Layer-by-Layer Self-Assembly Strategy for Surface Modification of Aramid Fibers to Enhance Interfacial Adhesion to Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
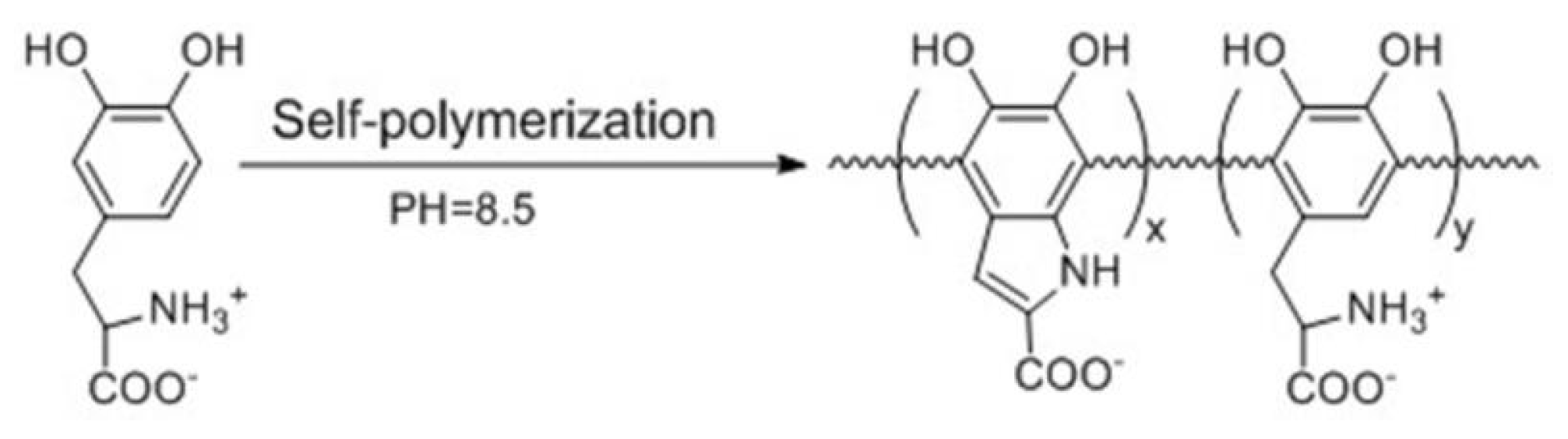
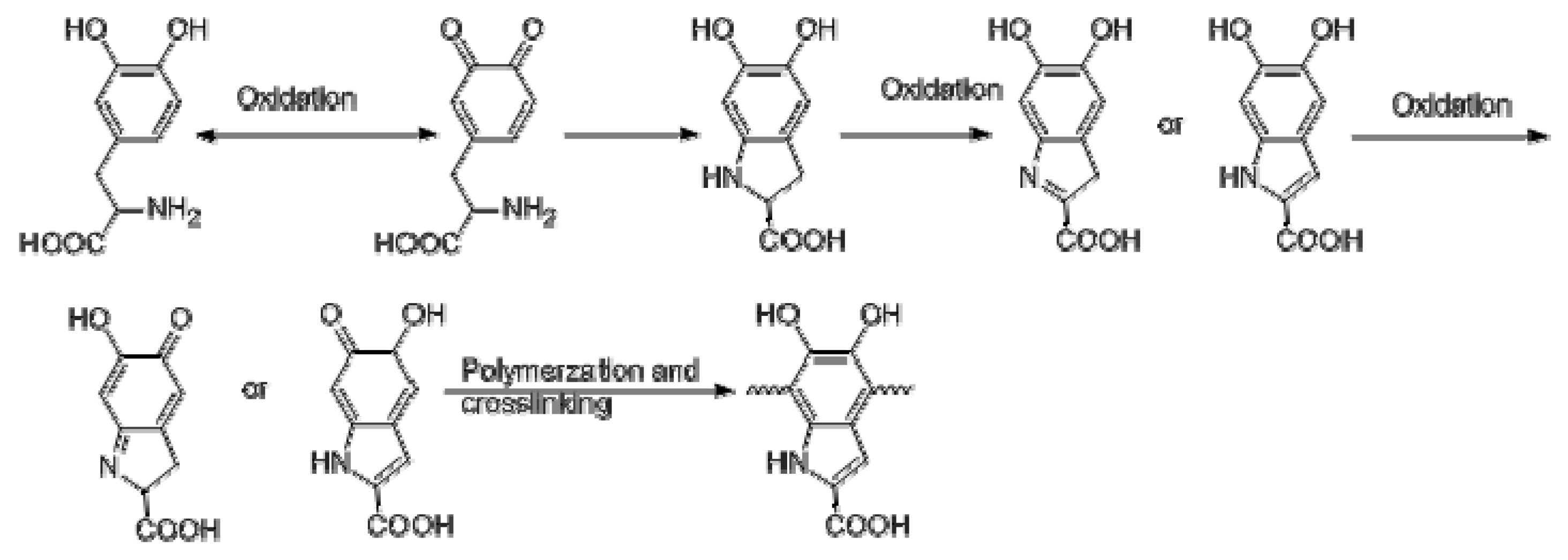
2.2. Preparation of l-PDOPA via Self-Polymerization of l-DOPA
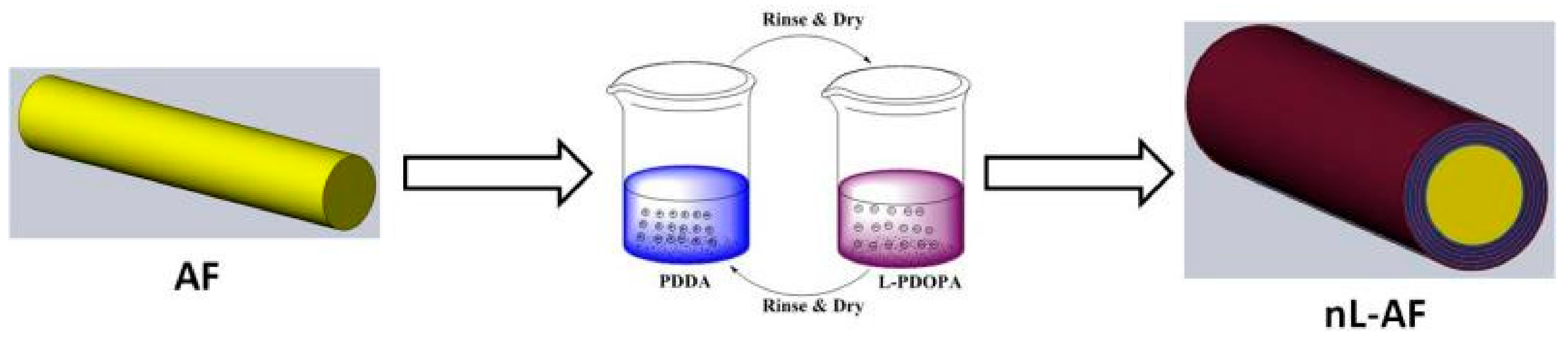
2.3. Layer-by-Layer Self-Assembly of (l-PDOPA) for Surface Modification of Aramid Fibers and Electrostatic Adsorption Acidification of Silane Coupling Agents (KH550)
2.4. Preparation of Aramid Fibers/Epoxy Resin Composites
2.5. Characterization
3. Results
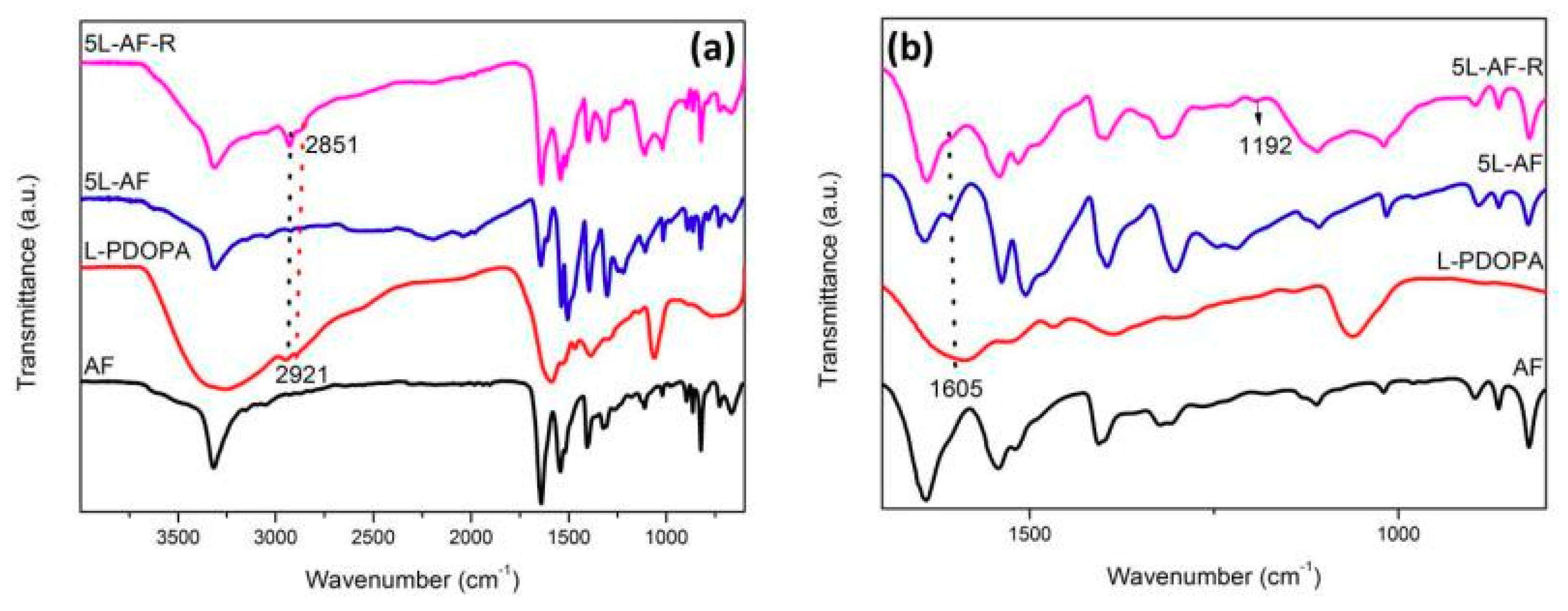
3.1. Spectroscopy Analysis
3.2. X-Ray Diffraction
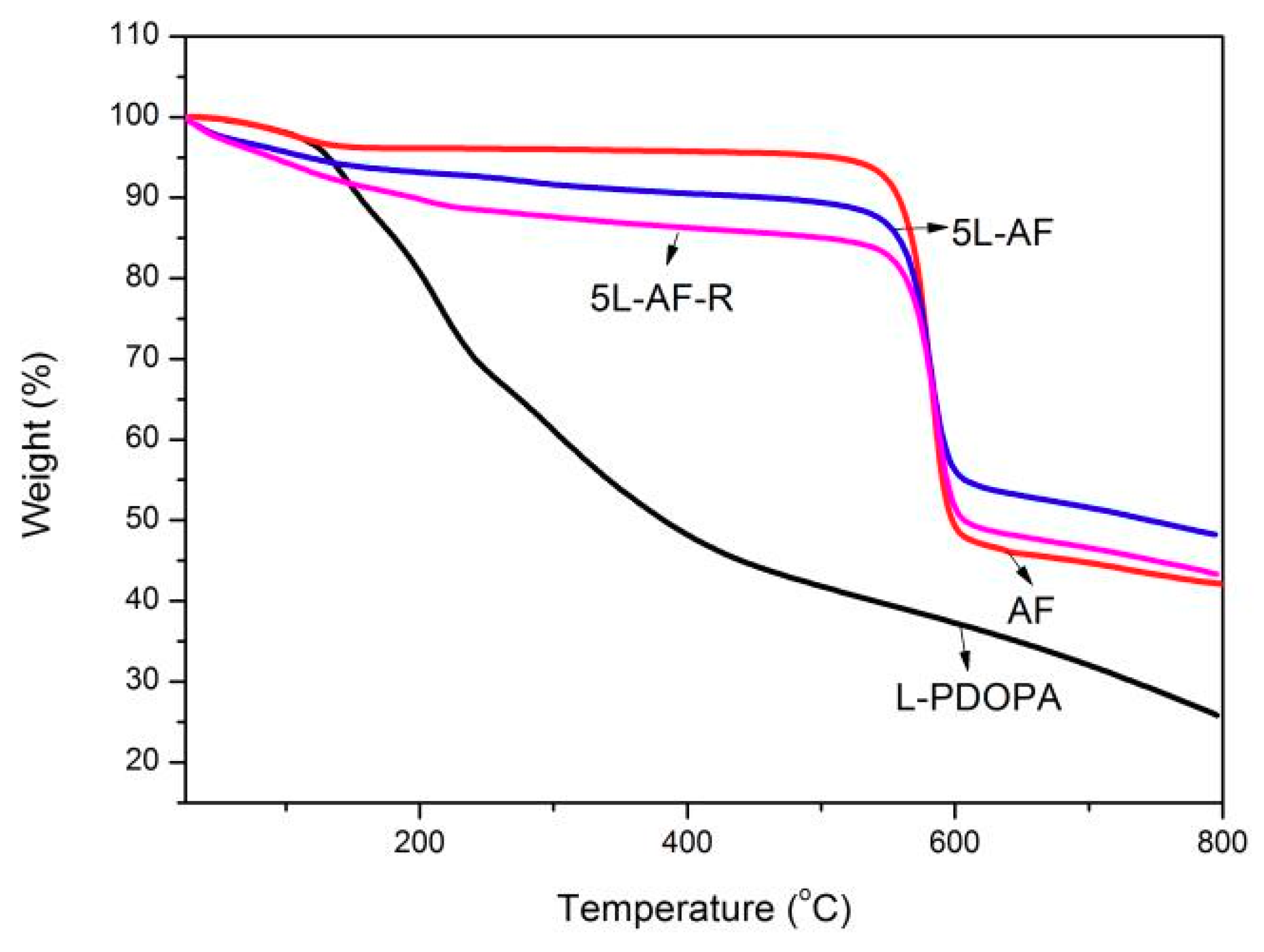
3.3. Thermogravimetric Analysis
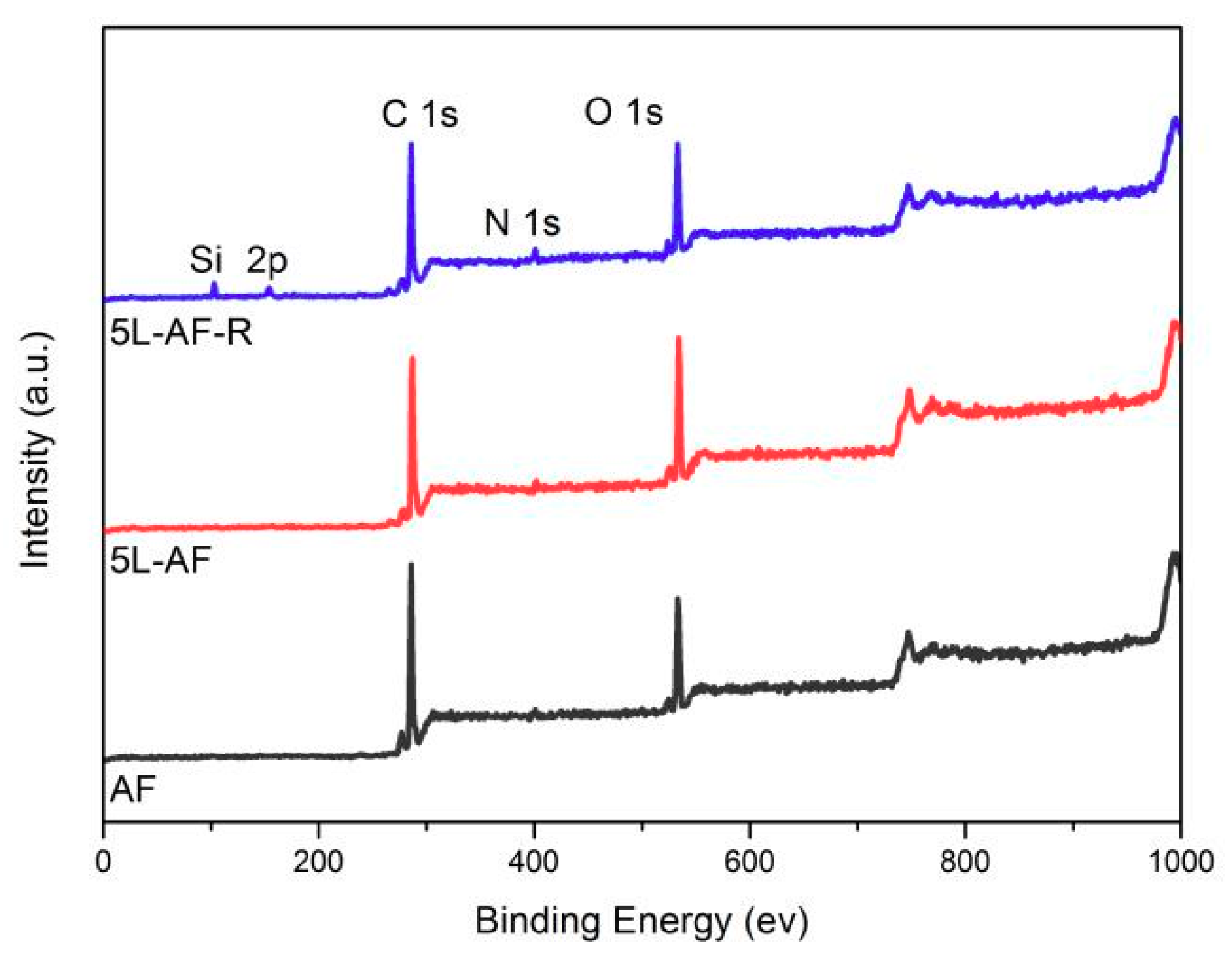
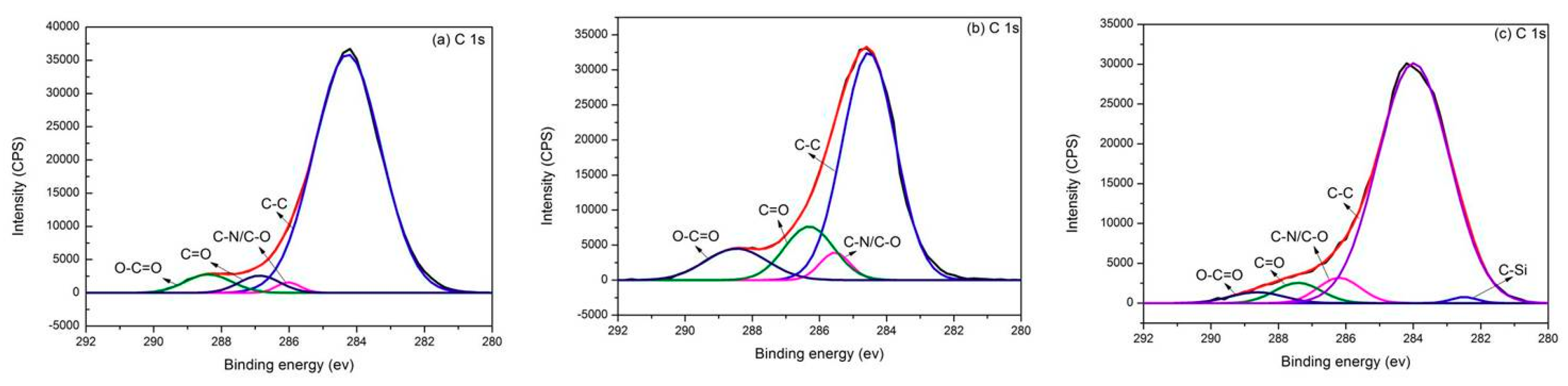
3.4. XPS Analysis
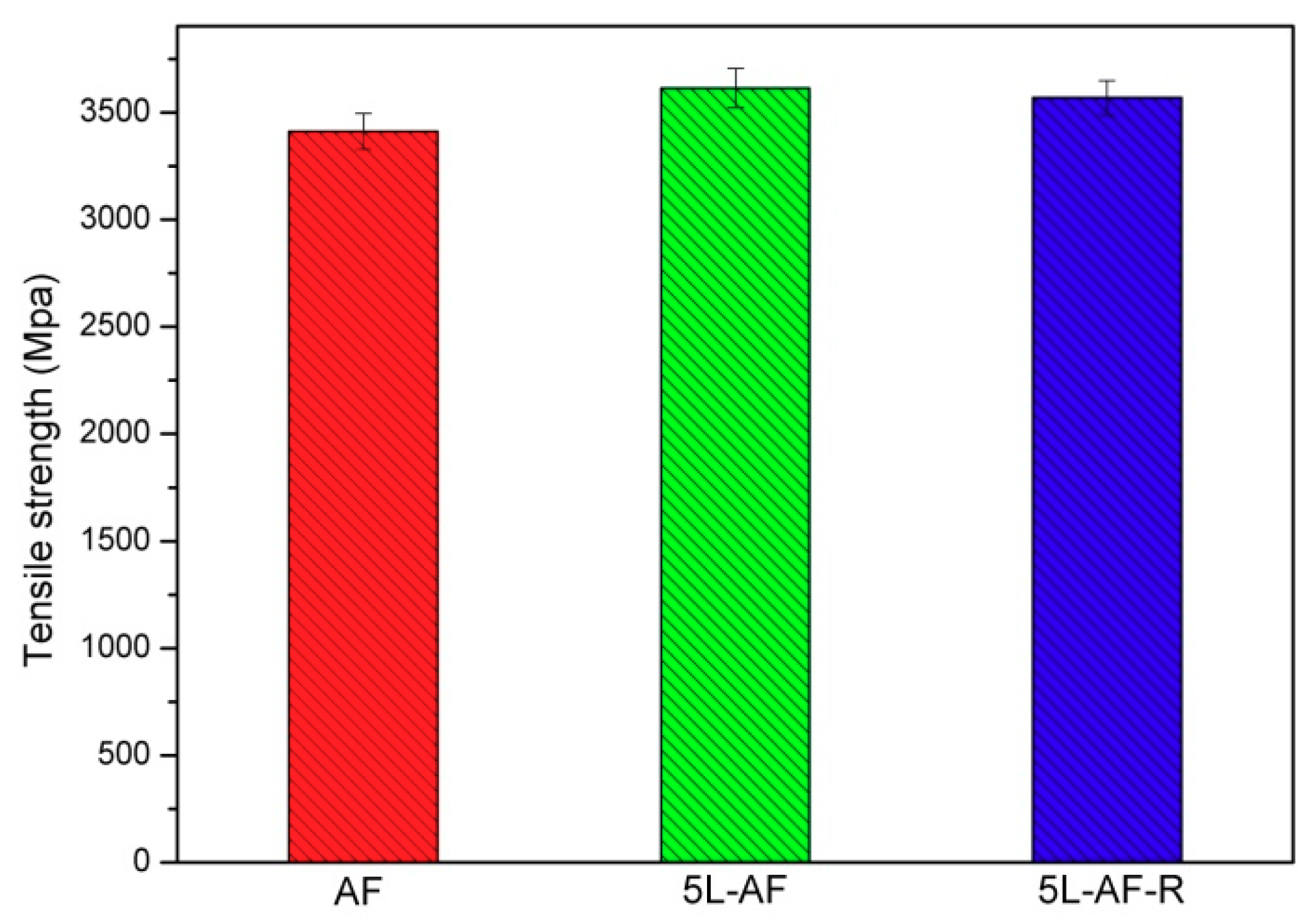
3.5. Single Fiber Tensile Strength of Aramid Fibers
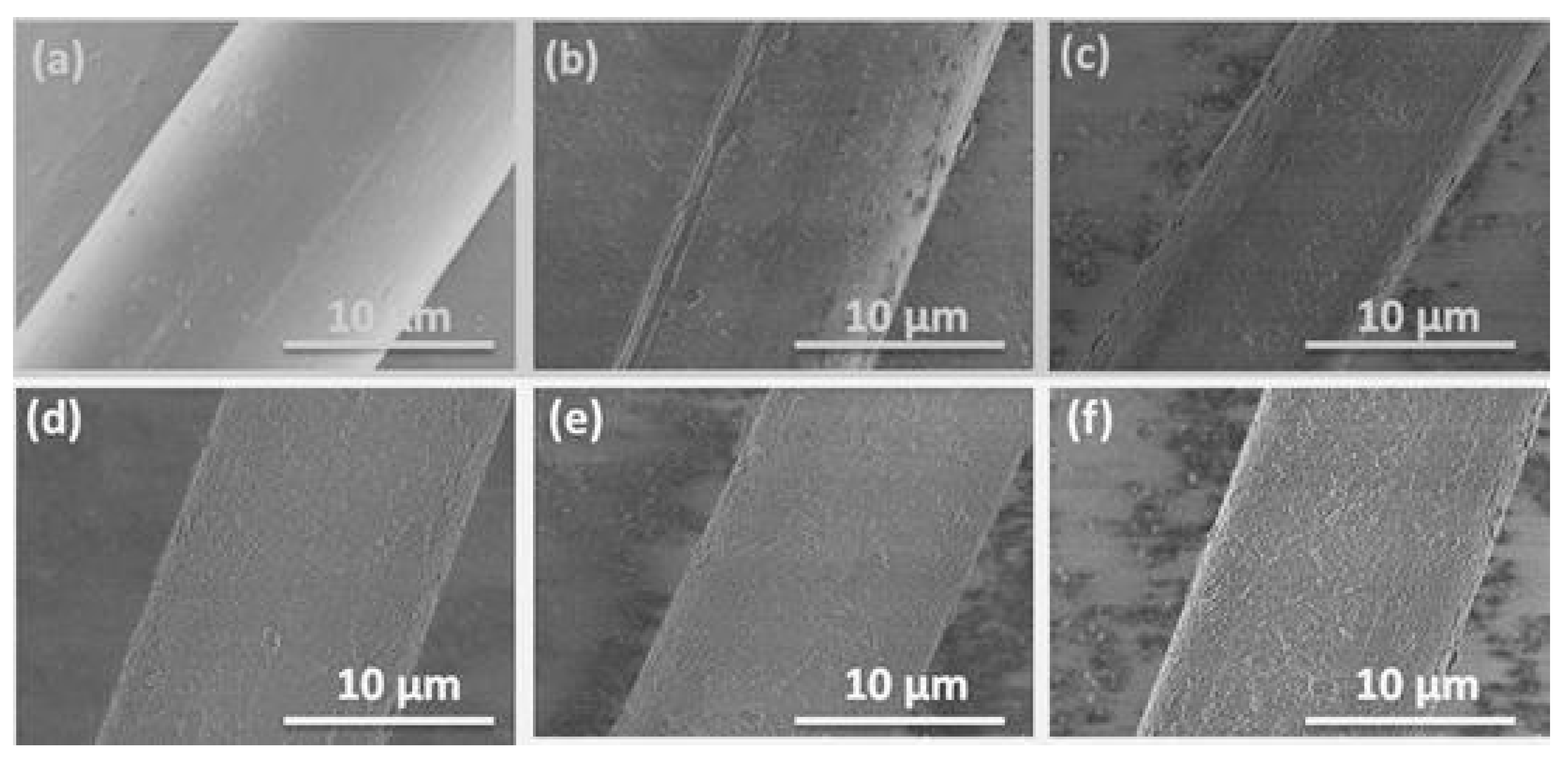
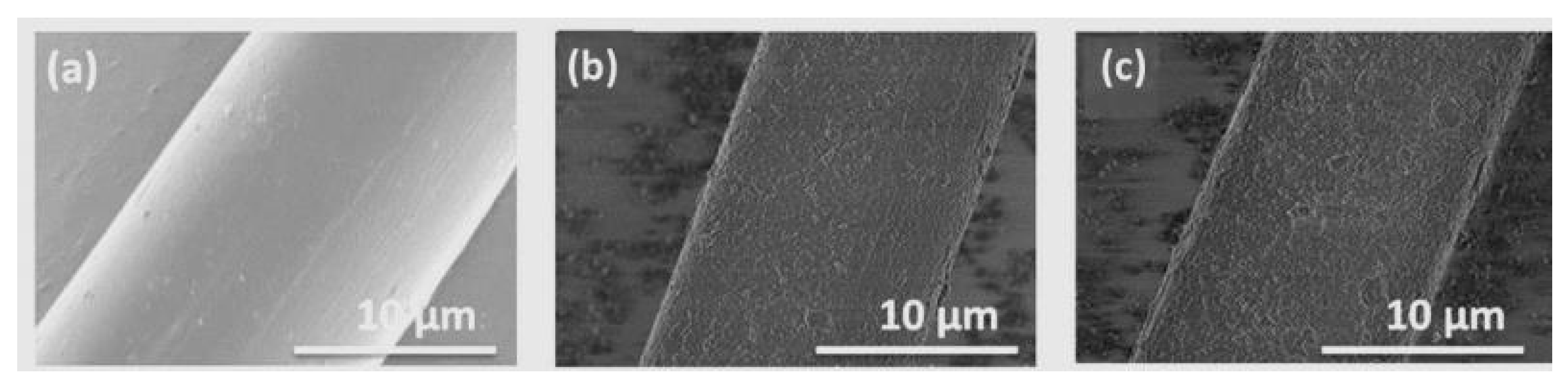
3.6. Surface Morphology of the Aramid Fibers
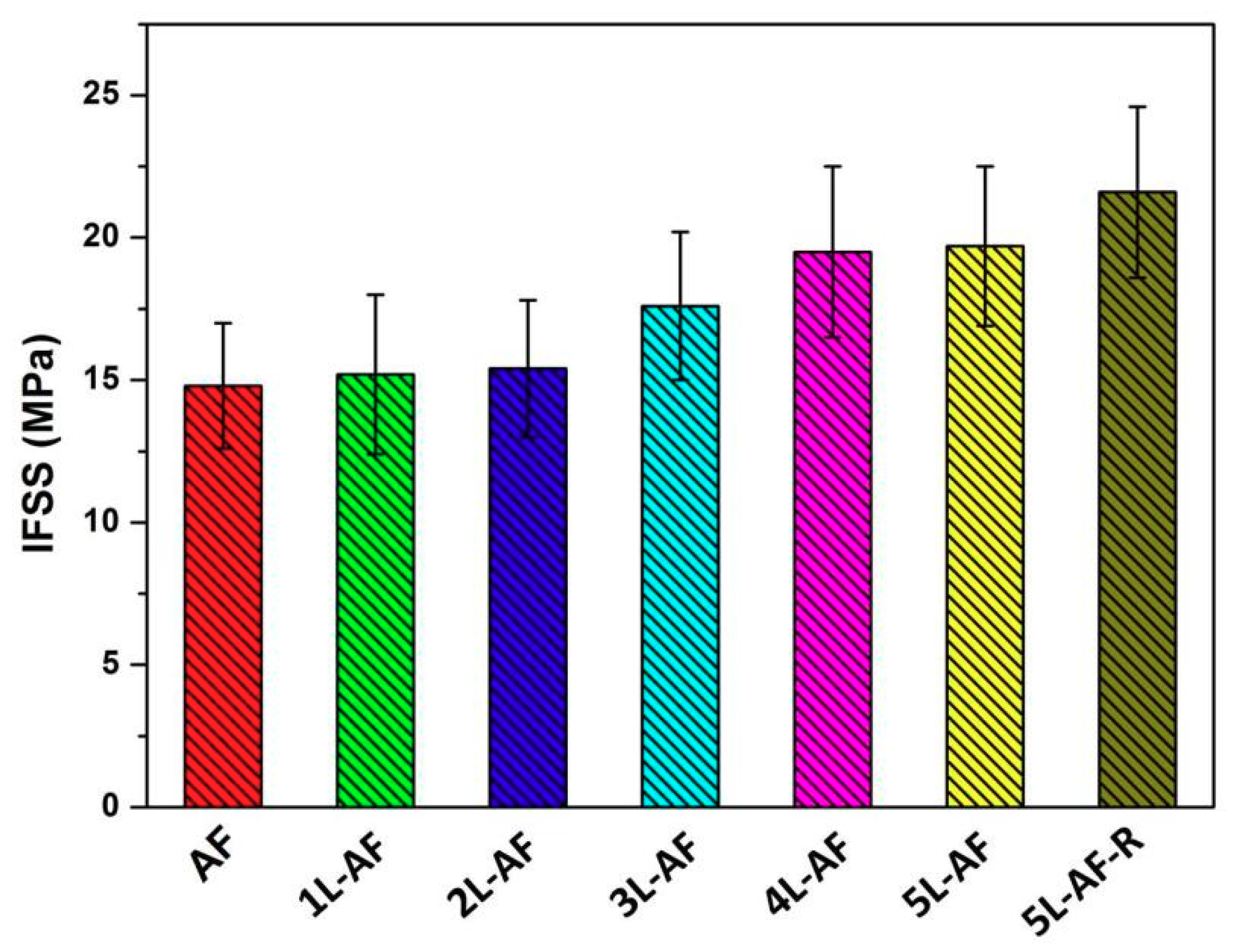
3.7. Interfacial Adhesion of Epoxy Resin to Aramid Fibers
3.8. Interlaminar Shear Strength (ILSS) of the Aramid Fibers Reinforced Epoxy Resin Composites
3.9. Fracture Morphology of the Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, M.L.; Kong, H.J.; Zhang, K.; Teng, C.Q.; Yu, M.H.; Liao, Y.Z. Simple synthesis of hydroxyl and ethylene functionalized aromatic polyamides as sizing agents to improve adhesion properties of aramid fiber/vinyl epoxy composites. Polymers 2017, 9, 143. [Google Scholar] [CrossRef]
