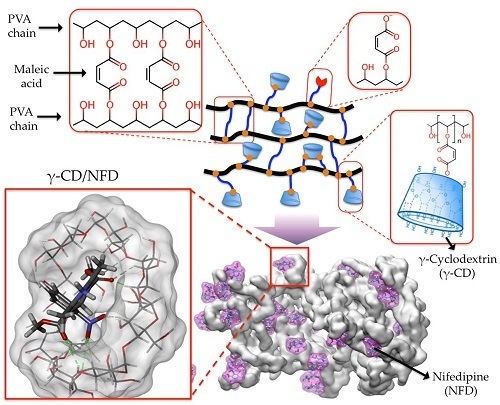
Rational Development of a Novel Hydrogel as a pH-Sensitive Controlled Release System for Nifedipine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Section
2.1.1. Designing and Building of the Molecular Structures
2.1.2. Inclusion Complexes (PVAchain-DA-γ-CD/NFD) Evaluation
2.1.3. Building of Hydrogel-NFD Systems and Molecular Dynamic (MD) Simulation Study
2.2. Experimental Section
2.2.1. Materials
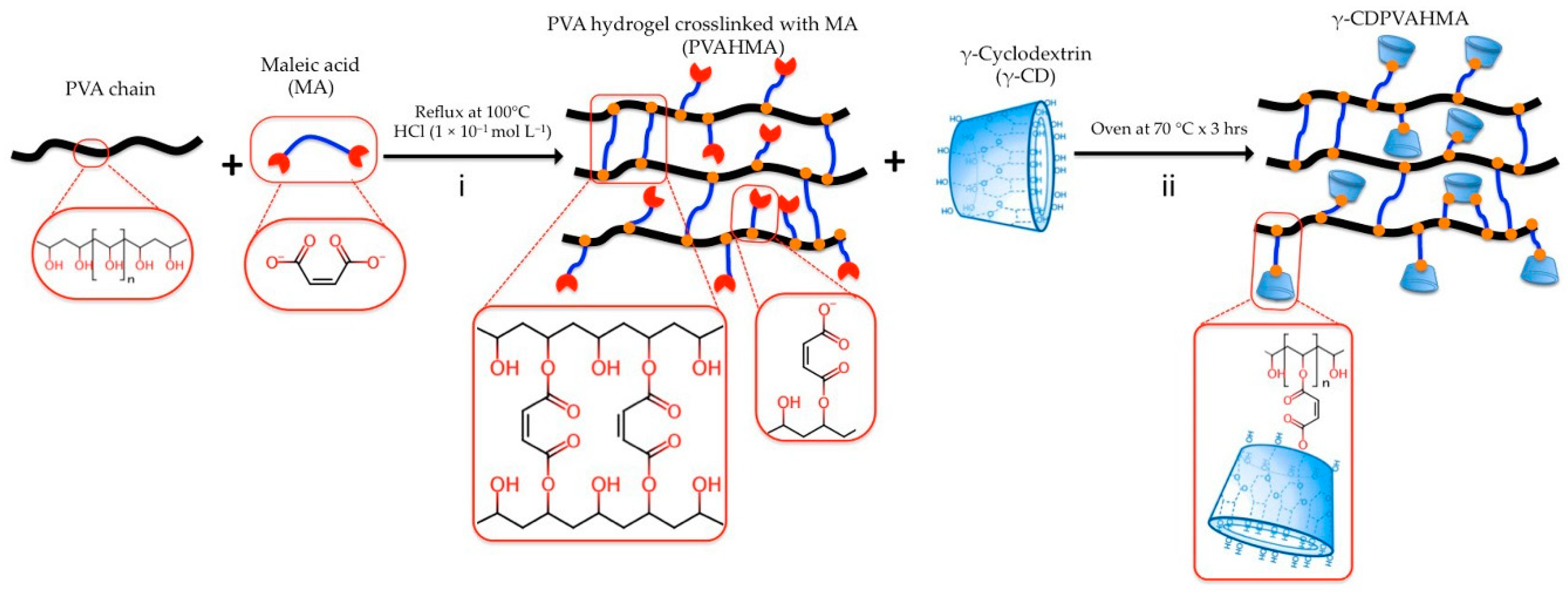
2.2.2. Synthesis of γ-CDPVAHMAs
2.2.3. Swelling Evaluation
