improved mechanical properties of resilin. To test the hypothesis, we first designed and biosynthesized two genetically engineered copolymers, each composed of CT and resilin consensus motifs of varying lengths. Second, thermoresponsiveness of the copolymers in solution was studied, and their thermo- and ion-sensitive gelation features were explored. Finally, gelation of the copolymers at physiologic temperature (37 °C) was examined, which resulted in hydrogels with pH-dependent release of a highly polar model drug in vitro.
2. Materials and Methods
2.1. Chemical and Materials
Ampicillin, β-mercaptoethanol, imidazole, and isopropyl-β-D-thiogalactopyranoside (IPTG) were purchased from Sangon Biotech (Shanghai, China). Tryptone and yeast extract were obtained from Oxoid (Basingstoke, Hampshire, UK). Ni-NTA agarose (catalog no. 30230) and rhodamine B (catalog no. 83689) were obtained from Qiagen (Hilden, Germany) and Sigma (St. Louis, MO, USA), respectively. Coomassie Brilliant Blue R-250 (catalog no. 161-0400) was purchased from Bio-Rad (Hercules, CA, USA). All other chemicals were of the highest purity available from commercial suppliers.
PrimeSTAR Max DNA polymerase for polymerase chain reaction (PCR) was obtained from Takara Biotechnology Co., Ltd. (Dalian, China). Restriction enzymes and T4 DNA ligase were obtained from New England Biolabs (Ipswich, MA, USA). TIANprep Mini Plasmid Kit, TIANgel Midi Purification Kit, and chemically competent cells of E. coli DH5α and BL21(DE3) were purchased from Tiangen Biotech Co., Ltd. (Beijing, China). Amicon Ultra-15 centrifugal filter units with Ultracel-3K membranes (3 kDa molecular weight cutoff) were obtained from Millipore (Billerica, MA, USA), and a Pierce BCA Protein Assay Kit (catalog no. 23225) was purchased from Thermo Fisher Scientific Inc. (Rockford, IL, USA).
2.2. Construction of Protein Expression Plasmids
Plasmid vector pET-19b (Novagen, Madison, WI, USA) was employed to construct plasmids for recombinant expression of protein polymers under the IPTG-inducible T7 promoter. First, DNA encoding the C-terminal domain of spider
N. clavipes major ampullate spidroin 1 was amplified from a template plasmid pUC57-MaSpIC [
18], using PrimeSTAR Max DNA polymerase and primers FcNdeNhe (5′-AATCATATGGCTAGCGTGGGCAGCGGCG-3′) and RcXhoSpe (5′-AATCTCGAGACTAGTGCCCAGCGCCTGATACA-3′). The PCR product was digested with restriction enzymes
NdeI and
XhoI, agarose gel purified, and cloned into vector pET-19b at the same sites, leading to plasmid pET19b-CT1. Next, the DNA fragment harboring 4 repeats of resilin-like sequence (GGRPSDSYGAPGGGN) was released from plasmid pET19b-R4 [
28] by double digest with the enzymes
SpeI and
PvuI, agarose gel purified, and then ligated with the 1.3 kb
NheI–
PvuI fragment of pET19b-CT1. The resulting plasmid was named pET19b-R4C, which allowed recombinant biosynthesis of a protein copolymer containing 4 repetitive units of resilin and the spidroin C-terminal domain. To biosynthesize another protein copolymer containing 8 repetitive units of resilin and the spidroin C-terminal domain, plasmid pET19b-R8C was created by ligating the 5.0 kb
SpeI–
PvuI fragment of pET19b-R4 with the 1.5-kb
PvuI–
NheI fragment of pET19b-R4C. The above copolymer expression plasmids were identified by restriction digest and further confirmed by DNA sequencing.