- Guan, Y.A.; Zheng, Y.J.; Cui, J.X.; Wan, X.H. Synthesis and characterization of graft copolymers based on poly (p-phenylene terphthalamide) backbone and well-defined polystyrene side chains. Chin. J. Polym. Sci. 2010, 28, 257–267. [Google Scholar] [CrossRef]
- Du, S.M.; Zhang, J.; Guan, Y.; Wan, X.H. Sequence effects on properties of the poly(p-phenylene terphthalamide)-based macroinitiators and their comb-like copolymers grafted by polystyrene side chains. Aust. J. Chem. 2013, 67, 39–48. [Google Scholar] [CrossRef]
- Su, M.; Gu, A.; Liang, G.; Li, Y. The effect of oxygen-plasma treatment on Kevlar fibers and the properties of Kevlar fibers/bismaleimide composites. Appl. Surf. Sci. 2011, 257, 3158–3167. [Google Scholar] [CrossRef]
- Kong, H.J.; Yang, P.; Teng, C.Q.; Yu, M.H. Surface modification of poly(p-phenylene terphthalamide) fibers with HDI assisted by supercritical carbon dioxide. RSC Adv. 2012, 258, 10168–10174. [Google Scholar] [CrossRef]
- Majumdar, A.; Butola, B.S.; Srivastava, A. Development of soft composites materials with improved impact resistance using Kevlar fabric and nano-silica based shear thickening fluid. Mater. Des. 2014, 54, 295–300. [Google Scholar] [CrossRef]