2.2.4. Fourier-Transform Infrared (FT-IR) Study
2.2.5. Thermal Gravimetric Analysis (TGA)
2.2.6. NFD Loading in γ-CDPVAHMA1, γ-CDPVAHMA2, and γ-CDPVAHMA3
2.2.7. Drug Release Evaluation of γ-CDPVAHMA1, γ-CDPVAHMA2 and γ-CDPVAHMA3
2.2.8. Cell Viability Assay
2.2.9. Statistical Analysis
3. Results and Discussions
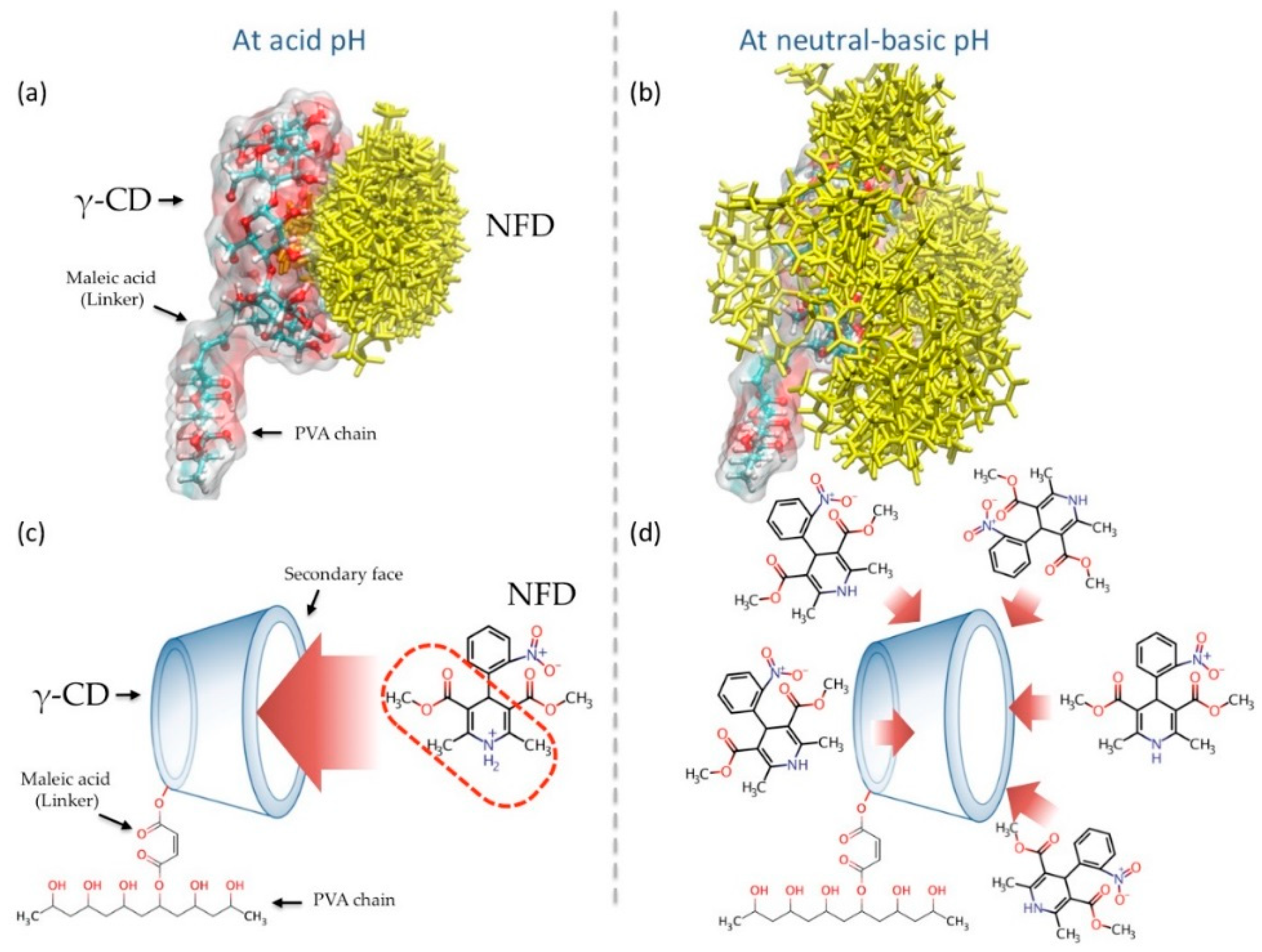
3.1. Inclusion Complexes (γ-CD/NFD) Study Through Interaction Energies Calculations
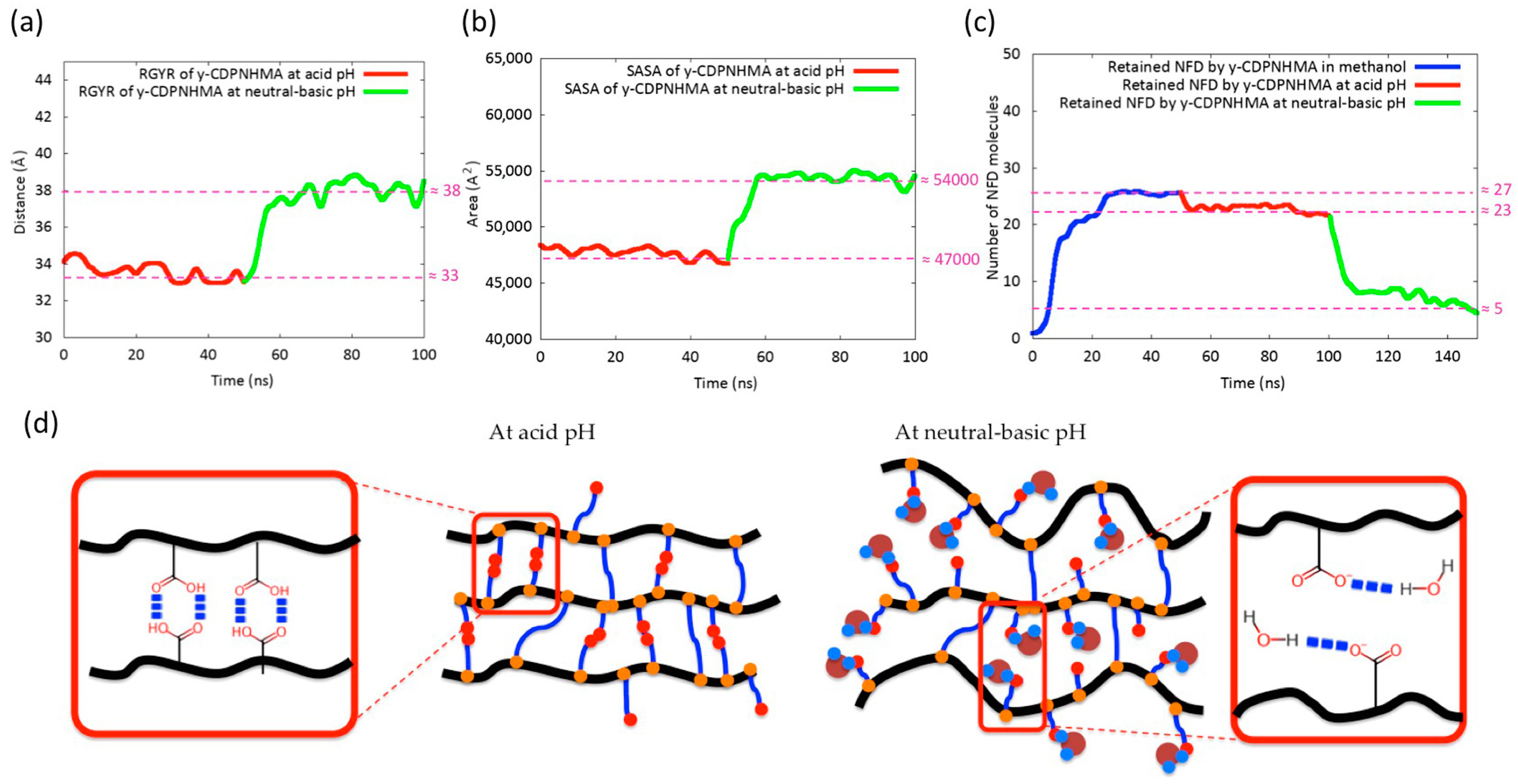
3.2. Molecular Dynamics (MD) Simulations Studies