2.3. Protein Expression, Purification, and Identification
The recombinant plasmids were transformed into the chemically competent cells of
E. coli BL21 (DE3), a common microbial expression host for the pET expression system. A single colony was picked on the selective Luria−Bertani (LB) solid medium supplemented with 100 µg mL
−1 of ampicillin, inoculated into a 15 mL tube containing 4 mL of selective liquid LB medium, and then cultured overnight at 37 °C and 220 rpm in a rotary shaker. Subsequently, 500 μL of the overnight culture was transferred into a 250 mL shake flask containing 50 mL of a minimal R/2 medium (pH 6.80) supplemented with 10 g L
−1 of glucose as a carbon source [
29]. The seed cultures, 250 mL in a total of 5 flasks, were incubated until the cell optical density at 600 nm (OD
600) reached ~3–4, and subsequently transferred into a 5 L jar fermenter (BIOTECH-5JG-7000; Shanghai BaoXing Bio-Engineering Equipment Co. Ltd., Shanghai, China) containing 2.5 L of fresh R/2 medium. The culture temperature was initially maintained at 37 °C and pH was kept at 6.80 by adding 28% (
w/
v) ammonia water. The dissolved oxygen (DO) concentration was kept above 40% of air saturation by automatically increasing the agitation speed from 200 to 800 rpm and by increasing the percentage of pure oxygen when agitation at 800 rpm was insufficient to maintain the DO level. A feeding solution containing 700 g L
−1 of glucose and 20 g L
−1 of MgSO
4·7H
2O was added to the bioreactor when the culture pH increased above 6.80 because of glucose depletion. When the cell OD
600 reached ~35–40, the temperature was downshifted to 30 °C, and IPTG was added to the fermenter at a final concentration of 1 mM. After induction at 30 °C for 6 h, the bacterial cells were harvested by centrifugation and stored at −40 °C before purification of the protein copolymers from the cell pellets.
For protein purification, the cell pellets were resuspended in 20 mM Tris-HCl buffer (pH 8.0) supplemented with 150 mM NaCl and 5 mM imidazole, and then lysed using an AH-1500 high-pressure homogenizer (ATS Engineering Ltd., Toronto, ON, Canada). The homogenate was centrifuged at 15,557 g for 20 min at 20 °C. The resulting supernatant was loaded onto an Ni-NTA agarose column that had been equilibrated with the above resuspension buffer. The column was washed and eluted with the Tris-HCl buffer supplemented with imidazole at 75 and 250 mM, respectively. The eluted proteins of interest were directly concentrated and changed into 20 mM phosphate buffer (pH 7.2) through ultrafiltration using Amicon Ultra centrifugal filter units (Millipore).
The purity of the purified protein polymers was verified with 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using a gel loading buffer with 1% β-mercaptoethanol, which is a reducing agent. The gels were stained with Coomassie Brilliant Blue R-250 and scanned using a Microtek Bio-5000plus Imaging Densitometer (Microtek, Shanghai, China). The protein concentrations were measured using a Pierce BCA Protein Assay Kit. All the protein samples were freshly prepared before use and maintained in the phosphate buffer (pH 7.2) unless otherwise specified. The molecular weights of the purified proteins were confirmed by using a Bruker Autoflex Speed matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometer (Bruker Daltonics, Billerica, MA, USA).
2.4. Characterization of Phase Transition
The phase transition behavior of each protein polymer was characterized by monitoring the absorbance of a protein solution at 350 nm (OD350) as a function of temperature on a Shimadzu UV-2600 UV-vis spectrophotometer (Shimadzu Corp., Kyoto, Japan) equipped with a constant-temperature water circulator (Model SDC-6; Scientz, Ningbo, China). Each protein at 50 mg mL−1 in 20 mM phosphate buffer (pH 7.2) was loaded into a Suprasil quartz micro cuvette (10 mm lightpath; Hellma, Müllheim, Germany). First, the samples were tested from 20 to 4 °C with a chilling rate of 1 °C min−1. Then, the temperature of the cuvettes was shifted back to 20 °C, and the moisture on the cuvette surface was cleaned up to eliminate its effect. Subsequently, OD350 of the samples was monitored from 20 to 85 °C with a heating rate of 1 °C min−1.