- Day, R.J.; Hewson, K.D.; Lovell, P.A. Surface modification and its effect on the interfacial properties of model aramid-fiber/epoxy composites. Compos. Sci. Technol. 2002, 62, 153–166. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, M.K.; Ma, T.J.; Lee, D.R. Effect of chemical treatment of Kevlar fibers on mechanical interfacial properties of composites. J. Colloid Interface Sci. 2002, 252, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Zhao, J.; Zhang, Y.; Guo, Z. Grafting modification of Kevlar fiber using horseradish peroxidase. Polym. Bull. 2006, 56, 507–515. [Google Scholar] [CrossRef]
- Gu, H. Tensile behaviours of quartz, aramid and glass filaments after NaCl treatment. Mater. Des. 2009, 30, 867–870. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.D.; Zhang, Z.Q.; Jiang, X.X.; Wu, L.N. Ultrasonic treatment of aramid fiber surface and its effect on the interface of aramid/epoxy composites. Appl. Surf. Sci. 2008, 28, 2594–2599. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Huang, Y.D.; Liu, L.; Wu, L. Surface modification of aramid fibers with ray radiation for improving interfacial bonding strength with epoxy resin. J. Appl. Polym. Sci. 2007, 2251–2262. [Google Scholar] [CrossRef]
- Xi, M.; Li, Y.L.; Shang, S.Y.; Li, D.H.; Yin, Y.X.; Dai, X.Y. Surface modification of aramid fiber by air DBD plasma at atmospheric pressure with continuous on-line processing. Surf. Coat. Technol. 2008, 202, 6029–6033. [Google Scholar] [CrossRef]