3.3. Preparation of γ-CDPVAHMAs
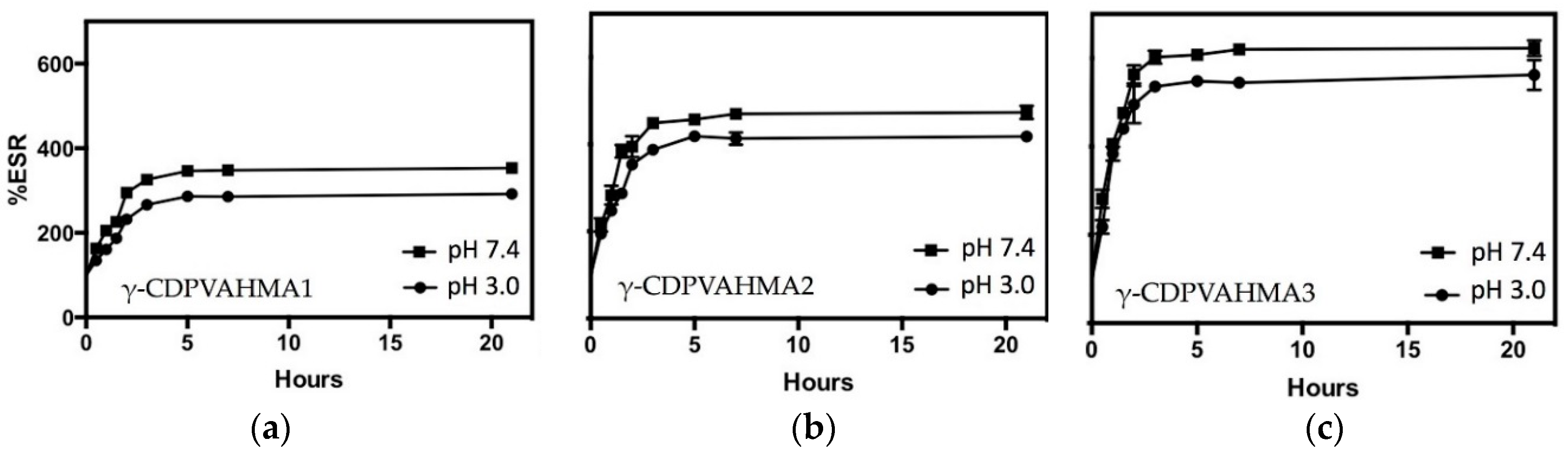
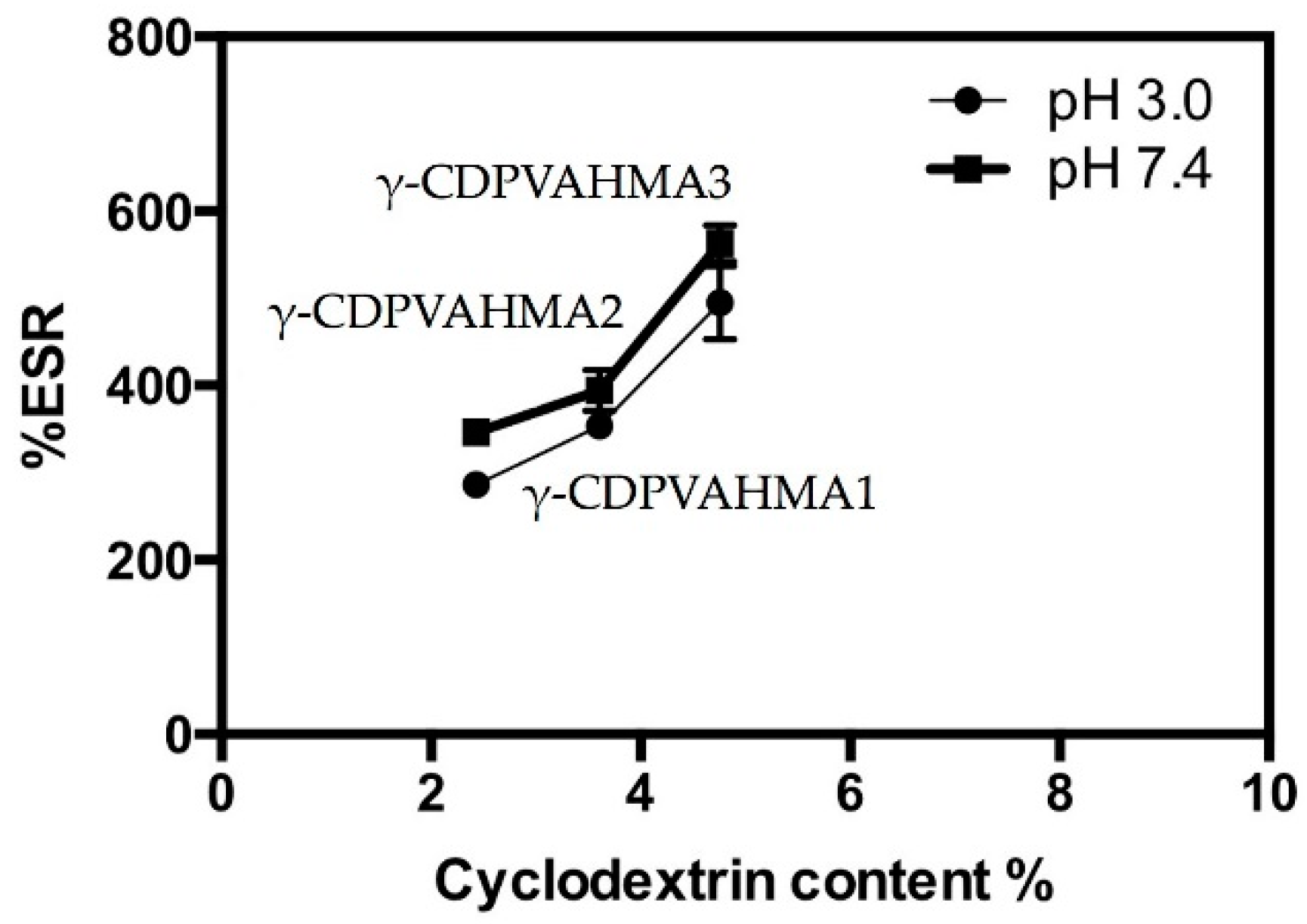
3.4. ESR Results
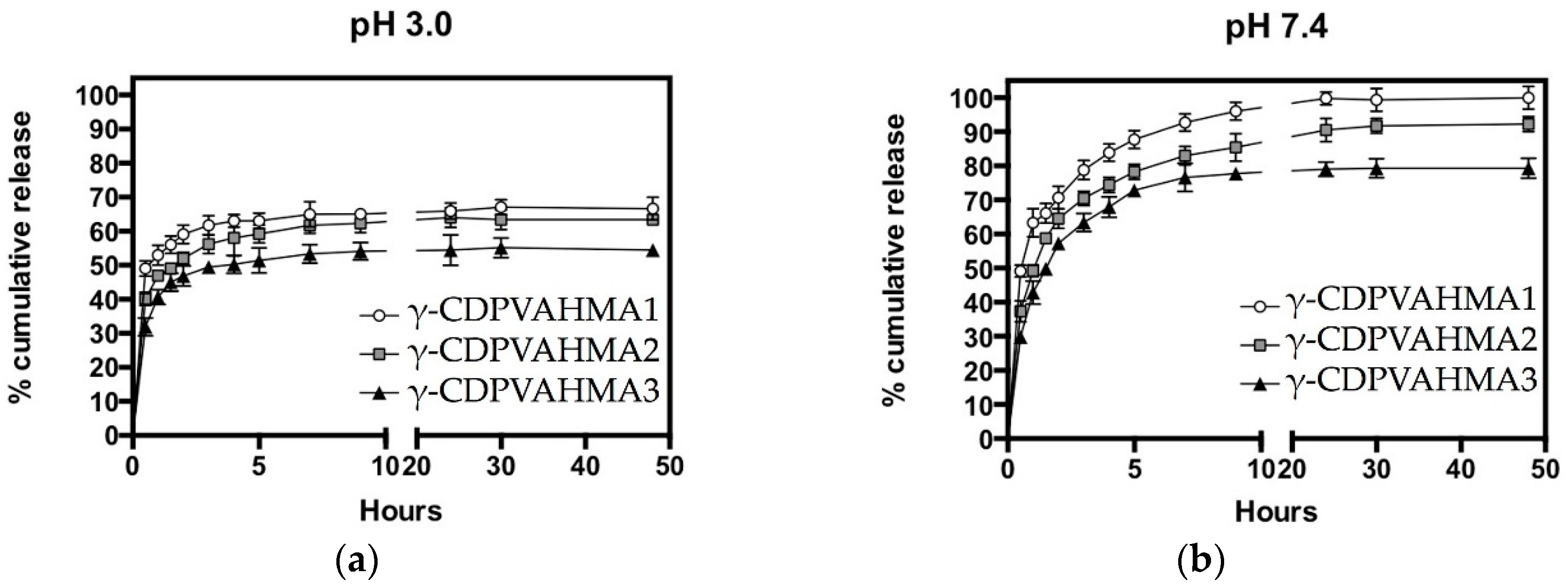
3.5. NFD Loading and In Vitro Release Behavior of γ-CDPVAHMAs
3.6. Photograph Analysis: Sample Preparation and Viewing
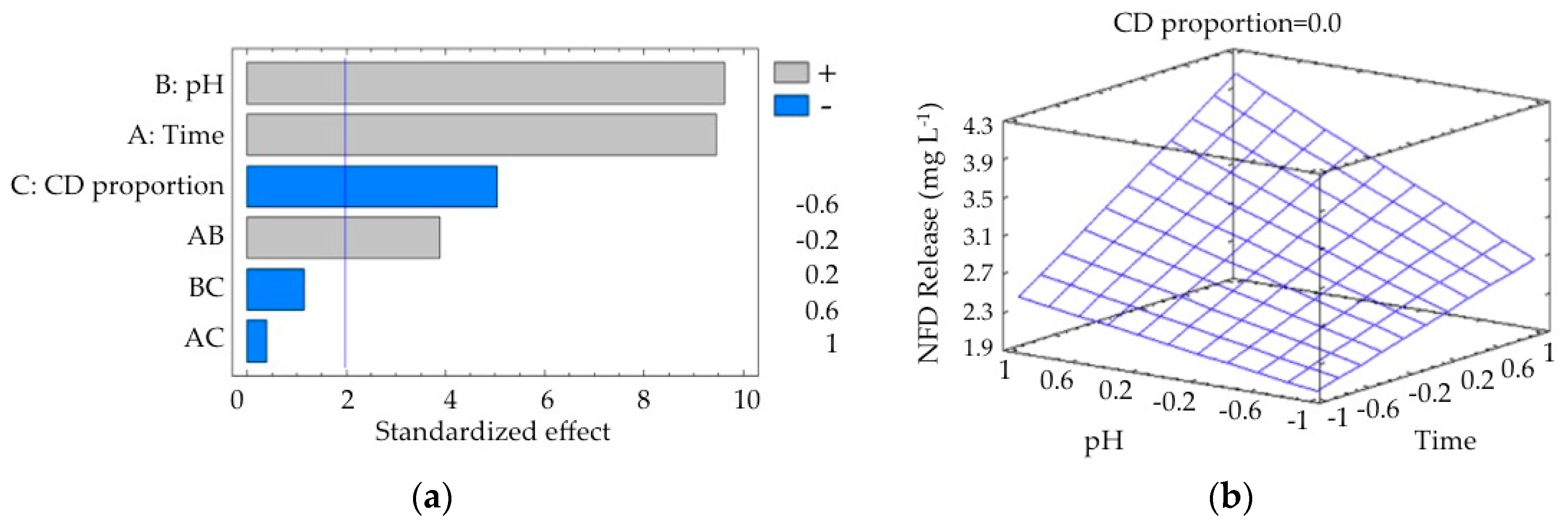
3.7. Statistical Analysis for Release of NFD by γ-CDPVAHMAs
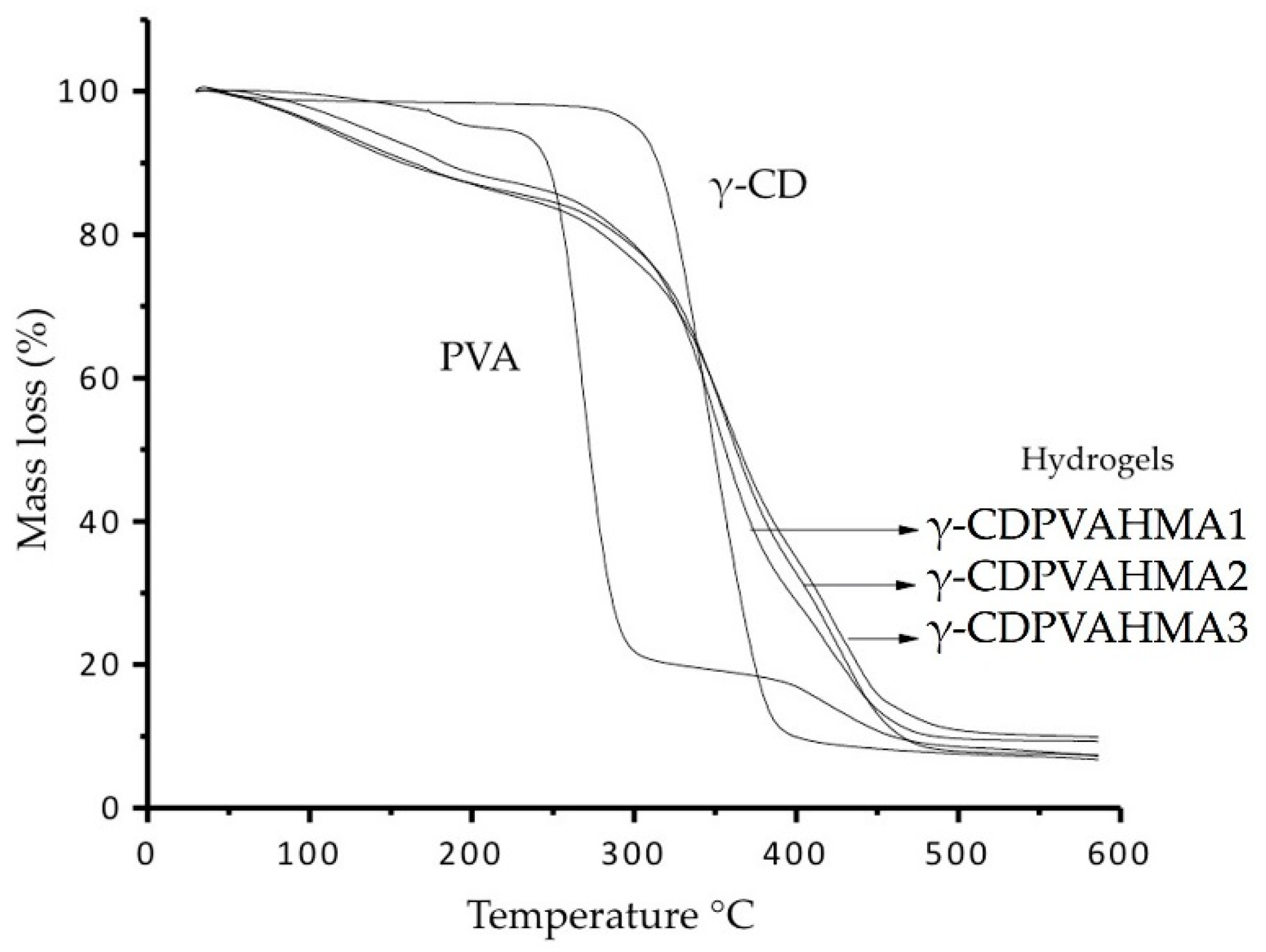
3.8. Thermogravimetric Analysis Results
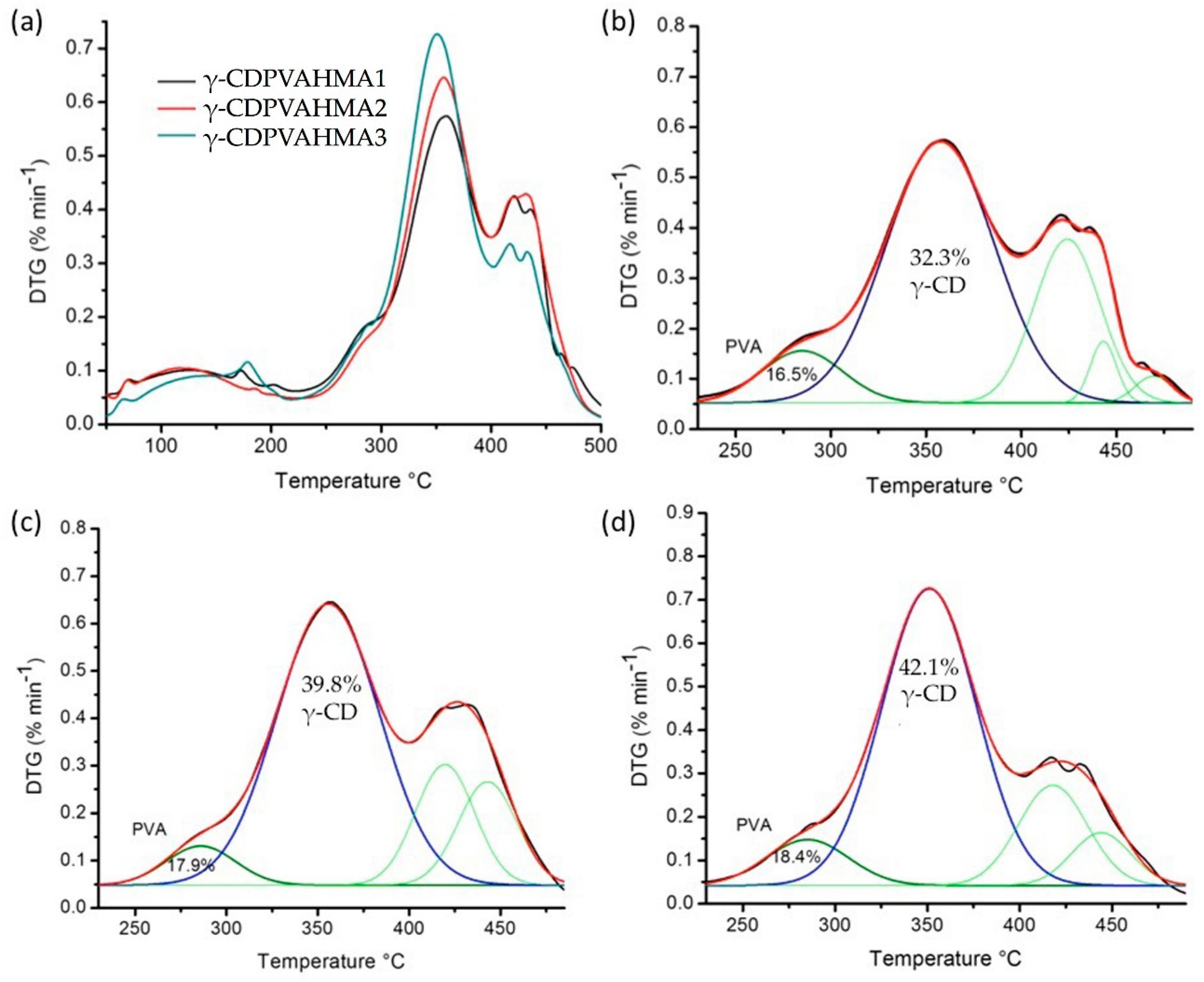
3.9. DTG Curves and Deconvolution Analysis