2.5. Atomic Force Microscopy
Specimens for atomic force microscopy (AFM) imaging were prepared by casting a drop (10 μL) of protein solution (1 µg mL−1 in 20 mM phosphate buffer, pH 7.2) onto a mica surface. The mica was then incubated at either 4, 37, or 65 °C for 10−12 h to allow drying, and then rinsed gently with Milli-Q H2O. The samples were subsequently allowed to dry at the respective temperatures before analysis. The AFM images were acquired in tapping mode under ambient conditions using a multimode AFM (Bruker, Dresden, Germany) with a Nanoscope IIIa scanning probe controller (Digital Instruments, Santa Barbara, CA, USA). The AFM images were collected with a scanning area of 5 × 5 μm2 and further analyzed with Nanoscope v5.30 software (Bruker).
2.6. Thermoresponsive Hydrogel Formation
Each copolymer solution (200 μL) at a protein concentration of 15% (w/v) in 20 mM phosphate buffer (pH 7.2) was transferred into a glass tube and precooled to 4 °C. The tubes were first incubated at 4 °C for 10 min, and inverted to test the formation of self-supporting hydrogels. Incubation and inversion tests were then performed at temperatures ranging from 10 to 85 °C. Pictures of the inverted tubes were taken by a Canon EOS 550D digital camera (Canon, Tokyo, Japan) and processed to the same size by using Meitu version 4.0.1.2002 software (Xiamen, China).
2.7. Rheological Measurements
Rheological measurements of the protein samples were performed on a stress-controlled AR-G2 rheometer (TA Instruments, New Castle, DE, USA) with a 40 mm diameter plate-on-plate geometry. After instrument calibration, 500 μL of a freshly prepared protein solution was loaded onto the bottom plate at 25 °C. The top plate was then lowered to a gap distance of 350 μm. Hydrogenated silicone oil was added to the edge of the plate to avoid dehydration. After equilibration at 2 °C for 10 min, the measurements were performed at temperatures ranging from 2 to 85 °C with a heating rate of 2 °C min−1. The storage modulus (G′) and loss modulus (G″) were recorded as a function of temperature at a strain of 1% and a frequency of 1 Hz. In another setup, time sweeps were carried out at 37 °C with a strain of 1% and a frequency of 1 Hz.
2.8. pH-Responsive Drug Release Assay
Rhodamine B was used as a model polar drug to examine drug release from the protein hydrogels in vitro. Briefly, 90 μL of either R4C or NcCT at 16.7% (w/v) in 20 mM phosphate buffer (pH 7.2) was mixed with 10 μL of rhodamine B solution at 0.5 mg mL−1 on 96-well cell culture plates (Nest Biotechnology Co., Ltd., Wuxi, China). The mixtures were incubated statically at 37 °C overnight to allow the formation of hydrogels. Release of rhodamine B was initiated by dropping on the hydrogel surface 200 μL of phosphate buffered saline (PBS) with pH at 7.2, 6.0, or 5.0. A 20 μL aliquot of the PBS solution was sampled from the wells at predetermined time points, and an equal volume of warmed fresh buffer at each corresponding pH was added back to the wells. Rhodamine B concentrations in the sampled solutions were quantified using an external standard calibration curve. The rhodamine B fluorescence intensities at 627 nm with excitation at 553 nm were measured on a SpectraMax M5 fluorescence microplate reader (Molecular Devices Corp., Sunnyvale, CA, USA). Data are represented as the average of 3 biological replicates with standard deviation.
2.9. Circular Dichroism Spectroscopy
Far-UV circular dichroism (CD) spectra were acquired using a JASCO J-815 spectropolarimeter coupled with a JASCO PTC-432S Peltier temperature controller (Tokyo, Japan). Each protein (0.2 mg mL−1 in 20 mM phosphate buffer, pH 7.2) was loaded into a 1 mm path length quartz cuvette and equilibrated at 2 °C for 10 min before measurements. The CD data at 222 nm were collected from 2 to 85 °C with a heating rate of 2 °C min−1. The CD spectra were measured 3 times and averaged. The data fitting was carried out by using Origin 9.0 software (OriginLab Corp., Northampton, MA, USA).