- Jia, C.X.; Chen, P.; Liu, W.; Lin, B.; Wang, Q. Surface treatment of aramid fiber by air dielectric barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2011, 257, 4265–4270. [Google Scholar] [CrossRef]
- Li, S.; Han, K.Q.; Rong, H.P.; Li, X.Z.; Yu, M.H. Surface modification of aramid fibers via ammonia-plasma treatment. J. Appl. Polym. Sci. 2014, 40250, 1–6. [Google Scholar] [CrossRef]
- Wang, C.X.; Du, M.; Lv, J.C.; Zhou, Q.Q.; Ren, Y.; Liu, G.L.; Gao, D.W.; Jin, L.M. Surface modification of aramid fiber by plasma induced vapor phase graft polymerization of acrylic acid. I. Influence of plasma conditions. Appl. Surf. Sci. 2015, 349, 333–342. [Google Scholar] [CrossRef]
- Gu, R.X.; Yu, J.R.; Hu, C.C.; Chen, L.; Zhu, J.; Hu, Z.M. Surface treatment of para-aramid fiber by argon dielectric barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2012, 258, 10168–10174. [Google Scholar] [CrossRef]
- Shirazi, M.; Rooij, M.B.; Talma, A.G.; Noordermeer, K.W.M. Adhesion of RFL-coating aramid fibers to elastomers: The role of elastomer-latex compatibility. J. Adhes. Sci. Technol. 2013, 27, 1886–1898. [Google Scholar] [CrossRef]
- Chen, J.R.; Zhu, Y.F.; Ni, Q.Q.; Fu, Y.Q.; Fu, X. Surface modification and characrterization of aramid fibers with hybrid coating. Appl. Surf. Sci. 2014, 321, 103–108. [Google Scholar] [CrossRef]