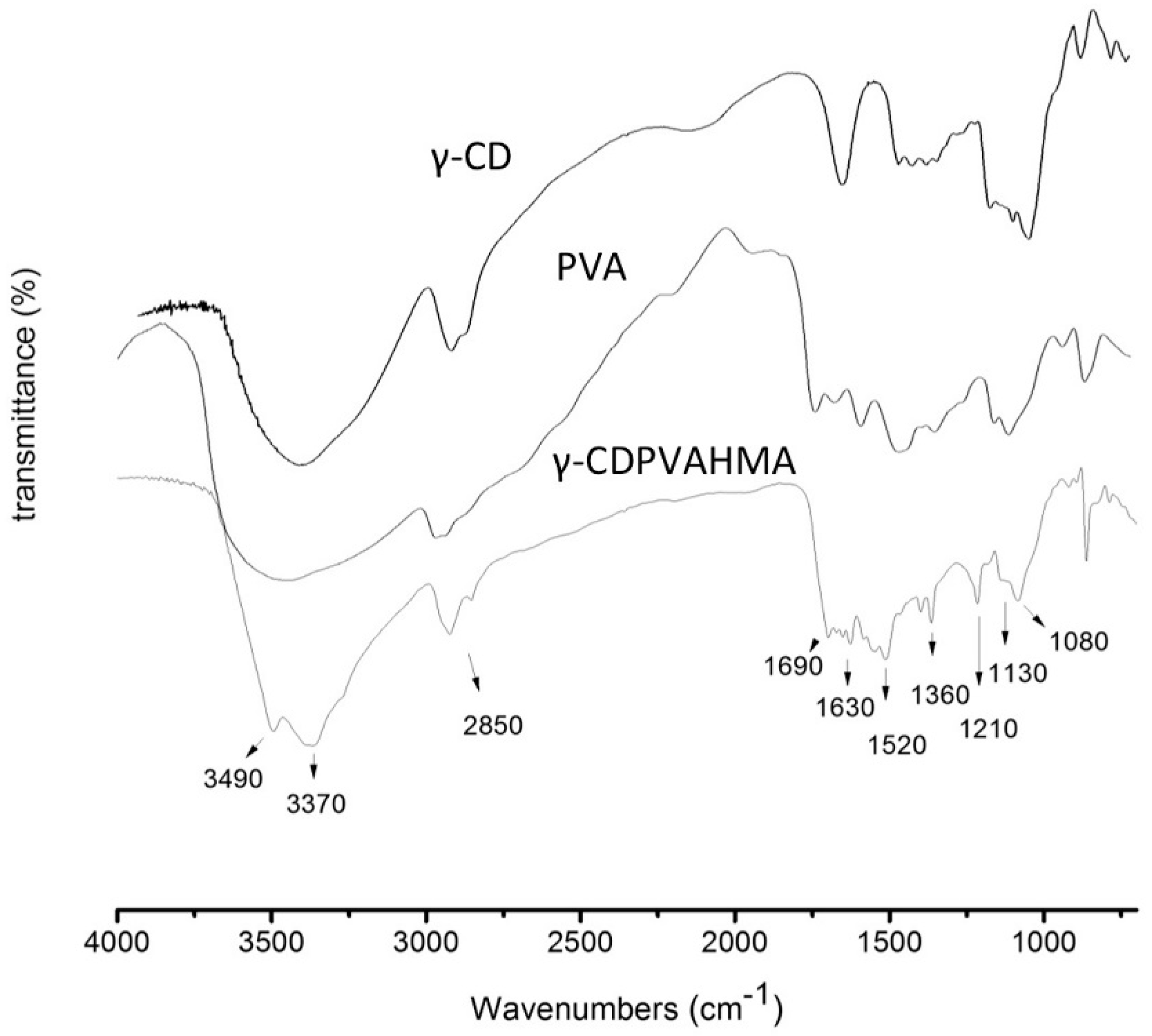
3.10. FT-IR Results
3.11. Evaluation of γ-CDPVAHMAs Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Babu, V.R.; Sairam, M.; Hosamani, K.M.; Aminabhavi, T.M. Preparation of sodium alginate–methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr. Polym. 2007, 69, 241–250. [Google Scholar] [CrossRef]
- Derakhshandeh, K.; Soleymani, M. Formulation and in vitro evaluation of nifedipinecontrolled release tablet: Influence of combination of hydrophylic and hydrophobic matrix forms. Asian J. Pharm. 2014, 4, 185–193. [Google Scholar] [CrossRef]
- Minami, J.; Numabe, A.; Andoh, N.; Kobayashi, N.; Horinaka, S.; Ishimitsu, T.; Matsuoka, H. Comparison of once-daily nifedipine controlled-release with twice-daily nifedipine retard in the treatment of essential hypertension. Br. J. Clin. Pharmacol. 2004, 57, 632–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Center for Biotechnology Information. PubChem Compound Database. CID = 4485. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4485 (accessed on 4 May 2018).