- Du, S.M.; Wang, W.B.; Yan, Y.; Zhang, J.; Tian, M.; Zhang, L.Q.; Wan, X.H. A facile synthetic route to poly(p-phenylene terephthalamide) with dual functional groups. Chem. Commun. 2014, 50, 9929–9931. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Qi, X.; Guan, Y.; Zhang, F.; Zhang, J.; Yan, C.; Zhu, Y.D.; Wan, X.H. Synthesis and properties of poly(p-phenylene terephthalamide) bearing both polar and unsaturated substituents introduced via claisen rearrangement reaction. J. Polym. Sci. Part. A Polym. Chem. 2016, 54, 2050–2059. [Google Scholar] [CrossRef]
- Zhou, L.F.; Yuan, L.; Guan, Q.B.; Gu, A.J.; Liang, G.Z. Building unique surface structure on aramid fibers through a green layer-by-layer self-assembly technique to develop new high performance fibers with greatly improved surface activity, thermal resistance, mechanical properties and UV resistance. Appl. Surf. Sci. 2017, 411, 34–45. [Google Scholar] [CrossRef]
- Xu, J.; Yang, L.; Hu, X.; Xu, S.; Wang, J.; Feng, S. The effect of polysaccharide types on adsorption properties of LbL assembled multilayer films. Soft Matter. 2015, 11, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.T.; Huang, H.T.; Du, S.; Huo, Y.D.; He, J.X.; Cui, S.Z. Removal of copper ions from aqueous solution by adsorption onto novel polyelectrolyte film-coated nanofibrous silk fibroin non-wovens. Appl. Surf. Sci. 2015, 345, 169–174. [Google Scholar] [CrossRef]
- Khan, F.; Liu, P.P.; Xu, F.J.; Ma, Y.; Qiu, Y.P. Dye aggregation in layer-by-layer dyeing of cotton fabrics. RSC Adv. 2016, 6, 20286–20293. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Fan, X.R. Layer-by-layer self-assembly of TiO2 sol on wool to improve its anti-ultraviolet and anti-ageing properties. J. Sol.-Gel Sci. Technol. 2012, 62, 338–343. [Google Scholar] [CrossRef]
- Uğur, Ş.S.; Sarııšık, M.; Aktaş, A.H. Nano-TiO2 based multilayer film deposition on cotton fabrics for UV-protection. Fibers Polym. 2011, 12, 190–196. [Google Scholar] [CrossRef]
- Uğur, Ş.S.; Sarııšık, M.; Aktaş, A.H.; Uçar, M.Ç.; Erden, E. Modifying of cotton fabric surface with nano-ZnO multilayer films by layer-by-layer deposition method. Nanoscale Res. Lett. 2010, 5, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Uğur, Ş.S.; Sarııšık, M.; Aktaş, A.H. Nano-Al2O3 multilayer film deposition on cotton fabrics by layer-by-layer deposition method. Mater. Res. Bull. 2011, 46, 1202–1206. [Google Scholar] [CrossRef]
- Song, B.; Meng, L.H.; Huang, Y.D. Preparation and characterization of (POSS/TiO2)n multi-coatings based on PBO fibers surface for improvement of UV resistance. Fibers Polym. 2013, 14, 375–381. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, Y.X.; Chen, S.X.; Wang, W.C.; Tian, M.; Ning, N.Y.; Zhang, L.Q. Highly efficient mussel-like inspired modification of aramid fibers by UV-accelerated catechol/polyamine deposition followed chemical grafting for high-performance polymer composites. Chem. Eng. J. 2017, 314, 583–593. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.H.; Zhang, L.Q.; Wang, W.C.; Tian, M. Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl. Mater. Interf. 