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Schanuel, F.S.; Santos, K.S.R.; Monte-Alto-Costa, A.; de Oliveira, M.G. Combined Nitric Oxide-releasing Poly(vinyl alcohol) Film/F127 Hydrogel for Accelerating Wound Healing. Colloids Surf. B Biointerfaces 2015, 130, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Mellace, S.; Pellegrino, M.; Ricchio, E.; Mauro, L.; Andò, S.; Picci, N.; Trombino, S. Biocompatible Targeting Hydrogels for Breast Cancer Treatment. Mini Rev. Med. Chem. 2016, 16, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Mellati, A.; Dai, S.; Bi, J.; Jina, B.; Zhang, H. A biodegradable thermosensitive hydrogel with tuneable properties for mimicking three-dimensional microenvironments of stem cells. RSC Adv. 2014, 4, 63951–63961. [Google Scholar] [CrossRef]
- Bordi, F.; Paradossi, G.; Rinaldi, C.; Ruzicka, B. Chemical and Physical Hydrogels: Two Casesystems Studied by Quasi Elastic Light Scattering. Phys. A 2002, 304, 119–128. [Google Scholar] [CrossRef]
- Valdes, O.; Avila-Salas, F.; Marican, A.; Fuentealba, N.; Villaseñor, J.; Arenas-Salinas, M.; Argandoña, Y.; Durán-Lara, E.F. Methamidophos Removal from Aqueous Solutions Using a Super Adsorbent Based on Crosslinked Poly(vinyl alcohol) Hydrogel. J. Appl. Polym. Sci. 2017, 135. [Google Scholar] [CrossRef]
- Yin, L.; Fei, L.; Tang, C.; Yin, C. Synthesis, Characterization, Mechanical Properties and Biocompatibility of Interpenetrating Polymer Network–super-porous Hydrogel Containing Sodium Alginate. Polym. Int. 2007, 56, 1563–1571. [Google Scholar] [CrossRef]
- Qinyuan, C.; Yang, J.; Xinjun, Y. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Gupta, N.V.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J. Pharm. Res. 2012, 11, 481–493. [Google Scholar] [PubMed]
- Ninawe, P.R.; Parulekar, S.J. Drug Loading into and Drug Release from pH- and Temperature-Responsive Cylindrical Hydrogels. Biotechnol. Prog. 2011, 27, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Sloand, J.N.; Gaffey, A.C.; Venkataraman, C.M.; Wang, Z.; Trubelja, A.; Hammer, D.A.; Atluri, P.; Burdick, J.A. Injectable, Guest–Host Assembled Polyethylenimine Hydrogel for siRNA Delivery. Biomacromolecules 2017, 18, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Marican, A.; Avila-Salas, F.; Valdés, O.; Wehinger, S.; Villaseñor, J.; Fuentealba, N.; Arenas-Salinas, M.; Argandoña, Y.; Carrasco-Sánchez, V.; Durán-Lara, E.F. Rational Design, Synthesis and Evaluation of γ-CD-Containing Cross-Linked Polyvinyl Alcohol Hydrogel as a Prednisone Delivery Platform. Pharmaceutics 2018, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, E13. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.; Hong, B.; Nam, S.Y.; Jung, S.Y.; Rhim, J.W.; Lee, S.B.; Moon, G.Y. Swelling Behavior and Drug Release of Poly(vinyl alcohol) Hydrogel Cross-Linked with Poly(acrylic acid). Macromol. Res. 2008, 16, 189–193. [Google Scholar] [CrossRef]
- Escobar-Sierra, D.M.; Perea-Mesa, Y.P. Manufacturing and Evaluation of Chitosan, PVA and Aloe Vera hydrogels for Skin Applications. DYNA 2017, 84, 134–142. [Google Scholar] [CrossRef]
- Oliveira, R.N.; McGuinness, G.B.; Ramos, M.E.; Kajiyama, C.E.; Thiré, R.M. Properties of PVA Hydrogel Wound-Care Dressings Containing UK Propolis. Macromol. Symp. 2016, 368, 122–127. [Google Scholar] [CrossRef]
- Chen, P.H.; Kuo, T.Y.; Liu, F.H.; Hwang, Y.H.; Ho, M.H.; Wang, D.M.; Lai, J.Y.; Hsieh, H.J. Use of Dicarboxylic Acids to Improve and Diversify the Material Properties of Porous Chitosan Membranes. J. Agric. Food Chem. 2008, 56, 9015–9021. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Teshirogi, T. Cyclodextrin-grafted Chitosan Hydrogels for Controlled Drug Delivery. Int. J. Biol. Macromol. 2015, 72, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Engelke, A.; Wenz, G. Solubilizing Steroidal Drugs by β-cyclodextrin Derivatives. Int. J. Pharm. 2017, 531, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- ChemAxon Ltd. MarvinSketch Program Version 17.29 (For OSX); ChemAxon Ltd.: Budapest, Hungary, 2018; Available online: https://chemaxon.com/products/marvin (accessed on 10 January 2018).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.; Burant, J. Gaussian 16, Revision, A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Fan, C.F.; Olafson, B.D.; Blanco, M. Application of molecular simulation to derive phase diagrams of binary mixtures. Macromolecules 1992, 25, 3667–3676. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Avila-Salas, F.; Sandoval, C.; Caballero, J.; Guiñez-Molinos, S.; Santos, L.S.; Cachau, R.E.; González-Nilo, F.D. Study of Interaction Energies Between the PAMAM Dendrimer and Nonsteroidal Anti-inflammatory Drug Using a Distributed Computational Strategy and Experimental Analysis by ESI-MS/MS. J. Phys. Chem. B 2012, 116, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Avila-Salas, F.; Santos, L.S.; Iturmendi, N.; Moine, V.; Cheynier, V.; Saucier, C. Rosé Wine Fining Using Polyvinylpolypyrrolidone: Colorimetry, Targeted Polyphenomics, and Molecular Dynamics Simulations. J. Agric. Food Chem. 2017, 65, 10591–10597. [Google Scholar] [CrossRef] [PubMed]
- Avila-Salas, F.; Marican, A.; Villaseñor, J.; Arenas-Salinas, M.; Argandoña, Y.; Caballero, J.; Durán-Lara, E.F. In-Silico Design, Synthesis and Evaluation of a Nanostructured Hydrogel as a Dimethoate Removal Agent. Manomaterials 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. MOPAC2016; Version 16.111L (LINUX); Stewart Computational Chemistry (SCC): Colorado Springs, CO, USA, 2016; Available online: http://openmopac.net/downloads.html (accessed on 21 March 2018).
- Case, D.A.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Greene, D.; Homeyer, N.; et al. AMBER 2017; University of California: San Francisco, CA, USA, 2017; Available online: http://ambermd.org/#AmberTools (accessed on 28 April 2018).
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. Software News and Update Packmol: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- DE Shaw Research. Schrödinger Release: Desmond/Maestro, Molecular Dynamics System, Release 2017-4, Maestro Version 11.4.011; DE Shaw Research: New York, NY, USA, 2017. [Google Scholar]
- Han, M.; Chen, P.; Yang, X. Molecular Dynamics Simulation of PAMAM Dendrimer in Aqueous Solution. Polymer 2015, 46, 3481–3488. [Google Scholar] [CrossRef]
- Vergara-Jaque, A.; Comer, J.; Monsalve, L.; González-Nilo, F.D.; Sandoval, C. Computationally Efficient Methodology for Atomic-level Characterization of Dendrimer-Drug Complexes: A Comparison of Amine-and Acetyl-terminated PAMAM. J. Phys. Chem. B 2013, 117, 6801–6813. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C. Gnuplot 5.2: An Interactive Plotting Program, Official Gnuplot Documentation, 2018. Available online: http://www.gnuplot.info/docs_5.2/Gnuplot_5.2.pdf (accessed on 5 May 2018).
- BIOVIA Discovery Studio Visualizer Software Version 2017 R2 for Windows; Accelrys Software Inc.: San Diego, CA, USA, 2018; Available online: http://www.accelrys.com (accessed on 3 May 2018).
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1984, 65, 55–63. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, K.; Wang, L.; Yang, X.; Zhu, S. Cyclodextrin-containing Hydrogels as an Intraocular Lens for Sustained Drug Release. PLoS ONE 2017, 12, e0189778. [Google Scholar] [CrossRef] [PubMed]
- Kipcak, A.S.; Ismail, O.; Doymaz, I.; Piskin, S. Modeling and Investigation of the Swelling Kinetics of Acrylamide-Sodium Acrylate Hydrogel. J. Chem. 2014, 2014, 281063. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Database. CID = 444266. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/444266 (accessed on 24 May 2018).