2014, 6, 21730–21738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ai, K.L.; Lu, L.H. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, X.K.; Yuan, W.C.; Brown, L.J.; Wang, D.Y. Confined flocculation of ionic pollutants by poly(L-dopa)-based polyelectrolyte complexes in hydrogel beads for three-dimensional, quantitative, efficient water decontamination. Langmuir 2015, 31, 6351–6366. [Google Scholar] [CrossRef] [PubMed]
- Sóvágó, I.; Kállay, C.; Várnagy, K. Peptides as complexing agents: Factors influencing the structure and thermodynamic stability of peptide complex. Coord. Chem. Rev. 2012, 256, 2225–2233. [Google Scholar] [CrossRef]
- Kirwan, K.J.; Fawell, P.D.; Bronswijk, W. In situ FTIR-ATR examination of poly (acrylic acid) adsorbed onto hematite at low PH. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Xiong, S.Q.; Wang, Y.; Zhu, J.; Yu, J.R.; Hu, Z.M. Mussel-adhesive-inspired fabrication of multifunctional silver nanoparticle assemble. Langmuir 2015, 31, 5504–5512. [Google Scholar] [CrossRef] [PubMed]




| Fiber | Crystallinity (%) | d-Spacing (Å) | |
|---|---|---|---|
| 110 | 200 | ||
| AF | 55.5 | 4.31 | 3.87 |
| 5L-AF | 49.8 | 4.38 | 3.94 |
| 5L-AF-R | 42.9 | 4.33 | 3.84 |
| Sample | Initial Decomposition Temperature (°C) | Maximum Decomposition Temperature (°C) | Residual Amount (%) |
|---|---|---|---|
| AF | 533 | 580 | 42.0 |
| 5L-AF | 528 | 575 | 48.2 |
| 5L-AF-R | 530 | 575 | 43.4 |
| Samples | Atomic Concentration (%) | Atomic Ratio | |||
|---|---|---|---|---|---|
| C | N | O | O/C | N/C | |
| AF | 78.4 | 1.2 | 20.4 | 0.26 | 0.015 |
| 5L-AF | 72.5 | 2.2 | 25.3 | 0.35 | 0.03 |
| 5L-AF-R | 71.2 | 2.9 | 20.5 | 0.29 | 0.04 |
| Samples | Content of Functional Groups (%) | |||
|---|---|---|---|---|
| C-C | C-N/C-O | C=O | O-C=O | |
| AF | 89.8 | 1.4 | 3.9 | 4.8 |
| 5L-AF | 69.7 | 5.0 | 15.8 | 9.5 |
| 5L-AF-R | 76.4 | 5.0 | 14.4 | 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, B.; Kong, H.; Yu, M.; Qin, M.; Teng, C. Layer-by-Layer Self-Assembly Strategy for Surface Modification of Aramid Fibers to Enhance Interfacial Adhesion to Epoxy Resin. Polymers 2018, 10, 820. https://doi.org/10.3390/polym10080820
Li Z, Liu B, Kong H, Yu M, Qin M, Teng C. Layer-by-Layer Self-Assembly Strategy for Surface Modification of Aramid Fibers to Enhance Interfacial Adhesion to Epoxy Resin. Polymers. 2018; 10(8):820. https://doi.org/10.3390/polym10080820
Chicago/Turabian StyleLi, Zhaomin, Baihua Liu, Haijuan Kong, Muhuo Yu, Minglin Qin, and Cuiqing Teng. 2018. "Layer-by-Layer Self-Assembly Strategy for Surface Modification of Aramid Fibers to Enhance Interfacial Adhesion to Epoxy Resin" Polymers 10, no. 8: 820. https://doi.org/10.3390/polym10080820
APA StyleLi, Z., Liu, B., Kong, H., Yu, M., Qin, M., & Teng, C. (2018). Layer-by-Layer Self-Assembly Strategy for Surface Modification of Aramid Fibers to Enhance Interfacial Adhesion to Epoxy Resin. Polymers, 10(8), 820. https://doi.org/10.3390/polym10080820