- Ionov, L. Hydrogel-based actuators: Possibilities and limitations. Mater. Today 2014, 17, 494–503. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly(acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef]
- Bayomi, M.A.; Abanumay, K.A.; Al-Angary, A.A. Effect of inclusion complexation with cyclodextrins on photostability of nifedipine in solid state. Int. J. Pharm. 2002, 243, 107–117. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the Importance and Mechanisms of Burst Release in Matrix-controlled Drug Delivery Systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Crini, G. Studies on Adsorption of Dyes on Beta-cyclodextrin Polymer. Bioresour. Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.K. A thermal degradation mechanism of polyvinyl alcohol/silicananocomposites. Polym. Degrad. Stab. 2007, 92, 1061–1071. [Google Scholar] [CrossRef]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. X-ray diffraction analysis of poly (vinyl alcohol) hydrogels, obtained by freezing and thawing techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A. Characterization of poly (vinyl alcohol)/poly (ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Bezrodna, T.; Puchkovska, G.; Shymanovska, V.; Baran, J.; Ratajczak, H. IR-analysis of H-bonded H2O on the pure TiO2 surface. J. Mol. Struct. 2004, 700, 175–181. [Google Scholar] [CrossRef]



| Chemical Characteristics and Properties | Detail | |
|---|---|---|
| Chemical Structure | 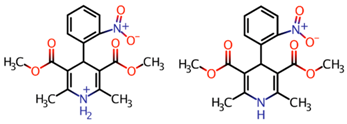 At acid pH At neutral and basic pH | |
| Molecular formula | C17H19N2O6 | C17H18N2O6 |
| Appearance | Yellow crystals | |
| Solubility in water (20–25 °C) | Insoluble | |
| Mol. Wt. | 346.339 g mol−1 | |
| Melting point | 172–174 °C | |
| Wavelength (λ, nm) | 240 nm | |
| Hydrogel Formulation | γ-CD Proportion (%) | Copolymer Concentration % PVA/MA w/w | Hydrogel at 25 °C |
|---|---|---|---|
| γ-CDPVAHMA1 | 2.43 | 20% | Yes |
| γ-CDPVAHMA2 | 3.61 | 20% | Yes |
| γ-CDPVAHMA3 | 4.76 | 20% | Yes |
| Id. | Hydrogel Block | Block/NFD Average ΔE at pH 3.0 kcal mol−1 | Block/NFD Average ΔE at pH 7.4 kcal mol−1 | Difference of Average ΔE pH 3.0–pH 7.4 kcal mol−1 |
|---|---|---|---|---|
| 1 | PVAchain-Oxalic Acid-γCD | −2.99 ± 0.02 | −2.38 ± 0.03 | −0.61 |
| 2 | PVAchain-Malonic Acid-γCD | −3.15 ± 0.04 | −2.45 ± 0.02 | −0.70 |
| 3 | PVAchain-Succinic Acid-γCD | −3.30 ± 0.02 | −2.41 ± 0.04 | −0.89 |
| 4 | PVAchain-Malic Acid-γCD | −3.76 ± 0.04 | −2.39 ± 0.04 | −1.37 |
| 5 | PVAchain-Fumaric Acid-γCD | −3.21 ± 0.02 | −2.34 ± 0.03 | −0.87 |
| 6 | PVAchain-Maleic Acid-γCD | −3.98 ± 0.04 | −2.01 ± 0.02 | −1.97 |
| 7 | PVAchain-Citraconic Acid-γCD | −3.25 ± 0.03 | −2.38 ± 0.04 | −0.87 |
| 8 | PVAchain-Itaconic Acid-γCD | −3.61 ± 0.04 | −2.45 ± 0.02 | −1.16 |
| 9 | PVAchain-Tartaric Acid-γCD | −2.96 ± 0.03 | −2.13 ± 0.05 | −0.83 |
| 10 | PVAchain-Glutaric Acid-γCD | −2.99 ± 0.02 | −2.54 ± 0.03 | −0.45 |
| 11 | PVAchain-Adipic Acid-γCD | −3.10 ± 0.02 | −2.63 ± 0.03 | −0.47 |
| 12 | PVAchain-Pimelic Acid-γCD | −3.25 ± 0.05 | −2.87 ± 0.02 | −0.38 |
| 13 | PVAchain-Suberic Acid-γCD | −3.33 ± 0.03 | −2.89 ± 0.04 | −0.44 |
| 14 | PVAchain-Azelaic Acid-γCD | −3.42 ± 0.03 | −2.95 ± 0.05 | −0.47 |
| 15 | PVAchain-Phthalic Acid-γCD | −3.67 ± 0.02 | −2.98 ± 0.02 | −0.69 |
| 16 | PVAchain-Isophthalic Acid-γCD | −3.75 ± 0.04 | −2.75 ± 0.03 | −1.00 |
| 17 | PVAchain-Terephthalic Acid-γCD | −3.79 ± 0.05 | −2.89 ± 0.04 | −0.90 |
| 18 | PVAchain-2,5-pyridine Acid-γCD | −3.68 ± 0.06 | −2.45 ± 0.05 | −1.23 |
| 19 | PVAchain-Aspartic Acid-γCD | −3.98 ± 0.02 | −3.69 ± 0.03 | −0.29 |
| 20 | PVAchain-Glutamic Acid-γCD | −3.87 ± 0.03 | −3.65 ± 0.02 | −0.22 |
| Composite | Amount of loaded NFD (mg g Dried Hydrogel−1) Concentration of Aqueous Soaking Solution 0.08 mg mL−1 |
|---|---|
| γ-CDPVAHMA1 | 4.02 ± 0.32a |
| γ-CDPVAHMA2 | 4.49 ± 0.49a |
| γ-CDPVAHMA3 | 5.01 ± 0.5a |
| Time of Release (h) | pH | γ-CD proportion (%) | NFD Release (mg L−1 ± SD) (n = 3) |
|---|---|---|---|
| 0 (−1) | 3 (−1) | 2.43 (−1) | ND * |
| 0.5 (−0.979) | 3 (−1) | 2.43 (−1) | 1.94 ± 0.09 |
| 1 (−0.958) | 3 (−1) | 2.43 (−1) | 2.10 ± 0.11 |
| 1.5 (−0.938) | 3 (−1) | 2.43 (−1) | 2.22 ± 0.10 |
| 2 (−0.917) | 3 (−1) | 2.43 (−1) | 2.34 ± 0.11 |
| 3 (−0.875) | 3 (−1) | 2.43 (−1) | 2.44 ± 0.11 |
| 4 (−0.833) | 3 (−1) | 2.43 (−1) | 2.49 ± 0.07 |
| 5 (−0.792) | 3 (−1) | 2.43 (−1) | 2.49 ± 0.09 |
| 7 (−0.708) | 3 (−1) | 2.43 (−1) | 2.57 ± 0.15 |
| 9 (−0.625) | 3 (−1) | 2.43 (−1) | 2.57 ± 0.06 |
| 24 (0) | 3 (−1) | 2.43 (−1) | 2.61 ± 0.10 |
| 30 (0.25) | 3 (−1) | 2.43 (−1) | 2.65 ± 0.09 |
| 48 (1) | 3 (−1) | 2.43 (−1) | 2.64 ± 0.13 |
| 0 (−1) | 3 (−1) | 3.61 (0.0129) | ND * |
| 0.5 (−0.979) | 3 (−1) | 3.61 (0.0129) | 1.59 ± 0.08 |
| 1 (−0.958) | 3 (−1) | 3.61 (0.0129) | 1.86 ± 0.06 |
| 1.5 (−0.938) | 3 (−1) | 3.61 (0.0129) | 1.94 ± 0.05 |
| 2 (−0.917) | 3 (−1) | 3.61 (0.0129) | 2.06 ± 0.08 |
| 3 (−0.875) | 3 (−1) | 3.61 (0.0129) | 2.22 ± 0.11 |
| 4 (−0.833) | 3 (−1) | 3.61 (0.0129) | 2.30 ± 0.21 |
| 5 (−0.792) | 3 (−1) | 3.61 (0.0129) | 2.34 ± 0.11 |
| 7 (−0.708) | 3 (−1) | 3.61 (0.0129) | 2.44 ± 0.09 |
| 9 (−0.625) | 3 (−1) | 3.61 (0.0129) | 2.47 ± 0.11 |
| 24 (0) | 3 (−1) | 3.61 (0.0129) | 2.53 ± 0.11 |
| 30 (0.25) | 3 (−1) | 3.61 (0.0129) | 2.51 ± 0.12 |
| 48 (1) | 3 (−1) | 3.61 (0.0129) | 2.51 ± 0.06 |
| 0 (−1) | 3 (−1) | 4.76 (1) | ND * |
| 0.5 (−0.979) | 3 (−1) | 4.76 (1) | 1.25 ± 0.13 |
| 1 (−0.958) | 3 (−1) | 4.76 (1) | 1.61 ± 0.09 |
| 1.5 (−0.938) | 3 (−1) | 4.76 (1) | 1.78 ± 0.11 |
| 2 (−0.917) | 3 (−1) | 4.76 (1) | 1.85 ± 0.12 |
| 3 (−0.875) | 3 (−1) | 4.76 (1) | 1.96 ± 0.08 |
| 4 (−0.833) | 3 (−1) | 4.76 (1) | 1.99 ± 0.11 |
| 5 (−0.792) | 3 (−1) | 4.76 (1) | 2.03 ± 0.15 |
| 7 (−0.708) | 3 (−1) | 4.76 (1) | 2.11 ± 0.11 |
| 9 (−0.625) | 3 (−1) | 4.76 (1) | 2.14 ± 0.10 |
| 24 (0) | 3 (−1) | 4.76 (1) | 2.16 ± 0.18 |
| 30 (0.25) | 3 (−1) | 4.76 (1) | 2.18 ± 0.11 |
| 48 (1) | 3 (−1) | 4.76 (1) | 2.16 ± 0.07 |
| 0 (−1) | 7.4 (1) | 2.43 (−1) | ND * |
| 0.5 (−0.979) | 7.4 (1) | 2.43 (−1) | 1.94 ± 0.07 |
| 1 (−0.958) | 7.4 (1) | 2.43 (−1) | 2.50 ± 0.17 |
| 1.5 (−0.938) | 7.4 (1) | 2.43 (−1) | 2.62 ± 0.11 |
| 2 (−0.917) | 7.4 (1) | 2.43 (−1) | 2.80 ± 0.13 |
| 3 (−0.875) | 7.4 (1) | 2.43 (−1) | 3.12 ± 0.11 |
| 4 (−0.833) | 7.4 (1) | 2.43 (−1) | 3.32 ± 0.10 |
| 5 (−0.792) | 7.4 (1) | 2.43 (−1) | 3.47 ± 0.10 |
| 7 (−0.708) | 7.4 (1) | 2.43 (−1) | 3.67 ± 0.10 |
| 9 (−0.625) | 7.4 (1) | 2.43 (−1) | 3.80 ± 0.11 |
| 24 (0) | 7.4 (1) | 2.43 (−1) | 3.95 ± 0.08 |
| 30 (0.25) | 7.4 (1) | 2.43 (−1) | 3.93 ± 0.13 |
| 48 (1) | 7.4 (1) | 2.43 (−1) | 3.96 ± 0.13 |
| 0 (−1) | 7.4 (1) | 3.61 (0.0129) | ND * |
| 0.5 (−0.979) | 7.4 (1) | 3.61 (0.0129) | 1.48 ± 0.12 |
| 1 (−0.958) | 7.4 (1) | 3.61 (0.0129) | 1.95 ± 0.07 |
| 1.5 (−0.938) | 7.4 (1) | 3.61 (0.0129) | 2.32 ± 0.02 |
| 2 (−0.917) | 7.4 (1) | 3.61 (0.0129) | 2.55 ± 0.12 |
| 3 (−0.875) | 7.4 (1) | 3.61 (0.0129) | 2.79 ± 0.09 |
| 4 (−0.833) | 7.4 (1) | 3.61 (0.0129) | 2.94 ± 0.09 |
| 5 (−0.792) | 7.4 (1) | 3.61 (0.0129) | 3.10 ± 0.09 |
| 7 (−0.708) | 7.4 (1) | 3.61 (0.0129) | 3.28 ± 0.11 |
| 9 (−0.625) | 7.4 (1) | 3.61 (0.0129) | 3.38 ± 0.16 |
| 24 (0) | 7.4 (1) | 3.61 (0.0129) | 3.58 ± 0.14 |
| 30 (0.25) | 7.4 (1) | 3.61 (0.0129) | 3.63 ± 0.09 |
| 48 (1) | 7.4 (1) | 3.61 (0.0129) | 3.65 ± 0.09 |
| 0 (−1) | 7.4 (1) | 4.76 (1) | ND * |
| 0.5 (−0.979) | 7.4 (1) | 4.76 (1) | 1.18 ± 0.06 |
| 1 (−0.958) | 7.4 (1) | 4.76 (1) | 1.69 ± 0.14 |
| 1.5 (−0.938) | 7.4 (1) | 4.76 (1) | 1.97 ± 0.05 |
| 2 (−0.917) | 7.4 (1) | 4.76 (1) | 2.27 ± 0.06 |
| 3 (−0.875) | 7.4 (1) | 4.76 (1) | 2.51 ± 0.10 |
| 4 (−0.833) | 7.4 (1) | 4.76 (1) | 2.69 ± 0.12 |
| 5 (−0.792) | 7.4 (1) | 4.76 (1) | 2.88 ± 0.07 |
| 7 (−0.708) | 7.4 (1) | 4.76 (1) | 3.03 ± 0.16 |
| 9 (−0.625) | 7.4 (1) | 4.76 (1) | 3.08 ± 0.07 |
| 24 (0) | 7.4 (1) | 4.76 (1) | 3.13 ± 0.09 |
| 30 (0.25) | 7.4 (1) | 4.76 (1) | 3.14 ± 0.11 |
| 48 (1) | 7.4 (1) | 4.76 (1) | 3.14 ± 0.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Salas, F.; Rodriguez Nuñez, Y.A.; Marican, A.; Castro, R.I.; Villaseñor, J.; Santos, L.S.; Wehinger, S.; Durán-Lara, E.F. Rational Development of a Novel Hydrogel as a pH-Sensitive Controlled Release System for Nifedipine. Polymers 2018, 10, 806. https://doi.org/10.3390/polym10070806
Avila-Salas F, Rodriguez Nuñez YA, Marican A, Castro RI, Villaseñor J, Santos LS, Wehinger S, Durán-Lara EF. Rational Development of a Novel Hydrogel as a pH-Sensitive Controlled Release System for Nifedipine. Polymers. 2018; 10(7):806. https://doi.org/10.3390/polym10070806
Chicago/Turabian StyleAvila-Salas, Fabián, Yeray A. Rodriguez Nuñez, Adolfo Marican, Ricardo I. Castro, Jorge Villaseñor, Leonardo S. Santos, Sergio Wehinger, and Esteban F. Durán-Lara. 2018. "Rational Development of a Novel Hydrogel as a pH-Sensitive Controlled Release System for Nifedipine" Polymers 10, no. 7: 806. https://doi.org/10.3390/polym10070806
APA StyleAvila-Salas, F., Rodriguez Nuñez, Y. A., Marican, A., Castro, R. I., Villaseñor, J., Santos, L. S., Wehinger, S., & Durán-Lara, E. F. (2018). Rational Development of a Novel Hydrogel as a pH-Sensitive Controlled Release System for Nifedipine. Polymers, 10(7), 806. https://doi.org/10.3390/polym10070806